Abstract
Laminin (Ln)-332 consists of α3, β3, and γ2 chains, which mediate epithelial cell adhesion to the basement membrane. Ln-γ2, a component of Ln-332, is frequently expressed as a monomer in the invasion front of several types of malignant tissues without simultaneous expression of Ln-α3 and/or Ln-β3 chains. Moreover, monomeric Ln-γ2 induces tumor cell proliferation and migration in vitro. These unique biological activities indicate that monomeric Ln-γ2 could be a candidate biomarker for early cancer surveillance. However, the present immune method for monomeric Ln-γ2 detection can only predict its expression, since no antibody that specifically reacts with monomeric γ2, but not with heterotrimeric γ2 chain, is commercially available. We have, therefore, developed monoclonal antibodies to specifically detect monomeric Ln-γ2, and devised a highly sensitive method to measure serum monomeric Ln-γ2 levels using a fully automated chemiluminescent immunoassay (CLIA). We evaluated its diagnostic value in sera from patients with several digestive cancers, including hepatocellular carcinoma (HCC), and found serum monomeric Ln-γ2 to be a clinically available biomarker for HCC surveillance. The combination of monomeric Ln-γ2 and prothrombin induced by Vitamin K Absence II (PIVKA-II) may be more sensitive for clinical diagnosis of HCC than any currently used combination.
Keywords: hepatocellular carcinoma, monomeric laminin-γ2, biomarker, α-fetoprotein, prothrombin induced by Vitamin K Absence II, surveillance
1. Laminin-332
Extracellular matrix (ECM) proteins in basement membranes (BMs), such as collagen and laminin, play an important role in cell-cell adhesion to maintain epithelial structures in vitro [1]. Among these, Ln-332 (formerly called laminin-5) is a major macromolecule in the epithelial BMs and comprises of three polypeptide chains: Ln-α3, -β3, and -γ2 (Figure 1) [2,3]. In normal epithelium and cancer tissues, heterotrimeric Ln-332 exhibits dual functions of adhesion and migration, and plays important roles in maintaining the static epithelial structure and epithelial cell turnover. These physiopathology functions of Ln-332 are critically regulated by interaction with integrins as a laminin receptor. Integrins α3β1 and α6β4 are a major ligand for Ln-332 and bind the C-terminal domain of Ln-α3 chain, named LG1-3, to promote cellular adhesion and migration [3,4]. Moreover Ln-332 is essential for anchoring epithelia to BMs, and its interaction with integrin α6β4 causes the assembly of hemidesmosomes and immobilization of epithelial cells onto the underlying BMs [5].
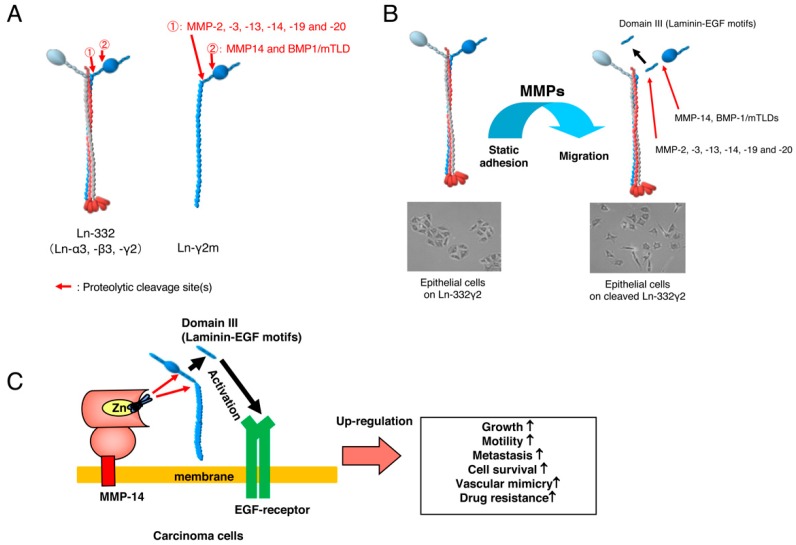
Figure 1.
(A) The γ2 chain of Ln-332 trimer and monomeric Ln-γ2 chains (Ln-α3 chain: red, Ln-β3 chain: grey, Ln-γ2 chain: blue). Proteolytic processing sites of γ2 chain by MMPs (①) or MMP-14 (②) (B) Proteolytic processing of Ln-γ2 chain occurs at cell surface for subsequent regulatory cellular functions (red arrows). Proteolytic processing of γ2 chain converts Ln-332 function from static adhesion to migratory substance. (C) Membrane type-1 matrix metalloproteinase (MMP14) cleaves Ln-γ2 chin at both sites and releases the Domain III (laminin-EGF motif) from cancer cells (red arrows), resulting in upregulation of malignant progression of tumor cells through EGFR activation. Figure 1B,C are modified from Koshikawa et al. [8], and Shenk et al. [9].
So far, it has been reported that many proteases are involved in the processing of each Ln-332 chain [6]. Interestingly, Giannelli et al. reported for the first time that proteolytic processing of the γ2 chain of Ln-332 stimulates mammary epithelial cell motility [7]. Proteolytic cleavage of the γ2 chain of Ln-332 trimer is crucial to convert its static form to a migratory form, since proteolytic Ln-γ2 fragment, Domain III, acts as an EGF-receptor (EGFR) ligand and promotes cell motility [8,9]. Besides integrins, the short arm of Ln-γ2 chain binds a transmembrane heparan sulfate proteoglycan, syndecan-1 and regulates cellular adhesion and migration through Ln-332 by suppressing integrin β4 phosphorylation (Figure 1) [10].
2. Monomeric Laminin-γ2
Heterotrimeric Ln-332 expression is observed as a single layer on BMs in both normal epithelium and carcinoma tissues in vivo; however, Ln-332 expression is generally reduced in advanced tumors [11,12] (Figure 1 and Figure 2). Although the mechanism of Ln-332 down-regulation has not been fully elucidated, each laminin chain is observed either in monomeric or dimeric form in tumor-stage dependent manner. Thus, monomeric and/or dimeric laminin chains are believed to be specific biomarkers for malignant tumors [13]. In this review, we focus on the monomeric Ln-γ2 chain, which has been suggested to be involved in tumor cell invasion. The monomeric Ln-γ2 chain was originally identified in conditioned medium obtained from gastric carcinoma cells, and its expression was detected by immunohistochemistry in budding or disseminating tumor cells in gastric carcinoma tissues (Figure 2) [14]. Many lines of evidence have reported similar results in pancreatic, gastric, tongue, colorectal, lung, cervical, and esophageal carcinomas (Table 1) [14,15,16,17,18,19].
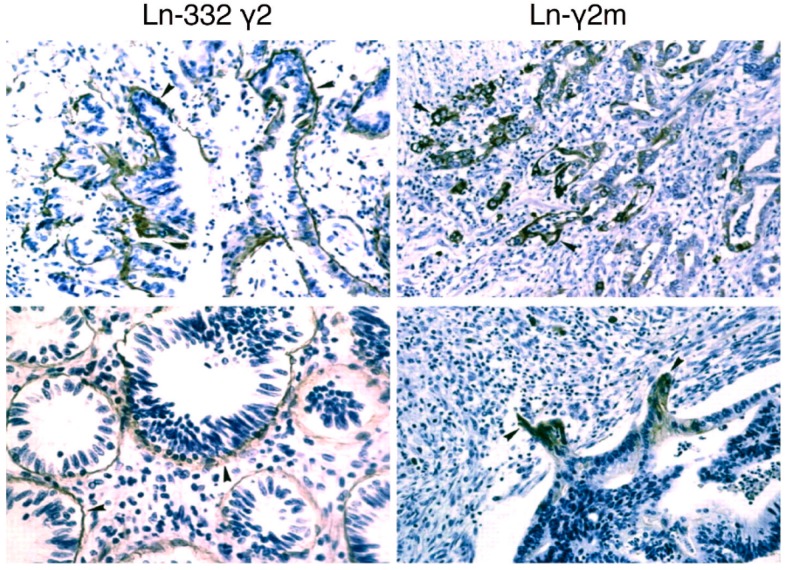
Figure 2.
Detection of the γ2 chain of Ln-332 trimer and monomeric Ln-γ2 (Ln-γ2m) chain in gastric carcinoma in vivo. The γ2 chain of Ln-332 trimer was detected in the basement membranes of carcinoma tissues, whereas monomeric γ2 chain was selectively detected in the leading edge of carcinomas. Arrowheads, positive signal for the monomeric Ln-γ2 chain. Magnification: 200×. Figure 2 is modified from Koshikawa et al. [14].
Table 1.
Reported studies of expression of Ln-γ2 in cancer of gastrointestinal tract.
| Organ | Detection Rate by IHC | Detection Rate by Serological Assay | References |
|---|---|---|---|
| esophagus | 44% (44/100) | N/A | [18] |
| stomach | 23% (35/153) | N/A | [20] |
| colon | 65% (29/39) | N/A | [21] |
| liver | 63% (25/40) | CLIA, 63% (36/57) | [22,23] |
| pancreas | 53% (8/15) | ELISA, 72% (36/50) | [16,24] |
| biliary tract | 57% (35/61) | N/A | [25] |
N/A; not available.
Similar to the γ2 chain of Ln-332 trimer, the monomeric Ln-γ2 chain is also cleaved by proteases. Membrane type-1 matrix metalloproteinase (MT1-MMP/MMP-14) cleaves domain III of Ln-γ2 chain, which contains a laminin-EGF-like motif and promotes malignant cancer progression via EGFR and its downstream signaling (Figure 1) [26].
3. Development of a Specific Antibody against Monomeric Ln-γ2 Chain
Since monomeric Ln-γ2 chain is believed to be a promising target for invasive cancers, previous studies have extensively reported the expression and localization of monomeric Ln-γ2 chain in cancer tissues [2,14,21,25,27,28,29]. For detecting monomeric Ln-γ2 chain by an immunoassay, two different laminin chain antibodies (Abs) (e.g., anti-Ln-α3 and Ln-γ2 mAbs) are required. For example, existence of the monomeric Ln-γ2 chain can be determined from Ln-α3 (negative) and Ln-γ2 (positive) immunostaining. The present immune method for monomeric Ln-γ2 detection only predicts its expression, since no antibody that specifically reacts with monomeric γ2, but not with the γ2 chain of Ln-332 trimer, is commercially available. Moreover, immunoassay with multiple antibodies is complicated and exhibits low detection sensitivity. Due to these major reasons, monomeric Ln-γ2 has not been applied in clinical cancer diagnosis yet.
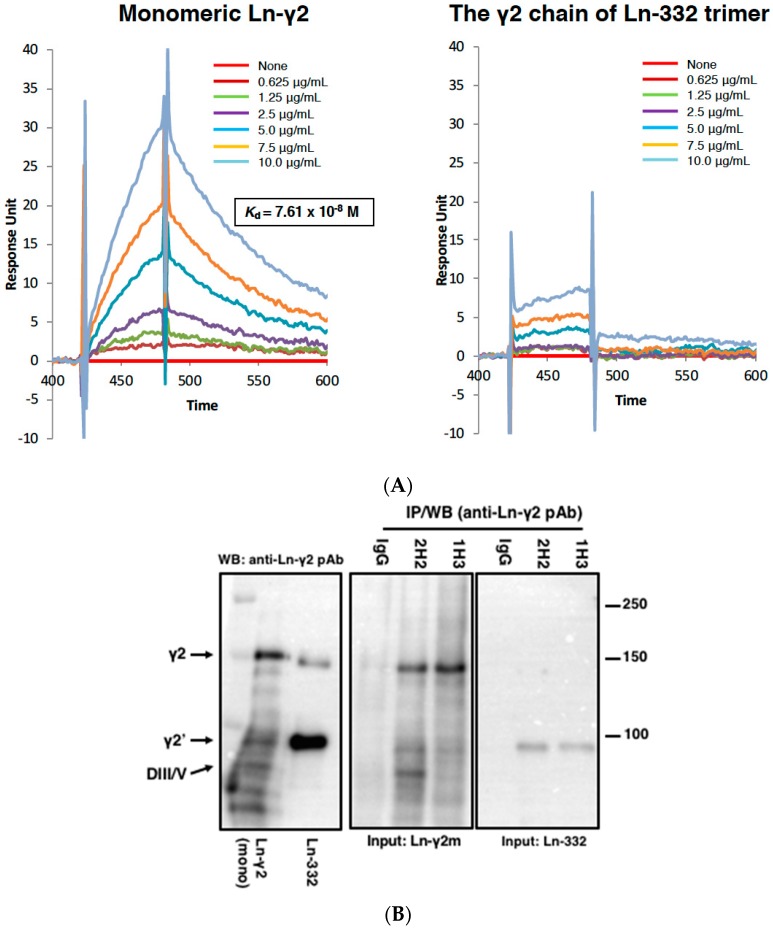
To overcome this limitation, we generated specific antibodies against monomeric Ln-γ2, and subsequently isolated two different hybridoma clones (1H3 and 2H2 mAbs) using screening assays such as enzyme-linked immunosorbent assay (ELISA) and western blotting [30]. The equilibrium dissociation constant (Kd) of 2H2 mAb for monomeric Ln-γ2 was determined to be 7.61 × 10−8 M using surface plasmon resonance (SPR), though the same for the γ2 chain of Ln-332 trimer was not detected (Figure 3A). Therefore, 2H2 mAb was established, for the first time, as a powerful tool for the specific detection of monomeric Ln-γ2, and could be used for immunoassays, including immunoprecipitation, ELISA, immunohistostaining, and western blotting (Figure 3B,C).
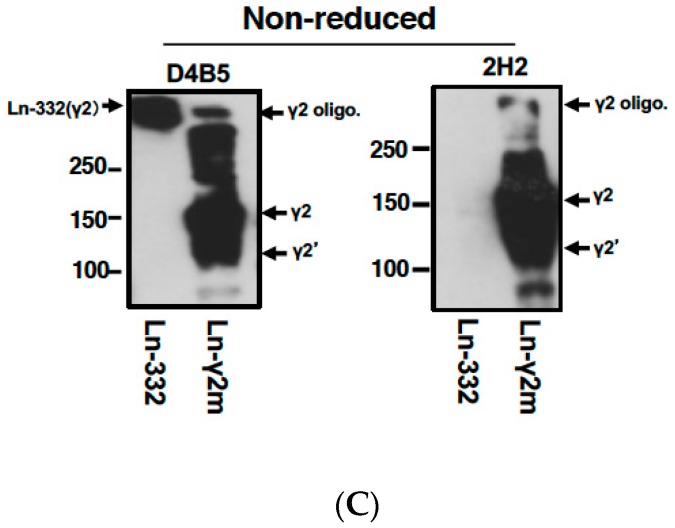
Figure 3.
(A) The sensorgrams of SPR analysis for interactions between 2H2 mAb and monomeric Ln-γ2 (left), and 2H2 mAb and Ln-332 (right); 2H2 reacts with monomeric Ln-γ2 chain but not with the γ2 chain of Ln-332 trimer. (B) Immunoprecipitation (IP) of Ln-γ2 chain by 1H3 and 2H2 mAbs. Purified monomeric Ln-γ2 or Ln-332 (0.5 μg) protein was incubated with mouse immunoglobulin (IgG), 1H3 mAb, or 2H2 mAb (2 μg/mL each). Antibody-antigen complexes were then precipitated and subjected to western blotting (WB) using anti-Ln-γ2 polyclonal antibody (pAb). In addition to the intact form of Ln-γ2 (140 kDa), NH2-terminal (70 kDa) and COOH-terminal (100 kDa) fragments, processed by MMP-14 or mTLDs (Figure 1, right ②), are detected and indicated as γ2, DIII/IV, and γ2’, respectively. Left, sample proteins (monomeric Ln-γ2 and Ln-332) used for the assay were directly analyzed by WB using anti-Ln-γ2 pAb. Figure 3B is from Koshikawa et al. [30]. (C) Use of 2H2 and D4B5 mAbs in WB analysis. D4B5 is a commercially available anti-Ln-γ2 mAb. Purified monomeric Ln-γ2 (1 μg) and Ln-332 trimer (3 μg) proteins were separated by 7.5% SDS-PAGE and blotted on PVDF membranes under non-reducing conditions, and then detected either by D4B5 or 2H2 mAb. Arrows, Ln-γ2 homo-oligomer (γ2 oligo), monomer (γ2), its processed fragment (γ2’), and Ln-332.
4. Detection of Ln-γ2 or Its N-Terminal Domain Fragment in Serum Specimens of Patients with Cancer
Katayama et al. established a sandwich ELISA for Ln-γ2 Domain IV–V fragment using a mAb against the DIV-V fragment [31,32], and evaluated it in serum specimens of patients with cancer. The sandwich ELISA revealed that patients with head and neck squamous cell carcinoma, hepatocellular carcinoma, bile duct carcinoma, gallbladder carcinoma, and pancreatic carcinoma with liver metastasis exhibited higher serum Ln-γ2 DIV-V fragment levels than healthy volunteers. Moreover, the study revealed that patients with benign digestive diseases also showed higher DIV-V levels than healthy volunteers. Furthermore, it was possible to monitor the lung epithelial repair process by measuring the serum Ln-γ2 DIV-V level in patients with acute lung injury [33]. Presumably, in sandwich ELISA, the mAb reacts with DIV-V fragments derived from both heteromeric and monomeric Ln-γ2 chains and cannot distinguish between patients with cancer and those with inflammatory diseases that release Ln-332 from BMs through ECM degradation.
A recent study by Kosanam et al. evaluated Ln-γ2 in the serum of patients with pancreatic carcinoma using a commercially available ELISA kit [24]; the serum Ln-γ2 level in patients with pancreatic carcinoma was found to be significantly elevated compared to that in patients with chronic pancreatitis and healthy volunteers in three cohort studies. This result strongly suggests that monomeric Ln-γ2 might be a potential candidate biomarker for cancer.
5. Establishment of an Automated Chemiluminescent Immunoassay (CLIA)
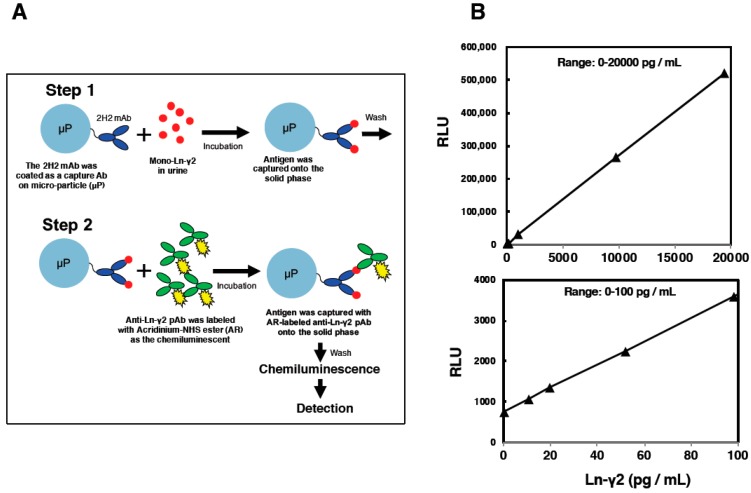
Based on previous findings, we established a detection system using a chemiluminescent immunoassay (CLIA) for estimating serum monomeric Ln-γ2 levels using 2H2 mAb, and applied it for clinical cancer diagnosis. The CLIA is a highly sensitive protein detection system with low background, compared to sandwich ELISA, and is a common biomarker assay in clinical diagnosis (Figure 4A). The 2H2 mAb was introduced in CLIA assay to eliminate cross-reaction with the γ2 chain of Ln-332 trimer directly [22,34]. The CLIA assay with 2H2 mAb can reduce false-positive reactions and enhance the diagnostic accuracy. Indeed, a standard curve using recombinant monomeric Ln-γ2 protein shows that the CLIA can measure from 0 to 20,000 pg/mL of the protein (Figure 4B). The lower detection limit is 10 pg/mL, and serum monomeric Ln-γ2 levels in healthy volunteer’s average to 44.3 ± 17.6 pg/mL (mean ± SD) [22]. These data collectively indicate that measurement efficiency of the CLIA exceeds that of sandwich ELISA (Table 2). Therefore, we evaluated the serum monomeric Ln-γ2 level in serum specimens of patients with digestive cancers, using CLIA, and found it to be a promising biomarker for early detection of hepatocellular carcinoma (HCC), as described below.
Figure 4.
(A). Detection method for Chemiluminescent immunoassay (CLIA) (B), and its standard curve at different ranges using recombinant monomeric Ln-γ2 protein. RLU; Relative Light Unit.
Table 2.
Comparison of measurement efficiency between LISA and CLIA.
| ELISA | Automated CLIA | |
|---|---|---|
| Dynamic range | Narrow *1 | Wide *2 |
| Detection sensitivity | Low | High |
| Diagnostic accuracy | Low | High |
| Background | High | Low |
| Throughput | Low | High |
| Reaction time | > 2.5 h | 30 min |
*1: Note that dynamic range of ELISA is 15–4000 pg/mL *2, and CLIA is 10–20,000 pg/mL.
6. Hepatocellular Carcinoma Surveillance and Biomarkers
There has been remarkable development in therapeutic options for HCC recently. Nevertheless, curative options are only feasible in case of early diagnosis. Screening programs in an increased-risk population could lead to more frequent detection of HCC at early stages and reduce HCC mortality [35]. The subjects of regular HCC surveillance include patients with chronic hepatitis B virus (HBV), chronic hepatitis C virus, and non-viral liver cirrhosis. Tests that can be used in HCC surveillance include serological and imaging examinations. Ultrasonography is the most widely used method for HCC surveillance. In Taiwan, residents aged between 45 and 69 years, with a high prevalence of hepatitis B surface antigen (HBsAg), were invited to community-based abdominal ultrasonography screening for HCC, followed by subsequent reduction in HCC-related mortality compared to that in those who were not invited [36]. The American Association for the Study of Liver Diseases has recommended six monthly ultrasonography screening for HCC [37]. Ultrasonography is not invasive, but is relatively expensive and operator- and patient-dependent. In contrast, serological biomarkers can be used at relatively low costs, without any burden to the patient. α-fetoprotein (AFP) is the most frequently used biomarker for HCC worldwide. Screening with six monthly AFP assays in HBV-positive individuals resulted in earlier diagnosis of HCC, but did not affect five-year survival [38]. Randomized controlled trial indicated that biannual screening with combination of AFP and ultrasound reduced HCC-mortality in individuals with HBV infection or history of chronic hepatitis. Patients with early and surgically respectable stages of HCC were found significantly more often in the screened group than in the control [35]. Surveillance program of patients with liver cirrhosis combined AFP and ultrasound to prolong survival rate of patients with HCC-mortality [39]. Thus, the Japan Society of Hepatology has recommended surveillance with six monthly ultrasonography and biomarker assays every three to four months for high-risk populations [40].
In surveillance, biomarkers are used to complement imaging tests, alone or in combination. AFP and prothrombin induced by Vitamin K Absence II (PIVKA-II), also known as des-gamma-carboxy prothrombin, are the most commonly used biomarkers for HCC surveillance. Currently, the recommended clinical cut-off values are 20 ng/mL for AFP and 40 mAU/mL for PIVKA-II. A case-control study in patients with chronic HBV infection with or without HCC, showed sensitivity 57.5% and specificity 88% for AFP, and sensitivity 51.9% and specificity 97% for PIVKA-II [41]. A recent systematic review has indicated sensitivity 59% and specificity 86% for AFP, and sensitivity 63% and specificity 91% for PIVKA-II [42]. AFP is the most commonly used biomarker; however, small HCCs are not always associated with elevated AFP in serum, and even among large HCCs, only about 80% show elevated AFP. Therefore, an appropriate combination of these markers might increase sensitivity. Combination of AFP and PIVKA-II increased the sensitivity to 78.3% [41]. Among early stage HCC, the receiver operating characteristic area under curve (ROC curve AUC) of PIVKA-II, AFP, and combination of both markers were 0.84, 0.68, and 0.83, respectively; the combination of PIVKA-II and AFP did not seem to be better than PIVKA-II in detecting early stages of HCC [42]. Development of more effective biomarker combination would be required for early HCC diagnosis.
Biomarker monitoring is also useful in detecting HCC recurrence after therapeutic intervention. Positive AFP and PIVKA-II status became negative at 6 months post-hepatectomy in 80.3% and 99.6% patients, respectively. AFP and PIVKA-II levels in patients showing recurrence in ≤6 months correlated with the levels measured before hepatectomy [43]. Postoperative AFP level is, therefore, a useful tool for predicting recurrence after curative hepatectomy. A positive level of post-operative AFP might suggest a site of residual viable cancer [44].
7. Serum Monomeric Ln-γ2 as a Novel Biomarker for Hepatocellular Carcinoma
Although heterotrimeric Ln-332 is not detected in normal hepatic tissues [29], increased expression of monomeric Ln-γ2 in HCC tissue has been shown to be associated with a more proliferative and metastatic phenotype [23]. In HCC, invasive tumor cells secrete TGF-β1, which triggers invasiveness and motility in Ln-332 by inducing the expression of the transmembrane integrin receptor α3β1. Ln-332 upregulates the expression of the transcriptional repressors Snail and Slug, which induce the EMT together with TGF-β1, and downregulating E-cadherin, followed by translocation of β-catenin to the nucleus [45]. According to a previous report, indeed all HCC cells tested expresses Ln-γ2, MT1-MMP and MMP2. It is believed that the Ln-γ2 processing presumably occurs and plays roles in their growth, motility and invasiveness through the EGFR activation [46].
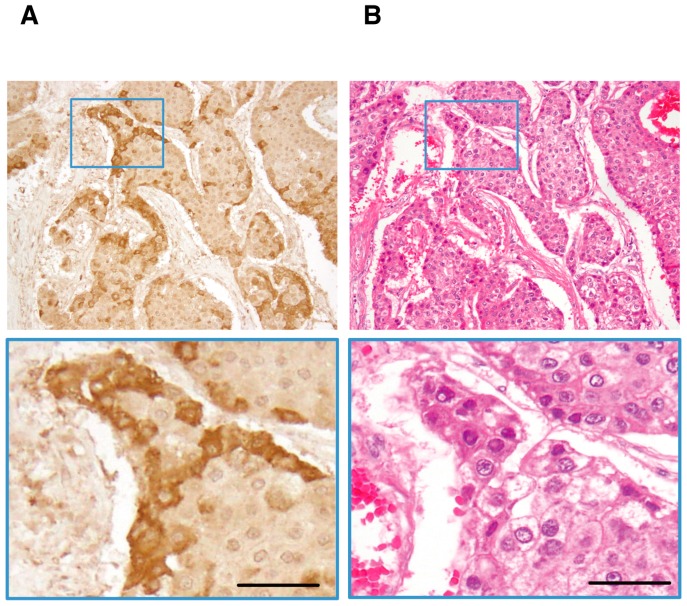
As shown in Figure 5, cytoplasmic staining of Ln-γ2 was observed in surgically removed HCC nodules. Ln-γ2 immunoreactivites are preferentially observed in marginal-moderately to poorly differentiated parts of HCC nodules [22], as is observed in gastric carcinoma tissue [14]. Monomeric Ln-γ2 is also expressed in human HCC-derived cell lines. Recent report indicated that Ln-γ2 is highly found in HCCs expressing the biliary marker keratin 19 [47]. Aberrant activation of Wnt/β-catenin signaling is a common genetic abnormality in human HCC [48]. Previous studies on the mechanism of Ln-γ2 expression had demonstrated that its gene and protein expression are up-regulated in gastric and colon cancer cells by transcriptional factor 4 (TCF4)/β-catenin and/or Wnt-5a [20,49]. Moreover, a study on comparative genomic hybridization (CGH), using cancer specimens, demonstrated that copy number of the gene encoding Ln-γ2 is frequently increased in both early and advanced stages of hepatocellular carcinoma (HCC) and lung squamous cell carcinoma [27]. Furthermore, expression of Ln-332 was seen to be lost in many types of cancers due to gene methylation [50,51,52,53,54]. These reports together support the increased expression of monomeric Ln-γ2 in HCC tissue.
Figure 5.
(A). Immunohistochemical staining of Ln-γ2 in HCC. A representative example of positive staining for Ln-γ2. (B). HE staining. Scale bars, 50 μm. Figure 5 is modified from Kiyokawa et al. [22].
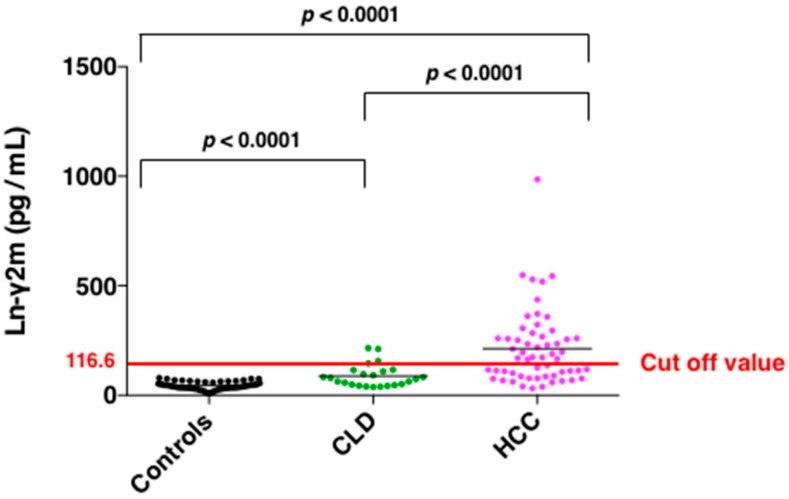
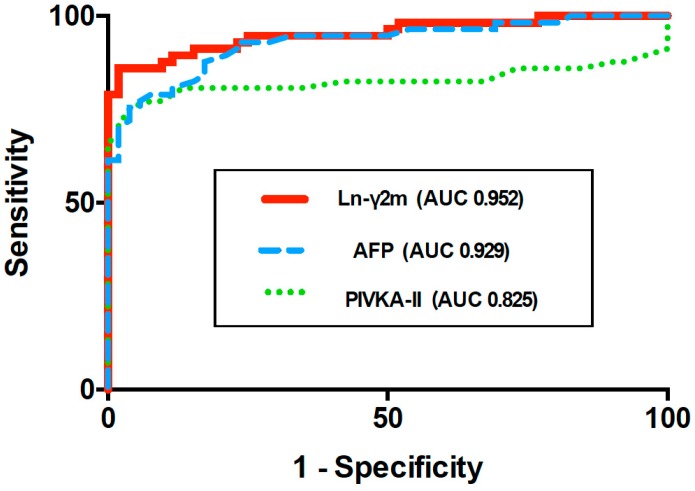
Since monomeric Ln-γ2, rather than heterotrimeric Ln-γ2, is expressed preferentially in HCC nodules, we have evaluated the diagnostic value of monomeric Ln-γ2, AFP, and PIVKA-II in sera from patients with HCC and chronic liver diseases (CLD) using the above-mentioned automated CLIA along with ARCHITECT [22]. A significant increase in monomeric Ln-γ2 levels was observed in patients with HCC compared to patients with CLD and healthy volunteers (Figure 6). ROC curve AUC of monomeric Ln-γ2, PIVKA-II, and AFP were 0.952, 0.825, and 0.929, respectively, when comparing healthy volunteers and patients with HCC. The discriminative ability of monomeric Ln-γ2 significantly surpassed that of PIVKA-II, and was as effective as AFP (Figure 7). When discriminating patients with non-malignant CLD from those with HCC, ROC curve AUC of monomeric Ln-γ2, PIVKA-II, and AFP were 0.793, 0.845, and 0.788 respectively [22]. Therefore, serum monomeric Ln-γ2 seems to be more effective than AFP in differentiating patients with HCC from those with CLD.
Figure 6.
Scatter plot of Ln-γ2 concentrations determined in serum samples from healthy subjects (n = 52), patients with CLD (n = 24), and patients with HCC (n = 57). The optimal cutoff value for Ln-γ2 to distinguish between HCC and non-malignant CLD is 116.6 pg/mL. Figure 6 is modified from Kiyokawa et al. [22].
Figure 7.
ROC curve AUC of monomeric Ln-γ2, PIVKA-II, and AFP in patients with HCC versus healthy volunteers. Figure 7 is modified from Kiyokawa et al. [22].
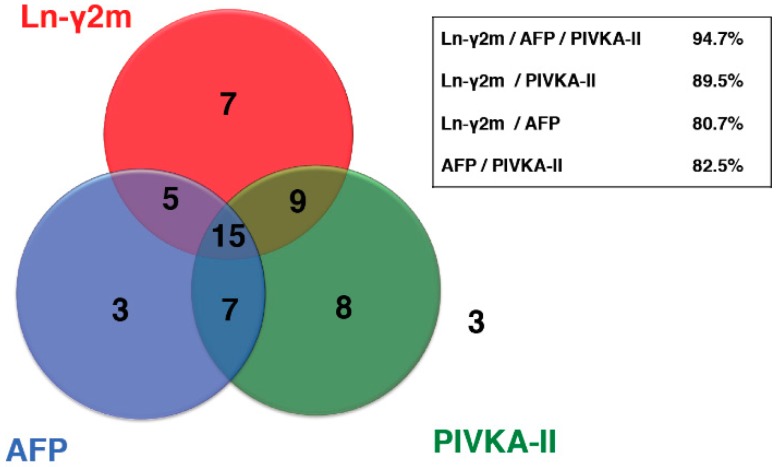
In addition, the positivity rate in patients with HCC for the combination of Ln-γ2 and PIVKA-II was 89.5%, whereas that for monomeric Ln-γ2 and AFP was 80.7%, and for PIVKA-II and AFP was 82.5%. The combination of Ln-γ2 and PIVKA-II seemed to make a more sensitive pair of biomarkers compared to a conventional marker (Figure 8).
Figure 8.
Serum monomeric Ln-γ2 levels were measured in 57 patients with HCC. HCC positive rates, obtained when combining two biomarkers, were compared. Three patients were negative for all three biomarkers. HCC detection rates for the combination of Ln-γ2 and PIVKA-II, Ln-γ2 and AFP, and PIVKA-II and AFP, were 89.5% (51/57), 82.5% (47/57), and 80.7% (46/57), respectively. A combination of all three markers was detected in 54/57 patients (94.7%). Figure 8 is modified from Kiyokawa et al. [22].
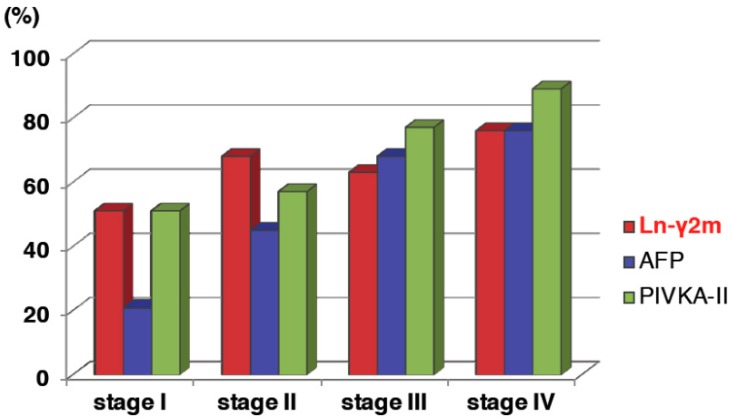
Increase of monomeric Ln-γ2 levels is observed with the stepwise progression of CLD, and according to tumor stages. The optimal cutoff value for Ln-γ2 to distinguish between HCC and nonmalignant CLD was 116.6 pg/mL. Positivity rate of monomeric Ln-γ2 in patients with HCC for each TMN stage was 50% in stage I, 67% in stage II, 62% in stage III, and 75% in stage IV, respectively, whereas that of AFP was 20% in stage I, 44% in stage II, 67% in stage III, and 75% in stage IV, respectively, and of PIVKA-II was 50% in stage I, 56% in stage II, 76% in stage III, and 88% in stage IV, respectively (Figure 9) [32]. Positivity rate of monomeric Ln-γ2 is clearly higher than AFP and comparable to PIVKA-II. Among patients with early-stage HCC (T1 or T2; the T factor includes three criteria: solitary tumor, maximum tumor diameter < 2 cm and no vascular invasion. T1 meets all three criteria, T2 meets two of the three criteria), the positivity rates of monomeric Ln-γ2 may be higher than AFP or PIVKA-II. Taken together, these results indicate the potential clinical applicability of monomeric Ln-γ2 for the detection of early-stage HCC. Besides being a diagnostic marker, it would be of particular interest, in the future, to examine the potential of serum monomeric Ln-γ2 as a biomarker to monitor therapeutic effects.
Figure 9.
Comparison of the biomarker-positive rate in HCC by tumor stages. Figure 9 is modified from Kiyokawa et al. [22].
8. Conclusions
Monomeric Ln-γ2 was identified as a biomarker, which is specifically expressed on the cancer invasion front. Although monomeric Ln-γ2 has long been of interest as a potential biomarker for cancer diagnosis owing to its unique biological features, development of an assay system for Ln-γ2 single chain faced many obstacles, considering that Ln-γ2 is a part of Ln-332 trimer and most antibodies that react with Ln-γ2 chain also recognize the Ln-332 trimer. We have therefore developed mAbs that specifically detect monomeric Ln-γ2. Previous research has indicated important roles of Ln-γ2/Ln-322 in pathophysiology of HCC. Using this tool, we have thus further developed highly sensitive CLIA for serum monomeric Ln-γ2. Serum monomeric Ln-γ2 may be considered a clinically available biomarker for HCC surveillance. Moreover, the combination of monomeric Ln-γ2 and PIVKA-II may become a sensitive tool for clinical diagnosis of HCC at early stages, hence preventing HCC-related deaths.
Acknowledgments
We are grateful to Ritsuko Oikawa and Chiaki Okuse (St. Marianna University) for their valuable discussions.
Abbreviations
| AFP | α-fetoprotein |
| BM | basement membrane |
| PIVKA-II | prothrombin induced by Vitamin K Absence II |
| CLIA | chemiluminescent immunoassay |
| CLD | chronic liver disease |
| ECM | extracellular matrix |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| IP | Immunoprecipitation |
| Ln-332 | laminin 332 |
| Ln-γ2 | laminin-γ2 |
| mAb | monoclonal antibody |
| pAb | polyclonal antibody |
| ROC curve AUC | receiver operating characteristic area under curve |
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (15K08655) (H.Y.), a Medical Research and Development Programs Focused on Technology Transfer: Adaptable and Seamless Technology Transfer Program through Target-Driven Research and Development (A-STEP) (M.N, N.K. and M.S.), and a Project for Development of Innovative Research on Cancer Therapeutics (P-DIRECT) (N.K.) from The Japan Agency for Medical Research and Development (AMED): a Grant-in-Aid for Scientific Research on Innovative Areas (17H06329) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), a Grant-in-Aid for Scientific Research (C) (17K09027), and a Core-to Core program (Establishing International Research Network of Mathematical Oncology) from the Japan Society for the Promotion Science, and Abbott Laboratories (N.K.).
Conflicts of Interest
N.K. received research funding from Abbott Laboratories (Chicago, IL, USA). M.N., E.Y., and T.Y. are employees of Abbott, Japan. No potential competing interests were disclosed by other authors.
References
- 1.Colognato H., Yurchenco P.D. Form and function: The laminin family of heterotrimers. Dev. Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki K. Laminin-5 (laminin-332): Unique biological activity and role in tumor growth and invasion. Cancer Sci. 2006;97:91–98. doi: 10.1111/j.1349-7006.2006.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guess C.M., Quaranta V. Defining the role of laminin-332 in carcinoma. Matrix Biol. 2009;28:445–455. doi: 10.1016/j.matbio.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang M., Koshikawa N., Schenk S., Quaranta V. The LG3 module of laminin-5 harbors a binding site for integrin α3β1 that promotes cell adhesion, spreading, and migration. J. Biol. Chem. 2001;276:33045–33053. doi: 10.1074/jbc.M100798200. [DOI] [PubMed] [Google Scholar]
- 5.Gaietta G., Redelmeier T.E., Jackson M.R., Tamura R.N., Quaranta V. Quantitative measurement of α6β1 and α6β4 integrin internalization under cross-linking conditions: A possible role for α6 cytoplasmic domains. J. Cell Sci. 1994;107:3339–3349. doi: 10.1242/jcs.107.12.3339. [DOI] [PubMed] [Google Scholar]
- 6.Rousselle P., Beck K. Laminin 332 processing impacts cellular behavior. Cell Adh. Migr. 2013;7:122–134. doi: 10.4161/cam.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannelli G., Falk-Marziller J., Schiraldi O., Stetler-Stevenson W.G., Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 8.Koshikawa N., Giannelli G., Cirulli V., Miyazaki K., Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. Erratum in 2000, 151, 479–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenk S., Hintermann E., Bilban M., Koshikawa N., Hojilla C., Khokha R., Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J. Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa T., Tsubota Y., Hashimoto J., Kariya Y., Miyazaki K. The short arm of laminin γ2 chain of laminin-5 (laminin-332) binds syndecan-1 and regulates cellular adhesion and migration by suppressing phosphorylation of integrin β4 chain. Mol. Biol. Cell. 2007;18:1621–1633. doi: 10.1091/mbc.e06-09-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter P.M., Sivadas P., Hua S.S., Xiao C., Gutierrez A.B., Ngo T., Gershon P.D. Migration of breast cancer cell lines in response to pulmonary laminin 332. Cancer Med. 2017;6:220–234. doi: 10.1002/cam4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao J., Jackson L., Calaluce R., McDaniel K., Dalkin B.L., Nagle R.B. Investigation into the mechanism of the loss of laminin 5 (α3β3γ2) expression in prostate cancer. Am. J. Pathol. 2001;158:1129–1135. doi: 10.1016/S0002-9440(10)64060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J., Zhang H., Luo J., Wu X., Li X., Zhao X., Zhou D., Yu S. Overexpression of α3, β3 and γ2 chains of laminin-332 is associated with poor prognosis in pancreatic ductal adenocarcinoma. Oncol. Lett. 2018;16:199–210. doi: 10.3892/ol.2018.8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koshikawa N., Moriyama K., Takamura H., Mizushima H., Nagashima Y., Yanoma S., Miyazaki K. Overexpression of laminin γ2 chain monomer in invading gastric carcinoma cells. Cancer Res. 1999;59:5596–5601. [PubMed] [Google Scholar]
- 15.Takahashi S., Hasebe T., Oda T., Sasaki S., Kinoshita T., Konishi M., Ochiai T., Ochiai A. Cytoplasmic expression of laminin γ2 chain correlates with postoperative hepatic metastasis and poor prognosis in patients with pancreatic ductal adenocarcinoma. Cancer. 2002;94:1894–1901. doi: 10.1002/cncr.10395. [DOI] [PubMed] [Google Scholar]
- 16.Soini Y., Määttä M., Salo S., Tryggvason K., Autio-Harmainen H. Expression of the laminin γ2 chain in pancreatic adenocarcinoma. J. Pathol. 1996;180:290–294. doi: 10.1002/(SICI)1096-9896(199611)180:3<290::AID-PATH661>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima N., Sakamoto M., Hirohashi S. Expression of laminin-5-γ-2 chain in intraductal papillary-mucinous and invasive ductal tumors of the pancreas. Mod. Pathol. 2001;14:404–409. doi: 10.1038/modpathol.3880326. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto H., Itoh F., Iku S., Hosokawa M., Imai K. Expression of the γ2 chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Clin. Cancer Res. 2001;7:896–900. [PubMed] [Google Scholar]
- 19.Garg M., Kanojia D., Okamoto R., Jain S., Madan V., Chien W., Sampath A., Ding L.W., Xuan M., Said J.W., et al. Laminin-5γ-2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration, and invasion by modulating signaling of EGFR. J. Clin. Endocrinol. Metab. 2014;99:E62–E72. doi: 10.1210/jc.2013-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto H., Kitadai Y., Yamamoto H., Oue N., Ohdan H., Yasui W., Kikuchi A. Laminin γ2 Mediates Wnt5a-induced invasion of gastric cancer cells. Gastroenterology. 2009;137:242–252. doi: 10.1053/j.gastro.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Sordat I., Bosman F.T., Dorta G., Rousselle P., Aberdam D., Blum A.L., Sordat B. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J. Pathol. 1998;185:44–52. doi: 10.1002/(SICI)1096-9896(199805)185:1<44::AID-PATH46>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 22.Kiyokawa H., Yasuda H., Oikawa R., Okuse C., Matsumoto N., Ikeda H., Watanabe T., Yamamoto H., Itoh F., Otsubo T., et al. Serum monomeric laminin-γ2 as a novel biomarker for hepatocellular carcinoma. Cancer Sci. 2017;108:1432–1439. doi: 10.1111/cas.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannelli G., Fransvea E., Bergamini C., Marinosci F., Antonaci S. Laminin-5 chains are expressed differentially in metastatic and nonmetastatic hepatocellular carcinoma. Clin. Cancer Res. 2003;9:3684–3691. [PubMed] [Google Scholar]
- 24.Kosanam H., Prassas I., Chrystoja C.C., Soleas I., Chan A., Dimitromanolakis A., Blasutig I.M., Ruckert F., Gruetzmann R., Pilarsky C., et al. Laminin, γ2 (LAMC2): A promising new putative pancreatic cancer biomarker identified by proteomic analysis of pancreatic adenocarcinoma tissues. Mol. Cell. Proteom. 2013;12:2820–2832. doi: 10.1074/mcp.M112.023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oka T., Yamamoto H., Sasaki S., Ii M., Hizaki K., Taniguchi H., Adachi Y., Imai K., Shinomura Y. Overexpression of β3/γ2 chains of laminin-5 and MMP7 in biliary cancer. World J. Gastroenterol. 2009;15:3865–3873. doi: 10.3748/wjg.15.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koshikawa N., Minegishi T., Sharabi A., Quaranta V., Seiki M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin γ2 chain. J. Biol. Chem. 2005;280:88–93. doi: 10.1074/jbc.M411824200. [DOI] [PubMed] [Google Scholar]
- 27.Niki T., Kohno T., Iba S., Moriya Y., Takahashi Y., Saito M., Maeshima A., Yamada T., Matsuno Y., Fukayama M., et al. Frequent co-localization of Cox-2 and laminin-5 γ2 chain at the invasive front of early-stage lung adenocarcinomas. Am. J. Pathol. 2002;160:1129–1141. doi: 10.1016/S0002-9440(10)64933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang S.G., Ha Y.R., Ko Y.H., Kang S.H., Joo K.J., Cho H.Y., Park H.S., Kim C.H., Kwon S.Y., Kim J.J., et al. Effect of laminin 332 on motility and invasion in bladder cancer. Kaohsiung J. Med. Sci. 2013;29:422–429. doi: 10.1016/j.kjms.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Mizushima H., Koshikawa N., Moriyama K., Takamura H., Nagashima Y., Hirahara F., Miyazaki K. Wide distribution of laminin-5 γ2 chain in basement membranes of various human tissues. Horm. Res. 1998;50(Suppl. 2):7–14. doi: 10.1159/000053118. [DOI] [PubMed] [Google Scholar]
- 30.Koshikawa N., Minegishi T., Nabeshima K., Seiki M. Development of a new tracking tool for the human monomeric laminin-γ2 chain in vitro and in vivo. Cancer Res. 2008;68:530–536. doi: 10.1158/0008-5472.CAN-07-5269. Erratum in 2016, 76, 762. [DOI] [PubMed] [Google Scholar]
- 31.Katayama M., Funakoshi A., Sumii T., Sanzen N., Sekiguchi K. Laminin γ2-chain fragment circulating level increases in patients with metastatic pancreatic ductal cell adenocarcinomas. Cancer Lett. 2005;225:167–176. doi: 10.1016/j.canlet.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 32.Kuratomi Y., Sato S., Monji M., Shimazu R., Tanaka G., Yokogawa K., Inoue A., Inokuchi A., Katayama M. Serum concentrations of laminin γ2 fragments in patients with head and neck squamous cell carcinoma. Head Neck. 2008;30:1058–1063. doi: 10.1002/hed.20838. [DOI] [PubMed] [Google Scholar]
- 33.Katayama M., Ishizaka A., Sakamoto M., Fujishima S., Sekiguchi K., Asano K., Betsuyaku T., Kotani T., Ware L.B., Matthay M.A., et al. Laminin γ2 fragments are increased in the circulation of patients with early phase acute lung injury. Intensive Care Med. 2010;36:479–486. doi: 10.1007/s00134-009-1719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa M., Karashima T., Kamada M., Yoshida E., Yoshimura T., Nojima M., Inoue K., Shuin T., Seiki M., Koshikawa N. Development of a fully automated chemiluminescence immunoassay for urine monomeric laminin-γ2 as a promising diagnostic tool of non-muscle invasive bladder cancer. Biomark. Res. 2017;5:29. doi: 10.1186/s40364-017-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B.H., Yang B.H., Tang Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 36.Yeh Y.P., Hu T.H., Cho P.Y., Chen H.H., Yen A.M., Chen S.L., Chiu S.Y., Fann J.C., Su W.W., Fang Y.J., et al. Evaluation of abdominal ultrasonography mass screening for hepatocellular carcinoma in Taiwan. Hepatology. 2014;59:1840–1849. doi: 10.1002/hep.26703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruix J., Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J.G., Parkin D.M., Chen Q.G., Lu J.H., Shen Q.J., Zhang B.C., Zhu Y.R. Screening for liver cancer: Results of a randomized controlled trial in Qidong, China. J. Med. Screen. 2003;10:204–209. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 39.Bolondi L., Sofia S., Siringo S., Gaiani S., Casali A., Zironi G., Piscaglia F., Gramantieri L., Zanetti M., Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: A cost effectiveness analysis. Gut. 2001;48:251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Japan Society of Hepatology; [(accessed on 6 January 2019)]. Clinical Practice Guidelines for Hepatocellular Carcinoma 2013. Available online: http://www.jsh.or.jp/English/examination_en. [Google Scholar]
- 41.Yoon Y.J., Han K.H., Kim D.Y. Role of serum prothrombin induced by vitamin K absence or antagonist-II in the early detection of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Scand. J. Gastroenterol. 2009;44:861–866. doi: 10.1080/00365520902903034. [DOI] [PubMed] [Google Scholar]
- 42.Li C., Zhang Z., Zhang P., Liu J. Diagnostic accuracy of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatocellular carcinoma: A systematic review. Hepatol. Res. 2014;44:E11–E25. doi: 10.1111/hepr.12201. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto K., Imamura H., Matsuyama Y., Hasegawa K., Beck Y., Sugawara Y., Makuuchi M., Kokudo N. Significance of α-fetoprotein and des-γ-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann. Surg. Oncol. 2009;16:2795–2804. doi: 10.1245/s10434-009-0618-y. [DOI] [PubMed] [Google Scholar]
- 44.Nobuoka D., Kato Y., Gotohda N., Takahashi S., Nakagohri T., Konishi M., Kinoshita T., Nakatsura T. Postoperative serum α-fetoprotein level is a useful predictor of recurrence after hepatectomy for hepatocellular carcinoma. Oncol. Rep. 2010;24:521–528. doi: 10.3892/or_00000888. [DOI] [PubMed] [Google Scholar]
- 45.Rani B., Cao Y., Malfettone A., Tomuleasa C., Fabregat I., Giannelli G. Role of the tissue microenvironment as a therapeutic target in hepatocellular carcinoma. World J. Gastroenterol. 2014;20:4128–4140. doi: 10.3748/wjg.v20.i15.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giannelli G., Bergamini C., Fransvea E., Marinosci F., Quaranta V., Antonaci S. Human hepatocellular carcinoma (HCC) cells require both α3β1 integrin and matrix metalloproteinases activity for migration and invasion. Lab. Investig. 2001;81:613–627. doi: 10.1038/labinvest.3780270. [DOI] [PubMed] [Google Scholar]
- 47.Govaere O., Wouters J., Petz M., Vandewynckel Y.P., Van den Eynde K., Van den Broeck A., Verhulst S., Dollé L., Gremeaux L., Ceulemans A., et al. Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. J. Hepatol. 2016;64:609–617. doi: 10.1016/j.jhep.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Fujimoto A., Furuta M., Totoki Y., Tsunoda T., Kato M., Shiraishi Y., Tanaka H., Taniguchi H., Kawakami Y., Ueno M., et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat. Genet. 2016;48:500–509. doi: 10.1038/ng.3547. [DOI] [PubMed] [Google Scholar]
- 49.Hlubek F., Jung A., Kotzor N., Kirchner T., Brabletz T. Expression of the invasion factor laminin γ2 in colorectal carcinomas is regulated by β-catenin. Cancer Res. 2001;61:8089–8093. [PubMed] [Google Scholar]
- 50.Hashimoto K., Mori N., Tamesa T., Okada T., Kawauchi S., Oga A., Furuya T., Tangoku A., Oka M., Sasaki K. Analysis of DNA copy number aberrations in hepatitis C virus-associated hepatocellular carcinomas by conventional CGH and array CGH. Mod. Pathol. 2004;17:617–622. doi: 10.1038/modpathol.3800107. [DOI] [PubMed] [Google Scholar]
- 51.Sathyanarayana U.G., Maruyama R., Padar A., Suzuki M., Bondaruk J., Sagalowsky A., Minna J.D., Frenkel E.P., Grossman H.B., Czerniak B., et al. Molecular detection of noninvasive and invasive bladder tumor tissues and exfoliated cells by aberrant promoter methylation of laminin-5 encoding genes. Cancer Res. 2004;64:1425–1430. doi: 10.1158/0008-5472.CAN-03-0701. [DOI] [PubMed] [Google Scholar]
- 52.Sathyanarayana U.G., Padar A., Huang C.X., Suzuki M., Shigematsu H., Bekele B.N., Gazdar A.F. Aberrant promoter methylation and silencing of laminin-5-encoding genes in breast carcinoma. Clin. Cancer Res. 2003;9:6389–6394. [PubMed] [Google Scholar]
- 53.Sathyanarayana U.G., Padar A., Suzuki M., Maruyama R., Shigematsu H., Hsieh J.T., Frenkel E.P., Gazdar A.F. Aberrant promoter methylation of laminin-5-encoding genes in prostate cancers and its relationship to clinicopathological features. Clin. Cancer Res. 2003;9:6395–6400. [PubMed] [Google Scholar]
- 54.Sathyanarayana U.G., Toyooka S., Padar A., Takahashi T., Brambilla E., Minna J.D., Gazdar A.F. Epigenetic inactivation of laminin-5-encoding genes in lung cancers. Clin. Cancer Res. 2003;9:2665–2672. [PubMed] [Google Scholar]