Abstract
Pituitary adenoma (PA) is prevalent in the general population. Due to its severe complications and aggressive infiltration into the surrounding brain structure, the effective management of PA is required. Till now, no drug has been approved for treating non-functional PA, and the removal of cancerous cells from the pituitary is still under experimental investigation. Due to its superior specificity and safety profile, immunotherapy stands as one of the most promising strategies for dealing with PA refractory to the standard treatment, and various studies have been carried out to discover immune-related gene markers as target candidates. However, the lists of gene markers identified among different studies are reported to be highly inconsistent because of the greatly limited number of samples analyzed in each study. It is thus essential to substantially enlarge the sample size and comprehensively assess the robustness of the identified immune-related gene markers. Herein, a novel strategy of direct data integration (DDI) was proposed to combine available PA microarray datasets, which significantly enlarged the sample size. First, the robustness of the gene markers identified by DDI strategy was found to be substantially enhanced compared with that of previous studies. Then, the DDI of all reported PA-related microarray datasets were conducted to achieve a comprehensive identification of PA gene markers, and 66 immune-related genes were discovered as target candidates for PA immunotherapy. Finally, based on the analysis of human protein–protein interaction network, some promising target candidates (GAL, LMO4, STAT3, PD-L1, TGFB and TGFBR3) were proposed for PA immunotherapy. The strategy proposed together with the immune-related markers identified in this study provided a useful guidance for the development of novel immunotherapy for PA.
Keywords: pituitary adenomas, immunotherapy, immune-related gene markers, transcriptomics
1. Introduction
Pituitary adenoma (PA) accounts for approximately 8–15% of intracranial tumors [1,2,3] and its prevalence is estimated to be ~14–22% by autopsy and radiological studies [4,5,6]. Due to its aggressive infiltration into the surrounding brain structure and its severe complications (such as Cushing’s disease, hyperprolactinemia, and acromegaly), the effective managements of PA are still required [7,8,9], which involves trans-sphenoidal surgery, radiotherapy, and medicine [10]. Trans-sphenoidal surgery is reported as sometimes contraindicated or ineffective [11], and the application of radiotherapies is greatly limited by its subsequent hypopituitarism [11]. So far, medical therapy has been expected and emerged to provide the treatment of enhanced efficacy, safety and tolerance [11,12,13], and several drugs have already been approved by U.S. FDA (Food and Drug Administration) for treating certain complications of PA [14,15,16]. For example, cabergoline, octreotide, and pasireotide have FDA approval for the management of hyperprolactinemia, acromegaly, and Cushing’s disease, respectively [17,18,19,20]. Apart from the drugs aiming at PA complication, temozolomide is found to penetrate well into central nervous system, and therefore has been tested in clinical trial (Phase II) for treating PAs [21,22,23,24].
However, those approved therapeutics primarily focus on managing the complication of PA rather than PA itself [17,18], which are incapable of removing the tumor cells and are ineffective in turning the pituitary back to its normal state [25]. The therapeutics designed for the direct treatment of PA are primarily chemotherapy [21], kinase inhibitor [26,27,28,29], and monoclonal antibody [30,31,32]. Till now, chemotherapy and kinase inhibitors have emerged as the most promising candidates with temozolomide and lapatinib in clinical developments [21,26,33], but they suffer from severe myelotoxicity, mental disturbances or cardiac dysrhythmia that can substantially affect the approval processes of these clinical candidates [34,35,36]. So far, no effective drug has been developed for treating non-functional pituitary tumors, and the removal of cancerous cells directly from the pituitary is still under experimental investigation [37,38,39]. It is thus crucial to discover a new strategy for PA treatment. With the advent of immunotherapy [40,41], the management of PA base for this new strategy has become the focus of current research [1,30]. In other words, due to its high specificity and good safety profiles [42], immunotherapy stands as one of the most promising alternatives for treating PAs that are resistant or refractory to the standard treatments [43,44,45,46].
Although no immunotherapy of PA has been approved yet, various studies have been carried out to discover immune-related genes or target candidates. Particularly, as an immunomodulatory peptide, protein galanin (GAL) is substantially suppressed in the tissue of PA patients compared with those in healthy pituitaries [47]. Increased levels (both mRNA and protein) of programmed death-ligand 1 (PD-L1, the immunosuppressive protein) are observed in tumor tissue of PA patients [43], which can induce immune evasion by desensitizing the recognition and elimination of cancer cells via CD8+ lymphocytes [48]. Transforming growth factor-beta (TGFB) was discovered as one of the chief immune suppressive mediators of prolactin (PRL) secretions and PA proliferation [49,50]. Apart from the above studies, a variety of transcriptomics analyses (demonstrated in Table 1) have been conducted to identify the differentially expressed genes (DEGs) between PA patients and normal ones [51,52,53,54]. Due to their Omics-based nature, these analyses can accelerate more effectively the discovery of target candidates compared with previous studies. However, because of the limited number of both the patients and controls in each analysis (~4–16 samples for patients and ~0–9 for healthy individuals shown in Table 1), the lists of DEGs identified from different analyses are reported to be highly inconsistent [55,56], which asks for substantial enhancements of the robustness of the identified PA biomarkers [57,58]. Moreover, no Omics study has been conducted to reveal immune-related genes and mechanisms underlying PA’s development, which is essential for the discovery of promising targets for PA immunotherapy [59]. Thus, it is urgently needed to systematically identify the immune-related DEGs based on the comprehensive analysis of the PA-based Omics datasets with significantly enlarged sample size (for both PA patients and healthy individuals) [60].
Table 1.
Pituitary adenomas related microarray datasets collected for analysis in this study.
| GEO ID | Samples PA:NP a | Description of the Collected Datasets for Studying on | Microarray Platform | Reference of the Studied Datasets |
|---|---|---|---|---|
| GSE2175 | 4:1 | GeneChip arrays of 1 healthy and 4 pituitary adenoma samples | HG-U133A | [61] |
| GSE22812 | 13:0 | Genomic hybridization arrays of 13 pituitary adenoma samples | GE Healthcare | [53] |
| GSE26966 | 14:9 | GeneChip arrays of 9 healthy and 14 pituitary adenoma samples | HG-U133_Plus_2 | [62] |
| GSE36314 | 4:3 | Genome arrays of 3 healthy and 4 pituitary adenoma samples | HG_U95Av2 | [63] |
| GSE4237 | 10:0 | Affymetrix oligo arrays of 10 pituitary adenoma samples | HG-U133_Plus_2 | [52] |
| GSE46311 | 16:0 | Affymetrix Human Gene arrays of 16 pituitary adenoma samples | HuGene-1_0-st | [51] |
| GSE51618 | 7:3 | Agilent arrays of 3 healthy and 7 pituitary adenoma samples | Agilent-hgug4112a | [64] |
a PAs: pituitary adenomas; NP: normal pituitary.
In this study, a strategy of direct data integration (DDI) was proposed to combine available PA microarray datasets for significantly enlarging the sample size. To test the impact of the DDI strategy on the classification ability and the robustness of identified DEGs, its performance and disease relevance were first evaluated by comparing with previously published datasets. Then, all currently available PA-related microarray datasets were directly integrated to achieve comprehensive identification of DEGs between PA patients and healthy individuals. Finally, the immune-related genes were annotated from DEGs, which could be further studied as target candidates for PA immunotherapy. The strategy proposed together with the immune-related DEGs identified in this study provided a useful guidance for future immunotherapy.
2. Results and Discussion
2.1. The Level of PA-Relevance of the DEGs Identified by Different Analytical Strategies
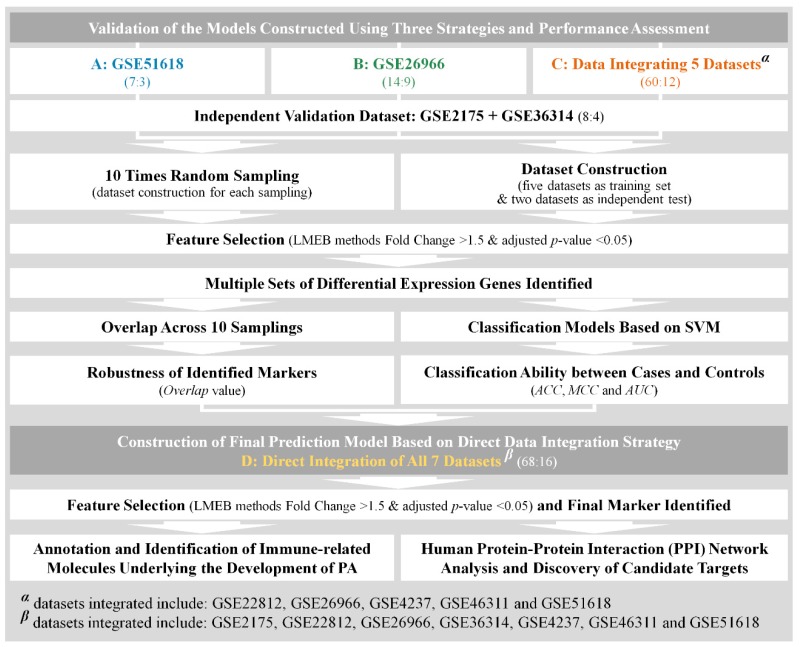
Several studies have been conducted to identify the DEG capable of distinguishing PA patients from healthy people [51,52,53]. Due to their limited number of disease and healthy samples, the DEGs identified in different studies are reported to be highly inconsistent [55], which requires substantial enhancement in the robustness of the identified DEGs [57,58]. Thus, it is necessary to evaluate the impact of sample size on the robustness and disease relevance of the identified DEGs. In this study, three analytical strategies were proposed based on the construction of three datasets. As illustrated in Figure 1, the datasets included: (A) GSE51618, (B) GSE26966, (C) DDI of five datasets GSE22812, GSE26966, GSE4237, GSE46311, and GSE51618. Clearly, the sample size of dataset C (60 cases and 12 controls) is significantly larger than that of the remaining two (seven cases and three controls for dataset A; 14 cases and six controls for dataset B). By using this DDI strategy, it is now feasible to discuss the effectiveness of this strategy on enhancing the robustness of identified DEGs and systematically assess the impact of sample size on the resulting DEGs.
Figure 1.
A schematic representation of the direct data integration (DDI) strategy adopted in this study. Four datasets (A: GSE51618, B: GSE26966, C: Data Integrating 5 Datasets and D: Direct Integration of All 7 Datasets) were labeled by blue, green, orange and yellow color, respectively. LMEB: Linear models and empirical Bayes; ACC: accuracy; MCC: Matthews correlation coefficient; AUC: area under the curve.
As the first assessment, the level of PA relevance was reviewed and discussed for three different analytical strategies. Three lists of DEGs were identified using three different strategies by the linear models and empirical Bayes (LMEB, fold change > 1.5 and adjusted p-value < 0.05). Top-ranked DEGs (top five for upregulated and another top five for downregulated) and their relevance to PAs were reviewed by comprehensive literature review. As shown in Supplementary Table S1, 3, 4, and 7 of the top 10 DEGs (identified by datasets A, B, and C, respectively) had been reported to be relevant to PAs. Moreover, 32, 56, and 43 KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways were enriched using DEGs identified from datasets A, B, and C, respectively. The top 10 ranked pathways and their relevance to PA were comprehensively reviewed. As shown in Supplementary Table S2, 3, 4, and 7 of the top 10 ranked pathways (identified by datasets A, B, and C, respectively) had been reported to be closely relevant to PAs. Furthermore, top 10 GO (gene ontology) molecular functions (MFs) and biological processes (BPs) and their relevance to PA were comprehensively reviewed. As shown in Supplementary Tables S3 and S4, 5, 5, and 7 of the top 10 ranked BPs and 4, 4, and 6 of the top 10 ranked MFs (identified by datasets A, B, and C, respectively) had been reported to be relevant to PAs. Overall, these findings indicated that all three strategies used in this study showed a certain level of PA relevance in their identified DEGs. The DDI strategy presented a higher percentage of PA-relevant DEGs, pathways, and GO terms, which indicated the enhanced disease relevance of this strategy comparing to that of the single dataset-based one.
2.2. Evaluating the Classification Abilities of the DEGs Identified by Different Analytical Strategies
Classification models are frequently constructed in current transcriptomics studies for predicting samples of various disease status [65,66] or assessing the reliability of identified gene markers [67,68]. The capacity of the constructed classification model was evaluated by various metrics including accuracy (ACC), sensitivity (SEN), specificity (SPE), Matthews correlation coefficient (MCC), receiver operating characteristics (ROC), and area under ROC curve (AUC value) [69,70,71]. As shown in Table 2, there was great variation in each assessment metric among the analytical strategies. Particularly, ACCs, SENs, SPEs, and MCCs were in the ranges of ~0.67–0.92, ~0.50–0.88, ~0.75–1.00, and ~0.35–0.84 among strategies, respectively. The metrics ACC and MCC were frequently used in current Omics study to evaluate correctness [72] and stability [73] of the constructed models. As demonstrated in Table 2, the ACC of DDI strategy (0.92) was substantially higher than that of the single dataset-based strategies (both are 0.67). Similar to ACC, MCC of DDI strategy (0.84) was discovered to be higher than that of the other two (0.35 and 0.50, respectively).
Table 2.
Prediction ability of three classifiers. These classifiers were build using GSE51618, GSE26966 and the combination of GSE22812, GSE26966, GSE4237, GSE46311, and GSE51618. Prediction ability was assessed by the integration of GSE36314 and GSE2175 as the independent validation dataset.
| Datasets | TP a | FN a | TN a | FP a | ACC b | SEN b | SPE b | MCC b | AUC c |
|---|---|---|---|---|---|---|---|---|---|
| A: GSE51618 | 4 | 4 | 4 | 0 | 0.67 | 0.50 | 1.00 | 0.50 | 0.75 |
| B: GSE26966 | 5 | 3 | 3 | 1 | 0.67 | 0.63 | 0.75 | 0.35 | 0.72 |
| C: DDI Strategy | 7 | 1 | 4 | 0 | 0.92 | 0.88 | 1.00 | 0.84 | 0.97 |
a TP: true positive; TN: true negative; FP: false positive; FN: false negative. b ACC: accuracy; SEN: sensitivity; SPE: specificity; MCC: Matthews correlation coefficient. c AUC: area under the curve.
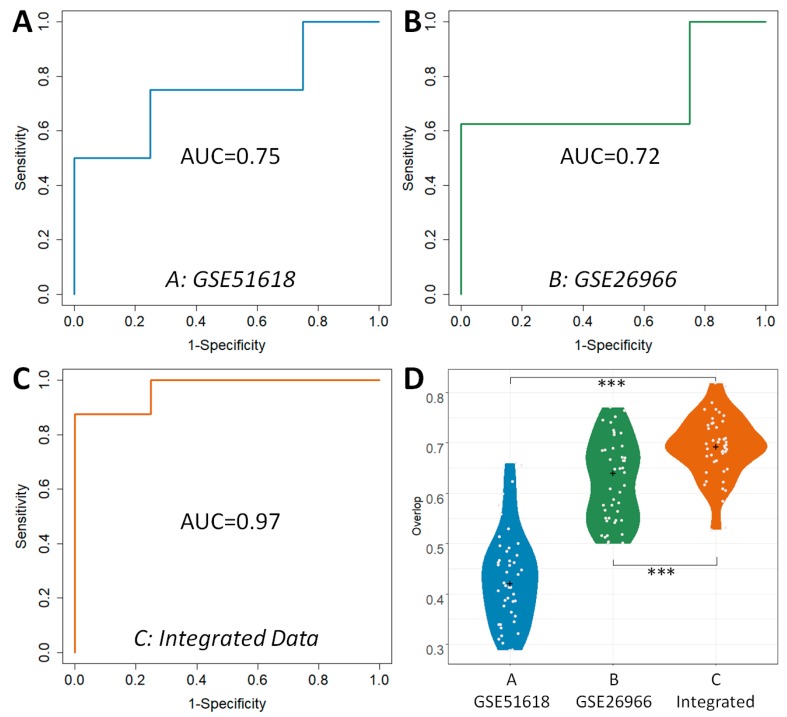
Apart from ACC and MCC, the ROC and AUC were two other popular metrics widely used to assess ability for classification, which were acknowledged to achieve comprehensive performance evaluations. As shown in Figure 2, the ROC curves and AUC values of three strategies were compared. Grey diagonals represented an invalid model with the corresponding AUC value equaled 0.5. As shown in Figure 2, the AUC value of the DDI strategy (0.97) was substantially higher than that of the others (0.75 and 0.72, respectively), which were similar to the results assessed by ROC curves. In conclusion, this finding indicated that classification correctness (assessed by ACC, ROC, and AUC) and prediction stability (evaluated by MCC) of DDI strategy were found consistently better compared with those strategies based on single dataset.
Figure 2.
Classification capacity (assessed by AUC (area under the curve)) and robustness (evaluated by Overlap value) of three classifiers. Classifiers were build using (A) GSE51618, (B) GSE26966, and (C) combination of GSE22812, GSE26966, GSE4237, GSE46311, and GSE51618. Statistical comparative analyses of the overlap values among the strategies based on these three datasets were also provided (D). The + presents the median of all overlap values, and *** presents the p value < 0.001.
2.3. Comparative Analysis on the Robustness of the DEGs Identified by Different Analytical Strategies
Apart from prediction capacity simultaneously assessed by classification correctness and prediction stability, the robustness of identified DEGs was widely accepted as an additional key metric with its underlying theory distinct from that of prediction ability [69,74]. So far, overlap value had been recognized as the quantitative measure of the robustness of identified DEGs [75]. The higher overlap values represented the more robust DEGs identified by a given strategy. Herein, one sub-dataset was first generated by randomly selecting 2/3 of both cases and controls in the studied dataset, and ten iterations of the same procedure generated ten sub-datasets. For each sub-dataset, a list of DEGs were then identified by LMEB method (fold change > 1.5 and adjusted p-value < 0.05), and the value of overlap between any two sub-datasets was calculated using their corresponding lists of DEGs. In total, there were 45 () overlap values denoting all possible combinations between any two sub-datasets. Finally, overlap values of three different analytical strategies were compared. As shown in the upper section of Table 3, the total numbers of DEGs identified by ten sub-datasets together with the median values of overlap were provided. It was obvious that the total numbers of identified DEGs among ten sub-datasets varied significantly (from 370 to 1310). The median overlap of DDI strategy (0.69) was found larger than that of the other strategies (0.64 and 0.42, respectively).
Table 3.
The robustness of the identified markers assessed by 10 random sampling datasets.
| Dataset | A: GSE51618 | B: GSE26966 | C: DDI Strategy | |
|---|---|---|---|---|
| Overlap Median across 10 Samplings | 0.42 | 0.64 | 0.69 | |
| No. of DEGs Identified by the nth Sampling Dataset (n=) | 1 | 230 | 244 | 175 |
| 2 | 149 | 312 | 147 | |
| 3 | 179 | 332 | 140 | |
| 4 | 319 | 262 | 134 | |
| 5 | 284 | 328 | 132 | |
| 6 | 633 | 349 | 282 | |
| 7 | 361 | 278 | 171 | |
| 8 | 283 | 295 | 172 | |
| 9 | 377 | 243 | 223 | |
| 10 | 585 | 295 | 192 | |
| No. of DEGs Identified | 1310 | 370 | 410 | |
| No. (Percent) of DEGs Co-discovered by N Sampling Datasets | 10 | 56 (0.04) | 52 (0.14) | 71 (0.17) |
| ≥9 | 73 (0.06) | 63 (0.17) | 90 (0.22) | |
| ≥8 | 87 (0.07) | 75 (0.20) | 107 (0.26) | |
| ≥7 | 103 (0.08) | 87 (0.24) | 119 (0.29) | |
| ≥6 | 129 (0.10) | 104 (0.28) | 133 (0.32) | |
Compared with the median value of overlap, the statistical difference of 45 overlap values between different analytical strategies was more meaningful to reveal their level of robustness. Therefore, the comprehensive statistical comparison of robustness among strategies was conducted and illustrated in Figure 2D. Overlap values of three strategies were colored in blue, green and orange. Apart from the enhanced median value of overlap by DDI strategy, all overlap values of DDI strategy were found significantly higher (p-value < 0.05) than that of the other two. Particularly, as illustrated in Figure 2D, the statistical differences between DDI strategy and others (p-value) were all lower than 1.00 × 10−3 (1.89 × 10−24 between A and C; 5.56 × 10−5 between B and C). Moreover, the majority of the overlap values of DDI strategy were larger than 0.6, while that of the other strategies were lower than 0.6. This finding indicated that the DDI strategy performed better than others in the robustness of the identified DEGs. Additionally, Table 3 (lower section) provided the numbers and percentages of DEGs simultaneously discovered by N (N ≥ 6, ≥7, ≥8, ≥9, =10) sub-datasets. It was very clearly that the robustness of the DEGs identified by DDI strategy was much better than other two strategies in terms of both the number and percentage of co-identified DEGs. Particularly, about one third of the DEGs of DDI strategy were identified by over five sub-datasets, which was substantially higher than that of other two (28% and 10%, respectively). 17% of the DEGs of DDI strategy could be identified by all sub-datasets, which was still significantly higher than that of other two (14% and 4%, respectively).
2.4. Annotating the Immune-Related DEGs and Candidate Target Discovery for PA Immunotherapy
The discussion above testified the effectiveness of the adopted feature selection algorithm and revealed the superior robustness and classification ability of the proposed DDI strategy compared with that based on the single dataset. To maximumly enhance the prediction power of constructed model, the most comprehensive set of PA-related microarray data were collected (Table 1) and integrated to form data D (Figure 1). To the best of our knowledge, the dataset D was the largest one among current PA-related transcriptomic analyses. Using LMEB method (fold change > 1.5 and adjusted p-value < 0.05), a total of 370 DEGs were discovered. As shown in Table 4, the top-ranked DEGs (top-20 for up-regulation & another top-20 for down-regulation) were provided, and the identified DEGs that could not be found by the other two strategies were highlighted in bold font. These top-ranked DEGs and their relevance to PAs were reviewed by comprehensive literature review, and a variety of DEGs were identified as relevant to PAs by experiments. For example, the mRNA expression of TSHB was found to be absent in most PA patients [76]. Different from normal pituitary, the GAL was reported to be rarely expressed in somatotroph adenoma and prolactinoma [77]. The expression of GAL was substantially suppressed in the tissue of PA patients compared with that in healthy pituitary [47]. The CEL was identified to regulate the hormone secretion in adenomatous pituitary cells [78]. The high expression of PRL and under-expression of RPS29 were observed in pituitary adenomas [79]. The c-Fos overexpression was found to be much less common in pituitary tumors and did not necessarily correlate with the ability of tumor to become invasive [80]. Pathway analysis facilitated the discovery of PMAIP1 as important in the tumorigenesis and progression of PAs [81]. The serum AGR2 level was significantly higher in the serum of PA patients than the patients with other sellar lesions, which suggests AGR2 a potential marker for PAs’ diagnosis [82]. By annotating those 370 DEGs using GO terms, 66 (17.8%) DEGs were discovered as closely immune-related. Table 5 provided a full list of the immune-related DEGs together with their fold changes and p-values. Due to their close relationship with human immune system and the differential expression between PA patients and healthy individuals, they are potential target candidates for PA immunotherapy.
Table 4.
Top 20 up- and downregulated DEGs identified by combining all seven datasets in Table 1. These DEGs that could not be identified by both GSE62966 and GSE51618 dataset were highlighted in bold font.
| No. | Entrez | Symbol | LogFC | p-Value | GES62966 | GSE51618 |
|---|---|---|---|---|---|---|
| 1 | 5443 | POMC | −3.85 | 2.79 × 10−18 | −8.00 | −7.09 |
| 2 | 7252 | TSHB | −3.26 | 6.65 × 10−16 | −5.96 | −8.07 |
| 3 | 51,083 | GAL | −2.69 | 4.22 × 10−21 | −5.10 | −8.03 |
| 4 | 1056 | CEL | −2.22 | 9.21× 10−10 | −5.73 | −2.23 |
| 5 | 3240 | HP | −2.21 | 1.06 × 10−9 | −4.10 | - |
| 6 | 3397 | ID1 | −2.08 | 3.85 × 10−16 | −3.10 | −3.84 |
| 7 | 5446 | PON3 | −2.00 | 2.01 × 10−7 | −3.46 | - |
| 8 | 5617 | PRL | −1.93 | 6.41 × 10−3 | −7.33 | - |
| 9 | 1410 | CRYAB | −1.88 | 1.31 × 10−12 | −2.57 | - |
| 10 | 4885 | NPTX2 | −1.87 | 8.75 × 10−9 | −4.18 | - |
| 11 | 6161 | RPL32 | −1.83 | 1.05 × 10−2 | - | - |
| 12 | 4821 | NKX2-2 | −1.82 | 3.36 × 10−7 | −6.26 | −0.83 |
| 13 | 2697 | GJA1 | −1.64 | 5.94 × 10−8 | −3.73 | - |
| 14 | 5105 | PCK1 | −1.61 | 1.74 × 10−5 | −6.43 | −2.38 |
| 15 | 6235 | RPS29 | −1.54 | 1.18 × 10−2 | - | - |
| 16 | 12 | SERPINA3 | −1.48 | 4.62 × 10−4 | −4.15 | - |
| 17 | 2353 | FOS | −1.45 | 2.85 × 10−7 | −3.80 | - |
| 18 | 5366 | PMAIP1 | −1.44 | 4.11 × 10−7 | −3.85 | −2.39 |
| 19 | 6146 | RPL22 | −1.42 | 2.91 × 10−2 | - | - |
| 20 | 10,551 | AGR2 | −1.41 | 5.24 × 10−5 | −2.47 | −3.75 |
| 21 | 22,999 | RIMS1 | 0.78 | 3.05 × 10−2 | - | - |
| 22 | 8573 | CASK | 0.78 | 5.10 × 10−6 | 0.85 | 1.48 |
| 23 | 5795 | PTPRJ | 0.78 | 1.01 × 10−2 | - | - |
| 24 | 1272 | CNTN1 | 0.80 | 7.88 × 10−5 | 1.70 | - |
| 25 | 22,858 | ICK | 0.81 | 1.53 × 10−2 | - | - |
| 26 | 23,390 | ZDHHC17 | 0.81 | 4.94 × 10−3 | - | - |
| 27 | 51,755 | CDK12 | 0.81 | 3.45 × 10−2 | - | - |
| 28 | 4684 | NCAM1 | 0.81 | 5.74 × 10−3 | 0.74 | - |
| 29 | 4863 | NPAT | 0.81 | 1.07 × 10−2 | - | - |
| 30 | 57,125 | PLXDC1 | 0.82 | 6.76 × 10−4 | 0.76 | - |
| 31 | 9472 | AKAP6 | 0.83 | 1.75 × 10−3 | 0.86 | - |
| 32 | 23,101 | MCF2L2 | 0.83 | 2.02 × 10−2 | - | - |
| 33 | 868 | CBLB | 0.84 | 3.45 × 10−2 | - | - |
| 34 | 8490 | RGS5 | 0.84 | 3.25 × 10−4 | 1.41 | - |
| 35 | 1006 | CDH8 | 0.85 | 2.18 × 10−3 | 2.94 | - |
| 36 | 55,752 | SEPT11 | 0.87 | 1.13 × 10−2 | - | - |
| 37 | 5149 | PDE6H | 0.89 | 1.67 × 10−2 | 1.36 | - |
| 38 | 1641 | DCX | 0.91 | 6.76 × 10−4 | 3.14 | - |
| 39 | 29,899 | GPSM2 | 0.91 | 1.73 × 10−2 | 1.04 | - |
| 40 | 23,305 | ACSL6 | 0.91 | 3.51 × 10−3 | 0.90 | - |
Table 5.
Immune-related DEGs identified in this study with fold change, p-value, and their representative biological processes and molecular functions.
| Entrez ID | Gene Symbol | LogFC | p-Value | Representative GO Biological Processes and Molecular Functions |
|---|---|---|---|---|
| 51,083 | GAL | −2.69 | 4.22 × 10−21 | negative regulation of immune system process; regulation of immune process |
| 3240 | HP | −2.21 | 1.06 × 10−9 | immune system process |
| 2353 | FOS | −1.45 | 2.85 × 10−7 | positive regulation of immune system process; regulation of immune response |
| 5366 | PMAIP1 | −1.44 | 4.11 × 10−7 | immune effector process; immune system process |
| 6146 | RPL22 | −1.42 | 2.91 × 10−2 | immune system development; immune system process |
| 7060 | THBS4 | −1.33 | 7.19 × 10−6 | positive regulation of immune system process; regulation of immune process |
| 10,410 | IFITM3 | −1.28 | 8.39 × 10−6 | innate immune response; immune effector process; immune system process |
| 2488 | FSHB | −1.28 | 5.95 × 10−3 | regulation of immune system process |
| 133 | ADM | −1.28 | 4.34 × 10−7 | humoral immune response; immune response; immune system process |
| 2719 | GPC3 | −1.22 | 3.09 × 10−9 | immune system development; immune system process |
| 7852 | CXCR4 | −1.22 | 3.24 × 10−9 | immune system process |
| 347 | APOD | −1.21 | 2.78 × 10−5 | negative regulation of immune system process; regulation of immune process |
| 9353 | SLIT2 | −1.21 | 6.81 × 10−10 | negative regulation of immune system process; regulation of immune process |
| 8324 | FZD7 | −1.21 | 1.74 × 10−8 | immune system development; immune system process |
| 2197 | FAU | −1.19 | 1.35 × 10−2 | humoral immune response; innate immune response in mucosa |
| 4783 | NFIL3 | −1.14 | 8.44 × 10−5 | immune response; immune system process |
| 6218 | RPS17 | −1.13 | 2.70 × 10−2 | immune system process |
| 5950 | RBP4 | −1.08 | 1.79 × 10−2 | positive regulation of production of molecular mediator of immune response |
| 5919 | RARRES2 | −1.06 | 4.34 × 10−7 | positive regulation of immune system process; regulation of immune process |
| 3669 | ISG20 | −1.03 | 8.23 × 10−8 | innate immune response; immune effector process; immune system process |
| 3059 | HCLS1 | −1.02 | 6.70 × 10−5 | positive regulation of immune system process; regulation of immune process |
| 6279 | S100A8 | −1.01 | 1.99 × 10−3 | innate immune response; immune response; immune system process |
| 710 | SERPING1 | −1.00 | 6.70 × 10−6 | adaptive immune response; b cell mediated immunity; humoral immune response |
| 716 | C1S | −1.00 | 5.90 × 10−4 | adaptive/innate immune response; Leukocyte mediated immunity |
| 7056 | THBD | −1.00 | 2.52 × 10−6 | immune system process |
| 715 | C1R | −0.98 | 3.31 × 10−6 | adaptive/innate immune response; positive regulation of immune system process |
| 1672 | DEFB1 | −0.92 | 1.44 × 10−4 | humoral immune response; innate immune response in mucosa |
| 2669 | GEM | −0.89 | 2.22 × 10−4 | immune response; immune system process |
| 1958 | EGR1 | −0.86 | 2.45 × 10−3 | innate immune response; immune response; immune system development |
| 6662 | SOX9 | −0.86 | 3.43 × 10−4 | negative regulation of immune system process; regulation of immune process |
| 3426 | CFI | −0.84 | 6.05 × 10−10 | adaptive immune response; leukocyte/lymphocyte mediated immunity |
| 7412 | VCAM1 | −0.84 | 6.87 × 10−7 | innate immune response; positive regulation of immune system process |
| 1366 | CLDN7 | −0.84 | 4.27 × 10−3 | regulation of immune effector process; regulation of immune system process |
| 3726 | JUNB | −0.84 | 2.43 × 10−5 | immune system development; immune system process |
| 9796 | PHYHIP | −0.82 | 2.78 × 10−3 | regulation of immune effector process; regulation of immune system process |
| 7049 | TGFBR3 | −0.81 | 3.58 × 10−3 | immune response; immune system development; immune system process |
| 3958 | LGALS3 | −0.80 | 4.63 × 10−2 | innate immune response; negative regulation of immune effector process |
| 3485 | IGFBP2 | −0.78 | 1.23 × 10−3 | positive regulation of immune system process; regulation of immune process |
| 1051 | CEBPB | −0.75 | 2.38 × 10−3 | negative regulation of immune system process; regulation of immune process |
| 302 | ANXA2 | −0.72 | 1.55 × 10−2 | immune system development; immune system process |
| 5806 | PTX3 | −0.72 | 2.46 × 10−3 | innate immune response; immune effector process; immune system process |
| 3320 | HSP90AA1 | −0.71 | 1.90 × 10−2 | positive regulation of immune system process; activation of immune response |
| 7704 | ZBTB16 | −0.69 | 3.81 × 10−2 | negative/positive regulation of immune system process |
| 8543 | LMO4 | −0.69 | 1.35 × 10−2 | immune system development; immune system process |
| 6223 | RPS19 | −0.69 | 1.25 × 10−2 | regulation of innate immune response; immune system development |
| 4057 | LTF | −0.68 | 3.41 × 10−2 | humoral immune response; innate immune response in mucosa |
| 100,133,941 | CD24 | −0.65 | 5.68 × 10−3 | positive regulation of immune system process; immune system process |
| 684 | BST2 | −0.64 | 8.65 × 10−3 | humoral/innate immune response; negative regulation of immune response |
| 23,543 | RBFOX2 | 0.59 | 5.42 × 10−3 | regulation of immune system process |
| 5747 | PTK2 | 0.59 | 1.12 × 10−2 | activation of immune response; positive regulation of immune response |
| 2047 | EPHB1 | 0.60 | 8.01 × 10−3 | immune system process; immunological synapse formation |
| 27,242 | TNFRSF21 | 0.60 | 4.66 × 10−3 | adaptive/humoral immune response; negative regulation of immune process |
| 8754 | ADAM9 | 0.61 | 1.68 × 10−2 | immune system process |
| 3175 | ONECUT1 | 0.64 | 1.36 × 10−3 | immune system development; immune system process |
| 54,861 | SNRK | 0.64 | 1.61 × 10−2 | immune system development; immune system process |
| 801 | CALM1 | 0.64 | 6.76 × 10−4 | immune response regulating cell surface receptor signaling pathway |
| 51,752 | ERAP1 | 0.65 | 2.84 × 10−2 | regulation of innate immune response; regulation of immune system process |
| 7456 | WIPF1 | 0.65 | 1.59 × 10−3 | activation of immune response; immune system process |
| 4982 | TNFRSF11B | 0.66 | 3.67 × 10−2 | immune response; immune system process |
| 6362 | CCL18 | 0.67 | 3.72 × 10−2 | innate immune response; immune response; immune system process |
| 30,849 | PIK3R4 | 0.67 | 2.02 × 10−2 | activation/positive regulation of innate immune response |
| 5594 | MAPK1 | 0.71 | 2.18 × 10−3 | regulation of immune system process; immune system development |
| 8563 | THOC5 | 0.71 | 7.07 × 10−3 | immune system development; immune system process |
| 8473 | OGT | 0.72 | 1.16 × 10−2 | positive regulation of immune system process |
| 5795 | PTPRJ | 0.78 | 1.01 × 10−2 | negative/positive regulation of immune system process |
| 4684 | NCAM1 | 0.81 | 5.74 × 10−3 | innate immune response; immune response; immune system process |
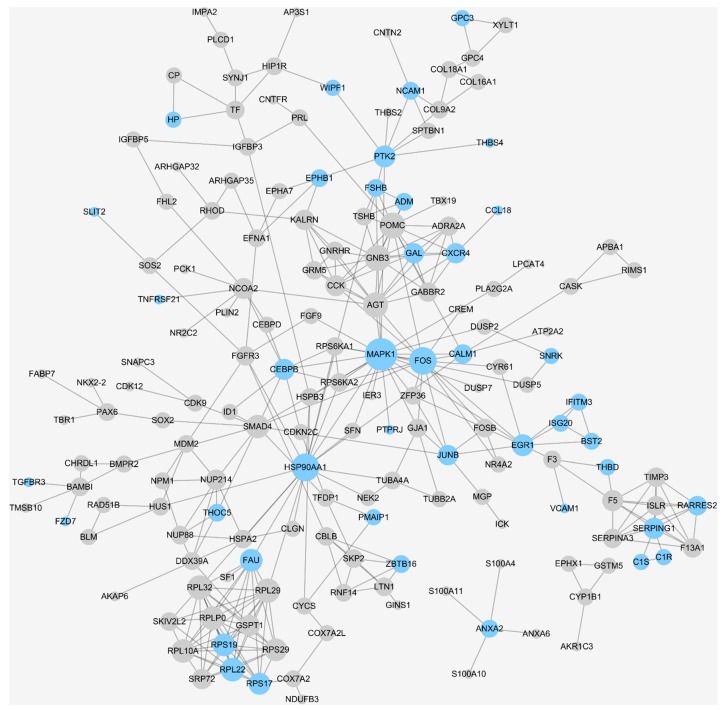
The human protein–protein interaction (PPI) network analyses (Figure 3) further revealed relations among the identified DEGs and immune-related ones (highlighted in blue). Among the identified target candidates for PA immunotherapy, MAPK1, FOS and HSP90AA1 were the top three immuno-candidates with the highest connectivity (network degree) to other human proteins. Moreover, galanin (GAL) was identified as greatly downregulated DEGs in the pituitary glands of PA patients. Due to it immunomodulatory property in PA, the GAL could be expected as a promising target candidate for PA immunotherapy, and this finding was in accordance to previous work [47]. The dysregulation of LMO4 was identified in this study, which induced STAT3 activation in brain [83] and subsequently regulated the expression of programmed death-ligand 1 (PD-L1) [84]. Since the upregulated expression of immunosuppressive protein PD-L1 was observed in the pituitary gland of PA patients [43], the inhibition of LMO4/STAT3/PD-L1 signaling could block the immune evasion by recovering the recognition and elimination of cancer cells via CD8+ lymphocytes [48]. Furthermore, the transforming growth factor-beta (TGFB) was reported to be one of the chief immune suppressive mediators of PRL secretion and PA proliferation [49,50]. Its receptor, TGFBR3, was also discovered in this study as a DEG, which could be another target candidate for developing novel PA immunotherapy.
Figure 3.
Human protein–protein interaction (PPI) network constructed based on 370 DEGs identified in this study. The PPI information was collected from the 2017 version of the STRING database and visualized using the Cytoscape software package. The diameter of each protein was defined by its network degree, and the proteins colored in blue were identified to be “immune-related” in this study.
3. Materials and Methods
3.1. Construction of Analytical Datasets and Discovery of Differentially Expressed Genes
Pituitary glands were wildly recognized as the major locus of PA’s dysfunction [63]. In this study, a variety of microarray studies based on the tissues of pituitary gland were thus collected through searching “pituitary adenoma”, “pituitary tumor”, “pituitary neoplasm”, “pituitary macroadenoma”, “pituitary carcinoma”, and “pituitary cancer” in the Gene Expression Omnibus (GEO) [85]. As a result, seven independent microarray studies were collected. As shown in Table 1, each dataset comprises a cohort of PA patients and/or another cohort of healthy controls. Among these studies, a limited number of both diseased and healthy samples were reported (~4–16 samples for the PA patients and ~0–9 samples for healthy people). In this study, four analytical datasets were constructed for (1) evaluating the effectiveness of the adopted feature selection algorithm, (2) conducting a performance comparison between the DDI strategy and the strategy of a single dataset, and (3) constructing the final model for identifying the immune-related molecule underlying PA’s development. In particular, the four analytical datasets included (as illustrated in Figure 1 and Table 1): (A) GSE51618, (B) GSE26966, (C) DDI of five datasets, and (D) DDI of seven datasets. Datasets (A–C) were used for validating feature selection method and comparing the performances of DDI strategy and the strategy of single dataset, and dataset D was applied to identify the target candidates for the immunotherapy of PA.
The direct data integration (DDI) strategy combining multiple datasets was carried out in the R environment (available online: http://www.r-project.org). The raw data (the CEL file) was first read, log transformed, and normalized, and all parameters were set as default. Then, outliers in each dataset were checked and removed, and all probe sets were mapped to their corresponding genes by Bioconductor [86]. Average expression value was retained if one gene was mapped to multiple probes. To remove the batch effect among datasets, Z score transformation [87,88,89] (provided in Equation (1)) was used to adjust the gene expression levels in each dataset.
| (1) |
where refers to the raw intensity of each gene, indicates the average intensity of all genes within single experiment and represents the standard deviation (SD) of all expression intensities in one array. After this process, the mean Z score for each array became zero with SD equaling one. The DDI strategy was applied to construct the dataset C (integrating GSE22812, GSE26966, GSE4237, GSE46311, and GSE51618) and D (integrating GSE2175, GSE22812, GSE26966, GSE36314, GSE4237, GSE46311, and GSE51618). As a result, the dataset C contained 60 PA patients and 12 healthy people, and dataset D contained 68 PA patients and 16 healthy individuals (Figure 1). The differentially expressed genes (DEGs) between the PA patients and healthy individuals were identified using the linear models and empirical Bayes (LMEB) [90] by the R package limma [91]. Herein, the genes with fold change > 1.5 and adjusted p-value < 0.05 were identified as the differentially expressed genes between case and control samples (Figure 1).
3.2. Validation of the Constructed Prediction Models Based on Different Analytical Datasets
A systematic validation and comparison of the prediction models constructed based on three analytical datasets (A–C) was conducted by evaluating the relevance level to PAs of the identified DEGs and by assessing the robustness of marker discovery and the classification ability of the constructed models.
3.2.1. The Level of Relevance of the Identified DEGs to Pituitary Adenoma
For complex disorders like PA, the identified DEGs are expected to contain a substantial percentage of PA-related genes [92], and a certain number of irrelevant genes may be inevitably selected due to measurement variability. Herein, a comprehensive literature review was performed to investigate the PA relevance of the top-ranked DEGs. Moreover, the pathway enrichment analyses on the identified DEGs were also conducted to identify the significantly overrepresented KEGG pathways using hypergeometric tests (adjusted p-value < 0.05) [93]. According to another comprehensive literature review on the top-ranked pathways playing key roles in PA, the level of PA relevance of the pathways enriched by three different strategies were compared. Finally, the gene ontology (GO) enrichment analyses using biological process (BP) and molecular function (MF) by the identified DEGs were conducted to identify the significantly overrepresented GO terms using hypergeometric tests (adjusted p-value < 0.05) [93]. The level of PA relevance of the top-ranked GO terms was also reviewed, analyzed, and compared among three different strategies.
3.2.2. The Classification Capacity of the Identified DEGs Assessed by the Independent Validation Dataset
The classification abilities of the DEGs identified by three different strategies (Dataset A, B and C shown in Figure 1) were assessed and compared by predicting PA outcomes of the independent validation dataset containing eight PA patients and four healthy controls. To statistically maximize both the prediction power of the constructed models and the validation effectiveness, the independent validation dataset was constructed by two datasets with the smallest amount of case and control samples: GSE2175 (four cases and one control) and GSE36314 (four cases and three controls). The classification models were constructed by the classifier of support vector machine (SVM) [94]. A variety of popular assessment metrics were adopted, which included true positive (TP), true negative (TN), false positive (FP), false negative (FN), accuracy (ACC), sensitivity (SEN), specificity (SPE), Matthews correlation coefficient (MCC), receiver operating characteristics (ROC) curve, and the area under ROC curve (AUC). All these metrics were frequently used in current Omics researches [95,96,97,98].
3.2.3. The Robustness of the Identified DEGs among Different Sampling Groups
The robustness of the identified DEGs among different samplings groups was widely accepted to be another metric for assessing the constructed prediction models [69]. Particularly, overlap value between two sampling groups was applied to quantify the robustness of the identified DEGs [75]. In this study, a sub-dataset was first generated by randomly selecting 2/3 samples of the original dataset, and ten iterations of this selection procedure resulted in ten sub-datasets. For each sub-dataset, a list of DEGs were identified by LMEB (fold change > 1.5 and adjusted p-value < 0.05), and the overlap value between any two sub-datasets was calculated using their corresponding lists of DEGs identified. In total, there were 45 () overlap values denoting all possible combinations between any two sub-datasets. Finally, the overlap values of three different strategies were compared to assess their robustness of the identified DEGs.
3.3. Annotating the Immune-Related DEGs and Candidate Target Discovery for PA Immunotherapy
Due to the substantially enhanced robustness and classification capacity of the DDI strategy discovered in this study, the DDI of all seven datasets (Table 1) was conducted to achieve the most comprehensive discovery of DEG between patients and healthy individuals. The method applied for data integration and batch effects removal was shown in the first section of Materials and Methods section. The samples integrated in constructing the final prediction model included 68 PA patients and 16 healthy individuals. To the best of our knowledge, this newly integrated dataset is the largest one among the current PA-related transcriptomic analyses. Based on the comprehensive dataset D integrated above (Figure 1), DEGs were further identified using the LMEB method (fold change > 1.5 and adjusted p-value < 0.05). Then, GO terms were used to annotate and identify immune-related DEGs. Moreover, the human protein–protein interaction (PPI) network analysis was introduced to explore the connection among identified DEGs [99]. In particular, the STRING database [100] was adopted to the construct human PPI network, and only PPI data of high confidence levels (>0.7) were applied in this study. The identified DEGs together with the STRING human PPI network were input into Cytoscape [101] for visualization, and only the identified DEGs were displayed (round circles). The immune-related DEGs annotated by GO terms were colored by light blue.
4. Conclusions
In this study, the direct data integration strategy was proposed, and its classification ability and robustness were assessed in this study. Compared with the traditional strategy based on a single dataset, the DDI strategy was found to greatly enhance the robustness of identified DEGs, which may be attributed to the significant enlargement of the sample size. Based on the comprehensive analysis of the available PA-related transcriptomics data, 370 DEGs were identified, and 66 immune-related DEGs were annotated and proposed as target candidates for PA immunotherapy. Using the PPI network analysis and literature review, several promising candidate targets (GAL, LMO4, STAT3, PD-L1, TGFB and TGFBR3) were further proposed for PA immunotherapy.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (81872798), the National Key Research and Development Program of China (2018YFC0910500), Innovation Project on Industrial Generic Key Technologies of Chongqing (cstc2015zdcy-ztzx120003), and Fundamental Research Funds for the Central Universities (2018QNA7023, 10611CDJXZ238826, 2018CDQYSG0007, CDJZR14468801, CDJKXB14011).
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-0067/20/1/151/s1.
Author Contributions
F.Z. conceived the idea and supervised the work. Q.Y. collected the multiple datasets and completed data integration. Q.Y., Y.W., S.Z. and J.T. performed research and developed the tool. Q.Y., F.L., J.Y., Y.L. (Yi Li) and W.W.X. wrote the original scripts and performed analysis. J.F., B.L. and Y.L. (Yongchao Luo) finished the visualization of data, and F.Z. performed writing-review and editing of the manuscript. All authors reviewed and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Salomon M.P., Wang X., Marzese D.M., Hsu S.C., Nelson N., Zhang X., Matsuba C., Takasumi Y., Ballesteros-Merino C., Fox B.A., et al. The epigenomic landscape of pituitary adenomas reveals specific alterations and differentiates among acromegaly, cushing’s disease and endocrine-inactive subtypes. Clin. Cancer Res. 2018;24:4126–4136. doi: 10.1158/1078-0432.CCR-17-2206. [DOI] [PubMed] [Google Scholar]
- 2.Zhu H., Guo J., Shen Y., Dong W., Gao H., Miao Y., Li C., Zhang Y. Functions and mechanisms of tumor necrosis factor-alpha and noncoding rnas in bone-invasive pituitary adenomas. Clin. Cancer Res. 2018;24:5757–5766. doi: 10.1158/1078-0432.CCR-18-0472. [DOI] [PubMed] [Google Scholar]
- 3.Tu G., Fu T., Yang F., Yao L., Xue W., Zhu F. Prediction of glun2b-ct1290-1310/dapk1 interaction by protein(-)peptide docking and molecular dynamics simulation. Molecules. 2018;23:3018. doi: 10.3390/molecules23113018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nota N.M., Wiepjes C.M., de Blok C.J.M., Gooren L.J.G., Peerdeman S.M., Kreukels B.P.C., den Heijer M. The occurrence of benign brain tumours in transgender individuals during cross-sex hormone treatment. Brain. 2018;141:2047–2054. doi: 10.1093/brain/awy108. [DOI] [PubMed] [Google Scholar]
- 5.Han Z.J., Xue W.W., Tao L., Zhu F. Identification of novel immune-relevant drug target genes for alzheimer’s disease by combining ontology inference with network analysis. CNS Neurosci. Ther. 2018;24:1253–1263. doi: 10.1111/cns.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu T., Zheng G., Tu G., Yang F., Chen Y., Yao X., Li X., Xue W., Zhu F. Exploring the binding mechanism of metabotropic glutamate receptor 5 negative allosteric modulators in clinical trials by molecular dynamics simulations. ACS Chem. Neurosci. 2018;9:1492–1502. doi: 10.1021/acschemneuro.8b00059. [DOI] [PubMed] [Google Scholar]
- 7.Mehta G.U., Lonser R.R. Management of hormone-secreting pituitary adenomas. Neuro. Oncol. 2017;19:762–773. doi: 10.1093/neuonc/now130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue W., Yang F., Wang P., Zheng G., Chen Y., Yao X., Zhu F. What contributes to serotonin-norepinephrine reuptake inhibitors’ dual-targeting mechanism? The key role of transmembrane domain 6 in human serotonin and norepinephrine transporters revealed by molecular dynamics simulation. ACS Chem. Neurosci. 2018;9:1128–1140. doi: 10.1021/acschemneuro.7b00490. [DOI] [PubMed] [Google Scholar]
- 9.Wang P., Fu T., Zhang X., Yang F., Zheng G., Xue W., Chen Y., Yao X., Zhu F. Differentiating physicochemical properties between ndris and snris clinically important for the treatment of adhd. Biochim. Biophys. Acta Gen. Subj. 2017;1861:2766–2777. doi: 10.1016/j.bbagen.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Molitch M.E. Diagnosis and treatment of pituitary adenomas: A review. JAMA. 2017;317:516–524. doi: 10.1001/jama.2016.19699. [DOI] [PubMed] [Google Scholar]
- 11.Castinetti F., Regis J., Dufour H., Brue T. Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat. Rev. Endocrinol. 2010;6:214–223. doi: 10.1038/nrendo.2010.4. [DOI] [PubMed] [Google Scholar]
- 12.Wang P., Zhang X., Fu T., Li S., Li B., Xue W., Yao X., Chen Y., Zhu F. Differentiating physicochemical properties between addictive and nonaddictive adhd drugs revealed by molecular dynamics simulation studies. ACS Chem. Neurosci. 2017;8:1416–1428. doi: 10.1021/acschemneuro.7b00173. [DOI] [PubMed] [Google Scholar]
- 13.Yang F.Y., Fu T.T., Zhang X.Y., Hu J., Xue W.W., Zheng G.X., Li B., Li Y.H., Yao X.J., Zhu F. Comparison of computational model and X-ray crystal structure of human serotonin transporter: Potential application for the pharmacology of human monoamine transporters. Mol. Simul. 2017;43:1089–1098. doi: 10.1080/08927022.2017.1309653. [DOI] [Google Scholar]
- 14.Li Y.H., Yu C.Y., Li X.X., Zhang P., Tang J., Yang Q., Fu T., Zhang X., Cui X., Tu G., et al. Therapeutic target database update 2018: Enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Res. 2018;46:D1121–D1127. doi: 10.1093/nar/gkx1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu F., Li X.X., Yang S.Y., Chen Y.Z. Clinical success of drug targets prospectively predicted by in silico study. Trends Pharmacol. Sci. 2018;39:229–231. doi: 10.1016/j.tips.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Tang J., Zhang Y., Fu J., Wang Y., Li Y., Yang Q., Yao L., Xue W., Zhu F. Computational advances in the label-free quantification of cancer proteomics data. Curr. Pharm. Des. 2018 doi: 10.2174/1381612824666181102125638. [DOI] [PubMed] [Google Scholar]
- 17.Frara S., Maffezzoni F., Mazziotti G., Giustina A. Current and emerging aspects of diabetes mellitus in acromegaly. Trends Endocrinol. Metab. 2016;27:470–483. doi: 10.1016/j.tem.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Zhu F., Han B., Kumar P., Liu X., Ma X., Wei X., Huang L., Guo Y., Han L., Zheng C., et al. Update of ttd: Therapeutic target database. Nucleic Acids Res. 2010;38:D787–D791. doi: 10.1093/nar/gkp1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng G., Yang F., Fu T., Tu G., Chen Y., Yao X., Xue W., Zhu F. Computational characterization of the selective inhibition of human norepinephrine and serotonin transporters by an escitalopram scaffold. Phys. Chem. Chem. Phys. 2018;20:29513–29527. doi: 10.1039/C8CP06232C. [DOI] [PubMed] [Google Scholar]
- 20.Li X.X., Yin J., Tang J., Li Y., Yang Q., Xiao Z., Zhang R., Wang Y., Hong J., Tao L., et al. Determining the balance between drug efficacy and safety by the network and biological system profile of its therapeutic target. Front. Pharmacol. 2018;9:1245. doi: 10.3389/fphar.2018.01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Losa M., Bogazzi F., Cannavo S., Ceccato F., Curto L., De Marinis L., Iacovazzo D., Lombardi G., Mantovani G., Mazza E., et al. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J. Neurooncol. 2016;126:519–525. doi: 10.1007/s11060-015-1991-y. [DOI] [PubMed] [Google Scholar]
- 22.Yang H., Qin C., Li Y.H., Tao L., Zhou J., Yu C.Y., Xu F., Chen Z., Zhu F., Chen Y.Z. Therapeutic target database update 2016: Enriched resource for bench to clinical drug target and targeted pathway information. Nucleic Acids Res. 2016;44:D1069–D1074. doi: 10.1093/nar/gkv1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu F., Shi Z., Qin C., Tao L., Liu X., Xu F., Zhang L., Song Y., Liu X., Zhang J., et al. Therapeutic target database update 2012: A resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012;40:D1128–D1136. doi: 10.1093/nar/gkr797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormack A.I., Wass J.A., Grossman A.B. Aggressive pituitary tumours: The role of temozolomide and the assessment of mgmt status. Eur. J. Clin. Investig. 2011;41:1133–1148. doi: 10.1111/j.1365-2362.2011.02520.x. [DOI] [PubMed] [Google Scholar]
- 25.Riebold M., Kozany C., Freiburger L., Sattler M., Buchfelder M., Hausch F., Stalla G.K., Paez-Pereda M. A c-terminal hsp90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of cushing disease. Nat. Med. 2015;21:276–280. doi: 10.1038/nm.3776. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Shlomo A., Cooper O. Role of tyrosine kinase inhibitors in the treatment of pituitary tumours: From bench to bedside. Curr. Opin. Endocrinol. Diabetes Obes. 2017;24:301–305. doi: 10.1097/MED.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J., Wang P., Yang H., Zhou J., Li Y., Li X., Xue W., Yu C., Tian Y., Zhu F. Comparison of fda approved kinase targets to clinical trial ones: Insights from their system profiles and drug-target interaction networks. Biomed. Res. Int. 2016;2016:2509385. doi: 10.1155/2016/2509385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu F., Ma X.H., Qin C., Tao L., Liu X., Shi Z., Zhang C.L., Tan C.Y., Chen Y.Z., Jiang Y.Y. Drug discovery prospect from untapped species: Indications from approved natural product drugs. PLoS ONE. 2012;7:e39782. doi: 10.1371/journal.pone.0039782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y.H., Wang P.P., Li X.X., Yu C.Y., Yang H., Zhou J., Xue W.W., Tan J., Zhu F. The human kinome targeted by fda approved multi-target drugs and combination products: A comparative study from the drug-target interaction network perspective. PLoS ONE. 2016;11:e0165737. doi: 10.1371/journal.pone.0165737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin A.L., Jonsson P., Tabar V., Yang T.J., Cuaron J., Beal K., Cohen M., Postow M., Rosenblum M., Shia J., et al. Marked Response of a Hypermutated ACTH-Secreting Pituitary Carcinoma to Ipilimumab and Nivolumab. J. Clin. Endocrinol. Metab. 2018;103:3925–3930. doi: 10.1210/jc.2018-01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu F., Zheng C.J., Han L.Y., Xie B., Jia J., Liu X., Tammi M.T., Yang S.Y., Wei Y.Q., Chen Y.Z. Trends in the exploration of anticancer targets and strategies in enhancing the efficacy of drug targeting. Curr. Mol. Pharmacol. 2008;1:213–232. doi: 10.2174/1874467210801030213. [DOI] [PubMed] [Google Scholar]
- 32.Tao L., Zhu F., Xu F., Chen Z., Jiang Y.Y., Chen Y.Z. Co-targeting cancer drug escape pathways confers clinical advantage for multi-target anticancer drugs. Pharmacol. Res. 2015;102:123–131. doi: 10.1016/j.phrs.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Li Y.H., Li X., Hong J., Wang Y., Fu J., Yang H., Yu C., Li F., Hu J., Xue W.W., et al. Clinical trials, progression-speed differentiating features, and swiftness rule of the innovative targets of first-in-class drugs. Brief. Bioinform. 2019 doi: 10.1093/bib/bby130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kourelis T.V., Buckner J.C., Gangat N., Patnaik M.M. Temozolomide induced bone marrow suppression—A single institution outcome analysis and review of the literature. Am. J. Hematol. 2015;90:E183–E184. doi: 10.1002/ajh.24066. [DOI] [PubMed] [Google Scholar]
- 35.Li X.F., Li X.X., Li Y.H., Yu C.Y., Xue W.W., Hu J., Li B., Wang P.P., Zhu F. What makes species productive of anti-cancer drugs? Clues from drugs’ species origin, druglikeness, target and pathway. Anticancer Agents Med. Chem. 2018 doi: 10.2174/1871520618666181029132017. [DOI] [PubMed] [Google Scholar]
- 36.Xue W., Wang P., Tu G., Yang F., Zheng G., Li X., Li X., Chen Y., Yao X., Zhu F. Computational identification of the binding mechanism of a triple reuptake inhibitor amitifadine for the treatment of major depressive disorder. Phys. Chem. Chem. Phys. 2018;20:6606–6616. doi: 10.1039/C7CP07869B. [DOI] [PubMed] [Google Scholar]
- 37.Yagnik G., Jahangiri A., Chen R., Wagner J.R., Aghi M.K. Role of a p53 polymorphism in the development of nonfunctional pituitary adenomas. Mol. Cell Endocrinol. 2017;446:81–90. doi: 10.1016/j.mce.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu F., Han L., Zheng C., Xie B., Tammi M.T., Yang S., Wei Y., Chen Y. What are next generation innovative therapeutic targets? Clues from genetic, structural, physicochemical, and systems profiles of successful targets. J. Pharmacol. Exp. Ther. 2009;330:304–315. doi: 10.1124/jpet.108.149955. [DOI] [PubMed] [Google Scholar]
- 39.Xue W., Fu T., Zheng G., Tu G., Zhang Y., Yang F., Tao L., Yao L., Zhu F. Recent advances and challenges of the drugs acting on monoamine transporters. Curr. Med. Chem. 2018 doi: 10.2174/0929867325666181009123218. [DOI] [PubMed] [Google Scholar]
- 40.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 41.Zheng G., Xue W., Yang F., Zhang Y., Chen Y., Yao X., Zhu F. Revealing vilazodone’s binding mechanism underlying its partial agonism to the 5-ht1a receptor in the treatment of major depressive disorder. Phys. Chem. Chem. Phys. 2017;19:28885–28896. doi: 10.1039/C7CP05688E. [DOI] [PubMed] [Google Scholar]
- 42.Smyth E.C., Cervantes A. Immunotherapy is not for all comers in chemotherapy-refractory advanced gastric cancer. Better predictive biomarkers are needed. Ann. Oncol. 2018;29:2027–2028. doi: 10.1093/annonc/mdy331. [DOI] [PubMed] [Google Scholar]
- 43.Wang P., Wang T., Yang Y., Yu C., Liu N., Yan C. Detection of programmed death ligand 1 protein and cd8+ lymphocyte infiltration in plurihormonal pituitary adenomas: A case report and review of the literatures. Medicine. 2017;96:e9056. doi: 10.1097/MD.0000000000009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaliszczak M., van Hechanova E., Li Y., Alsadah H., Parzych K., Auner H.W., Aboagye E.O. The hdac6 inhibitor c1a modulates autophagy substrates in diverse cancer cells and induces cell death. Br. J. Cancer. 2018;119:1278–1287. doi: 10.1038/s41416-018-0232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue W., Wang P., Li B., Li Y., Xu X., Yang F., Yao X., Chen Y.Z., Xu F., Zhu F. Identification of the inhibitory mechanism of fda approved selective serotonin reuptake inhibitors: An insight from molecular dynamics simulation study. Phys. Chem. Chem. Phys. 2016;18:3260–3271. doi: 10.1039/C5CP05771J. [DOI] [PubMed] [Google Scholar]
- 46.Zheng G., Xue W., Wang P., Yang F., Li B., Li X., Li Y., Yao X., Zhu F. Exploring the inhibitory mechanism of approved selective norepinephrine reuptake inhibitors and reboxetine enantiomers by molecular dynamics study. Sci. Rep. 2016;6:26883. doi: 10.1038/srep26883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang R., Gundlach A.L., Holmes F.E., Hobson S.A., Wynick D., Hokfelt T., Kofler B. Physiology, signaling, and pharmacology of galanin peptides and receptors: Three decades of emerging diversity. Pharmacol. Rev. 2015;67:118–175. doi: 10.1124/pr.112.006536. [DOI] [PubMed] [Google Scholar]
- 48.Alsaab H.O., Sau S., Alzhrani R., Tatiparti K., Bhise K., Kashaw S.K., Iyer A.K. Pd-1 and pd-l1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camilletti M.A., Ferraris J., Abeledo-Machado A., Converse A., Faraoni E.Y., Pisera D., Gutierrez S., Thomas P., Diaz-Torga G. Participation of membrane progesterone receptor alpha in the inhibitory effect of progesterone on prolactin secretion. J. Neuroendocrinol. 2018;30:e12614. doi: 10.1111/jne.12614. [DOI] [PubMed] [Google Scholar]
- 50.Vinay D.S., Ryan E.P., Pawelec G., Talib W.H., Stagg J., Elkord E., Lichtor T., Decker W.K., Whelan R.L., Kumara H., et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Lekva T., Berg J.P., Fougner S.L., Olstad O.K., Ueland T., Bollerslev J. Gene expression profiling identifies esrp1 as a potential regulator of epithelial mesenchymal transition in somatotroph adenomas from a large cohort of patients with acromegaly. J. Clin. Endocrinol. Metab. 2012;97:E1506–E1514. doi: 10.1210/jc.2012-1760. [DOI] [PubMed] [Google Scholar]
- 52.Hussaini I.M., Trotter C., Zhao Y., Abdel-Fattah R., Amos S., Xiao A., Agi C.U., Redpath G.T., Fang Z., Leung G.K., et al. Matrix metalloproteinase-9 is differentially expressed in nonfunctioning invasive and noninvasive pituitary adenomas and increases invasion in human pituitary adenoma cell line. Am. J. Pathol. 2007;170:356–365. doi: 10.2353/ajpath.2007.060736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wierinckx A., Roche M., Raverot G., Legras-Lachuer C., Croze S., Nazaret N., Rey C., Auger C., Jouanneau E., Chanson P., et al. Integrated genomic profiling identifies loss of chromosome 11p impacting transcriptomic activity in aggressive pituitary prl tumors. Brain Pathol. 2011;21:533–543. doi: 10.1111/j.1750-3639.2011.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang F., Zheng G., Fu T., Li X., Tu G., Li Y.H., Yao X., Xue W., Zhu F. Prediction of the binding mode and resistance profile for a dual-target pyrrolyl diketo acid scaffold against hiv-1 integrase and reverse-transcriptase-associated ribonuclease h. Phys. Chem. Chem. Phys. 2018;20:23873–23884. doi: 10.1039/C8CP01843J. [DOI] [PubMed] [Google Scholar]
- 55.Sav A., Rotondo F., Syro L.V., Scheithauer B.W., Kovacs K. Biomarkers of pituitary neoplasms. Anticancer Res. 2012;32:4639–4654. [PubMed] [Google Scholar]
- 56.Suhardja A., Kovacs K., Greenberg O., Scheithauer B.W., Lloyd R.V. Prognostic indicators in pituitary tumors. Endocr. Pathol. 2005;16:1–9. doi: 10.1385/EP:16:1:001. [DOI] [PubMed] [Google Scholar]
- 57.Di Ieva A., Rotondo F., Syro L.V., Cusimano M.D., Kovacs K. Aggressive pituitary adenomas--diagnosis and emerging treatments. Nat. Rev. Endocrinol. 2014;10:423–435. doi: 10.1038/nrendo.2014.64. [DOI] [PubMed] [Google Scholar]
- 58.Raverot G., Castinetti F., Jouanneau E., Morange I., Figarella-Branger D., Dufour H., Trouillas J., Brue T. Pituitary carcinomas and aggressive pituitary tumours: Merits and pitfalls of temozolomide treatment. Clin. Endocrinol. 2012;76:769–775. doi: 10.1111/j.1365-2265.2012.04381.x. [DOI] [PubMed] [Google Scholar]
- 59.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S., et al. Genetic basis for clinical response to ctla-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mistry M., Gillis J., Pavlidis P. Genome-wide expression profiling of schizophrenia using a large combined cohort. Mol. Psychiatry. 2013;18:215–225. doi: 10.1038/mp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris D.G., Musat M., Czirjak S., Hanzely Z., Lillington D.M., Korbonits M., Grossman A.B. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. Eur. J. Endocrinol. 2005;153:143–151. doi: 10.1530/eje.1.01937. [DOI] [PubMed] [Google Scholar]
- 62.Michaelis K.A., Knox A.J., Xu M., Kiseljak-Vassiliades K., Edwards M.G., Geraci M., Kleinschmidt-DeMasters B.K., Lillehei K.O., Wierman M.E. Identification of growth arrest and DNA-damage-inducible gene beta (GADD45beta) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology. 2011;152:3603–3613. doi: 10.1210/en.2011-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tong Y., Zheng Y., Zhou J., Oyesiku N.M., Koeffler H.P., Melmed S. Genomic characterization of human and rat prolactinomas. Endocrinology. 2012;153:3679–3691. doi: 10.1210/en.2012-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu S.Y., Hong L.C., Feng J., Wu Y.T., Zhang Y.Z. Integrative proteomics and transcriptomics identify novel invasive-related biomarkers of non-functioning pituitary adenomas. Tumour Biol. 2016;37:8923–8930. doi: 10.1007/s13277-015-4767-2. [DOI] [PubMed] [Google Scholar]
- 65.Maudsley S., Devanarayan V., Martin B., Geerts H., Brain Health Modeling I. Intelligent and effective informatic deconvolution of “big data” and its future impact on the quantitative nature of neurodegenerative disease therapy. Alzheimers Dement. 2018;14:961–975. doi: 10.1016/j.jalz.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 66.Date Y., Kikuchi J. Application of a deep neural network to metabolomics studies and its performance in determining important variables. Anal. Chem. 2018;90:1805–1810. doi: 10.1021/acs.analchem.7b03795. [DOI] [PubMed] [Google Scholar]
- 67.Song L., Zhuang P., Lin M., Kang M., Liu H., Zhang Y., Yang Z., Chen Y., Zhang Y. Urine metabonomics reveals early biomarkers in diabetic cognitive dysfunction. J. Proteome Res. 2017;16:3180–3189. doi: 10.1021/acs.jproteome.7b00168. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Z., Shen X., Tu J., Zhu Z.J. Large-scale prediction of collision cross-section values for metabolites in ion mobility-mass spectrometry. Anal. Chem. 2016;88:11084–11091. doi: 10.1021/acs.analchem.6b03091. [DOI] [PubMed] [Google Scholar]
- 69.Hart C.D., Vignoli A., Tenori L., Uy G.L., Van To T., Adebamowo C., Hossain S.M., Biganzoli L., Risi E., Love R.R., et al. Serum metabolomic profiles identify er-positive early breast cancer patients at increased risk of disease recurrence in a multicenter population. Clin. Cancer Res. 2017;23:1422–1431. doi: 10.1158/1078-0432.CCR-16-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu C.Y., Li X.X., Yang H., Li Y.H., Xue W.W., Chen Y.Z., Tao L., Zhu F. Assessing the performances of protein function prediction algorithms from the perspectives of identification accuracy and false discovery rate. Int. J. Mol. Sci. 2018;19:183. doi: 10.3390/ijms19010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou W., Meng X., Zhao A., Zhao W., Pan J., Tang J., Huang Y., Li H., Jia W., Liu F., et al. Development of multimarker diagnostic models from metabolomics analysis for gestational diabetes mellitus (gdm) Mol. Cell. Proteom. 2018;17:431–441. doi: 10.1074/mcp.RA117.000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alonso A., Julia A., Vinaixa M., Domenech E., Fernandez-Nebro A., Canete J.D., Ferrandiz C., Tornero J., Gisbert J.P., Nos P., et al. Urine metabolome profiling of immune-mediated inflammatory diseases. BMC Med. 2016;14:133. doi: 10.1186/s12916-016-0681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu F., Chi L., Ru H., Parvez F., Slavkovich V., Eunus M., Ahmed A., Islam T., Rakibuz-Zaman M., Hasan R., et al. Arsenic exposure from drinking water and urinary metabolomics: Associations and long-term reproducibility in bangladesh adults. Environ. Health Perspect. 2018;126:017005. doi: 10.1289/EHP1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valikangas T., Suomi T., Elo L.L. A systematic evaluation of normalization methods in quantitative label-free proteomics. Brief. Bioinform. 2018;19:1–11. doi: 10.1093/bib/bbw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C., Gong B., Bushel P.R., Thierry-Mieg J., Thierry-Mieg D., Xu J., Fang H., Hong H., Shen J., Su Z., et al. The concordance between rna-seq and microarray data depends on chemical treatment and transcript abundance. Nat. Biotechnol. 2014;32:926–932. doi: 10.1038/nbt.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jameson J.L., Klibanski A., Black P.M., Zervas N.T., Lindell C.M., Hsu D.W., Ridgway E.C., Habener J.F. Glycoprotein hormone genes are expressed in clinically nonfunctioning pituitary adenomas. J. Clin. Investig. 1987;80:1472–1478. doi: 10.1172/JCI113228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leung B., Iisma T.P., Leung K.C., Hort Y.J., Turner J., Sheehy J.P., Ho K.K. Galanin in human pituitary adenomas: Frequency and clinical significance. Clin. Endocrinol. 2002;56:397–403. doi: 10.1046/j.1365-2265.2002.01486.x. [DOI] [PubMed] [Google Scholar]
- 78.La Rosa S., Vigetti D., Placidi C., Finzi G., Uccella S., Clerici M., Bartolini B., Carnevali I., Losa M., Capella C. Localization of carboxyl ester lipase in human pituitary gland and pituitary adenomas. J. Histochem. Cytochem. 2010;58:881–889. doi: 10.1369/jhc.2010.956169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zebracka-Gala J., Rudnik A., Hasse-Lazar K., Larysz D., Jarzab M., Krajewska J., Bazowski P., Jarzab B. Molecular classification of pituitary adenomas: In search for criteria useful for high-throughput studies. Endokrynol. Pol. 2016;67:148–156. doi: 10.5603/EP.a2016.0024. [DOI] [PubMed] [Google Scholar]
- 80.Woloschak M., Roberts J.L., Post K. C-myc, c-fos, and c-myb gene expression in human pituitary adenomas. J. Clin. Endocrinol. Metab. 1994;79:253–257. doi: 10.1210/jcem.79.1.8027238. [DOI] [PubMed] [Google Scholar]
- 81.Jiang Z., Gui S., Zhang Y. Analysis of differential gene expression by bead-based fiber-optic array in nonfunctioning pituitary adenomas. Horm. Metab. Res. 2011;43:325–330. doi: 10.1055/s-0031-1271748. [DOI] [PubMed] [Google Scholar]
- 82.Tohti M., Li J., Tang C., Wen G., Abdujilil A., Yizim P., Ma C. Serum agr2 as a useful biomarker for pituitary adenomas. Clin. Neurol. Neurosurg. 2017;154:19–22. doi: 10.1016/j.clineuro.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Zhou X., Gomez-Smith M., Qin Z., Duquette P.M., Cardenas-Blanco A., Rai P.S., Harper M.E., Tsai E.C., Anisman H., Chen H.H. Ablation of lmo4 in glutamatergic neurons impairs leptin control of fat metabolism. Cell. Mol. Life Sci. 2012;69:819–828. doi: 10.1007/s00018-011-0794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marzec M., Zhang Q., Goradia A., Raghunath P.N., Liu X., Paessler M., Wang H.Y., Wysocka M., Cheng M., Ruggeri B.A., et al. Oncogenic kinase npm/alk induces through stat3 expression of immunosuppressive protein cd274 (pd-l1, b7-h1) Proc. Natl. Acad. Sci. USA. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M., et al. Ncbi geo: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tippmann S. Programming tools: Adventures with r. Nature. 2015;517:109–110. doi: 10.1038/517109a. [DOI] [PubMed] [Google Scholar]
- 87.Lazar C., Meganck S., Taminau J., Steenhoff D., Coletta A., Molter C., Weiss-Solis D.Y., Duque R., Bersini H., Nowe A. Batch effect removal methods for microarray gene expression data integration: A survey. Brief. Bioinform. 2013;14:469–490. doi: 10.1093/bib/bbs037. [DOI] [PubMed] [Google Scholar]
- 88.Fu J., Tang J., Wang Y., Cui X., Yang Q., Hong J., Li X., Li S., Chen Y., Xue W., et al. Discovery of the consistently well-performed analysis chain for swath-ms based pharmacoproteomic quantification. Front. Pharmacol. 2018;9:681. doi: 10.3389/fphar.2018.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang J., Fu J., Wang Y., Li B., Li Y.H., Yang Q., Cui X., Hong J., Li X., Chen Y.Z., et al. Anpela: Analysis and performance-assessment of the label-free quantification workflow for metaproteomic studies. Brief. Bioinform. 2019 doi: 10.1093/bib/bby127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smyth G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3:3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 91.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for rna-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grames M.S., Dayton R.D., Jackson K.L., Richard A.D., Lu X., Klein R.L. Cre-dependent aav vectors for highly targeted expression of disease-related proteins and neurodegeneration in the substantia nigra. FASEB J. 2018;32:4420–4427. doi: 10.1096/fj.201701529RR. [DOI] [PubMed] [Google Scholar]
- 93.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pochet N., De Smet F., Suykens J.A., De Moor B.L. Systematic benchmarking of microarray data classification: Assessing the role of non-linearity and dimensionality reduction. Bioinformatics. 2004;20:3185–3195. doi: 10.1093/bioinformatics/bth383. [DOI] [PubMed] [Google Scholar]
- 95.Li B., Tang J., Yang Q., Cui X., Li S., Chen S., Cao Q., Xue W., Chen N., Zhu F. Performance evaluation and online realization of data-driven normalization methods used in lc/ms based untargeted metabolomics analysis. Sci. Rep. 2016;6:38881. doi: 10.1038/srep38881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murakami Y., Spriggs R.V., Nakamura H., Jones S. Piranha: A server for the computational prediction of rna-binding residues in protein sequences. Nucleic Acids Res. 2010;38:W412–W416. doi: 10.1093/nar/gkq474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simurina M., de Haan N., Vuckovic F., Kennedy N.A., Stambuk J., Falck D., Trbojevic-Akmacic I., Clerc F., Razdorov G., Khon A., et al. Glycosylation of immunoglobulin g associates with clinical features of inflammatory bowel diseases. Gastroenterology. 2018;154:1320–1333. doi: 10.1053/j.gastro.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li B., Tang J., Yang Q., Li S., Cui X., Li Y., Chen Y., Xue W., Li X., Zhu F. Noreva: Normalization and evaluation of ms-based metabolomics data. Nucleic Acids Res. 2017;45:W162–W170. doi: 10.1093/nar/gkx449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brindisi M., Senger J., Cavella C., Grillo A., Chemi G., Gemma S., Cucinella D.M., Lamponi S., Sarno F., Iside C., et al. Novel spiroindoline hdac inhibitors: Synthesis, molecular modelling and biological studies. Eur. J. Med. Chem. 2018;157:127–138. doi: 10.1016/j.ejmech.2018.07.069. [DOI] [PubMed] [Google Scholar]
- 100.Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P., et al. The string database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.