Abstract
Salvia species find widespread application in food and pharmaceutical products owing to their large polyphenol content. The main polyphenols in Chinese Salvia species are phenolic acids and flavonoids, which exhibit anti-oxygenation, anti-ischemia-reperfusion injury, anti-thrombosis, anti-tumour, and other therapeutic effects. However, there are few peer-reviewed studies on polyphenols in Chinese Salvia species, especially flavonoids. This review is a systematic, comprehensive collation of available information on the biosynthesis, chemistry, and pharmacology of Chinese Salvia species. We believe that our study makes a significant contribution to the literature because this review provides a detailed literary resource on the currently available information on various polyphenolic components of Chinese Salvia species, including their bioactivities and structures. In addition, the study provides information that would encourage further investigation of this plant material as a natural resource with potential for a broad range of applications in various industries, such as the food and pharmaceutical industries.
Keywords: biosynthesis, chemistry, polyphenols, phenolic acids, pharmacology, Salvia
1. Introduction
The genus Salvia is a dominant genus of the Lamiaceae family, which is extensively distributed in China (84 species). The primary phytochemical constituents of Chinese Salvia species include sesquiterpenes (e.g., plebeiolide C and trijugins A), diterpenoids (e.g., tanshinone IIA and dihydrotanshinone I), triterpenoids (e.g., 1β,11α-dihydroxyolean-18-en-3-one and 1β,11α,20-trihydroxy-lupan-3-one) [1], phenolic acids (e.g., 3,4-dihydroxycinnamicacid (caffeic acid) and 3-[3,4-dihydroxyphenyl]lactic acid (danshensu)) [2], flavonoids (e.g., luteolin and gardenin-B) [3], alkaloids (e.g., isosalvamines C and salvianan) [4], and saccharides [5]. The main bioactive ingredients in Salvia species are polyphenolic compounds (phenolic acids and flavonoids). Phenolic acids have diverse structures (monomers, dimers, trimers, tetramers, and multimers) and are present in high concentrations, which has important taxonomic significance for Chinese Salvia species [2]. For example, the monomer caffeic acid is the basic phenolic acid compound. The caffeic acid dimer, rosmarinic acid, is considered the chemotaxonomic marker at the subfamily level in Lamiaceae [6]. In addition, phenolic acids mediate the major pharmacological activities of Chinese Salvia species, such as anti-oxygenation [7], anti-ischemia-reperfusion injury [8], and anti-thrombosis [9] effects. Owing to their high nutritional value, Chinese Salvia species play an important role in the food [10,11], pharmaceutical [12,13], and cosmetic industries [14].
Polyphenols are a class of important secondary metabolites with multiple phenolic hydroxyl groups, and include flavonoids, phenolic acids, stilbenes, and tannins (hydrolysable and condensed) [15], which are mainly synthesized by the phenylpropanoid metabolic pathway [16]. They possess various pharmacological activities, such as anti-cardiovascular [17], anti-oxidation [18], anti-inflammatory [19], and anti-tumour [20] effects, and are widely distributed among Chinese Salvia species [21]. There has been increasing attention on research and development of Chinese Salvia species because of the nutritional potential of polyphenols.
This review focuses on the chemical and pharmacological properties of polyphenols in Chinese Salvia species. Moreover, it highlights the biosynthetic pathways of polyphenols.
2. Biosynthetic Pathway of Phenolic Acids
The biosynthetic pathways of polyphenols include the shikimic acid and phenylpropanoid metabolism pathways [22]. The polyphenols in Salvia species are mainly produced by the phenylpropanoid metabolic pathway [16,23,24], and most derivatives have similar basic structures [25]. Phenylalanine and tyrosine are precursor compounds of the phenylpropanoid metabolic pathway, and their biosynthetic pathways constitute two parallel branches of this pathway, which involve five rate-limiting enzymes [26,27]. The enzymes are phenylalanine ammonia-lyase (PAL; a key regulatory enzyme in plant metabolism), cinnamic acid-4-hydroxylase (C4H) and 4-coumarate: coenzyme A (CoA) ligase (a key regulatory enzyme in the phenylalanine branch), tyrosine aminotransferase (the first key enzyme and rate-limiting enzyme in the tyrosine metabolism pathway), and rosmarinic acid synthase (a key enzyme in catalytic synthesis) [28,29].
The phenolic acids of Salvia species are caffeic acid derivatives, which are mostly formed by esterification of caffeic acid with danshensu [2,30]. Caffeic acid belongs to the class of phenylpropionic acids [31], and is the basic structural unit of phenolic acids [32]. The precursor compound of caffeic acid is phenylalanine, which produces caffeic acid through the action of PAL and C4H enzymes. It plays a central role in the phenylpropanoid metabolic pathway, and is a precursor compound of rosmarinic acid [28]. Studies have speculated that, in the main synthetic route of rosmarinic acid, caffeic acid is first catalysed to caffeoyl CoA. Subsequently, caffeoyl CoA and 4-hydroxyphenyllactic acid are catalysed by hydroxycinnamoyl-CoA: hydroxyphenyllactate hydroxycinnamoyl transferase (rosmarinic acid synthase (RAS)) to generate caffeoyl-4′-hydroxyphenyllactic acid (caffeoyl-4′-HPLA). Finally, it is catalysed by CYP98A14 to rosmarinic acid [33]. Salvianolic acid E is formed by rosmarinic acid under the action of enzymes and other reactions, and then transformed into salvianolic acid B and other compounds. This observation suggests that rosmarinic acid is the core constituent unit of a series of complex phenolic acids, such as salvianolic acids [34,35,36]. The synthesis of rosmarinic acid has great significance for the formation of complex phenolic compounds [25].
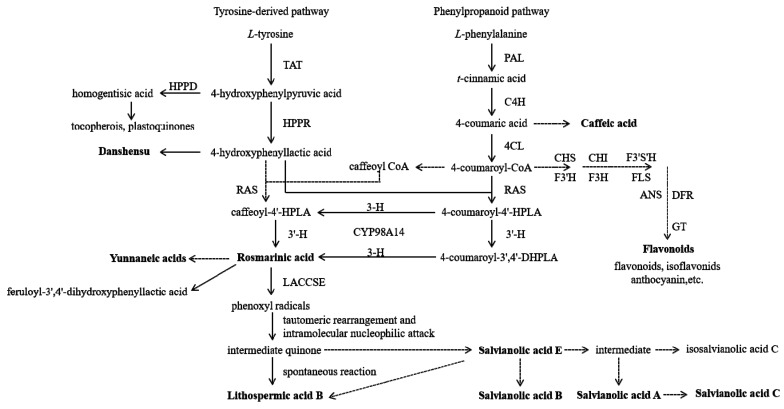
Moreover, the phenylpropanoid metabolic pathway is an important upstream pathway for producing flavonoids (e.g., anthocyanins, flavonoids, and isoflavonoids) [37,38,39,40]. The biosynthetic pathway of phenolic acids in Salvia species is shown in Figure 1.
Figure 1.
The biosynthetic pathway of phenolic acids in Salvia species. TAT: tyrosine aminotransferase; HPPR: 4-hydroxyphenylpyruvate reductase; HPPD: 4-hydroxyphenylpyruvated dioxygenase; PAL: phenylalanine ammonia-lyase; C4H: cinnamic acid 4-hydroxylase; 4CL: 4-coumarate-CoA ligase; RAS: hydroxycinnamoyl-CoA: hydroxyphenyllactate hydroxycinnamoyl transferase; caffeoyl-4′-HPLA: caffeoyl-4′-hydroxyphenyllactic acid; 4-coumaroyl-4′-HPLA: 4-coumaroyl-4′-hydroxyphenyllactic acid; 4-coumaroyl-3′,4′-DHPLA: 4-coumaroyl-3′,4′-dihydroxyphenyllactic acid; 3-H: hydroxycinnamoyl-hydroxyphenyllactate 3-hydroxylase, 3′-H: hydroxycinnamoyl-hydroxyphenyllactate 3′-hydroxylase. Solid line: the verified biosynthesis process; dotted line: proposed biosynthesis processes.
3. Chemical Constituents and Structure of Polyphenols
The polyphenolics in Chinese Salvia species are phenolic acids, flavonoids, and anthocyanins [41]. Phenolic acids are one of the main active components [42].
3.1. Phenolic Acids
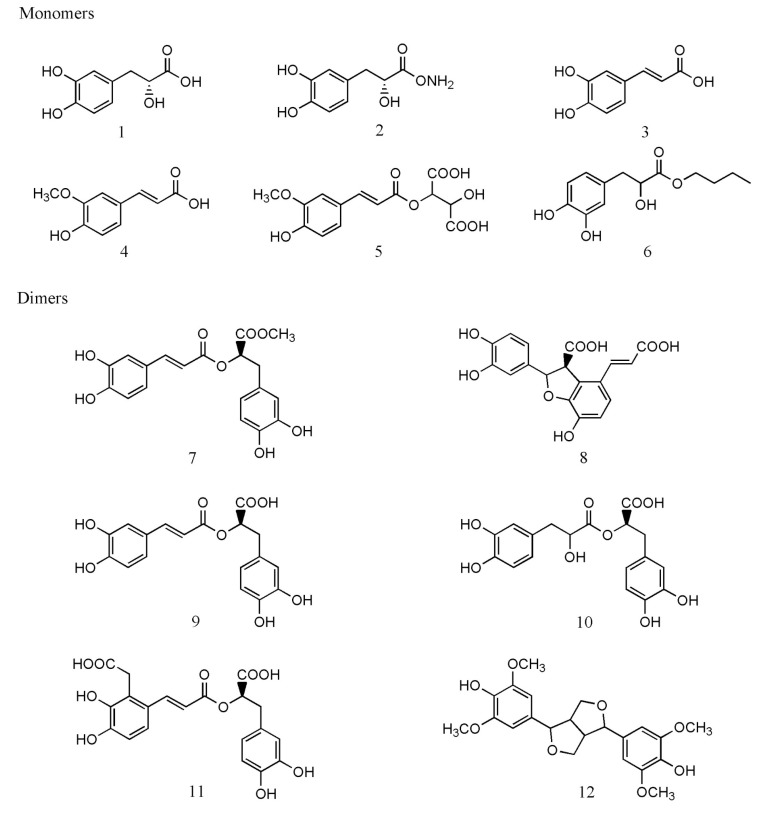
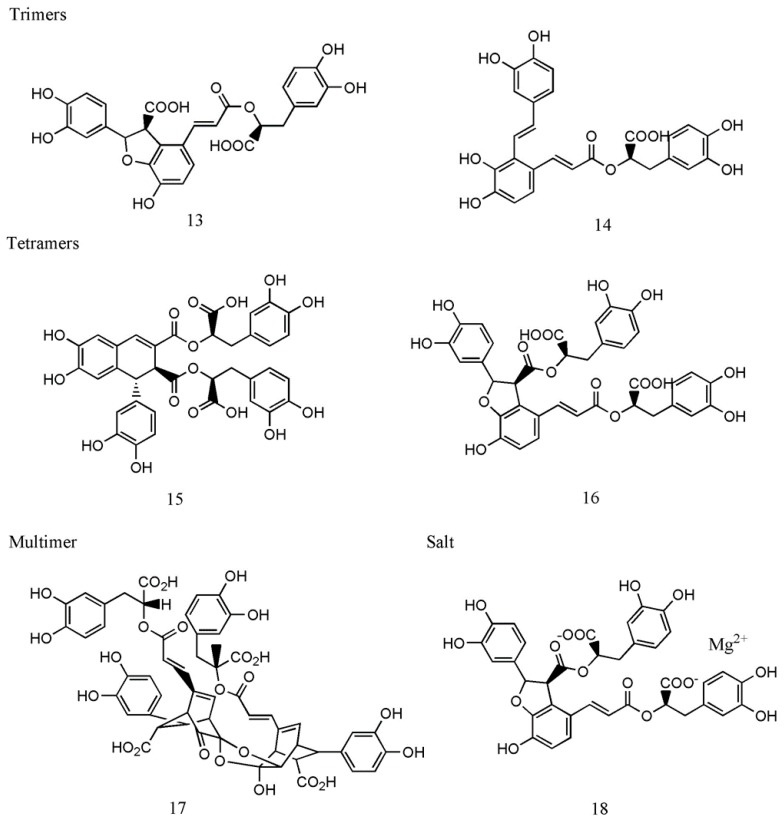
Caffeic acid and danshensu are the structural units of phenolic acids in Salvia species [2,43]. Phenolic acids can be classified as caffeic acid monomers, dimers, trimers, tetramers, and multimers based on their polymerization degree [2,44,45,46]. Phenolic acids and their representative structures are shown in Table 1 and Figure 2, respectively.
Table 1.
The phenolic acids in Salvia species.
| NO. | Structures | Compound | Species | References |
|---|---|---|---|---|
| 1 | Monomers | (2,3,4-trihydroxy-3-methyl)butyl-6-feruloylglucoside | S. officinalis | [55] |
| 2 | 1-caffeoyl-6-apiosyl-glucoside | S. officinalis | [56] | |
| 3 | (3-methoxy-4-glucosyloxyphenyl)-3-hydroxymethyl-5-(3-hydroxypropyl)-7-methoxy-2,3-dihydrobenzofuran | S. officinalis | [57] | |
| 4 | 3-(3,4-dihydroxyphenyl)lactic acid(danshensu) (1) | S. miltiorrhiza | [58] | |
| S. chinensis | [59] | |||
| S. prionitis | [60] | |||
| S. sonchifolia | [61] | |||
| 5 | 3-(3,4-dihydroxyphenyl)lactamide (2) | S. miltiorrhiza | [62] | |
| 6 | 3,4-dihydroxycinnamic acid(caffeic acid) (3) | S. miltiorrhiza | [63] | |
| S. bowleyana | [63] | |||
| S. chinensis | [59] | |||
| S. officinalis | [55] | |||
| S. plebeia | [64] | |||
| S. sonchifolia | [61] | |||
| 7 | 3-methoxy-4-hydroxybenzoic acid (vanillic acid) | S. officinalis | [65] | |
| 8 | 4-hydroxyacetophenone 4-(2-(5-syringoyl)apiosyl)glucoside | S. officinalis | [55] | |
| 9 | 4-hydroxybenzoic acid | S. officinalis | [66] | |
| 10 | 4-hydroxyphenyllactic | S. plebeia | [65,67] | |
| 11 | 6-caffeoyl-1-fructosyl-a-glucoside | S. officinalis | [56] | |
| 12 | 6-feruloyl-a-glucose | S. officinalis | [57] | |
| 13 | 6-feruloyl-b-glucose | S. officinalis | [57] | |
| 14 | ailanthoidol | S.miltiorrhiza | [68] | |
| 15 | coniferyl aldehyde | S. plebeia | [69] | |
| 16 | ferulic acid (4) | S. officinalis | [70] | |
| 17 | isoferulic acid | S. miltiorrhiza | [70] | |
| 18 | methyl 3,4-dihydroxyphenyllactate | S. plebeia | [71] | |
| 19 | m-hydroxybenzaldehyde | S. przewalskii | [72] | |
| 20 | mono-feruloyltartaric acid (5) | S. chinensis | [64] | |
| 21 | n-butyl 3,4-dihydroxyphenyllactate (6) | S. plebeia | [71] | |
| 22 | p-hydroxycinnamic acid | S. miltiorrhiza | [73] | |
| 23 | prionitiside A | S. prionitis | [60] | |
| 24 | prionitiside B | S. prionitis | [74] | |
| 25 | protocatechuic acid | S. miltiorrhiza | [75] | |
| S. sonchifolia | [55] | |||
| 26 | protocatechuic aldehyde | S. miltiorrhiza | [75] | |
| 27 | salvinal | S.miltiorrhiza | [76] | |
| 28 | salviaplebeiaside | S. plebeia | [77] | |
| 29 | Dimers | 1-hydroxypinoresinol 1-glucoside | S. officinalis | [57] |
| 30 | 2-(3-methoxy-4-hydroxyphenyl)-5-(3-hydroxypropyl)-7-methoxybenzofuran-3-carbaldehyde | S. miltiorrhiza | [68,78] | |
| 31 | isolariciresinol 3α-glucoside | S. officinalis | [79] | |
| 32 | isolariciresinol di(12-methylmyristate) | S. plebeia | [80] | |
| 33 | isosalvianolic acid C | S. cavaleriei | [59] | |
| 34 | methyl rosmarinate (7) | S.miltiorrhiza | [56] | |
| S. bowleyana | [63] | |||
| S. prionitis | [60] | |||
| 35 | prolithospermic acid (przewalskinic acid A) (8) | S.miltiorrhiza | [2] | |
| 36 | rosmarinic acid (9) | S. miltiorrhiza | [81] | |
| 37 | salvianic acid C (10) | S. miltiorrhiza | [2] | |
| 38 | S. cavaleriei | [60] | ||
| S. bowleyana | [63] | |||
| S. prionitis | [60] | |||
| salvianolic acid D (11) | S. miltiorrhiza | [70] | ||
| 39 | S. chinensis | [74] | ||
| salvianolic acid F | S.miltiorrhiza | [2] | ||
| 40 | salvianolic acid G | S.miltiorrhiza | [2] | |
| 41 | salvianolic acid N | S. yunnanensis | [82] | |
| 42 | salviaflaside | S. flava | [82] | |
| 43 | salviaflaside methyl ester | S. flava | [83] | |
| 44 | Syringaresinol (12) | S. plebeia | [84] | |
| 45 | Trimers | 9″-methyl lithospermate | S. miltiorrhiza | [81] |
| 46 | cis-lithospermic acid | S. yunnanensis | [85] | |
| 47 | dimethyl lithospermate | S. miltiorrhiza | [81] | |
| 48 | ethyl lithospermate | S.miltiorrhiza | [70] | |
| 49 | ethyl salvianolate A | S. yunnanensis | [60] | |
| 50 | feruloylisolariciresinol 12-methylmyristate | S. plebeia | [86] | |
| 51 | lithospermic acid (13) | S. miltiorrhiza | [81] | |
| 52 | lithospermic acid dimethyl ester | S. miltiorrhiza | [81] | |
| 53 | lithospermic acid monomethyl ester | S. miltiorrhiza | [81] | |
| 54 | methyl salvianolate A | S. yunnanensis | [85] | |
| 55 | methyl salvianolate I | S. officinalis | [79] | |
| 56 | methyl salvianolic acid C | S.miltiorrhiza | [87] | |
| 57 | sagecoumarin | S. officinalis | [79] | |
| 58 | salvianolic acid A (14) | S. miltiorrhiza | [88] | |
| 59 | S. cavaleriei | [60] | ||
| S. flava | [89] | |||
| S. yunnanensis | [85] | |||
| salvianolic acid C | S. miltiorrhiza | [90] | ||
| 60 | salvianolic acid H | S. cavaleriei | [91] | |
| 61 | salvianolic acid I | S. officinalis | [79] | |
| 62 | S. cavaleriei | [89] | ||
| salvianolic acid J | S. flava | [92] | ||
| 63 | salvianolic acid K | S. deserta | [79] | |
| 64 | salvianolic acid T | S. miltiorrhiza | [82] | |
| 65 | salvianolic acid U | S. miltiorrhiza | [82] | |
| 66 | yunnaneic acid C | S. yunnanensis | [51,93] | |
| 67 | yunnaneic acid D | S. yunnanensis | [51,93] | |
| 68 | yunnaneic acid E | S. yunnanensis | [94] | |
| 69 | yunnaneic acid F | S. yunnanensis | [94] | |
| 70 | Tetramers | 9′-monomethyl lithospermate B | S. przewalskii | [95] |
| 71 | 9‴-monomethyl lithospermate B | S. przewalskii | [95] | |
| 72 | S. miltiorrhiza | [96] | ||
| dimethyl lithospermate B | S. przewalskii | [95] | ||
| 73 | S. miltiorrhiza | [96] | ||
| ethyl lithospermate B | S. miltiorrhiza | [70] | ||
| 74 | Rabdosiin (15) | S. yunnanensis | [97] | |
| 75 | sagerinic acid | S. officinalis | [79] | |
| 76 | salvianolic acid B(lithospermic acid B) (16) | S. miltiorrhiza | [90] | |
| 77 | salvianolic acid E | S. miltiorrhiza | [70] | |
| 78 | salvianolic acid L | S. officinalis | [98] | |
| 79 | yunnaneic acid G | S. yunnanensis | [94] | |
| 80 | yunnaneic acid H | S. yunnanensis | [94] | |
| 81 | Multimers | yunnaneic acids A (17) | S. yunnanensis | [51,93] |
| 82 | yunnaneic acid B | S. yunnanensis | [51,93] | |
| 83 | Salts | ammonium-potassium lithospermate B | S. miltiorrhiza | [99] |
| 84 | sodium danshensu | S. miltiorrhiza | [73] | |
| 85 | magnesium lithospermate B (18) | S. miltiorrhiza | [99] |
Figure 2.
The representative structures of phenolic acids in Salvia species.
Caffeic acid monomers mainly consist of caffeic acid, danshensu, protocatechuic acid, and protocatechuic aldehyde. Caffeic acid is the basic compound of caffeic acid monomers [32]. Danshensu is also a basic component of caffeic acid derivatives in plant metabolites and the hydrolysate of caffeic acid [2,3,43,46,47]. Rosmarinic acid and salvianolic acids D, F, and G are caffeic acid dimers [43]. Rosmarinic acid is the simplest caffeic acid dimer, which was first isolated from Rosmarinus officinalis L. in 1958 [48]. It has a high taxonomic significance in Salvia species [6]. Salvianic acid C is synthesized by the condensation of two molecules of caffeic acid [3,36]. Caffeic acid trimers comprise the following members: salvianolic acid A, lithospermic acid, and yunnaneic acid C [2,3]. Structurally, salvianolic acid A has a similar structure to that of salvianolic acid F. It has been speculated that salvianolic acid A is the product of salvianolic acid F and danshensu synthesis [43,47,49]. Lithospermic acid is a typical trimer that is broadly distributed in Chinese Salvia species [6]. Caffeic acid tetramers can be regarded as derivatives of rosmarinic acid dimers [43]. They mainly contain salvianolic acid B (typical tetramer), salvianolic acid E, and yunnaneic acid G [25,36,47]. Salvianolic acid B has potential taxonomic value in Salvia species and represents the main active constituent [6]. Yunnaneic acid A and B are the caffeic acid multimer extracted from the roots of Salvia yunnanensis C. H. Wright. They are hexamers of caffeic acid and regarded as products of the combination of caffeic acid and rosmarinic acid. Yunnaneic acid B contains two units of yunnaneic acid C, while yunnaneic acid A is a combination of yunnan acid C and D [50,51].
Presently, three compounds of phenolic acid salts, namely sodium danshensu, magnesium lithospermate B, and ammonium-potassium lithospermate B, have been detected in Salvia species [43]. Magnesium lithospermate B and ammonium-potassium lithospermate B are magnesium and ammonium-potassium compounds of the tetramer salvianolic acid B. Magnesium lithospermate B has antioxidative [52], anti-liver injury [53], and anti-myocardial ischemia-reperfusion injury effects [54].
3.2. Other Compounds
In addition to phenolic acids, other phenolic compounds, such as flavonoids and anthocyanins, are also present in Salvia species [100,101,102]. Based on the biosynthesis pathway of phenolic acids, flavonoids are known to be produced through the phenylpropanoid pathway [37,38]. Currently, flavonoids are isolated from natural plants, such as Salvia plebeia R. Br. and Salvia miltiorrhiza Bunge [103]. Furthermore, research studies have reported the total flavonoid content in Salvia cavaleriei Levl. to be 14.69% [104].
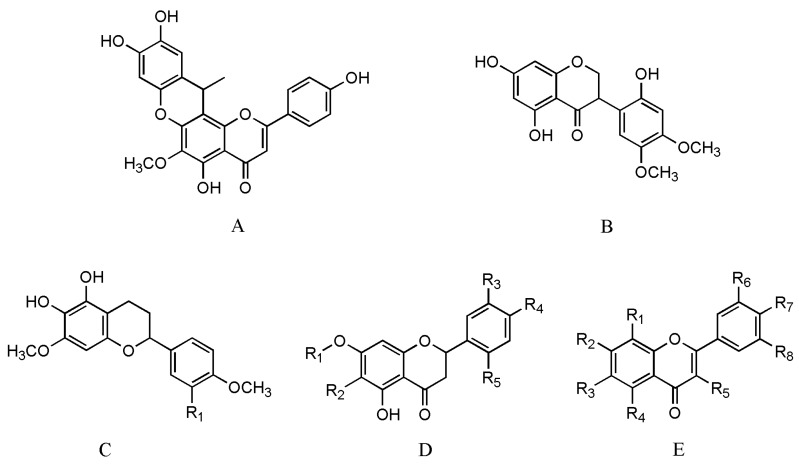
Moreover, Salvia species contain anthocyanin and tannin compounds. The compounds salvianin and monardalins are obtained from Salvia coccinea L. and Salvia splendens Ker-Gawl., which are good natural spices [105,106]. The flavonoids in Salvia species are shown in Table 2 and Figure 3.
Table 2.
The flavonoids in Salvia species.
| NO. | Compound | Structures | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Species | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 86 | neocafhispidulin | A | S. plebeia | [69] | ||||||||
| 87 | 2′-hydroxy-5′-methoxybiochanin A | B | S. plebeia | [69] | ||||||||
| 88 | 5,6-dihydroxy-7,4′-dimethoxyflavone | C | H | S. plebeia | [107] | |||||||
| 89 | 5,6,3′-trihydroxy-7,4′-dimethoxyflavone | OH | S. plebeia | [108] | ||||||||
| 90 | 5,7,3′-trihydroxy-4′-methoxyflavanone (hesperetin) | D | H | H | OH | OCH3 | H | S. officinalis | [65] | |||
| 91 | 6-methoxynaringenin | H | OCH3 | H | OH | H | S. plebeia | [107] | ||||
| 92 | 5,3′-dihydroxy-7,4′-dimethoxyflavanone | CH3 | H | OH | OCH3 | H | S. miltiorrhiza | [109] | ||||
| 93 | (2S)-5,7,4′-trihydroxy-6-methoxy-flavanone-7-O-β-D-glucopyran-oside | Glc | OCH3 | H | OH | H | S. plebeia | [108] | ||||
| 94 | 5,7,3′,4′-tetrahydroxy-6-methoxy-flavanone-7-O-β-D-glucopyran-oside | Glc | OCH3 | OH | OH | H | S. plebeia | [108] | ||||
| 95 | 5,6,7,4′-tertrahydroxyflavone | E | H | H | H | OH | H | H | OH | H | S. plebeia | [110] |
| 96 | luteolin | H | OH | H | OH | H | OH | OH | H | S. plebeia | [111] | |
| 97 | apigenin | H | OH | H | OH | H | H | OH | H | S. plebeia | [85] | |
| 98 | kaempferol | H | OH | H | OH | OH | H | OH | H | S. roborowskii | [112] | |
| 99 | -3′-methyl ether (isorhamnetin) | H | OH | H | OH | OH | H | OH | OCH3 | S. farinacea | [113] | |
| 100 | quercetin | H | OH | H | OH | OH | OH | OH | H | S. plebeia | [114] | |
| 101 | quercimelin | H | OH | H | OH | Rham | OH | OH | H | S. roborowskii | [115] | |
| 102 | myricitrin | H | OH | H | OH | Rham | OH | OH | OH | S. roborowskii | [112] | |
| 103 | rutin | H | OH | H | OH | Rham-Glc | OH | OH | H | S. roborowskii | [115] | |
| 104 | 6-hydroxyapigenin (scutellarein) | H | OH | OH | OH | H | H | OH | H | S. officinalis | [65] | |
| 105 | 6-hydroxyluteolin 5-O-glucoside | H | OH | OH | OGlc | H | H | OH | OH | S. tomentosa | [116] | |
| 106 | hispidulin | H | OH | OCH3 | OH | H | H | OH | H | S. plebeia | [108] | |
| 107 | pectolinarigenin | H | OH | OCH3 | OH | H | H | OCH3 | H | S. plebeia | [108] | |
| 108 | nepetin | H | OH | OCH3 | OH | H | OH | OH | H | S. officinalis | [117] | |
| 109 | jaceosidin | H | OH | OCH3 | OH | H | OCH3 | OH | H | S. plebeia | [108] | |
| 110 | eupatilin | H | OH | OCH3 | OH | H | OCH3 | OCH3 | H | S. plebeia | [114] | |
| 111 | gehkwahin | H | OCH3 | H | OH | H | H | OH | H | S. officinalis | [118] | |
| 112 | -7,4′-dimethyl ether | H | OCH3 | H | OH | H | H | OCH3 | H | S. officinalis | [3] | |
| 113 | kumalakenin | H | OCH3 | H | OH | OCH3 | H | OH | H | S. officinalis | [118] | |
| 114 | ayamin | H | OCH3 | H | OH | OCH3 | OH | OCH3 | H | S. officinalis | [118] | |
| 115 | sorbifolin | H | OCH3 | OH | OH | H | H | OH | H | S. plebeia | [108] | |
| 116 | cirsimaritin | H | OCH3 | OCH3 | OH | H | H | OH | H | S. officinalis | [118] | |
| 117 | cirsiliol | H | OCH3 | OCH3 | OH | H | H | OH | OH | S. plebeia | [107] | |
| 118 | eupatorin | H | OCH3 | OCH3 | OH | H | H | OCH3 | H | S. plebeia | [119] | |
| 119 | 5,6,7,4′-tetramethyl ether | H | OCH3 | OCH3 | OCH3 | H | H | OCH3 | H | S. officinalis | [120] | |
| 120 | cynaroside | H | OGlc | H | OH | H | OH | OH | H | S. plebeia | [114] | |
| 121 | hispidulin-7-O-D-glucoside | H | OGlc | OCH3 | OH | H | H | OH | H | S. plebeia | [69] | |
| 122 | nepitrin | H | OGlc | OCH3 | OH | H | OH | OH | H | S. plebeia | [108] | |
| 123 | cosmosiin | H | OGlu | H | OH | H | H | OH | H | S. deserta | [117] | |
| 124 | 6-hydroxyluteolin-7-glucoside | H | OGlc | OH | OH | H | OH | OH | H | S. plebeia | [110] | |
| 125 | nepetin-7-glucoside | H | OGlu | OCH3 | OH | H | OH | OH | H | S. plebeia | [111] | |
| 126 | homoplantaginin | H | OGlu | OCH3 | OH | H | H | OH | H | S. plebeia | [108] | |
| 127 | 6″-O-acetyl homoplantaginin | H | OGlu-Ac | OCH3 | OH | H | H | OH | H | S. plebeia | [108] | |
| 128 | luteolin 7-O-(4″,6″-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside | H | ORham-Glu | H | OH | H | H | OH | OH | S. splendens | [121] | |
| 129 | chrysoeriol-7-D-xyloside | H | Oxyloside | H | OH | H | OCH3 | OH | H | S. deserta | [118] | |
| 130 | salvitin | OH | OCH3 | H | OH | H | H | OH | H | S. Plebeia | [118] |
Figure 3.
The structures of flavonoids in Salvia species.
4. Pharmacological Activities of Phenolic Acids
Phenolic acids are the main active polyphenols in Chinese Salvia species with an active function in promoting health. Phenolic acids are rich in phenolic hydroxyl groups. Accordingly, representative compounds have been reported to possess a wide variety of activities, including anti-oxygenation [7], anti-ischemia-reperfusion injury [8], and anti-thrombosis [9] effects.
4.1. Anti-Oxygenation Activity
Oxidative damage is mainly caused by substances, such as free radicals, present inside and outside the cell. The inability to remove a large amount of free radicals in a timely manner causes dynamic imbalances in the redox system of the body. The antioxidant effects of phenolic acids involve three processes: free radical scavenging, inhibiting free radical generation, and anti-lipid peroxidation [8,122,123].
Phenolic acids possess radical scavenging activity. A study showed that, at 0–0.7 mmol·L−1, rosmarinic acid, caffeic acid, and danshensu were as effective as quercetin (positive control), which scavenged 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals in a concentration-dependent manner. In contrast, ferulic acid was less effective [124]. Moreover, other studies found that salvianolic acid B and danshensu exhibited higher scavenging activities against HO·, O2−, DPPH, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) than the other constituents. Among them, the half-maximal inhibitory concentration (IC50) of danshensu and salvianolic acid B were 94 and 102 μg·mL−1, respectively, and there was no obvious difference in the scavenging ability of hydroxyl radicals [7]. Thus, phenolic acids are considered to be hydroxyl radical scavengers.
The antioxidant activities of rosmarinic acid and salvianolic acid B in plant extracts were determined using DPPH radical scavenging, superoxide radical quenching, a β-carotene-linoleic acid system, and reductive potential assays. The data indicated that salvianolic acid B and rosmarinic acid, which were abundantly found in Salvia miltiorrhiza leaves, had strong antioxidant activity. Among them, salvianolic acid B was positively correlated with DPPH scavenging activity (correlation coefficient (r) = 0.693, P < 0.05), reducing ability (r = 0.748, P < 0.01), and inhibition of linoleic acid oxidation (r = 0.804, P < 0.01). It could be regarded as a new possible resource of natural phenolic antioxidants [125].
Studies have shown that the order of inhibition of spontaneous lipid peroxidation in liver tissue of mice by the following constituents is rosmarinic acid (minimum inhibitory concentration (MIC): 12.5 μg·mL−1, 54.5%), protocatechuic aldehyde (50.1%), chlorogenic acid (46.9%), caffeic acid (MIC: 50.0 μg·mL−1, 38.4%), ferulic acid (MIC: 50.0 μg·mL−1, 36.1%), danshensu (MIC: 50.0 μg·mL−1, 36.3%), and 3-hydroxycinnamic acid (32.2%). However, the protective effects of various constituents on hydrogen peroxide (H2O2)-induced liver lipid peroxidation in mice showed the following values: rosmarinic acid (MIC: 49.9 μg·mL−1, 99.0%), caffeic acid (98.9%), protocatechuic aldehyde (98.3%), chlorogenic acid (90.0%), ferulic acid (83.2%), danshensu (54.3%), and 3-hydroxycinnamic acid (42.1%). At the same time, the total radical reducing activities of the seven phenolic acids were determined to be consistent with the above results [126].
4.2. Anti-Myocardial/Cerebral Ischemia-Reperfusion Injury Activity
The phenolic acids in Salvia species are water-soluble components that are active against cardiovascular diseases. The pathophysiological mechanism of myocardial/cerebral ischemia-reperfusion injury is complex, and phenolic acids act against it through mechanisms such as regulation of active oxygen metabolism, inhibition of inflammatory reaction, apoptosis, and calcium overload [2,8].
Salvianolic acid B was found to effectively reduce myocardial ischemia-reperfusion injury. The experiment established an ischemia-reperfusion model by ligating the left circumflex artery in Sprague–Dawley (SD) rats. The effects of different doses of salvianolic acid B (20, 40, and 60 mg·d−1·kg−1) against myocardial ischemia-reperfusion injury were determined by measuring the concentration and apoptotic index of the plasma level of myocardial enzymes (cardiac troponins (CTn) I and creatine kinase-MB (CKMB)), malondialdehyde (MDA), endothelin (ET), nitric oxide (NO), and superoxide dismutase (SOD), and histological changes of the heart. The effects were superior in the salvianolic acid B high-dose group (60 mg·d−1·kg−1). The results showed that salvianolic acid B significantly increased the plasma CTn I, CKMB, MDA, and ET contents; decreased NO and T-SOD contents; reduced the infarct size; and improved myocardial ultrastructure changes. The study showed that salvianolic acid B protected against conditions such as myocardial ischemia-reperfusion injury by regulating active oxygen metabolism, reducing oxidative stress, and myocardial apoptosis [127].
Salvianolic acid A protected rats against cerebral ischemia-reperfusion injury by inhibiting the expression of matrix metalloproteinase (MMP)-9 and inflammatory response. Cerebral ischemia-reperfusion injury significantly upregulates the expression of MMP-9, leading to severe damage of the blood–brain barrier. Furthermore, it activates inflammatory response, produces oxygen free radicals, or releases lysosomal enzymes to damage tissue. Studies have shown that the level of MMP-9 in salvianolic acid A-treated groups (5, 10, and 20 mg·kg−1) was downregulated, tissue inhibitor of metalloproteinase-1 (TIMP-1) was upregulated, and damage to the blood–brain barrier was reduced. Salvianolic acid A inhibits activation of cerebral nuclear factor (NF)-κB p65 and reduces inflammatory response. Salvianolic acid A could be used as an effective drug to alleviate cerebral ischemia-reperfusion injury [128]. In addition, it can prevent myocardial ischemia-reperfusion injury under a high glucose condition by regulating the NADPH oxidase 2 (Nox2)/reactive oxygen species (ROS)/phosphorylated-c-Jun N-terminal kinase 2 (p-JNK2)/NF-κB pathway to reduce transient receptor potential cation channel, subfamily C, member 6 (TRPC6)/Ca2+ influx [129].
4.3. Anti-Thrombosis Activity
Thrombosis can occur anywhere in the blood circulation, causing acute myocardial infarction, cerebral infarction, and pulmonary thrombosis [130]. Presently, the strategies for preventing thrombosis mainly include improving blood rheology, anti-platelet aggregation, and protecting vascular endothelial cells [131].
Studies have shown that salvianolic acids in S. miltiorrhiza have anti-thrombotic effects and they act primarily by improving blood rheology, anti-platelet aggregation, and targeting P2Y1 or P2Y12 receptors, which are new target receptors for anti-platelet aggregation. It can be seen from the experimental results that salvianolic acid B (100 µmol·L−1) only antagonizes the action of platelet P2Y12 receptors, while salvianolic acid A and C (both 100 µmol·L−1) are P2Y1 and P2Y12 receptor inhibitors [9].
Caffeic acid can inhibit platelet-mediated thrombosis. High activation of platelets is one of the major causes of thrombosis. The adhesion and aggregation of platelets are enhanced in the activated state. The results demonstrated that caffeic acids have beneficial effects on aberrant platelet activation-related diseases. Caffeic acid (25–100 μmol·L−1) repressed ADP-induced platelet aggregation, P-selectin expression, ATP release, and Ca2+ mobilization. Furthermore, it attenuates the activation of p38, ERK, integrin αIIbβ3, and JNK. It could also increase the expression level of cAMP [132].
Danshensu (15–60 mg·kg−1) had better anti-thrombotic and antiplatelet therapeutic efficacy than other constituents. It mainly contributed to intensely and selectively suppressing the expression of cyclooxygenase (COX)-2 and balancing the ratio of thromboxane A2 (TXA2)/prostacyclin (PGI2) [133].
4.4. Anti-Liver Injury Activity
Liver damage is a common pathological manifestation of various liver diseases, and further progression leads to different degrees of hepatic cell necrosis, fatty liver, liver cirrhosis, and other liver diseases. Phenolic acids are potential anti-liver damage compounds. Liver protection can be achieved through the following mechanisms: elimination of free radicals, inhibition of lipid peroxidation, and inhibition of inflammatory cytokine expression [134,135].
Salvianolic acid B (15 or 30 mg·d−1·kg−1) protects liver cells by preserving lysosomal membrane integrity through scavenging ROS and enhancing lysosome-associated membrane protein 1 (LAMP1) expression. LAMP1 acts as a barrier to soluble hydrolase, preventing cell damage and death caused by the release of luminal contents into the cytoplasm. H2O2 attack significantly decreases LAMP1 expression, thereby disrupting the integrity of hepatocyte lysosomal membrane, leading to cell damage and death. Liver cells are protected by the anti-inflammatory and anti-oxidant properties of salvianolic acid B [136].
Salvianolic acid A protects against acute hepatic injury induced by concanavalin A (ConA) in mice. The results of the liver function indicators serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) showed that salvianolic acid A (15 or 25 mg·kg−1) significantly reduced ConA-induced ALT and AST activity. In addition, it reduced hepatotoxic cytokine levels, such as tumour necrosis factor (TNF)-α and interferon (IFN)-γ; ameliorated the increased NF-κB level and cleaved caspase-3; and reversed B-cell lymphoma-extra-large (Bcl-xL) expression. Notably, pretreatment with salvianolic acid A obviously upregulated the expression of SIRT1, which could alleviate acute hypoxic injury and metabolic liver disease. The study showed that the increase in SIRT1 was closely related to the p66 isoform (p66shc) of the growth factor adapter Shc. Other studies have shown that salvianolic acid A-alleviated ConA-induced hepatitis may be inhibited by the SIRT1-mediated p66shc pathway [137].
In addition, studies have shown that the water extract of S. miltiorrhiza (0.06–1 mg·mL−1) has the same hepatoprotective effect as salvianolic acid A does, and the mechanism may be related to the reduction of extracellular histone and related cytokines. A water extract of S. miltiorrhiza contained danshensu (8.2–130.5 μmol·L−1) and salvianolic acid B (3.3–53.5 μmol·L−1) as the main compounds that protected against liver injury. However, rosmarinic acid, protocatechuic aldehyde, and salvianolic acid A had no protective effects [138].
4.5. Anti-Tumour Activity
Phenolic acids have multitarget anti-tumour effects; however, their mechanisms are more complicated. They prevent the invasion of tumour cells by inducing apoptosis of tumour cells, regulating immune function, reversing multidrug resistance of cancer cells, targeting tumour microtubules to inhibit their proliferation and division, and inhibiting metastasis of cancer cells [139,140].
Salvianolic acid B inhibits the growth of human glioma U80 cells, which may be involved in p38-activation-mediated ROS production. These experimental results indicate that salvianolic acid B (1–100 μmol·L−1) significantly reduced the cell viability of U80 cells in a dose- and time-dependent manner. At the same time, it enhanced the production of intracellular ROS and induced apoptosis of U57 cells. The anti-tumour activity of salvianolic acid B in vivo was observed in a nude mouse xenograft model. Therefore, salvianolic acid B seems to be safe and effective, and this natural component could be developed into a potential therapeutic agent for glioma [141].
4.6. Others
Phenolic acids in Salvia species exert anti-hypertensive effects [142,143], improve memory and cognitive impairment [144,145], possess hypoglycaemic [146,147] and antiviral activities [148,149], and can be used to prevent and treat cataract [150,151]. These pharmacological activities of phenolic acids in Chinese Salvia species are listed in Table 3.
Table 3.
Other activities of phenolic acids in Salvia species
| Activity | Ingredient | Model | Treatment | Result | Reference |
|---|---|---|---|---|---|
| Anti-hypertension | Caffeic acid, Chlorogenic acid | Cyclosporine-induced hypertensive rats | 10 and 15 mg·d−1·kg−1 | Caffeic acid and chlorogenic acid significantly (p < 0.05) reduced systolic blood pressure (SBP) and heart rates (HR), activity of angiotensin-1-converting enzyme (ACE), acetylcholinesterase (AChE), butrylcholinesterase (BChE), and arginase in the treated hypertensive rats. | [142] |
| Caffeic acid and chlorogenic acid improved nitric oxide (NO) bioavailability, increased catalase activity, and reduced glutathione content while the MDA level was reduced. | |||||
| Salvianolic acid A (SalA) | Spontaneously hypertensive rats (SHR) | 2.5, 5, and 10 mg·d−1·kg−1 | The inward remodeling of the retinal vein was inhibited after treatment with SalA. | [143] | |
| SalA improved the endothelial-dependent vasodilatation of mesenteric vessels for SHR in vivo. | |||||
| Transendothelial electrical resistance (TEER) significantly increased the human umbilical vein endothelial cell line (HUVEC) monolayer treated with SalA. | |||||
| SalA exhibited an obvious protective effect on the HUVEC monolayer. | |||||
| Improve memory and cognitive impairment | Rosmarinic acid | Amyloid beta (Aβ) 42-induced echoic memory decline (Rat model of Alzheimer) | 50 mg·d−1·kg−1 | It decreased the levels of thiobarbituric acid reactive substances (TBARS) and 4-Hydroxy-2- nonenal (4-HNE) but increased the activity of antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px)) and glutathione levels. | [144] |
| Rosmarinic acid (RA) attenuated the increased Aβ staining and astrocyte activation. | |||||
| RA treatment reversed the Aβ42-related alterations in auditory event related potential (AERP) parameters. | |||||
| Total salvianolic acid (TSA) | Alzheimer′s disease modelAPPswe/PS1dE9 mice | 30 and 60 mg·d−1·kg−1, intraperitoneal (i.p.) injection | Treatment with TSA substantially decreased the low-density lipoprotein (LDL)-C level, and 60 mg·kg−1 TSA decreased the cholesterol (CHOL) level. | [145] | |
| The Aβ42 and Aβ40 levels in the hippocampus were decreased. | |||||
| Hypoglycemic | Salvianolic acid B (Sal B) | Multiple low-dose streptozotocin (MLDS)-induced diabetes in rat | 20 or 40 mg·kg−1 | They caused a significant decrease of the serum glucose (p < 0.05–0.01) and an improvement in the oral glucose tolerance test (OGTT). | [146] |
| Serum insulin was significantly higher in Sal B20- and Sal B40-treated diabetics and treatment of diabetics with Sal B40 significantly lowered MDA, raised GSH, and activity of catalase with no significant change of nitrite. | |||||
| The number of pancreatic islets and their area was significantly higher and apoptosis reactivity was significantly lower in the Sal B40-treated diabetic group versus diabetics. | |||||
| Water extract of S. libanotica | Animals were fed a high-fat diet | 50, 150, and 450 mg·kg−1 | A decrease in fasting serum glucose and an increase in fasting serum insulin and liver glycogen content. | [147] | |
| Intake produced a significant improvement in the serum high-density lipoprotein (HDL) and HDL/low-density lipoprotein (LDL) cholesterol ratio, as well as a decrease in abdominal fat. | |||||
| Antiviral | Protocatechuic aldehyde | Hepatitis B virus (HBV) replication in the HepG2 2.2.15 cell line | 24–48 μg·mL−1 | Protocatechuic aldehyde appeared to downregulate the secretion of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) as well as the release of HBV DNA from HepG2 2.2.15 in a dose- and time-dependent manner. | [148] |
| Duck hepatitis B virus (DHBV) replication in ducklings | 25, 50, or 100 mg·kg−1, intraperitoneally, twice daily | Protocatechuic aldehyde also reduced viremia in DHBV-infected ducks. | |||
| Magnesium lithospermate B | Enterovirus 71 (EV71) viral internal ribosome entry site (IRES)-mediated translation | 30 μg·mL−1 | Magnesium lithospermate B inhibited EV71 infection when they were added to rhabdomyosarcoma (RD) cells during the viral absorption stage. | [149] | |
| It had a low IC50 value of 0.09 mmol·L−1 and a high therapeutic index (TI) value of 10.52. | |||||
| 100 mg·mL−1 | Magnesium lithospermate B also reduced EV71 viral particle production and significantly decreased VP1 protein production. | ||||
| Rosmarinic acid | EV71 viral IRES-mediated translation | 30 μg·mL−1 | Rosmarinic acid inhibited EV71 infection when they were added to RD cells during the viral absorption stage. | ||
| It had an IC50 value of 0.50 mmol·L−1 and a TI value of 2.97. | |||||
| 100 mg·mL−1 | Rosmarinic acid also reduced EV71 viral particle production and significantly decreased VP1 protein production. | ||||
| Prevents and treats cataract | Danshensu | Selenite-induced cataractogenesis in cultured rat lens | 500 mmol·L−1 | Lens morphology: 75% of lenses were transparent, 25% developed only lesser amounts of cortical vacuolization. | [150] |
| Danshensu reduces MDA and restores GSH level and total sulfhydryl (SH) content in the lens. | |||||
| Increase of anti-oxidant enzymes (SOD, CAT) activities with danshensu. | |||||
| Protocatechualdehyde | Methylglyoxal-induced mitochondrial dysfunction in Human lens epithelial cells | 0.1, 1, and 10 μmol·L−1 | Protocatechualdehyde alleviated Methylglyoxal (MGO)-induced mitochondrial dysfunction and apoptosis in human lens epithelial cells (SRA01/04 cells). | [151] | |
| Protocatechualdehyde was capable of inhibiting MGO-mediated advanced glycation end products (AGEs) formation and blocking receptor of AGEs expression in SRA01/04 cells. |
5. Conclusions
In recent years, the antioxidant properties of polyphenolic compounds have encouraged their widespread used as supplements in food or pharmaceutical products. Salvia species are a rich source of polyphenols, whose application has been increasing widely used in many countries. The main polyphenolic compounds are phenolic acids (rosmarinic acid, salvianolic acid, and their derivatives), which are based on caffeic acid with compounds formed from two to four or more caffeic acid units. Most of the biological activities, such as anti-oxidant, anti-ischemia-reperfusion, and anti-thrombosis effects are attributed to phenolic acids. Nevertheless, in the study of the pharmacological mechanism of phenolic acids, detailed experiments have been conducted only on common compounds, such as rosmarinic acid, salvianolic acid A, and salvianolic acid B, whereas other compounds are less studied.
The flavonoids found in Chinese Salvia species are apigenin, acacetin, and luteolin. However, few studies have been conducted on flavonoids, including their phytochemistry and pharmacological effects. This is a shortcoming in the study of chemical constituents and pharmacological effects of Chinese Salvia species. The flavonoids in Chinese Salvia species should be analysed using modern analytical techniques, such as liquid chromatography-tandem mass spectrometry to supplement current knowledge on the chemical composition of phenolic acids.
Investigating the biosynthesis of polyphenols is a challenging task in Chinese Salvia species. The medicinal and economic values of phenolic acids make it particularly important to increase the content of phenolic acids in Chinese Salvia species. Presently, only the synthesis of upstream rosmarinic acid has been partially elucidated in the biogenic pathways of phenolic acids. The source pathways of other phenolic acids, such as salvianolic acid B downstream of rosmarinic acid, have not yet been elucidated. Therefore, elucidation of the conversion pathway of phenolic acid, particularly identifying key enzymes involved in transformation, and in-depth study of the biotransformation mechanism would promote the production and development of new phenolic acids.
Owing to the extensive use of Chinese Salvia species in food and medicine, it is necessary to explore polyphenols more comprehensively. In this review, we systematically summarized the biosynthesis, phytochemistry, and pharmacology of Chinese Salvia species, which provide a basis for its further development and utilization as a resource.
Funding
This work was financially supported by a grant (81541164) from the Chinese National Natural Science Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Xu J.P., Wei K.H., Zhang G.J., Lei L.J., Yang D.W., Wang W.L., Han Q.H., Xia Y., Bi Y.Q., Yang M., et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018;225:18–30. doi: 10.1016/j.jep.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Jiang R.W., Lau K.M., Hon P.M., Mak T., Woo K.S., Fung K.P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005;12:237–246. doi: 10.2174/0929867053363397. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y., Yeap F.L. Polyphenolics of Salvia—A review. Phytochemistry. 2002;59:117–140. doi: 10.1016/S0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B.B., Nie S.Q., Liang J.Y., Wu F.W., Feng F. Advances in chemical constituents and pharmacological activities of sage. Strait Pharm. J. 2014;26:1–5. [Google Scholar]
- 5.Zhang Z.F., Chen H.S., Li Z.R. Researches of constituents and bioactivity of Salvia spp. Chin. J. New Drugs. 2007;16:665–672. doi: 10.3321/j.issn:1003-3734.2007.09.003. [DOI] [Google Scholar]
- 6.Li M.H., Chen J.M., Peng Y., Xiao P.G. Distribution of phenolic acids in chinese Salvia plants. World Sci. Technol. 2008;10:46–52. doi: 10.1016/s1876-3553(09)60025-9. [DOI] [Google Scholar]
- 7.Zhao G.R., Zhang H.M., Ye T.X., Xiang Z.J., Yuan Y.J., Guo Z.X., Zhao L.B. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem. Toxicol. 2008;46:73–81. doi: 10.1016/j.fct.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Chang C.C., Chang Y.C., Hu W.L., Hung Y.C. Oxidative stress and Salvia miltiorrhiza in aging-associated cardiovascular diseases. Oxidative Med. Cell. Longev. 2016;2016:4797102. doi: 10.1155/2016/4797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X., Gao Z.G., Wu Y., Stevens R.C., Jacobson K.A., Zhao S. Salvianolic acids from antithrombotic Traditional Chinese Medicine Danshen are antagonists of human P2Y 1 and P2Y 12 receptors. Sci. Rep. 2018;8:11380. doi: 10.1038/s41598-018-29406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia D.S., Li R.S., Wen C.X., Xie X.L., Cui S.Z., Liu L.D., Bian J.B. Study on preparation technology and quality standard of Salvia miltiorrhiza tea. Food Res. Dev. 2017;38:67–70. doi: 10.3969/j.issn.1005-6521.2017.12.015. [DOI] [Google Scholar]
- 11.Zhang S.G., Xu F.H. Qu shan brand danshen liquor functional components-determination method of total saponins content. Shandong Food Ferment. 2004;3:50. [Google Scholar]
- 12.Shu Y., Li L.D., Li Y.K. Clinical research status and quality analysis of compound Salvia miltiorrhiza preparation for coronary heart disease. Pharmacol. Clin. Chin. Mater. Med. 2008;24:76–79. doi: 10.3969/j.issn.1001-859X.2008.06.037. [DOI] [Google Scholar]
- 13.Liu S., Wu J.R., Dan Z., Tan D., Zhang B. Meta-analysis on Randomized Controlled Trials of Compound Danshen Injection in Treatment for Acute Cerebral Infarction. Chin. J. Pharmacoepidemiol. 2017;26:465–470. [Google Scholar]
- 14.Xie N., Tang W.Y. Application of traditional Chinese medicine Salvia miltiorrhiza in modern cosmetics. Jilin J. Tradit. Chin. Med. 2018;38:700–702. [Google Scholar]
- 15.Del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kallscheuer N., Vogt M., Marienhagen J. A novel synthetic pathway enables microbial production of polyphenols independent from the endogenous aromatic amino acid metabolism. ACS Synth. Biol. 2017;6:410–415. doi: 10.1021/acssynbio.6b00291. [DOI] [PubMed] [Google Scholar]
- 17.Mattera R., Benvenuto M., Giganti M.G., Tresoldi I., Pluchinotta F.R., Bergante S., Tettamanti G., Masuelli L., Manzari V., Modesti A., et al. Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes. Nutrients. 2017;9:523. doi: 10.3390/nu9050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eghbaliferiz S., Iranshahi M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016;30:1379–1391. doi: 10.1002/ptr.5643. [DOI] [PubMed] [Google Scholar]
- 19.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brglez Mojzer E., Knez Hrnčič M., Škerget M., Knez Ž., Bren U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. 2016;21:901. doi: 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rungsimakan S., Rowan M.G. Terpenoids, flavonoids and caffeic acid derivatives from Salvia viridis L. cvar. Blue Jeans. Phytochemistry. 2014;108:177–188. doi: 10.1016/j.phytochem.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z.J., Wu J.Q., Ma Y., Wang P., Gu Z.X., Yang R.Q. Advances in biosynthesis, regulation and bioactivity of phenolic acids in plant food raw materials. Food Sci. 2018;39:321–328. [Google Scholar]
- 23.Schenck C.A., Maeda H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry. 2018;149:82–102. doi: 10.1016/j.phytochem.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W., Huang Q., Wu X., Zhou Z., Ding M., Shi M., Huang F., Li S., Wang Y., Kai G. Comprehensive transcriptome profiling of Salvia miltiorrhiza for discovery of genes associated with the biosynthesis of tanshinones and phenolic acids. Sci. Rep. 2017;7:10554. doi: 10.1038/s41598-017-10215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F. Master’s Thesis. Shaanxi Normal University; Xi’an, China: 2011. Effect of PAP2 transcription factor on synthesis of salvianolic acids. [Google Scholar]
- 26.Neta M., Moran O., Rinat O., Noga S.P., Biruk A., Aaron F., Gad G., Avichai P., David W., Michal O.S. Phenylalanine and tyrosine levels are rate-limiting factors in production of health promoting metabolites in Vitis vinifera cv. Gamay Red cell suspension. Front. Plant Sci. 2015;6:538. doi: 10.3389/fpls.2015.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Q., Sun M., Yuan T., Wang Y., Shi M., Lu S., Tang B., Pan J., Wang Y., Kai G. The AP2/ERF transcription factor SmERF1L1 regulates the biosynthesis of tanshinones and phenolic acids in Salvia miltiorrhiza. Food Chem. 2019;274:368–375. doi: 10.1016/j.foodchem.2018.08.119. [DOI] [PubMed] [Google Scholar]
- 28.Ma X.H., Ma Y., Tang J.F., He Y.L., Liu Y.C., Ma X.J., Shen Y., Cui G.H., Lin H.X., Rong Q.X., et al. The Biosynthetic Pathways of Tanshinones and Phenolic Acids in Salvia miltiorrhiza. Molecules. 2015;20:16235–16254. doi: 10.3390/molecules200916235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma P., Liu J., Zhang C., Liang Z. Regulation of Water-Soluble Phenolic Acid Biosynthesis in Salvia miltiorrhiza Bunge. Appl. Biochem. Biotechnol. 2013;170:1253–1262. doi: 10.1007/s12010-013-0265-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhao S.J., Zhang G.Y., Liu D., Hu Z.B. Advances in pharmacology and biosynthesis of water-soluble phenolic acids from Salvia miltiorrhiza. Chin. Tradit. Drug. 2004;3:107–110. doi: 10.7501/j.issn.0253-2670.2004.3.148. [DOI] [Google Scholar]
- 31.Razzaghi-Asl N., Garrido J., Khazraei H., Borges F., Firuzi O. Antioxidant properties of hydroxycinnamic acids: A review of structure- activity relationships. Curr. Med. Chem. 2013;20:4436–4450. doi: 10.2174/09298673113209990141. [DOI] [PubMed] [Google Scholar]
- 32.Zeng F., Chen Y.B., Xia C.N., Zhong G.X. Advances in the synthesis and bioactivity of caffeic acid derivatives. Zhejiang Chem. Ind. 2012;43:24–28. doi: 10.3969/j.issn.1006-4184.2012.08.007. [DOI] [Google Scholar]
- 33.Xu Z., Luo H., Ji A., Zhang X., Song J., Chen S. Global Identification of the Full-Length Transcripts and Alternative Splicing Related to Phenolic Acid Biosynthetic Genes in Salvia miltiorrhiza. Front. Plant Sci. 2016;7:100. doi: 10.3389/fpls.2016.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaram S., Tripathi A., Gupta D.K. Metabolic modeling of Rosmarinic acid biosynthetic pathway. Bioinformation. 2010;5:168–172. doi: 10.6026/97320630005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di P., Zhang L., Chen J., Tan H., Xiao Y., Dong X., Zhou X., Chen W. ¹³C tracer reveals phenolic acids biosynthesis in hairy root cultures of Salvia miltiorrhiza. ACS Chem. Biol. 2013;8:1537–1548. doi: 10.1021/cb3006962. [DOI] [PubMed] [Google Scholar]
- 36.Zeng H.T., Xu S.L., Zhu Y., Gu W., Qian D.W., Duan J.A. Research progress on biosynthesis pathway and regulation mechanism of salvianolic acids. Chin. Tradit. Drug. 2016;47:3324–3331. doi: 10.7501/j.issn.0253-2670.2016.18.028. [DOI] [Google Scholar]
- 37.Pandey R.P., Parajuli P., Koffas M.A.G., Sohng J.K. Microbial production of natural and non-natural flavonoids: Pathway engineering, directed evolution and systems/synthetic biology. Biotechnol. Adv. 2016;34:634–662. doi: 10.1016/j.biotechadv.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Koirala N., Thuan N.H., Ghimire G.P., Thang D.V., Sohng J.K. Methylation of flavonoids: Chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzyme Microb. Technol. 2016;86:103–116. doi: 10.1016/j.enzmictec.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Chen J.Y. Metabolic pathways of phenolic acids and phenylpropane from grape fruits in wine. China Agric. Univ. 2005 doi: 10.7666/d.y773545. [DOI] [Google Scholar]
- 40.Xing B.C. Master’s Thesis. Zhejiang Sci.-Tech. University; Hangzhou, China: 2015. Regulation of phenolic acids and tanshinones biosynthesis by bHLH and WD40 transcriptionfactors in Salvia miltiorrhiza Hairy roots. [Google Scholar]
- 41.Wu J.H., Wu Z.H., Pei J.G., Liu J., Sun Y.Z., Wu G.F., Peng S.M., Fu X.M. Research progress of polyphenols. Mod. Chin. Med. 2015;17:630–636. doi: 10.13313/j.issn.1673-4890.2015.6.025. [DOI] [Google Scholar]
- 42.Tohma H., Köksal E., Kılıç Ö., Alan Y., Yılmaz M.A., Gülçin İ., Bursal E., Alwasel S.H. RP-HPLC/MS/MS Analysis of the Phenolic Compounds, Antioxidant and Antimicrobial Activities of Salvia L. Species. Antioxidants. 2016;5:38. doi: 10.3390/antiox5040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou J., Fu J., Zhang Z.M., Zhu H.L. Modification of bioactivity and chemical structure of caffeic acid derivatives. Fudan Univ. J. Med. Sci. 2011;38:546–552. doi: 10.3969/j.issn.1672-8467.2011.06.017. [DOI] [Google Scholar]
- 44.Hu P., Luo G.A., Zhao Z., Jiang Z.H. Quality Assessment of Radix Salvia Miltiorrhizae. Chem. Pharm. Bull. (Tokyo) 2005;53:481–486. doi: 10.1248/cpb.53.481. [DOI] [PubMed] [Google Scholar]
- 45.Liang W.Y., Chen W.J., Yang G.H., Zhu D., Mao X., Shao Y.Y., Wu L.F., Zhang X.X., Zhang L.Z. Research progress of salvianolic acids. China J. Chin. Mater. Med. 2016;41:806–812. doi: 10.4268/cjcmm20160508. [DOI] [PubMed] [Google Scholar]
- 46.Bao Y.F., Shen C., Wang F. Research progress and development prospect of caffeic acid and its main derivatives. Nat. Prod. Res. Dev. 2018;10:1825–1833. doi: 10.16333/j.1001-6880.2018.10.028. [DOI] [Google Scholar]
- 47.Zeng G., Xiao H., Liu J., Liang X. Identification of phenolic constituents in radix Salvia miltiorrhizae by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2010;20:499–506. doi: 10.1002/rcm.2332. [DOI] [PubMed] [Google Scholar]
- 48.Kim G.D., Park Y.S., Jin Y.H., Park C.S. Production and applications of rosmarinic acid and structurally related compounds. Appl. Microbiol. Biotechnol. 2015;99:2083–2092. doi: 10.1007/s00253-015-6395-6. [DOI] [PubMed] [Google Scholar]
- 49.Choi H.G., Tran P.T., Lee J.H., Min B.S., Kim J.A. Anti-inflammatory activity of caffeic acid derivatives isolated from the roots of Salvia miltiorrhiza Bunge. Arch. Pharm. Res. 2018;41:1–7. doi: 10.1007/s12272-017-0983-1. [DOI] [PubMed] [Google Scholar]
- 50.Krzyzanowska-Kowalczyk J., Kolodziejczyk-Czepas J., Kowalczyk M., Pecio Ł., Nowak P., Stochmal A. Yunnaneic Acid B, a Component of Pulmonaria officinalis Extract, Prevents Peroxynitrite-Induced Oxidative Stress in vitro. J. Agric. Food Chem. 2017;65:3827–3834. doi: 10.1021/acs.jafc.7b00718. [DOI] [PubMed] [Google Scholar]
- 51.Griffith D.R., Botta L., St Denis T.G., Snyder S.A. Explorations of caffeic acid derivatives: Total syntheses of rufescenolide, yunnaneic acids C and D, and studies toward yunnaneic acids A and B. J. Org. Chem. 2014;79:88–105. doi: 10.1021/jo4023167. [DOI] [PubMed] [Google Scholar]
- 52.Liu X., Chen R., Shang Y., Jiao B., Huang C. Superoxide radicals scavenging and xanthine oxidase inhibitory activity of magnesium lithospermate B from Salvia miltiorrhiza. J. Enzyme Inhib. Med. Chem. 2009;24:663–668. doi: 10.1080/14756360802323829. [DOI] [PubMed] [Google Scholar]
- 53.Hase K., Kasimu R., Basnet P., Kadota S., Namba T. Preventive effect of lithospermate B from Salvia miltiorhiza on experimental hepatitis induced by carbon tetrachloride or D-galactosamine/lipopolysaccharide. Planta Med. 1997;63:22–26. doi: 10.1055/s-2006-957596. [DOI] [PubMed] [Google Scholar]
- 54.Quan W., Yin Y., Xi M., Zhou D., Zhu Y., Guan Y., Guo C., Wang Y., Duan J., Wen A. Antioxidant properties of magnesium lithospermate B contribute to the cardioprotection against myocardial ischemia/reperfusion injury in vivo and in vitro. J. Tradit. Chin. Med. 2013;33:85–91. doi: 10.1016/S0254-6272(13)60106-5. [DOI] [PubMed] [Google Scholar]
- 55.Wang M., Kikuzaki H., Zhu N., Sang S., Nakatani N., Ho CT. Isolation and structural elucidation of two new glycaosides from sage (Salvia officinalis L.) Agric. Food Chem. 2000;48:235–238. doi: 10.1021/jf990761p. [DOI] [PubMed] [Google Scholar]
- 56.Wang M., Shao Y., Li J., Zhu N., Rangarajan M., LaVoie E.J., Ho C.T. Antioxidative phenolic glycosides from sage (Salvia officinalis) J. Nat. Prod. 1999;62:454–456. doi: 10.1021/np980436g. [DOI] [PubMed] [Google Scholar]
- 57.Wang M., Li J., Rangarajan M., Shao Y., La Voie E.J., Huang T.C., Ho C.-T. Antioxidative phenolic compounds from sage (Salvia officinalis) J. Agric. Food Chem. 1998;46:4869–4873. doi: 10.1021/jf980614b. [DOI] [Google Scholar]
- 58.Zhang D.C., Wu W.L., Liu X.J., Pan D.J., Yang C.X., Zhang B.T. Salvia miltiorrhiza effective component research II. D (+) beta (3,4-dihydroxy phenyl) the structure of lactic acid. J. Shanghai First Med. Coll. 1980;5 [Google Scholar]
- 59.Qian T.-X., Li L.-N. Isosalvianolic acid C, a depside possessing a dibenzooxepin skeleton. Phytochemistry. 1992;31:1068–1070. doi: 10.1016/0031-9422(92)80081-O. [DOI] [Google Scholar]
- 60.Zhao L.M., Liang X.T., Li L.N. Prionitisides A and B, two phenolic glycosides from Salvia prionitis. Phytochemistry. 1996;42:899–901. doi: 10.1016/0031-9422(95)00967-1. [DOI] [Google Scholar]
- 61.Wu Z., Ouyang M., Yang C. Polyphenolic constituents of Salvia sonchifolia. Acta Botanica Yunnanica. 1999;21:393–398. [Google Scholar]
- 62.Kang H.S., Chung H.Y., Jung J.H., Kang S.S., Choi J.S. Antioxidant effect of Salvia miltiorrhiza. Arch. Pharm. Res. 1997;20:496. doi: 10.1007/BF02973947. [DOI] [PubMed] [Google Scholar]
- 63.Li J., Li L.N., Song W.Z. Study on chemical constituents of Salvia miltiorrhiza. Chin. Tradit. Herb. Drugs. 1994;25:347–349. doi: 10.7501/j.issn.0253-2670.1994.7.167. [DOI] [Google Scholar]
- 64.Jiang Y., Luo S., Zheng M. Active principles of Salvia plebeia. Chin. J. Pharm. 1987;18:349–351. [Google Scholar]
- 65.Cuvelier M.E., Richard H., Berset C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 1996;73:645–652. doi: 10.1007/BF02518121. [DOI] [Google Scholar]
- 66.El-Missiry M.M., Hussiney H.A., Ismail S.I., Radwan H.M., Rizk A.M. Constituents of plants growing in Qatar XXIV. Phytochemical investigation of Salvia aegyptiaca L. Qatar Univ. Sci. J. 1994;14:249–251. [Google Scholar]
- 67.Lu Y., Foo L.Y. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry. 2000;55:263–267. doi: 10.1016/S0031-9422(00)00309-5. [DOI] [PubMed] [Google Scholar]
- 68.Yang Z., Hon P.M., Chui K.Y., Xu Z.L., Chang H.M., Lee C.M., Cui Y.X., Wong H.N.C., Poon C.D., Fung B.M. Naturally occurring benzofuran: Isolation, structure elucidation and total synthesis of 5-(3-hydroxypropyl)-7-methoxy-2-(3′-methoxy-4′-hydroxyphenyl)-3-benzo[b]furancarbaldehyde, a novel adenosine A1 receptor ligand isolated from Salvia miltiorrhiza Bunge (danshen) Tetrahedron Lett. 1991;32:2061–2064. doi: 10.1002/chin.199206166. [DOI] [Google Scholar]
- 69.Weng X.C., Wang W. Antioxidant activity of compounds isolated from Salvia plebeia. Food Chem. 2000;71:489–493. doi: 10.1016/S0308-8146(00)00191-6. [DOI] [Google Scholar]
- 70.Ai C.B., Li L.N. Salvianolic acids d and e: Two new depsides from Salvia miltiorrhiza. Planta Med. 1992;58:197–199. doi: 10.1055/s-2006-961428. [DOI] [PubMed] [Google Scholar]
- 71.Liu L., Dai Y.Q., Xie G.Y., Li M.L., Feng Q.T., Qin M.J. Study on chemical constituents of litchi root. Chin. Pharm. J. 2014;16:1393–1396. doi: 10.11669/cpj.2014.16.007. [DOI] [Google Scholar]
- 72.Zhu L.P., Xiang C., Zhuang W.T., He J., Li P., Li B.P. Study on chemical constituents of sage ganci. Nat. Prod. Res. Dev. 2013;25:785–788. doi: 10.3969/j.issn.1001-6880.2013.06.013. [DOI] [Google Scholar]
- 73.Feng K., Zhao X., Zheng L.L., Liang Z.S. Analyis of Active Ingredients in Three Salvia Plant. J. Northwest For. Univ. 2010;25:140–143. [Google Scholar]
- 74.Li L.N. Water soluble active components of Salvia miltiorrhiza and related plants. J. Chin. Pharm. Sci. 1997;6:57–64. doi: 10.1253/circj.CJ-09-0327. [DOI] [Google Scholar]
- 75.Pan Y., Zhang L., Chen G. Separation and determination of protocatechuic aldehyde and protocatechuic acid in salivia miltorrhrza by capillary electrophoresis with amperometric detection. Analyst. 2001;126:1519–1523. doi: 10.1039/b009760h. [DOI] [PubMed] [Google Scholar]
- 76.Chang J.Y. Salvinal, a novel microtubule inhibitor isolated from Salvia miltiorrhizae Bunge (danshen), with antimitotic activity in multidrug-sensitive and -resistant human tumor cells. Mol. Pharmacol. 2004;65:77–84. doi: 10.1124/mol.65.1.77. [DOI] [PubMed] [Google Scholar]
- 77.Wu Y.B., Ni Z.Y., Shi Q.W., Dong M., Kiyota H., Gu Y.C., Cong B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012;112:5967–6026. doi: 10.1021/cr200058f. [DOI] [PubMed] [Google Scholar]
- 78.Fu J., Yang L.F., Liang J.M., Xu L., Zhao G., Fu Q. Advances on chemical constituents of Tibetan Salvia genus. China J. Chin. Mater. Med. 2017;42:3684–3695. doi: 10.19540/j.cnki.cjcmm.20170807.005. [DOI] [PubMed] [Google Scholar]
- 79.Lu Y.R., Foo L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry. 1999;51:91–94. doi: 10.1016/S0031-9422(98)00730-4. [DOI] [Google Scholar]
- 80.Plattner R.D., Powell R.G. A secoisolaricireinol branched fatty diester from Salvia plebeia seed. Phytochemistry. 1978;17:149–150. doi: 10.1016/S0031-9422(00)89701-0. [DOI] [Google Scholar]
- 81.Kohda H., Takeda O., Tanaka S., Yamasaki K., Yamashita A., Kurokawa T., Ishibashi S. Isolation of inhibitors of adenylate cyclase from dan-shen, the root of Salvia miltiorrhiza. Chem. Pharm. Bull. 1989;37:1287–1290. doi: 10.1248/cpb.37.1287. [DOI] [PubMed] [Google Scholar]
- 82.Li W., Zhou S.P., Jin Y.P., Huang X.F., Zhou W., Han M., Yu Y., Yan K.J., Li S.M., Ma X.H., et al. Salvianolic acids T and U: A pair of atropisomeric trimeric caffeic acids derivatives from root of Salvia miltiorrhiza. Fitoterapia. 2014;98:248–253. doi: 10.1016/j.fitote.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y.L. Master’s Thesis. BaoTou Medical College; BaoTou, China: 2016. The Investigation and Classification of Medicinal Plants from Salvia in China. [Google Scholar]
- 84.Liu H.Q., Wang G.K., Lin B.B., Huang J., Qin M.J. Chemical composition analysis of ethanol extract from litchi grass. J. Plant Resour. Environ. 2013;22:111–113. doi: 10.3969/j.issn.1674-7895.2013.02.16. [DOI] [Google Scholar]
- 85.Zhang Z.F., Peng Z.G., Gao L., Dong B., Li J.R., Li Z.Y., Chen H.S. Three new derivatives of anti-HIV-1 polyphenols isolated from Salvia yunnanensis. J. Asian Nat. Prod. Res. 2008;10:391–396. doi: 10.1080/10286020801966591. [DOI] [PubMed] [Google Scholar]
- 86.Powell R.G., Plattner R.D. Structure of a secoisolariciresinol diester from Salvia plebeia seed. Phytochemistry. 1976;15:1963–1965. doi: 10.1016/S0031-9422(00)88856-1. [DOI] [Google Scholar]
- 87.Zhu Z., Zhang H., Zhao L., Dong X., Li X., Chai Y., Zhang G. Rapid separation and identification of phenolic and diterpenoid constituents from Radix Salvia miltiorrhizae by high-performance liquid chromatography diode-array detection, electrospray ionization time-of-flight mass spectrometry and electrospray ionization quadrupole ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:1855–1865. doi: 10.1002/rcm.3023. [DOI] [PubMed] [Google Scholar]
- 88.Li L.N., Tan R., Chen W.M. Salvianolic acid A, a new depside from roots of Salvia miltiorrhiza. Planta Med. 1984;50:227–228. doi: 10.1055/s-2007-969684. [DOI] [PubMed] [Google Scholar]
- 89.Zhang H.J., Li L.N. Salvianolic Acid I: A New Depside from Salvia cavaleriei. Planta Med. 1994;60:70–72. doi: 10.1055/s-2006-959411. [DOI] [PubMed] [Google Scholar]
- 90.Ai C.B., Li L.N. Stereostructure of salvianolic acid b and isolation of salvianolic acid c from Salvia miltiorrhiza. J. Nat. Prod. 1988;51:145–149. doi: 10.1021/np50055a023. [DOI] [Google Scholar]
- 91.Zhang H., Li L.N. Salvianolic acid H, a new depside from Salvia cavalaries var. simplicifolia. Chin. Chem. Lett. 1993;4:501–504. [Google Scholar]
- 92.Ai C.B., Deng Q.H., Song W.Z., Li L.N. Salvianolic acid j, a depside from Salvia flava. Phytochemistry. 1994;37:907–908. doi: 10.1016/S0031-9422(00)90382-0. [DOI] [Google Scholar]
- 93.Tanaka T., Nishimura A., Kouno I., Nonaka G., Young T.J. Isolation and characterization of yunnaneic acids a−d, four novel caffeic acid metabolites from Salvia yunnanensis. J. Nat. Prod. 1996;59:843–849. doi: 10.1021/np960425s. [DOI] [Google Scholar]
- 94.Tanaka T., Nishimura A., Kouno I., Nonaka G., Yang C.R. Cheminform abstract: Four new caffeic acid metabolites, yunnaneic acids e-h, from Salvia yunnanensis. Cheminform. 2010;29 doi: 10.1002/chin.199812223. [DOI] [Google Scholar]
- 95.Wu Z.J., OuYang M.A. Polyphenols of Salvia miltiorrhiza. Plant Divers. 1999;21:512–516. doi: 10.3969/j.issn.2095-0845.1999.04.017. [DOI] [Google Scholar]
- 96.Ikeshiro Y., Mase I., Tomita Y. Abietane type diterpenoids from Salvia miltiorrhiza. Phytochemistry. 1989;28:3139–3141. doi: 10.1016/0031-9422(89)80294-8. [DOI] [Google Scholar]
- 97.Tezuka Y., Kasimu R., Basnet P., Namba T., Kadota S. Aldose reductase inhibitory constituents of the root of Salvia miltiorhiza Bunge. Chem. Pharm. Bull. (Tokyo) 1997;45:1306–1311. doi: 10.1248/cpb.45.1306. [DOI] [PubMed] [Google Scholar]
- 98.Lu Y., Foo L.Y. Salvianolic acid L, a potent phenolic antioxidant from Salvia officinalis. Tetrahedron Lett. 2001;42:8223–8225. doi: 10.1016/S0040-4039(01)01738-5. [DOI] [Google Scholar]
- 99.Yokozawa T., Chung H.Y., Lee T.W., Oura H., Tanaka T., Nonaka G., Nishioka I. Effect of magnesium lithospermate b on urinary excretion of arachidonate metabolites in rats with renal failure. Chem. Pharm. Bull. 1989;37:2766–2769. doi: 10.1248/cpb.37.2766. [DOI] [PubMed] [Google Scholar]
- 100.Mori M., Kondo T., Yoshida K. Cyanosalvianin, a supramolecular blue metalloanthocyanin, from petals of Salvia uliginosa. Phytochemistry. 2008;69:3151–3158. doi: 10.1016/j.phytochem.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 101.Ulubelen A., Oztürk S., Iśildatici S. A New Flavone from Sulviu trilobu L.f (Labiutue) J. Pharm. Sci. 1968;57:1037–1038. doi: 10.1002/jps.2600570630. [DOI] [PubMed] [Google Scholar]
- 102.Zahid M. Flavonoid glycosides from Salvia moorcroftiana wall. Carbohydr. Res. 2002;337:403–407. doi: 10.1016/S0008-6215(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 103.Al-Qudah M.A., Al-Jaber H.I., Abu Zarga M.H., Abu Orabi S.T. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry. 2014;99:115–120. doi: 10.1016/j.phytochem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 104.He K.Q., Lu W.Y., Li X.X., Zhao Z.Y., Yang Q. Determination of total flavonoids in sage from guizhou province. Guizhou Agric. Sci. 2013;41:77–79. doi: 10.3969/j.issn.1001-3601.2013.01.023. [DOI] [Google Scholar]
- 105.Kolodziejski J., Gill S., Mruk A., Surewicz-Szewczyk H. Variable content of ethereal oils and tannic compounds during the vegetation stage of Salvia officinalis L. Acta Pol. Pharm. 1963;20:269–276. [PubMed] [Google Scholar]
- 106.Petri G., Then M., Chanthabout L. Tannins and other polyphenolic compounds in the genus Salvia. Planta Med. 1988;54:575. doi: 10.1055/s-2006-962583. [DOI] [PubMed] [Google Scholar]
- 107.Kang W.J., Fu Y.B., Li D.H., Han G.H., Sun L., Li Z.L., Hua H.M. Chemical constituents of Salvia plebeia. Chin. Tradit. Herb. Drugs. 2015;45:1589–1592. doi: 10.7501/j.issn.0253-2670.2015.11.004. [DOI] [Google Scholar]
- 108.Jin M.R., Xu H., Duan C.H., Chou G.X. Two new flavones from Salvia plebeia. Nat. Prod. Res. 2015;29:1315–1322. doi: 10.1080/14786419.2014.999241. [DOI] [PubMed] [Google Scholar]
- 109.Chen C.C., Chen H.T., Chen Y.P., Hsu H.Y., Hsieh T.C. Isolation of the components of Salvia miltiorrhizae radix and their coronary dilator activities. Taiwan Yao Hsueh Tsa Chih. 1986;38:226–230. [Google Scholar]
- 110.Ren D.B., Qin Y.H., Yun Y.H., Lu H.M., Chen X.Q., Liang Y.Z. Separation of nine compounds from Salvia plebeia R.Br. using two-step high-speed counter-current chromatography with different elution modes. J. Sep. Sci. 2014;37:2118–2125. doi: 10.1002/jssc.201400293. [DOI] [PubMed] [Google Scholar]
- 111.Jin X.F., Lu Y.H., Wei D.Z., Wang Z.T. Chemical fingerprint and quantitative analysis of Salvia plebeia R.Br. by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2008;48:100–104. doi: 10.1016/j.jpba.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 112.Xing Y.X. Northwest Institute of Plateau Biology, Chinese Academy of Sciences; 2012. [(accessed on 2 January 2019)]. Chemical Composition Analysis and Ecological Study of Clematis from Qinghai Province. Available online: http://210.75.249.4/handle/363003/3498. [Google Scholar]
- 113.Kamel M.S., Desoky E.K., Abdallah O.M., Bishay D.W. Flavonol glycosides from leaves of Salvia farinacea Benth. Bull. Fac. Pharm. (Cairo Univ.) 1992;30:259–262. doi: 10.1007/pl00013097. [DOI] [Google Scholar]
- 114.Nugroho A., Kim M.H., Choi J., Baek N.I., Park H.J. In vivo sedative and gastroprotective activities of Salvia plebeia extract and its composition of polyphenols. Arch. Pharm. Res. 2012;35:1403–1411. doi: 10.1007/s12272-012-0810-7. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y., Li C., Zhang C.Z. Study on Chemical Constituents of Clematis Chinensis. Tradit. Chin. Med. Mater. 2002;25:792–793. doi: 10.13863/j.issn1001-4454.2002.11.010. [DOI] [PubMed] [Google Scholar]
- 116.Ulubelen A., Miski M., Mabry T.J. Further flavones and triterpenes and the new 6-hydroxyluteolin 5-β-d-glucoside from Salvia tomentosa. J. Nat. Prod. 1981;44:586–587. doi: 10.1021/np50017a014. [DOI] [Google Scholar]
- 117.Miski M., Neuman P., Mabry T.J. Flavonoids of Salvia tomentosa (Labiatae) J. Nat. Prod. (Lloydia) 1979;42:261–263. doi: 10.1021/np50003a002. [DOI] [Google Scholar]
- 118.Gu W.H. Flavonoids Compounds of Salvia Officinalis. Chin Tra and Herbal Drugs. 1981;12:41–48. [Google Scholar]
- 119.Gu L., Weng X. Antioxidant activity and components of Salvia plebeia R.Br. a Chinese herb. Food Chem. 2001;73:299–305. doi: 10.1016/S0308-8146(00)00300-9. [DOI] [Google Scholar]
- 120.Brieskorn C.H., Kapadia Z. Constituents of Salvia officinalis. XXIII: 5-Methoxysalvigenin in leaves of Salvia officinalis. Planta Med. 1979;35:376–378. doi: 10.1055/s-0028-1097234. [DOI] [Google Scholar]
- 121.Moharram A.E., Marzouk M.S., El-Shenawy S.M., Gaara A., El Kady W.M. Polyphenolic profile and biological activity of Salvia splendens leaves. J. Pharm. Pharmacol. 2012;64:1678–1687. doi: 10.1111/j.2042-7158.2012.01544.x. [DOI] [PubMed] [Google Scholar]
- 122.Kolac U.K., Ustuner M.C., Tekin N., Ustuner D., Colak E., Entok E. The Anti-Inflammatory and Antioxidant Effects of Salvia officinalis on Lipopolysaccharide-Induced Inflammation in Rats. J. Med. Food. 2017;20:1193–1200. doi: 10.1089/jmf.2017.0035. [DOI] [PubMed] [Google Scholar]
- 123.Loizzo M.R., Abouali M., Salehi P., Sonboli A., Kanani M., Menichini F., Tundis R. In vitro antioxidant and antiproliferative activities of nine, Salvia species. Nat. Prod. Res. 2014;28:2278–2285. doi: 10.1080/14786419.2014.939086. [DOI] [PubMed] [Google Scholar]
- 124.Adomakobonsu A.G., Chan S.L., Pratten M., Fry J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro. 2017;40:248–255. doi: 10.1016/j.tiv.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y., Li X., Wang Z. Antioxidant activities of leaf extract of Salvia miltiorrhiza Bunge and related phenolic constituents. Food Chem. Toxicol. 2010;48:0–2662. doi: 10.1016/j.fct.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 126.Zhang X.X., Zhang S., Huang X.Y., Li G., Wang Z.H., Ma C.Y. Anti-lipoperoxidation of Several Organic Polyphenol Acids in vitro. Coll. Life Sci. 2014;26:398–402. doi: 10.16333/j.1001-6880.2014.03.022. [DOI] [Google Scholar]
- 127.Xue L., Wu Z., Ji X.P., Gao X.Q., Guo Y.H. Effect and mechanism of salvianolic acid B on the myocardial ischemia-reperfusion injury in rats. Asian Pac. J. Trop. Med. 2014;7:280–284. doi: 10.1016/S1995-7645(14)60038-9. [DOI] [PubMed] [Google Scholar]
- 128.Zhang W., Song J.K., Zhang X., Zhou Q.M., He G.R., Xu X.N., Rong Y., Zhou W.X., Du G.H. Salvianolic acid A attenuates ischemia reperfusion induced rat brain damage by protecting the blood brain barrier through MMP-9 inhibition and anti-inflammation. Chin. J. Nat. Med. 2018;16:184–193. doi: 10.1016/S1875-5364(18)30046-3. [DOI] [PubMed] [Google Scholar]
- 129.Meng Y. Danshen (Salvia miltiorrhiza) Prevents Myocardial Ischemia Reperfusion Injury by Regulate Nox2/ROS/JNK2/NF-κB pathway and Recude TRPC6/Ca2+ influx under High Glucose Condition. Anhui Med. Univ. 2016;206 [Google Scholar]
- 130.Wang F.Q., Chen C., Xia Z.N., Yang F.Q. Application of Platelets in the Study of Promoting Blood Circulation and Removing Blood Stasis. Impurrity Chin. Tradit. Med. 2014;39:2993–3003. [PubMed] [Google Scholar]
- 131.Zhu H.L., Zhang D.W., Sun L.R., Ji J.B., Wu K. Effects of total salvianolic acid from Salvia miltiorrhiza f. alba on thromboangiitis obliterans in rats. Chin. Tradit. Herb. Drugs. 2012;43:1565–1569. [Google Scholar]
- 132.Lu Y., Li Q., Liu Y.Y., Sun K., Fan J.Y., Wang C.S., Han J.Y. Inhibitory effect of caffeic acid on ADP-induced thrombus formation and platelet activation involves mitogen-activated protein kinases. Sci. Rep. 2015;5:13824. doi: 10.1038/srep13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu C., Qi D., Lian W., Li Q.Z., Li H.J., Fan H.Y. Effects of Danshensu on Platelet Aggregation and Thrombosis: In Vivo Arteriovenous Shunt and Venous Thrombosis Models in Rats. PLoS ONE. 2014;9:e110124. doi: 10.1371/journal.pone.0110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chien C.F., Wu Y.T., Tsai T.H. Biological analysis of herbal medicines used for the treatment of liver diseases. Biomed. Chromatogr. 2011;25:21–38. doi: 10.1002/bmc.1568. [DOI] [PubMed] [Google Scholar]
- 135.Yuan Y., Wu Q., Shi J.S., Chen X.P. Advance in studies on hepatoprotective effect of Salvia miltiorrhiza and its main components. Impurrity Chin. Tradit. Med. 2015;40:558–593. [PubMed] [Google Scholar]
- 136.Zeng W., Shan W., Gao L., Gao D., Hu Y., Wang G., Zhang N., Li Z., Tian X., Xu W., et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci. Rep. 2015;5:16013. doi: 10.1038/srep16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu X., Hu Y., Zhai X., Liu M., Chen Z., Tian X.F., Zhang F., Gao D.Y., Ma X.C., Lv L., Yao J.H. Salvianolic acid A preconditioning confers protection against concanavalin A-induced liver injury through SIRT1-mediated repression of p66shc in mice. Toxicol. Appl. Pharmacol. 2013;273:68–76. doi: 10.1016/j.taap.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 138.Zhou X., Cheung C.M., Yang J.M., Or P.M., Lee W.Y., Yeung J.H. Danshen (Salvia miltiorrhiza) water extract inhibits paracetamol-induced toxicity in primary rat hepatocytes via reducing CYP2E1 activity and oxidative stress. J. Pharm. Pharmacol. 2015;67:980–989. doi: 10.1111/jphp.12381. [DOI] [PubMed] [Google Scholar]
- 139.Zhang W., Lu Y. Advances in studies on antitumor activities of compounds in Salvia miltiorrhiza. China J. Chin. Mater. Med. 2010;35:389. doi: 10.1002/(SICI)1097-0215(20000401)86:13.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 140.Garcia C.S., Menti C., Lambert A.P., Barcellos T., Moura S., Calloni C., Branco C.S., Salvador M., Roesch-Ely M., Henriques J.A. Pharmacological perspectives from Brazilian Salvia officinalis (Lamiaceae): Antioxidant, and antitumor in mammalian cells. Anais Da Academia Brasileira De Ciencias. 2016;88:281. doi: 10.1590/0001-3765201520150344. [DOI] [PubMed] [Google Scholar]
- 141.Wang Z.S., Luo P., Dai S.H., Liu Z.B., Zheng X.R., & Chen T. Salvianolic acid B induces apoptosis in human glioma U87 cells through p38-mediated ROS generation. Cell. Mol. Neurobiol. 2013;33:921–928. doi: 10.1007/s10571-013-9958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Agunloye O.M., Oboh G., Ademiluyi A.O., Ademosun A.O., Akindahunsi A.A., Oyagbemi A.A., Omobowale T.O., Ajibade T.O., Adedapo A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2018;109:450–458. doi: 10.1016/j.biopha.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 143.Teng F.K., Yin Y., Cui Y.J., Deng Y.P., Li D.F., Cho K., Zhang G., Lu A.P., Wu W.Y., Yang M., et al. Salvianolic acid A inhibits endothelial dysfunction and vascular remodeling in spontaneously hypertensive rats. Life Sci. 2015 doi: 10.1016/j.lfs.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 144.Gok D.K., Hidisoglu E., Ocak G.A., Er H., Acun A.D., Yargıcoglu P. Protective role of rosmarinic acid on amyloid beta 42-induced echoic memory decline: Implication of oxidative stress and cholinergic impairment. Neurochem. Int. 2018 doi: 10.1016/j.neuint.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 145.Shen L., Han B., Geng Y., Wang J., Wang Z., Wang M. Amelioration of cognitive impairments in APPswe/PS1dE9 mice is associated with metabolites alteration induced by total salvianolic acid. PLoS ONE. 2017;12:e0174763. doi: 10.1371/journal.pone.0174763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Raoufi S., Baluchnejadmojarad T., Roghani M., Ghazanfari T., Khojasteh F., Mansouri M. Antidiabetic potential of salvianolic acid B in multiple low-dose streptozotocin-induced diabetes. Pharm. Biol. 2015;53:1803–1809. doi: 10.3109/13880209.2015.1008148. [DOI] [PubMed] [Google Scholar]
- 147.Bassil M., Daher C.F., Mroueh M., Zeeni N. Salvia libanotica improves glycemia and serum lipid profile in rats fed a high fat diet. BMC Complement. Altern. Med. 2015;15:384. doi: 10.1186/s12906-015-0917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou Z., Zhang Y., Ding X.R., Chen S.H., Yang J., Wang X.J., Jia G.L., Chen H.S., Bo X.C., Wang S.Q. Protocatechuic aldehyde inhibiys hepatitis B virus replication both in vitro and in vivo. Antivir. Res. 2007;74:59–64. doi: 10.1016/j.antiviral.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 149.Chung Y.C., Hsieh F.C., Lin Y.J., Wu T.Y., Lin C.W., Lin C.T., Tang N.Y., Jinn T.R. Magnesium lithospermate B and rosmarinic acid, two compounds present in Salvia miltiorrhiza, have potent antiviral activity against enterovirus 71 infections. Eur. J. Pharmacol. 2015;755:127–133. doi: 10.1016/j.ejphar.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 150.Qi H.P., Wei S.Q., Zhang L.Q., Gao X.C., Yu N.N., Bi S., Cui H. Preventive effect of danshensu on selenite-induced cataractogenesis in cultured rat lens. Clin. Exp. Ophthalmol. 2013;41:172–179. doi: 10.1111/j.1442-9071.2012.02837.x. [DOI] [PubMed] [Google Scholar]
- 151.Wang Y.H., Han Y.P., Yu H.T., Pu X.P., Du G.H. Protocatechualdehyde prevents methylglyoxal-induced mitochondrial dysfunction and AGEs-RAGE axis activation in human lens epithelial cells. Eur. J. Pharmacol. 2014;738:374–383. doi: 10.1016/j.ejphar.2014.04.045. [DOI] [PubMed] [Google Scholar]