Abstract
Magnesium (Mg) is an essential nutrient element for plant growth and plays an important role in numerous physiological and biochemical processes. Mg deficiency inhibits plant growth and has become a growing problem for crop productions in agriculture. However, the molecular mechanisms for the resistance to Mg deficiency in plants were not well understood. In this study, we identified a Mg transporter gene OsMGT1 that confers resistance to Mg deficiency in rice (Oryza sativa). The expression of OsMGT1 was highly induced by Mg deficiency in shoots. Investigation of tissue expression patterns revealed that OsMGT1 was mainly expressed in the phloem region; however, Mg deficiency remarkably enhanced its expression in xylem parenchyma and mesophyll cells in shoots. Knockout of OsMGT1 resulted in a significant reduction in Mg content and biomass when grown at Mg-limited conditions. Furthermore, the sensitivity to low-Mg in mutants was intensified by excessive calcium supply. In addition, overexpression of OsMGT1 increased Mg content and biomass under low-Mg supply. In conclusion, our results indicate that OsMGT1 plays an important role in rice Mg import and is required for the resistance to Mg deficiency, which can be utilized for molecular breeding of low-Mg tolerant plants.
Keywords: OsMGT1, transporter, rice, Mg deficiency
1. Introduction
Magnesium (Mg) is an essential element for plant growth, development and reproductive success [1,2,3], which plays an important role in numerous physiological and biochemical processes, such as chlorophyll biosynthesis and degradation, photosynthetic CO2 assimilation, carbohydrate allocation, energy metabolism and ribosome aggregation [4,5,6,7,8]. Therefore, lack of Mg in plants reduces the photosynthetic rate, disrupts the distribution of carbohydrates from source to sink, inhibits the growth of plant organs and ultimately leads to a significant decline in crop productivity and quality [9,10]. Mg deficiency in plants may result from three following factors: First, Mg has a relatively larger hydrated radius in contrast to other cations, which makes it easier to be leached, particularly in acidic soils and sandy soils with low cation exchange capacity [11,12,13]. Second, with the increasing crop yield and multi-cropping, soil Mg supply cannot meet crop requirements, resulting in soil Mg depletion [14,15]. Third, the tremendous input of inorganic fertilizers and soil acidification lead to the antagonistic effect of other cations (H+, NH4+, Al3+, Mn2+) on plant Mg uptake [16]. Therefore, Mg deficiency has become a growing problem for many crop productions in agriculture.
In view of the biological significance and unique chemical property of Mg2+, the studies on Mg transporters, which mediate Mg2+ uptake, translocation and distribution are increasingly important [17,18]. Cation transporter gene families, such as MHX (Mg2+/H+ Exchanger), CNGC (Cyclic Nucleotide-Gated Channel), HKT (High-Affinity K+ Transport) and MRS2/MGT (Mitochondrial RNA Splicing 2/Magnesium Transporter) have been identified as Mg transporters in plants [19,20,21,22,23]. MHX is a unique vacuolar Mg transporter in Arabidopsis. The high expression of MHX in vascular tissues suggests its role in xylem loading or retrieval of Mg [19]. CNGCs are commonly known as Ca2+-permeable cation transport channels [24]; however, their properties of low cation selectivity suggest that they are also permeable to other cations, including K+, H+ and Mg2+ [25]. OsHKT2;4, a member of the OsHKT2 subfamily with Na+-K+ symport activity, functions as a low-affinity Mg2+ transporter in rice [23]. To date, the MRS2/MGT are the best-studied Mg2+ transporter gene family in plants [21,26], which are homologs of CorA in bacteria and Alr1 in yeast [27,28,29]. MRS2/MGT proteins form a funnel-shaped homopentamer and individually own two conserved transmembrane domains near their C-terminals [30,31,32]. Cytoplasmic Mg2+ is bound between monomers in the cytoplasmic domain for channel gating, while a conserved CorA motif of tripeptide (GMN) which appears at the end of first transmembrane helices, controls ion selectivity [31,32].
So far, the MRS2/MGT family has been revealed in several plant species, such as Arabidopsis, rice, soybean and maize [3,20,26,33]. Although most of them have Mg transport activity by functional complementation with yeast and bacteria mutants, the physiological roles in plants are largely different [21,26,34]. AtMGT6 and OsMGT1 are able to mediate Mg uptake in the roots of Arabidopsis and rice, respectively [35,36]. AtMGT6 confers both low- and high-Mg tolerance [36,37], whereas OsMGT1 mediates both Al and salt tolerance [35,38]. OsMGT2 and OsMGT6 in rice and AtMGT9 in Arabidopsis are mainly expressed in root vascular tissues, which are likely to be involved in the xylem loading during Mg translocation from roots to shoots [3,21]. AtMGT10 locates at the chloroplast envelope membrane for regulating Mg homeostasis in chloroplasts, which is crucial for chloroplast development, particularly under high light conditions [39,40,41]. Two mesophyll-abundant and tonoplast-localized transporters AtMGT2 and AtMGT3 are required for high vacuolar Mg storage through transport of Mg into vacuoles [42]. In addition, pollen development and male fertility require plenty of Mg influx, which are facilitated by AtMGT4, AtMGT5 and AtMGT9 in Arabidopsis [17,43,44,45].
There are 9 MGT homologs in the rice genome, but only one of them (OsMGT1) has been functionally studied [35,38]. Our previous studies have revealed that OsMGT1 is a plasma membrane-localized transporter, which is highly expressed in root tips and vascular tissues. Knockout of OsMGT1 results in decreased Mg uptake in the roots by a stable isotope 25Mg uptake experiment [35]. This evidence indicates that OsMGT1 is a transporter for root Mg uptake in rice. Furthermore, increasing Mg concentrations in the cytosol by OsMGT1 contributes to both higher Al and salt tolerance in rice [35,38], indicating diverse roles of OsMGT1 in response to abiotic stresses. However, whether OsMGTs in rice is involved in low-Mg tolerance is unknown. In this study, we firstly investigated the gene expression of all OsMGTs in both shoots and roots of rice, and observed that only the expression of OsMGT1 in the shoots was remarkably induced by Mg deficiency. Knockout of OsMGT1 resulted in much lower Mg accumulation and higher sensitivity to Mg deficiency, while overexpression of OsMGT1 enhanced the tolerance to Mg deficiency. Taken together, our results suggest that OsMGT1 plays an important role in rice growth under low-Mg stress.
2. Results
2.1. OsMGT1 Was Up-Regulated by Mg Deficiency
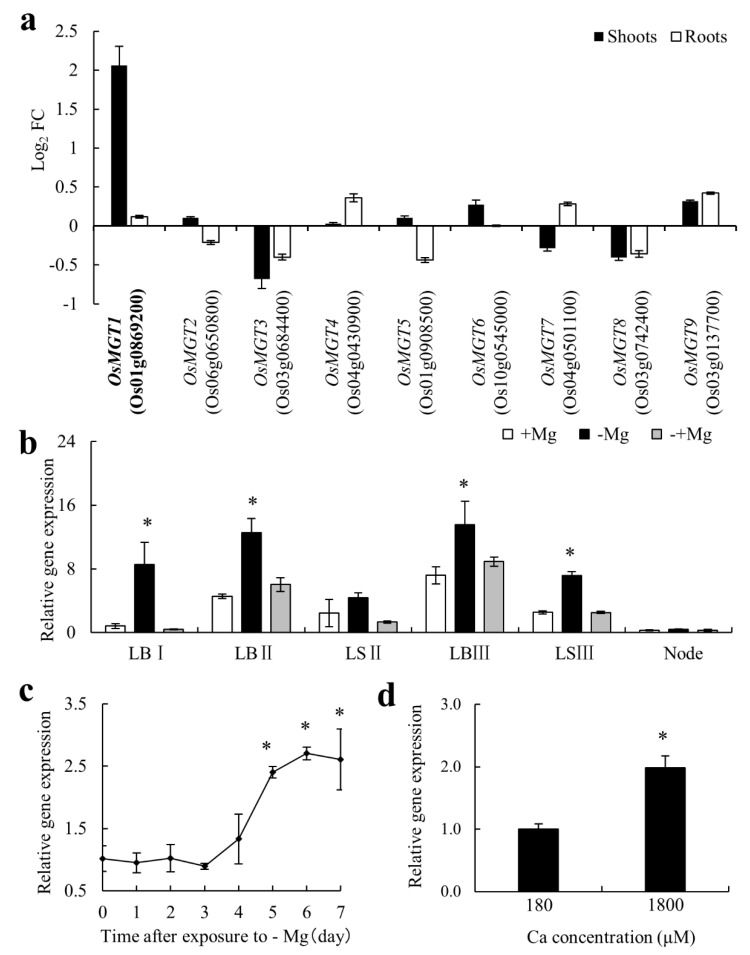
We removed Mg from nutrient solution in order to examine the response of OsMGTs to Mg deficiency in rice. Real-time RT-PCR results revealed that most of OsMGTs in rice have little response to Mg deficiency (Figure 1a). Among nine members, only OsMGT1 was significantly induced by Mg deficiency (Figure 1a). We found that its expression in the shoots was up-regulated by about 4 times after exposure to Mg deficiency for 7 days, whereas that in the roots was unaffected (Figure 1a). Analysis of shoot spatial expression showed that OsMGT1 in both leaf blade and leaf sheath was up-regulated in the absence of Mg, but recovered rapidly after the addition of Mg for 24 h (Figure 1b). A time-course experiment showed that the induction of OsMGT1 occurred at the fifth day after exposure to Mg deficiency and the expression kept a relatively high level after Mg induction (Figure 1c). Furthermore, the expression of OsMGT1 also can be enhanced by excessive calcium (Ca) supply under −Mg condition (Figure 1d).
Figure 1.
Gene expression pattern of OsMGT1 in response to Mg deficiency. Gene expression of all OsMGTS family members in both shoots and roots (a). FC, fold change of induced expression. Effect of Mg sufficiency (+Mg), Mg deficiency (−Mg) and resupply (−+Mg) on the expression of OsMGT1 in shoot tissues (b), including leaf blade (LB), leaf sheath (LS) and node. I to III is from young to old. Time-dependent expression of OsMGT1 in shoots after exposure to −Mg (c). Effect of excessive Ca on the expression of OsMGT1 under −Mg condition (d). The expression level was determined by real-time RT-PCR. OsActin was used as an internal standard. Data are means ± SD (n = 3). The asterisk indicates significantly different (p ≤ 0.05 by Tukey’s test).
2.2. Mg Deficiency Altered the Tissue Expression Pattern of OsMGT1
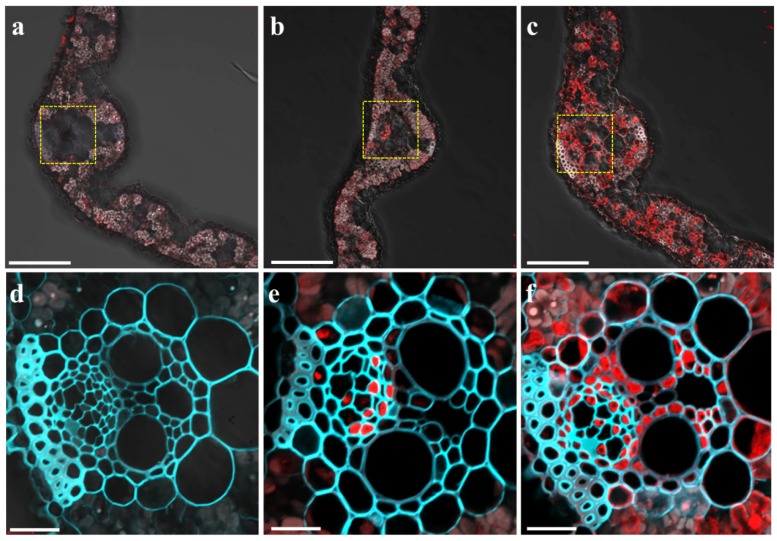
To examine the tissue and cell specificity of OsMGT1 expression in response to Mg deficiency, we performed immunostaining of the transgenic rice carrying the 2.5 kb promoter sequence of OsMGT1 fused with green fluorescent protein (GFP). The GFP antibody signal can be observed in the leaf blade of transgenic lines, but no signal was observed in wild type (WT) rice (Figure 2a,d), suggesting the high specificity of the GFP antibody. This signal was mainly in the phloem region of vascular bundles under Mg sufficient condition (Figure 2b,e). However, under Mg deficient condition, the signals were observed not only in the phloem region, but also xylem parenchyma cells and mesophyll cells in leaf blades (Figure 2c,f), indicating that Mg deficiency alters the tissue expression pattern of OsMGT1 in leaves.
Figure 2.
Tissue-specific and Mg-responsive expression of OsMGT1. Immunostaining with an anti-GFP was performed in the leaf blade of wild-type rice (a,d) and pOsMGT1-GFP transgenic rice under +Mg (b,e) and −Mg conditions (c,f). (d–f) are magnified images of yellow-dotted areas in (a–c) respectively. The red color represents the signal from the GFP antibody and cyan represents the signal from cell wall autofluorescence. Bars = 100 µm (a–c) and 20 µm (d–f).
2.3. Knockout of OsMGT1 Resulted in Higher Sensitivity to Mg Deficiency
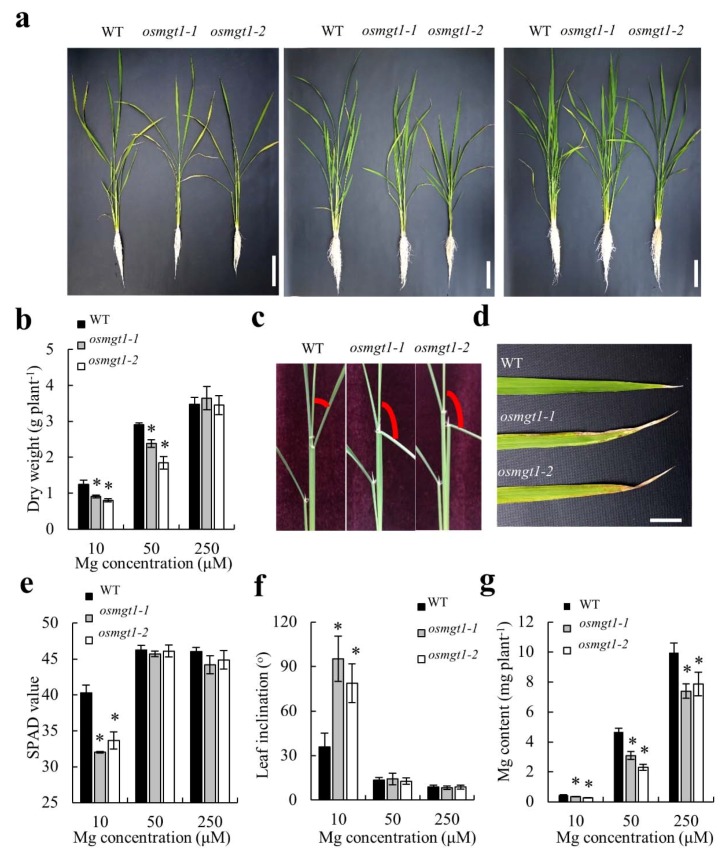
To investigate the physiological role of OsMGT1 in rice under Mg deficient condition, the WT and two independent OsMGT1 knockout lines were grown hydroponically with different concentrations of Mg supply. Under sufficient Mg (250 µM) supply, the growth was the same between the WT and two mutants (Figure 3a). However, under insufficient Mg (10 and 50 µM) supply, two mutants showed growth retardation compared with WT (Figure 3a), presenting a 20%–40% decrease in dry weight (Figure 3b). Furthermore, Mg deficient phenotypes such as leaf inclination and chlorosis were observed more evidently in two mutants (Figure 3c,d). The spectral plant analysis diagnostic (SPAD) values in fully expanded leaf of the mutants were remarkably lower than that of WT (Figure 3e). The angles of lamina joint in mutants became significantly larger than that in WT (Figure 3f). On the other hand, mineral analysis showed that Mg content was increased in both WT and mutants with increasing external Mg supply (Figure 3g). Nevertheless, two mutants showed much lower Mg content than WT at each Mg concentration (Figure 3g). All of these results indicate that OsMGT1 plays an important role in rice growth under Mg-limited conditions.
Figure 3.
Sensitivity of OsMGT1 knockout lines to Mg deficiency. Seedlings of both of WT and two OsMGT1 knockout lines were grown with different Mg concentrations (a). Growth conditions (a). Left, 10 µM; middle, 50 µM; right, 250 µM. Dry weight (b), leaf chlorosis (d), spectral plant analysis diagnostic (SPAD) value (e), Mg content (g) and leaf inclination (c,f). Data are means ± SD (n = 3). The asterisk shows a significant difference compared with WT (p ≤ 0.05 by Tukey’s test). Bars = 10 cm (a) and 2 cm (d).
2.4. Excessive Ca Aggravated Mg Deficiency in osmgt1 Mutants
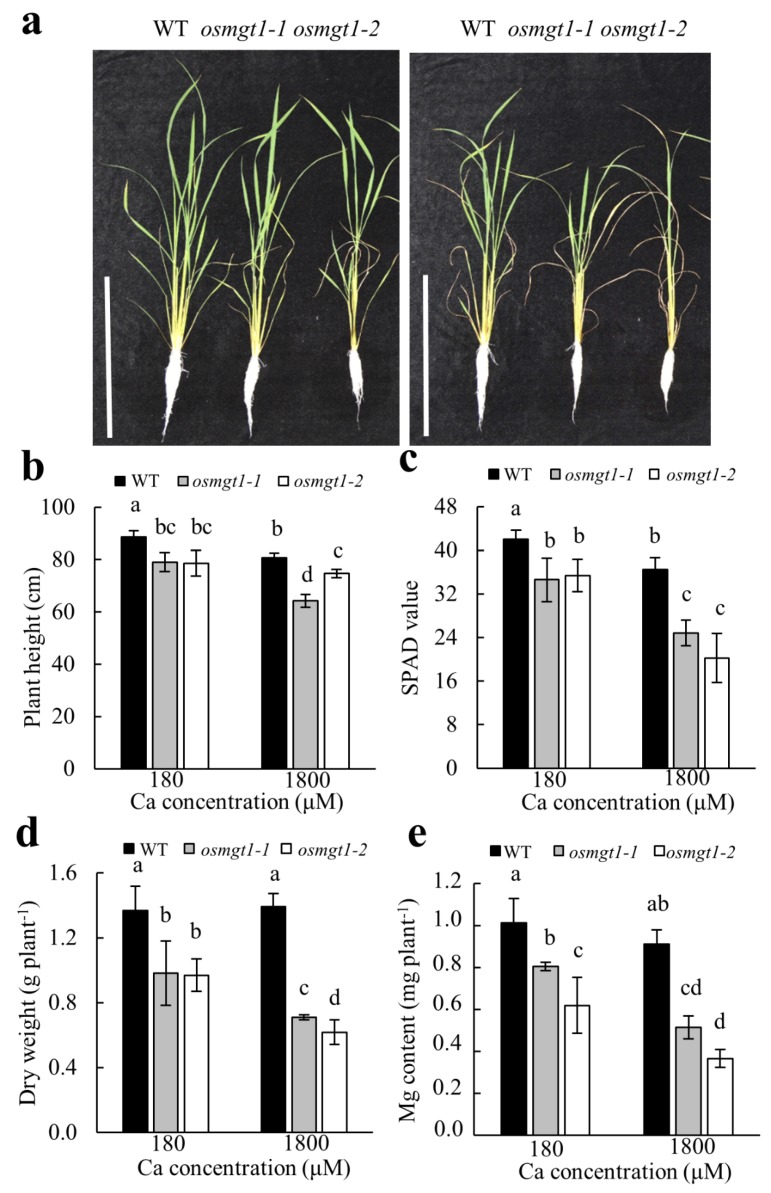
To test the effect of Ca on OsMGT1-mediated Mg transport, the WT and two mutants were grown in the nutrient solution containing normal Ca (180 µM) or high Ca (1800 µM) concentrations in the presence of low Mg (10 µM). Our results showed that high Ca did not affect the growth of WT, but significantly inhibited the growth of osmgt1 mutants under low-Mg conditions (Figure 4a). The parameters including plant height, SPAD value, dry weight and Mg content of the mutants were reduced more evidently by high Ca supply (Figure 4b–e), which suggests that the sensitivity to Mg deficiency in osmgt1 mutants can be aggravated by excessive Ca. By contrast, high Ca supply has little influence on these parameters in WT (Figure 4b–e), suggesting that OsMGT1 is also required for rice growth under Ca-aggravated Mg deficiency.
Figure 4.
Aggravation of Mg deficiency by excessive Ca in OsMGT1 knockout lines. Seedlings of both WT and two OsMGT1 knockout lines were exposed to normal (180 µM) or high (1800 µM) Ca concentration in the presence of low Mg (10 µM). Growth conditions (a). Left, 180 µM; right, 1800 µM (a). Plant height (b), SPAD value (c), dry weight (d) and Mg content (e). Data are means ± SD (n = 3). Means with different letters are significantly different (p ≤ 0.05 by Tukey’s test). Bar = 30 cm.
2.5. Overexpression of OsMGT1 Promoted Rice Growth Under Low Mg Stress
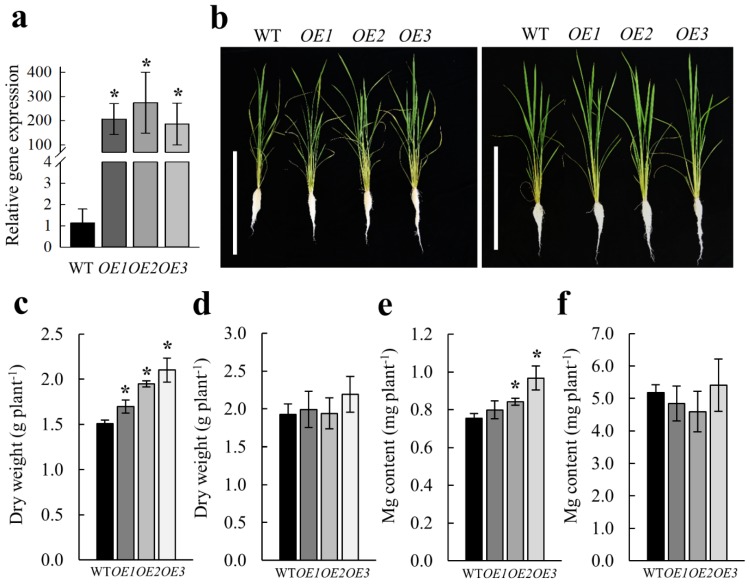
We generated three independent transgenic lines overexpressing OsMGT1 (Figure 5a), in order to explore its genetic potential. We compared the WT and three overexpression lines hydroponically in the nutrient solution containing deficient (10 µM) and sufficient (250 µM) Mg concentrations. Our results showed that overexpression of OsMGT1 resulted in better growth of rice plants under low-Mg conditions (Figure 5b), which was achieved by the increased Mg content and dry weight in transgenic lines (Figure 5c,e). By contrast, overexpression of OsMGT1 did not improve the rice growth under Mg sufficient conditions (Figure 5d,f).
Figure 5.
Overexpression of OsMGT1 improved rice growth under low-Mg conditions. The WT and three overexpressing lines (OE1, OE2 and OE3) were grown with deficient (10 µM) or sufficient (250 µM) Mg supply. Relative expression level of OsMGT1 in three overexpressing lines (a). The expression level was determined by real-time RT-PCR. OsActin was used as an internal standard. Growth conditions (b). Left, 10 µM; right, 250 µM. Dry weight (c,d) and Mg content (e,f) under deficient and sufficient Mg supply. Data are means ± SD (n = 3). The asterisk shows a significant difference compared with WT (p ≤ 0.05 by Tukey’s test). Bar = 50 cm.
3. Discussion
MRS2/MGT family members have been identified as main Mg transporter genes in both prokaryote and eukaryote [21,26,27,28,29]. However, unlike other elemental transporter genes, the expression of these genes is rarely up-regulated by Mg deficiency [46,47,48,49]. So far, only AtMGT6 in Arabidopsis roots has been revealed to be quickly induced by Mg deficiency, which is required for root Mg uptake [36]. In this study, we investigated the expression of all the OsMGTs in response to Mg deficiency in rice. Unexpectedly, none of them was able to be significantly induced by Mg deficiency for 7 days in rice roots (Figure 1a), suggesting that Mg transport systems in rice and Arabidopsis might be differently regulated. Notably, among nine members, only OsMGT1 in shoots were highly induced by Mg deficiency (Figure 1a). Furthermore, the induction of OsMGT1 by Mg deficiency is achieved by altering the tissue expression patterns in shoots. Under Mg sufficient condition, OsMGT1 is only expressed in the phloem region of shoot vascular bundle (Figure 2b,e). However, the expression was remarkably enhanced in xylem parenchyma and leaf mesophyll cells by Mg deficiency (Figure 2c,f). Since OsMGT1 is a plasma membrane-localized Mg transporter, we speculate that rice is able to enhance Mg acquisition by facilitating both xylem Mg unloading and Mg import into leaf mesophyll cells by OsMGT1, in order to overcome Mg deficiency in shoots. However, OsMGT1 in roots can be highly induced by Al toxicity and salt stress [25,50,51,52], suggesting that it has a more important role in the resistance to abiotic stresses than root Mg uptake. Indeed, knockout of OsMGT1 only reduces one-third amount of Mg in rice roots [25,50,51,52], suggesting that there are other transporters mediating Mg uptake in rice. In bacteria, the repressible Mg uptake is mediated by the MgtA and MgtB protein [53]. Unlike CorA, MgtA and MgtB are P-type ATPases that mediate Mg influx [54,55]. However, whether P-type ATPases are involved in Mg uptake in plants needs to be further clarified.
Comparison of WT and osmgt1 mutants revealed that the WT grew better than two osmgt1 mutants under low-Mg conditions (Figure 3a). The increased sensitivity to Mg deficiency in mutants was due to the much lower Mg content in plants (Figure 3g), which resulted in much severer chlorosis in the leaves and larger inclination in the lamina joint (Figure 3d,f). On the other hand, overexpression of OsMGT1 improved rice growth under low-Mg conditions, which is accompanied with increased dry weight and Mg content in overexpression lines (Figure 5c,e). These results indicate that OsMGT1 is required for the tolerance to Mg deficiency, and that it can be utilized for molecular breeding of low-Mg tolerant plants in the future.
Considering that Mg induced expression of OsMGT1 can be further enhanced by excessive Ca supply (Figure 1d), we compared WT and osmgt1 mutants under excessive Ca conditions. Interestingly, excessive Ca aggravated Mg deficiency in osmgt1 mutants, but not in WT (Figure 4a). Consistent with this phenotype, the plant height, SPAD value, dry weight and Mg content were decreased more evidently in osmgt1 mutants (Figure 4b–e). It is well-known that Ca has an inhibiting effect on Mg uptake, through competition for ion channels that directly inhibit Mg transport, or apoplastic binding sites that indirectly inhibit Mg transport [25,50,51,52]. Since MRS2/MGT transporters own unique structure characteristics that have high selectivity to Mg2+ [31,32], it is unlikely that OsMGT1 also has a high affinity to Ca2+. One possibility is that exogenously addition of Ca competitively reduced Mg apoplastic binding, leading to much lower Mg uptake and more severe Mg deficiency in osmgt1 mutants (Figure 4). By contrast, the WT is able to alleviate these stresses from low Mg and high Ca by upregulating OsMGT1. Therefore, our results suggest that upregulation of OsMGT1 by excessive Ca is an indirect response for rice to survive under a much more severe Mg deficient condition that is caused by excessive Ca.
Since OsMGT1 has diverse roles in abiotic stresses, a question remains as to whether OsMGT1 is able to transport other metals, such as Al, Na or Ca? However, Al3+ and Na+ have different ionic valency to Mg2+, and Ca2+ shows a different hydrated radius to that of Mg2+. It is unlikely that OsMGT1 shares equal affinity with these four metals. Although direct evidence is still needed to clarify the permeability of OsMGT1 to other metals, we speculate that Mg has strong interactions with these three metals, which are not through channel or transporter competition. Mg2+ competes with Al3+ for cellular oxygen donor compounds, competes with Ca2+ for apoplastic binding sites, and facilitates Na+ xylem retrieval by activating Na transporter OsHKT1;5.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Two Tos-17 insertion lines, NF0595 (osmgt1-1) and NE4528 (osmgt1-2) for OsMGT1, were obtained from the Rice Genome Resource Center in Japan. The homozygous lines were screened by PCR using specific primers as described in Chen, et al. [35]. In order to construct transgenic rice for overexpression of OsMGT1, the ORF of OsMGT1 was amplified by PCR using primers Ubi: OsMGT1F and Ubi: OsMGT1R (Table S1). The resulted fragment was cloned into KpnI/BamHI sites of pCAMBIA2300 vector driven by a ubiquitin promoter. The construct was introduced into the calluses of rice (cv Nipponbare) via Agrobacterium tumefaciens-mediated transformation [56].
Seeds of wild-type rice (cv Nipponbare), two knockout mutants, and overexpression lines were soaked in deionized water at 30 °C in the dark for 2 days and then transferred to a net floating on a 0.5 mM CaCl2 solution for 2 days, quarter strength Kimura B solution for 3 days, and half-strength Kimura B solution for 7 days as described in Yamaji and Ma [57]. The solution was renewed once every 2 days.
4.2. RNA Isolation and Gene Expression Analysis
In order to explore the effect of Mg deficiency on the expression of OsMGTs, a portion of 2-week-old rice seedlings were put into nutrient solution without Mg every day. After 7 days, the roots and shoots of all samples were separately harvested at the same time. After that, the additional 7 days Mg deficient seedlings were resupplied with 250 µM Mg for an additional day, and the samples including leaf blade, leaf sheath and node were separately harvested. In order to investigate the effect of excessive Ca on the expression of OsMGT1, 2-week-old rice seedlings were grown in the nutrient solution containing different concentrations of CaCl2 (180 µM and 1800 µM) in the presence of 10 µM Mg for 7 days, and the shoots were harvested.
Total RNA from rice tissues was extracted using the TransZol Up Plus RNA Kit (TransGen Biotech, Beijing, China). Half microgram of total RNA was used for first-strand cDNA synthesis using a TransScript One-Step gDNA Removal and cDNA Synthesis Super Mix (TransGen Biotech, Beijing, China) following the manufacturer’s instructions. The gene expression level was determined by real-time RT-PCR using the primers with TransStart Top Green qPCR Super Mix (TransGen Biotech, Beijing, China) on LightCycler 96 Real-Time PCR (Roche, Basel, Switzerland). Primers for real-time RT-PCR were listed in Table S1. OsActin was used as an internal control. Normalized relative expression was calculated by the ΔΔCt method.
4.3. Immunohistological Analysis of OsMGT1
The transgenic plants carrying promotorOsMGT1:GFP were generated as described in Chen, et al. [38]. The seedlings were grown hydroponically in nutrient solution with or without Mg for 7 days. The middle parts of the youngest fully expanded leaves were sampled for immunostaining according to the method modified from Chen, et al. [58]. The samples were fixed in a solution containing 4% (w/v) paraformaldehyde, 60 mM sucrose and 50 mM sodium cacodylate for 2 h, and then embedded in 5% agar. The samples were sectioned to 60 µm thickness and incubated in 10 mM PBS containing 0.3% (v/v) Triton X-100 for 2 h. The slides were then incubated with GFP antibodies (anti-green fluorescent protein, Thermo Fisher Scientific, Somerset, NJ, USA) and subsequently with secondary antibodies (Alexa Fluor 555, Molecular Probes, Eugene, OR, USA). We observed the sections with a laser scanning confocal microscope (LSM880, Carl Zeiss, Oberkochen, Germany).
4.4. Phenotypic Analysis
To compare the sensitivity to Mg deficiency between WT and osmgt1 mutants, 2-week-old seedlings of both WT (cv Nipponbare) and two OsMGT1 knockout lines were grown in the nutrient solution containing 10 µM, 50 µM or 250 µM Mg. After 45 days, the rice seedlings were photographed. The SPAD values of the youngest fully expanded leaves were measured by a chlorophyll meter (SPAD-502 Plus, Konica Minolta, Tokyo, Japan) and the angles of lamina joint in each leaf were recorded by protractor. The plants were sampled after the roots were washed with 5 mM CaCl2 for three times to remove the apoplastic cations. The dry weight of the plants was weighed after being dried in a 75 °C oven for 2 days. Mg was determined by ICP-MS as described below.
The effect of excessive Ca on Mg deficiency was investigated by exposing 2-week-old WT and two OsMGT1 knockout lines to a nutrient solution containing 180 µM or 1800 µM CaCl2 in the presence of 10 µM MgCl2 for 30 days. The rice seedlings were photographed. The plant height was measured by a ruler. The SPAD values of the youngest fully expanded leaves were measured using a chlorophyll meter. The dry weight of the plants was weighed after dried in 75 °C oven for 2 days. Mg was determined by ICP-MS as described below.
To investigate whether the tolerance to Mg deficiency is altered by overexpression of OsMGT1 in rice, 2-week-old seedlings of WT and three overexpression lines were exposed to a nutrient solution containing 10 µM or 250 µM Mg. After 30 days, the rice seedlings were photographed. The dry weight was weighed after dried in a 75 °C oven for 2 days. Mg was determined by ICP-MS as described below.
4.5. Mg Determination in Plant Tissues
After harvest, the tissues were dried at 75 °C for 2 days to constant weight and then were subjected to digestion with concentrated HNO3 (68%) at a temperature of up to 140 °C. Mg concentration in the digested solution was determined by inductively coupled plasma-mass spectrometry (ICP-MS 7900, Agilent Technologies, Santa Clara, CA, USA). The Mg content was calculated based on Mg concentration and dry weight.
5. Conclusions
Taken together, our results conclude that OsMGT1 is required for the resistance to Mg deficiency in rice through facilitating both Mg transfer in xylem and Mg import in mesophyll cells.
Supplementary Materials
The following are available online at http://www.mdpi.com/1422-0067/20/1/207/s1.
Author Contributions
Z.C. conceived and designed the experiments. L.Z., Y.P., X.T. and Z.C. performed and analyzed the experiments. J.L. constructed the overexpression vector and generated the overexpression lines. L.Z., and Z.C. wrote the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 31672218; 31872171); and the China National Key Program for Research and Development (grant number 2016YFD0100700). L.Z. were supported by the K+S scholarship from the International Magnesium Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Williams L., Salt D.E. The plant ionome coming into focus. Curr. Opin. Plant Biol. 2009;12:247–249. doi: 10.1016/j.pbi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marschner H. Marschner’s Mineral Nutrition of Higher Plants. 3rd ed. Academic Press; New York, NY, USA: 2012. pp. 165–170. [Google Scholar]
- 3.Chen Z.C., Peng W.T., Li J., Liao H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018;74:142–152. doi: 10.1016/j.semcdb.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Lin D.C., Nobel P.S. Control of photosynthesis by Mg2+ Arch. Biochem. Biophys. 1971;145:622–632. doi: 10.1016/S0003-9861(71)80022-X. [DOI] [PubMed] [Google Scholar]
- 5.Sperrazza J.M., Spremulli L.L. Quantitation of cation binding to wheat germ ribosomes: Influences on submit association equilibria and ribosome activity. Nucleic Acids Res. 1983;11:2665–2679. doi: 10.1093/nar/11.9.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce J., Lorimer G.H., Reddy G.S. Kinetic mechanism of ribulosebisphosphate carboxylase: Evidence for an ordered, sequential reaction. Biochemistry. 1986;25:1636–1644. doi: 10.1021/bi00355a029. [DOI] [Google Scholar]
- 7.Rissler H.M., Collakova E., DellaPenna D., Whelan J., Pogson B.J. Chlorophyll biosynthesis. Expression of a second Chl I gene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol. 2002;128:770–779. doi: 10.1104/pp.010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cakmak I., Kirkby E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008;133:692–704. doi: 10.1111/j.1399-3054.2007.01042.x. [DOI] [PubMed] [Google Scholar]
- 9.Cakmak I. Magnesium in crop production, food quality and human health. Plant Soil. 2013;368:1–4. doi: 10.1007/s11104-013-1781-2. [DOI] [Google Scholar]
- 10.Verbruggen N., Hermans C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil. 2013;368:87–99. doi: 10.1007/s11104-013-1589-0. [DOI] [Google Scholar]
- 11.Wilkinson S.R., Welch R.M., Mayland H.F., Grunes D.L. Magnesium in plants: Uptake, distribution, function and utilization by man and animals. Met. Ions Biol. Syst. 1990;26:33–56. [Google Scholar]
- 12.Maguire M.E., Cowan J.A. Magnesium chemistry and biochemistry. Biometals. 2002;15:203–210. doi: 10.1023/A:1016058229972. [DOI] [PubMed] [Google Scholar]
- 13.Grzebisz W. Magnesium-food and human health. J. Elemntol. 2011;16:299–323. doi: 10.5601/jelem.2011.16.2.13. [DOI] [Google Scholar]
- 14.Cakmak I., Yazici A.M. Magnesium: A forgotten element in crop production. Better Crops. 2010;94:23–25. [Google Scholar]
- 15.Rosanoff A. Changing crop magnesium concentrations: Impact on human health. Plant Soil. 2013;368:139–153. doi: 10.1007/s11104-012-1471-5. [DOI] [Google Scholar]
- 16.Gransee A., Führs H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil. 2013;368:5–21. doi: 10.1007/s11104-012-1567-y. [DOI] [Google Scholar]
- 17.Li L.G., Sokolov L.N., Yang Y.H., Li D.P., Ting J., Pandy G.K., Luan S. A mitochondrial magnesium transporter functions in Arabidopsis pollen development. Mol. Plant. 2008;1:675–685. doi: 10.1093/mp/ssn031. [DOI] [PubMed] [Google Scholar]
- 18.Hermans C., Conn S.J., Chen J.G., Xiao Q.Y., Verbruggen N. An update on magnesium homeostasis mechanisms in plants. Metallomics. 2013;5:1170–1183. doi: 10.1039/c3mt20223b. [DOI] [PubMed] [Google Scholar]
- 19.Shaul O., Hilgemann D.W., de-Almeida-Engler J., Montagu M.V., Inze´ D., Galili G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 1999;18:3973–3980. doi: 10.1093/emboj/18.14.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L.G., Tutone A.F., Drummond R.S.M., Gardner R.C., Luan S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell. 2001;13:2761–2775. doi: 10.1105/tpc.13.12.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebert M., Meschenmoser K., Svidova´ S., Weghuber J., Schweyen R., Eifler K., Lenz H., Weyand K., Knoop V. A root-expressed magnesium transporter of the MRS2/MGT gene family in Arabidopsis thaliana allows for growth in low-Mg2+ environments. Plant Cell. 2009;21:4018–4030. doi: 10.1105/tpc.109.070557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang R.J., Luan S. Regulation of calcium and magnesium homeostasis in plants: From transporters to signaling network. Curr. Opin. Plant Biol. 2017;39:97–105. doi: 10.1016/j.pbi.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C., Li H., Wang J., Zhang B., Wang W., Lin H., Luan S., Gao J., Lan W. The rice high-affinity k+ transporter OsHKT2;4 mediates Mg2+ homeostasis under high-Mg2+ conditions in transgenic Arabidopsis. Front. Plant Sci. 2017;8:1823–1836. doi: 10.3389/fpls.2017.01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan C.W.M., Schorrak L.M., Smith R.K., Jr., Bent A.F., Sussman M.R. A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol. 2003;132:728–731. doi: 10.1104/pp.102.019216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo K.M., Babourina O., Christopher D.A., Borsic T., Rengel Z. The cyclic nucleotide-gated channel AtCNGC10 transports Ca2+ and Mg2+ in Arabidopsis. Physiol. Plant. 2010;139:303–312. doi: 10.1111/j.1399-3054.2010.01366.x. [DOI] [PubMed] [Google Scholar]
- 26.Saito T., Kobayashi N.I., Tanoi K., Iwata N., Suzuki H., Iwata R., Nakanishi T.M. Expression and functional analysis of the CorA-MRS2-ALR-type magnesium transporter family in rice. Plant Cell Physiol. 2013;54:1673–1683. doi: 10.1093/pcp/pct112. [DOI] [PubMed] [Google Scholar]
- 27.Graschopf A., Stadler J.A., Hoellerer M.K., Eder S., Sieghardt M., Kohlwein S.D., Schweyen R.J. The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+ dependent control of its synthesis and degradation. J. Biol. Chem. 2001;276:16216. doi: 10.1074/jbc.M101504200. [DOI] [PubMed] [Google Scholar]
- 28.Niegowski D., Eshaghi S. The CorA family: Structure and function revisited. Cell. Mol. Life Sci. 2007;64:2564–2574. doi: 10.1007/s00018-007-7174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moomaw A.S., Maguire M.E. The unique nature of Mg2+ channels. Physiology. 2008;23:275–285. doi: 10.1152/physiol.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knoop V., Groth-Malonek M., Gebert M., Eifler K., Weyand K. Transport of magnesium and other divalent cations: Evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol. Genet. Genom. 2005;274:205–216. doi: 10.1007/s00438-005-0011-x. [DOI] [PubMed] [Google Scholar]
- 31.Lunin V.V., Dobrovetsky E., Khutoreskaya G., Zhang R.G., Joachimiak A., Doyle D.A., Bochkarev A., Maguire M.E., Edwards A.M., Koth C.M. Crystal structure of the CorA Mg2+ transporter. Nature. 2006;440:833–837. doi: 10.1038/nature04642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalmas O., Sompornpisut P., Bezanilla F., Perozo E. Molecular mechanism of Mg2+-dependent gating in CorA. Nat. Commun. 2014;5:3590. doi: 10.1038/ncomms4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H.Y., Du H.M., Huang K.F., Chen X., Liu T.Y., Gao S.B., Liu H.L., Tang Q.L., Rong T.Z., Zhang S.Z. Identification, and functional and expression analyses of the CorA/MRS2/MGT-type magnesium transporter family in maize. Plant Cell Physiol. 2016;57:1153–1168. doi: 10.1093/pcp/pcw064. [DOI] [PubMed] [Google Scholar]
- 34.Waters B.M. Moving magnesium in plant cells. New Phytol. 2011;190:510–513. doi: 10.1111/j.1469-8137.2011.03724.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z.C., Yamaji N., Motoyama R., Nagamura Y., Ma J.F. Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol. 2012;159:1624–1633. doi: 10.1104/pp.112.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao D.D., Chen J., Tian L.F., Liu Z., Yang L., Tang R., Li J., Lu C.Q., Yang Y.H., Shi J.S., et al. Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell. 2014;26:2234–2248. doi: 10.1105/tpc.114.124628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y.W., Mao D.D., Yang L., Qi J.L., Zhang X.X., Tang Q.L., Li Y.P., Tang R.J., Luan S. Magnesium transporter MGT6 plays an essential role in maintaining magnesium homeostasis and regulating high magnesium tolerance in Arabidopsis. Front. Plant Sci. 2018;9:274–287. doi: 10.3389/fpls.2018.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z.C., Yamaji N., Horie T., Che J., Li J., An G., Ma J.F. A magnesium transporter OsMGT1 plays a critical role in salt tolerance in rice. Plant Physiol. 2017;174:1837–1849. doi: 10.1104/pp.17.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drummond R.S.M., Tutone A., Li Y.C., Gardner R.C. A putative magnesium transporter AtMRS2-11 is localized to the plant chloroplast envelope membrane system. Plant Sci. 2006;170:78–89. doi: 10.1016/j.plantsci.2005.08.018. [DOI] [Google Scholar]
- 40.Sun Y., Yang R.N., Li L.G., Huang J.R. The magnesium transporter MGT10 is essential for chloroplast development and photosynthesis in Arabidopsis thaliana. Mol. Plant. 2017;10:1584–1587. doi: 10.1016/j.molp.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Liang S., Qi Y., Zhao J., Li Y., Wang R., Shao J., Liu X., An L., Yu F. Mutations in the Arabidopsis AtMRS2-11/AtMGT10/VAR5 gene cause leaf reticulation. Front. Plant Sci. 2017;8:2007–2019. doi: 10.3389/fpls.2017.02007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conn S.J., Conn V., Tyerman S.D., Kaiser B.N., Leigh R.A., Gilliham M. Magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium partitioning within Arabidopsis thaliana mesophyll vacuoles. New Phytol. 2011;190:583–594. doi: 10.1111/j.1469-8137.2010.03619.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen J., Li L.G., Liu Z.H., Yuan Y.J., Guo L.L., Mao D.D., Tian L.F., Chen L.B., Luan S., Li D.P. Magnesium transporter AtMGT9 is essential for pollen development in Arabidopsis. Cell Res. 2009;19:887–898. doi: 10.1038/cr.2009.58. [DOI] [PubMed] [Google Scholar]
- 44.Li J., Huang Y., Tan H., Yang X., Tian L., Luan S., Chen L.B., Li D.P. An endoplasmic reticulum magnesium transporter is essential for pollen development in Arabidopsis. Plant Sci. 2015;231:212–220. doi: 10.1016/j.plantsci.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Xu X.F., Wang B., Lou Y., Han W.J., Lu J.Y., Li D.D., Li L.G., Zhu J., Yang Z.N. Magnesium Transporter 5 plays an important role in Mg transport for male gametophyte development in Arabidopsis. Plant J. 2015;84:925–936. doi: 10.1111/tpj.13054. [DOI] [PubMed] [Google Scholar]
- 46.Hmiel S.P., Snavely M.D., Miller C.G., Maguire M.E. Magnesium transport in Salmonella typhimurium: Characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 1986;168:1444–1450. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermans C., Vuylsteke M., Coppens F., Craciun A., Inzé D., Verbruggen N. Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol. 2010;187:119–131. doi: 10.1111/j.1469-8137.2010.03258.x. [DOI] [PubMed] [Google Scholar]
- 48.Hermans C., Vuylsteke M., Coppens F., Cristescu S.M., Harren F.J.M., Inzé D., Verbruggen N. Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol. 2010;187:132–144. doi: 10.1111/j.1469-8137.2010.03257.x. [DOI] [PubMed] [Google Scholar]
- 49.Chen Z.C., Ma J.F. Magnesium transporters and their role in Al tolerance in plants. Plant Soil. 2013;368:51–56. doi: 10.1007/s11104-012-1433-y. [DOI] [Google Scholar]
- 50.Kinraide T.B., Parker D.R. Cation amelioration of aluminum toxicity in wheat. Plant Physiol. 1987;83:546–551. doi: 10.1104/pp.83.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas K.J., Rice C.V. Revised model of calcium and magnesium binding to the bacterial cell wall. BioMetals. 2014;27:1361–1370. doi: 10.1007/s10534-014-9797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yermiyahu U., Nir S., Ben-Hayyim G., Kafkafi U. Quantitative competition of calcium with sodium or magnesium for sorption sites on plasma membrane vesicles of melon (Cucumis melo L.) root cells. J. Membr. Biol. 1994;138:55–63. doi: 10.1007/BF00211069. [DOI] [PubMed] [Google Scholar]
- 53.Hmiel S.P., Snavely M.D., Florer J.B., Maguire M.E., Miller C.G. Magnesium transport in Salmonella typhimurium: Genetic characterization and cloning of three magnesium transport loci. J. Bacteriol. 1987;171:4742–4751. doi: 10.1128/jb.171.9.4742-4751.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tao T., Snavely M.D., Farr S.G., Maguire M.E. Magnesium transport in Salmonella typhimurium: mgtA encodes a Ptype ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J. Bacteriol. 1995;177:2654–2662. doi: 10.1128/jb.177.10.2654-2662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soncini F.C., García V.E., Solomon F., Groisman E.A. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: Identification of PhoP-regulated genes. J. Bacteriol. 1996;178:5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hiei Y., Komari T., Kubo T. Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol. Biol. 1997;35:205–218. doi: 10.1023/A:1005847615493. [DOI] [PubMed] [Google Scholar]
- 57.Yamaji N., Ma J.F. Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol. 2007;143:1306–1313. doi: 10.1104/pp.106.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Z.C., Yamaji N., Fujii-Kashino M., Ma J.F. A cation-chloride cotransporter gene is required for cell elongation and osmoregulation in rice. Plant Physiol. 2016;171:494–507. doi: 10.1104/pp.16.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.