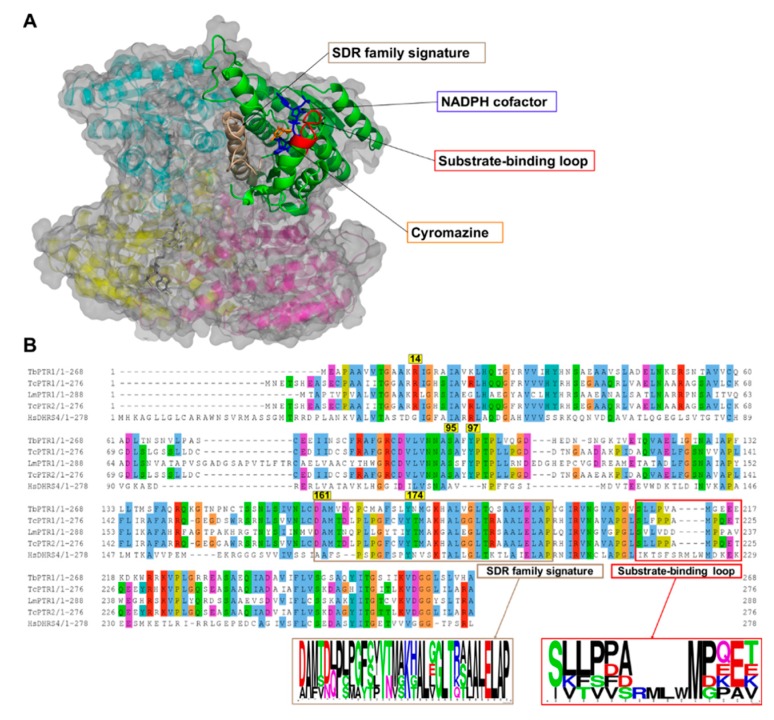
Figure 2.
A cartoon representation of the Trypanosoma brucei pteridine reductase 1 (TbPTR1) protein structure (PDB: 2X9N) and a multiple sequence alignment of T. brucei PTR1, T. cruzi PTR1, T. cruzi PTR2, L. major PTR1, and H. sapiens dehydrogenase/reductase (SDR family) member four (DHRS4). (A) The protein is colored by chain, with the NADPH cofactor colored blue and the co-crystallized ligand cyromazine colored orange. TbPTR1 is a tetramer and the α/β single domain subunit (chain A) is shown in green. The substrate binding loop is colored red and was composed of residues SER207–GLU215, while the SDR family signature, which is colored brown, was composed of residues ASP161–ALA193. (B) The multiple sequence alignment (MSA) showed notable conservation in the SDR family signature, as shown by the sequence logo of the extracted motif. The MSA also showed that within the substrate binding loop there was a four residue deletion that was present only among the trypanosomatids.