Abstract
Purpose of the review:
This review serves to update the reader on emerging data regarding a spectrum of drug-induced liver injury (DILI) outcomes that lie between complete resolution and acute liver failure. Such outcomes can range from mild chronic injury to late liver failure and mortality.
Recent findings:
Several large registries are maturing with large numbers of DILI cases thus shedding light on outcomes including chronic injury and late fatality. We cover definitions commonly used to describe resolution versus chronic injury and mortality due to DILI. We look at rates of occurrence for these different outcomes in major registries. Three specific types of chronic DILI that are illustrative but also easily missed by clinicians are also described.
Summary:
A small but important proportion of DILI cases do not resolve, going on to develop chronic injury and even liver failure. Defining and recognizing these cases is a challenge because DILI is rare, and chronic injury rarer still. Large registries are beginning to define these previously overlooked long term outcomes.
Keywords: chronic, hepatotoxicity, mortality, transplant, liver failure
Introduction:
The vast majority of drug induced liver injury (DILI) cases will resolve without long term sequelae as long as the offending agent is stopped and not taken again. On the other extreme lies drug-induced acute liver failure (ALF) leading to death or urgent need for liver transplant. DILI remains a leading cause of such acute liver failure (1). In the past, DILI was often considered in terms of just these two extremes of complete recovery and acute, fatal injury. However, outcomes can be much more varied including chronic injury that range from subclinical to severe and even fatal months or years after the injury. Chronic injuries that go undetected while on long term medications for months to years also occur. Studying this array of outcomes is challenging due to the relative rarity of DILI and lack of long term follow-up for a large number of cases. Moreover, documentation of injury, particularly from HDS products, remains poor leading to under-reporting and case acquisition biases. Thus, population based descriptions and incidence rates are even more difficult to obtain.
Nevertheless, large registries of DILI cases have been accruing cases for more than a decade now (2–4). While not always population based, they yield important clinical data on outcomes. Capitalizing on this emerging data will require clearer outcome definitions including chronic DILI and fatalities clearly attributable to DILI. For now, registries do provide an accurate description of the clinical array of outcomes and relative incidence amongst cases enrolled. If nothing else, these data should raise the level of awareness that DILI can have a variety of presentations as well as outcomes requiring diligence in follow-up.
Definitions:
While some liver injury outcomes may fall into established definitions such as acute liver failure (5, 6) or acute on chronic liver failure (7, 8), other outcomes are more nebulous to define. Even resolution can be open to debate. How long should one give for an injury to resolve before considering it chronic versus still resolving? If there is no known baseline of liver enzymes and bilirubin, what should the cut-off be to define resolution? Typically, complete normalization of liver biochemistries is considered resolution, but in this era of highly prevalent non-alcoholic fatty liver disease (NAFLD), this may not be reasonable. The upper limit of normal for liver biochemistries also varies between labs and which upper limit used will greatly effect descriptive data and future research on this topic (9). An acute injury from a drug may lead to residual fibrosis, portal hypertension, or worsening of a background liver disease. The biochemistries may fall, but the patient may be clearly worse off than before. Such cases are difficult to label as resolved even though the pathophysiology of the acute injury itself may be gone.
For now, most studies consider complete resolution as liver biochemistries falling to within normal limits (or prior baseline if known) without signs of persistent liver failure (e.g. portal hypertension) within 3–6 months of DILI onset. When enzymes do not fall by these criteria, chronic DILI, which was described histologically as early as 1999, becomes a concern (10). Ideally, chronic DILI would be best defined by histology in all cases. However, repeat biopsies in patients are not always done nor reported, particularly when the patient is feeling better and enzymes remain only modestly elevated. Fatal outcomes are somewhat arbitrarily divided into acute (< 6 months) versus late or chronic (> 6 months), with each having quite different phenotypic injuries and clinical courses. The role of DILI in these fatalities can also be quite varied and difficult to define, particular for deaths occurring beyond 6 months.
Chronic Drug-induced Liver Injury
Chronic liver injury from medications has been described for over 40 years (11–13). These earlier papers often focused on specific agents now well known to cause chronic injury (e.g. methyldopa, nitrofurantoin), particularly when taken for prolonged periods of time. However, in 1999, Aithal and Day described a cohort of patients who appeared to have chronic liver injury from a variety of medications and well after they’d been stopped (10). Using a single center histopathology database, they identified 44 hospitalized patients felt to have had DILI. Eleven had died or were lost to follow-up, but 33 were contacted and able to come back for evaluation after a median of 5 years (range 1–19). A remarkable 13 of 33 still had abnormal liver enzymes and/or abnormal imaging of the liver. Significant fibrosis on initial biopsy and delay in stopping the implicated agent were strongly associated with chronic damage. Only 4 of the 13 had repeat liver biopsies which showed a range of changes from mild non-specific inflammation to chronic hepatitis with fibrosis to ductopenia. Culprit medications were varied including some known to cause chronic liver injury with long term use (amiodarone, methyldopa, nitrofurantoin), but most of the agents were not classically associated with chronic injury (e.g. amoxicillin/clavulanate, diclofenac, halothane). Moreover, patients had been off the culprit medication for 1.8 to 19.5 years. Such a high rate of chronicity (nearly a third) is likely due to selection bias since all patients were hospitalized and had a biopsy during initially DILI onset. In other words, these were severe cases warranting hospitalization and biopsy.
In another study, DILI cases from the Swedish Adverse Drug Reactions Committee (SADRAC) registry were linked to the national hospitalization and death databases by Bjornson and Davidsdottir to yield a much lower rate of chronic DILI: 1.5% of 712 initially hospitalized cases spanning 11 years of follow-up (2). Here, only those cases re-hospitalized or dying with a liver related issue were identified and included for assessment. Twenty-three such cases were identified of which 11 were felt to have had “protracted DILI” and/or cryptogenic cirrhosis on chart review. Two died due to this cryptogenic cirrhosis, both 3 years after the initial injury. The 7 with protracted DILI without cirrhosis had follow up ranging from 6 to 19 years (median 13), and 6 of these had cholestatic injuries raising concerns for vanishing bile duct syndrome. Because this study looked at subsequent hospitalizations in a national database, most, if not all, severe chronic liver injuries or failures would be captured. Similar to the Aithal and Day study, the implicated agents were varied and prolonged use was significantly associated with chronic DILI. The much lower rate of 1.5% is likely due to the wider initial catchment of hospitalized DILI patients across a nation whether they had a biopsy or not, and analysis of only those who were later hospitalized with a liver related diagnosis. In other words, the denominator was larger and numerator relatively smaller.
The Registry of Hepatotoxicity in Southern Spain (aka Spanish Registry) has been collecting cases of DILI since April 1994 under the guidance of Raul Andrade and M. Isabel Lucena (14). Inpatient and outpatient cases of suspected DILI are referred by health care providers across Spain by well codified forms, interviews, questionnaires and required data. Cases are then fully reviewed and adjudicated for likelihood of DILI using the Rousell Uclaf Causality Assessment Method (RUCAM). Of 493 cases deemed definitely or probably DILI, 28 (5.7%) were considered to have chronic injury, defined by persistently abnormal liver biochemistries 3 months after agent discontinuation for hepatocellular injuries and 6 months for cholestatic or mixed (15). Similar to prior studies, cholestatic or mixed injuries were more common amongst the 28 chronic DILI cases compared to hepatocellular injuries (18 versus 10) and prolonged exposure to the culprit agent occurred in 60% of all chronic cases. Only 4 of the 28 finally recovered with normalization of enzymes, and it took 8 to 26 months to occur. The rest had persistence of abnormal liver biochemistries at 5 to 46 months, and there were a few clinically severe consequences. Three patients who had hepatocellular reactions had cirrhosis 16–25 months later. None of these 3 had known underlying liver disease and 2 had documented normal liver biochemistries prior to injury. Of the 18 with cholestatic or mixed injuries, 1 had vanishing bile ducts while another had ductopenia on biopsy. Remarkably, none of the 28 cases were due to agents classically associated with chronic damage such as nitrofurantoin, amiodarone or methotrexate. Here again the rate of 5.7% is dependent on the catchment of patients and how chronic DILI is defined. Unlike the prior two studies, the Spanish Registry includes non-hospitalized patients and has a relatively low threshold to be enrolled (> twice upper limit of normal for liver enzymes). Therefore this registry casts a wider net of cases with milder initial injury. Chronic injury was defined fairly early at 3–6 months and only required abnormal liver biochemistries.
The largest and most recent registry to look at chronic liver injury comes from the U.S. Drug-Induced Liver Injury Network (DILIN). Definition of chronic DILI was any abnormal liver biochemistries, abnormal liver histology or imaging signs of persistent liver injury at 6 months after enrollment. Fontana et al, reported chronic injury in 18.8% of 598 patients who were deemed to be at least probable DILI, and had survived the first 6 months of follow-up (16). Similar to previous data, cholestatic injuries were more common in the chronic injury group compared to the resolved group (43% versus 22%, p <0.01). Agents leading to chronic DILI varied widely and did not differ significantly from those who revolved the injury within 6 months. Together, antimicrobials and herbal/dietary supplements accounted for over half the chronic cases, 36.3% and 15.9%, respectively.
The DILIN went on to report the 12 month follow-up of these chronic DILI patients (17). Of the 113 patient who met chronic DILI criteria at 6 months, 99 patients came back for follow-up at 12 months. Interestingly, 75% (74 patients) still met criteria for chronic DILI at 12 months while the other 25% had finally resolved their injury. Thus, 12% (74 of 598) had signs of chronic DILI a full year later. The percentage drops slightly to 11% if the denominator includes those patients who died or needed transplant within 6 months. The predominance of cholestatic injuries persisted with 54% having presented with cholestastic injury in the chronic injury group versus 20% for those that resolved by 12 months. In this study, older age was also associated with chronic injury. SF-36 quality of life (QoL) scores for physical function were lower at onset and throughout follow-up for those with chronic injury compared to those that resolved, after controlling for age and gender. These lower QoL scores persisted out to 24 months, implying that chronic injury may cause significant morbidity well after the medication has been stopped. The DILIN’s higher 6 and 12 month rates of chronic injury may relate to enrollment of more severe cases. For example, aminotransferases had to be at least 5 times the upper limit of normal on two consecutive occasions, to qualify on aminotransferase levels alone, compared to the Spanish Registry which used a twice upper limit of normal threshold. Also, the DILIN included imaging and histology as possible criteria for chronic injury, while the Spanish registry used only liver biochemistries. However, it was not clear how many met imaging criteria alone.
Finally, the only truly population based study that has reported data on chronic injury comes from Iceland where complete capture of medication dispensation for the entire population is possible (18). While the focus of that study was on overall incidence of DILI, the authors did report a 7% (7 of 96) rate of persistently abnormal liver biochemistries at 6 months. However, no further data were given for these 7 patients.
Taken together these papers carry broad implications including a shift in our understanding of DILI outcomes from a binary result (fatal versus complete resolution) toward a spectrum that includes chronic injury. (Table 1) For the clinician, these data definitively demonstrate that chronic DILI can occur for a variety of medications, and not just the handful of agents classically associated with chronic hepatitis or fibrosis. Moreover, these chronic injuries can vary from asymptomatic lab tests abnormalities to significant morbidity and mortality. For the basic scientist, they open new perspectives on the pathophysiology of DILI that includes persistent perturbation in the liver and immune system. For the epidemiologist and clinical researcher, the registry data demand clearer definitions so that incidence and outcomes of chronic DILI can be better reported. The data also add DILI to the list of potential etiologies for cryptogenic liver disease. Currently we can only estimate the incidence of chronic DILI to be 5–10% with perhaps 10% of those having a protracted, severe or fatal course. And we suggest that a remote iatrogenic DILI needs to be considered when the etiology of chronic hepatitis, chronic bile duct injury or cirrhosis remains unclear despite an exhaustive evaluation.
Table 1:
Reported rates of chronic drug-induced liver injury
| Study | Rate of chronicity (n/N) | Study cohort | Definition of chronicity | Follow-up or time set for chronic determination | Population based |
|---|---|---|---|---|---|
| Aithal &Day(10) | 30% (13/44) | Hospitalized DILI cases at single center identified in histology database | Abnormal liver biochemistries and/or liver imaging at invited clinic follow up | 5 years (range 1–19) | No |
| SADRAC(2) | 1.5% (11/712) | Hospitalized DILI cases identified in a national hospital database | Abnormal liver biochemistries and/or cirrhosis unexplained on subsequent admission(s) | 13 years (range 6–19) | No |
| Spanish Registry(15) | 5.7% (28/493) | DILI cases referred from across Spain | Abnormal liver biochemistries | 3–6 months | No |
| DILIN(16, 17) | 12% (74/598) | DILI cases enrolled at 10 participating centers | Abnormal liver biochemistries, liver imaging or histology | 12 months | No |
| Iceland study(18) | 7% (7/96) | DILI cases from total population of Iceland | Abnormal liver biochemistries | 6 months | Yes |
Specific Chronic Injuries
There are specific signatures of chronic injury worth noting. Agents leading to chronic hepatitis with autoimmune features have been reported for some time (e.g. nitrofurantoin, minocycline, alpha-methyldopa) (13, 19, 20). Similarly, drugs like methotrexate, amiodarone and estrogen modulators (e.g. tamoxifen) leading to chronic fatty liver are well described (21–24). These agents develop chronic injury primarily due to long term use. Such chronic injuries will not be covered here. The reader is encouraged to access the references given and two reviews for more information (25, 26). Instead, we cover three specific chronic injuries that are less well described or have newer data. These three can present well after the agents are stopped.
Non-cirrhotic portal hypertension due to nodular regenerative hyperplasia:
Patients with DILI may rarely present with non-cirrhotic portal hypertension leading to variceal bleeding or ascites with preserved hepatic synthetic function. Nodular regenerative hyperplasia (NRH) may be present on a liver biopsy (Figure 1), but other times histology is unrevealing. Several medications including oral contraceptives, anti-neoplastic drugs and immunosuppressive agents have been implicated (27, 28). Due to the indolent development of portal hypertension from stellate cell stimulation and liver regeneration, latency periods can be long and well after drug discontinuance. Azathioprine is used in the treatment of inflammatory bowel disease (IBD) and autoimmune hepatitis (AIH) (29, 30). Portal hypertension development in these diseases is often assumed due to associated primary sclerosing cholangitis or cirrhosis, but occasionally azathioprine-induced NRH is present instead. Oxaliplatin is used for stage III colon cancer and has also been associated with portal hypertension due to NRH (31, 32). Patients often present several years after completing adjuvant chemotherapy with this agent. (Figure 1)
Figure 1:
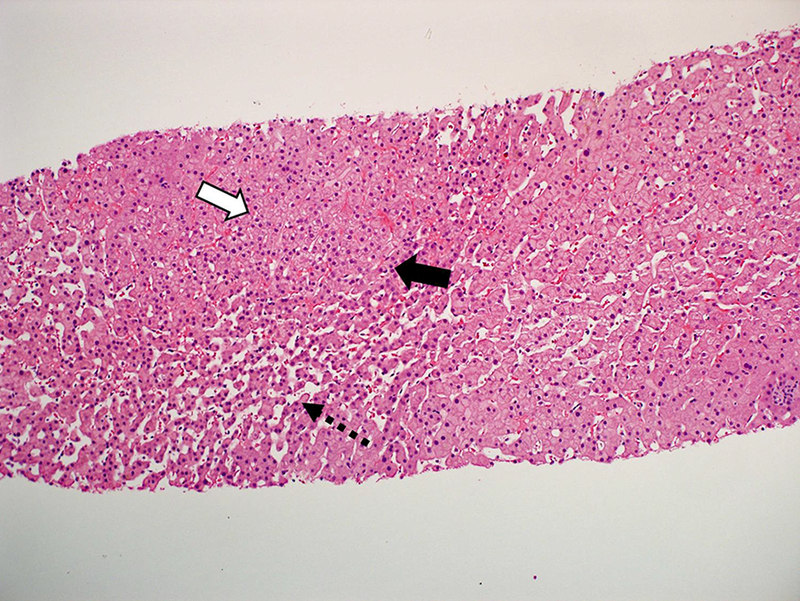
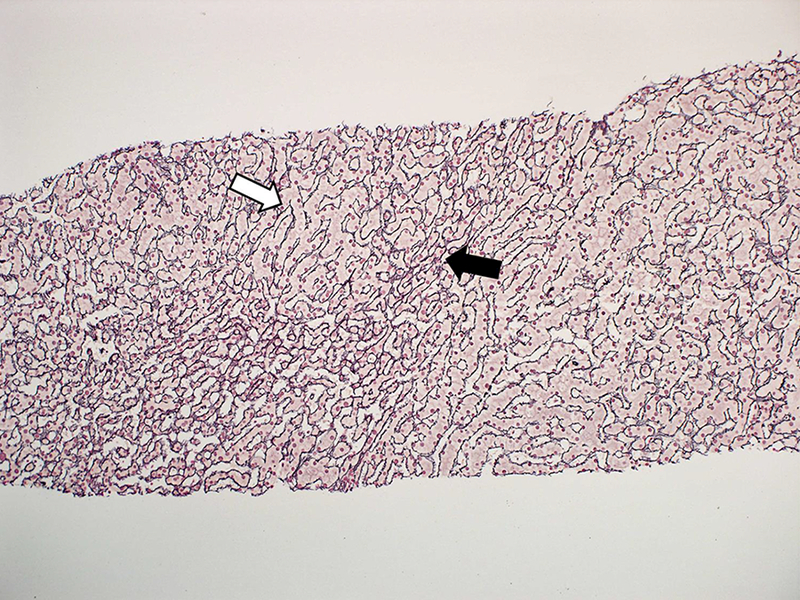
Needle liver biopsy from a 60 year old woman who presented with varcieal bleeding 3.5 years after taking oxaliplatin for Stage III colon cancer. Biopsy shows nodular regenerative hyperplasia without cirrhosis. (a) Hypertrophic (white arrow) and atrophic hepatocytes (black arrow) with sinudoidal dilation (dashed arrow) typical of nodular regenerative hyperplasia. (H&E stain) (b) Reticulin stain highlighting hypertrophy (white arrow) and atrophy (black arrow).
Vanishing bile duct syndrome
Cholestatic drug-induced liver injury is a rare but clinically important cause of chronic destruction and loss of small ducts (33). Extensive bile duct loss and sometimes complete disappearance on liver biopsy, is referred to as the vanishing bile duct syndrome (VBDS). VBDS is diagnosed mainly in patients with prolonged cholestatic liver injury, often with jaundice (34–36). It can be progressive leading to cirrhosis and death (33–35). While VBDS has been associated with a long list of agents, adequate attribution and documentation can be difficult and is not found in all reports. The prototype drug leading to this rare outcome is chlorpromazine which is well-known to cause acute cholestatic liver injury (37–39).
A recent study from DILIN analyzed a relatively large cohort of patients with bile duct loss and VBDS (36). Over the first 10 years of the DILIN study, 1056 patients fulfilled the pre-determined enrollment criteria for DILI. Approximately 30% had a liver biopsies and 7% of those had bile duct loss. Fourteen had severe bile duct loss (< 50% of portal areas with bile ducts) and 12 mild bile duct loss. The vast majority of the patients (96%) presented with jaundice and 77% with itching. Bile ducts were present in 64% of portal areas in biopsies from patients with benign outcome in contrast to only 17% of portal areas having bile ducts in the biopsies from patients with poor outcome. Overall 19% liver-related mortality was observed in patients with bile duct loss, whereas only 6.2% overall mortality was found in the overall DILIN cohort with approximately 50% of those being liver-related (40). Liver transplantation was observed in 8% in the VBDS cohort and 4% in the overall DILIN cohort. Chronicity, defined as elevation of liver tests at 6 months of follow-up was observed in 94% of the patients with bile duct loss vs. 47% in patients with other histological patterns (36). Thus, although very rare the outcome of patients who suffer from VBDS can be severe.
Large bile duct damage
Idiosyncratic DILI has recently been associated with sclerosing cholangitis demonstrated on cholangiography (41–44). Such secondary sclerosing cholangitis has an identifiable etiology in contrast to primary sclerosing cholangitis (45). During the last couple decades, a growing number of causes of secondary sclerosing cholangitis have been identified such as eosinophilic infiltration of bile ducts, IgG4 associated cholangitis and sclerosing cholangitis in critically ill patients (46). Hepatic intra-arterial infusion of chemotherapy drugs was reported to lead to sclerosing cholangitis more than 30 years ago (47, 48). Since then several case reports of idiosyncratic DILI causing secondary sclerosing cholangitis have been published (41, 43, 49, 50).
A recent study of cholangiographies among unselected patients with DILI from a prospective study in Iceland suggested that up to 10% of cases may have sclerosing cholangitis-like changes on magnetic resonance cholangiography (42). Recently, these findings were reproduced in a study from the DILIN cohort (44). Among 56 DILI patients who had been investigated with biliary imaging during the diagnostic work-up of the liver injury, four cases (7%) had drug-induced secondary sclerosing cholangitis. Of these four cases, one was due to moxifloxacin, another atorvastatin, and two herbal supplements. One of the four needed liver transplantation. The limitations of that study was the small number of cases and that only a small minority of patients had undergone biliary imaging. Therefore, it is not clear to what extent secondary sclerosing cholangitis may be found in chronic DILI with cholestatic injury overall.
Long term fatalities
DILI is one of the leading causes of acute liver failure leading to death or need for transplant. Such fatalities occurring within 6 months of onset are more easily reported upon due to the shorter outcome and dramatic decline in liver function. Most registries and reviews report an overall fatality rate of about 5–10%, with most of these being due to ALF (3, 40, 51, 52). There are fewer data on fatalities occurring more than 6 months post-injury, yet clearly they occur. As mentioned previously, cases of chronic injury leading to cirrhosis and liver failure are well described in case reports and registries. However, registries without dedicated long-term follow-up of patients may not be able to fully describe these late deaths or miss them altogether. Determining the precise role DILI plays in these later deaths can be particularly challenging without such long-term follow-up.
The US DILIN prospectively follows all patients with clinic visits and blood tests for up to 2 years. Therefore, the group looked at all fatalities occurring within 2 years of liver injury (53). All fatalities who had at least probable DILI were reviewed systematically by a subgroup of 8 DILIN investigators. The group assessed how much the DILI lead to the fatality. Each case was assigned as either DILI having a primary, contributory or no role in the death. For primary role cases, the course of the liver failure leading to death or transplant were further categorized by time course and pattern of injury. When DILI played only a contributory role or no role in a fatality, a cause of death was assigned.
Of the 1089 cases adjudicated as having at least probable DILI, there were 86 (7.6%) fatalities that were due to the liver injury primarily or partially within the 2 years of follow-up. If limited to only cases presenting with jaundice, the fatality rate climbed to 9.5% (68 of 712). Sixty-eight (82%) of the 86 patients died or required transplant as a direct result of hepatotoxicity, and of these 68, the majority 59 died of liver failure within 6 months. However, the remaining 9 developed a more chronic injury as a direct result of the DILI and eventually died of liver failure during longer term follow-up (6–24 months after presentation). DILI contributed significantly to death by other cause in 15 other patients. These 15 patients died a median of 68 days after the initial injury and tended to be older compared to those who died as a direct result of the liver injury (59 yr. vs. 51, p = 0.06). Most common causes of death were malignancy, sepsis, and severe skin reactions (e.g. DRESS) and most died within 3 months. However, one patient died more than 6 months after the DILI led to worsening of underlying NAFLD. Thus, DILI led to late (> 6 months) mortality, either directly or indirectly, in 10 patients (1% of the total cohort). While not always included in DILI outcome studies, such contributions to late deaths are not trivial. For an aging United States population with increasing NAFLD and polypharmacy, DILI’s contribution to long-term mortality is likely to grow and should be tallied toward the burden of disease. Indeed, DILI risk is directly related to the number of medications prescribed (18).
Conclusion:
Long term outcomes after drug-induced liver injury (DILI) remain ill defined. While the majority of DILI patients do recover without sequelae, registry data convincingly show that chronic liver injury does occur well after discontinuance of the inciting agent. Longer exposure to the agent and cholestatic injuries seem to increase the risk of chronicity. Of these chronic injuries, outcomes can vary widely from asymptomatic elevation in liver enzymes to vanishing bile duct syndrome, portal hypertension, cirrhosis and liver failure. The agents leading to such chronic injury are varied including herbal supplements, antibiotics, non-steroidal antiinflammatory agents and ant-epileptics.
The explanation for this chronicity may lie in perturbations in the immune system that are long standing and effecting bile ducts, vascular structures or both. However, validated mechanistic data are lacking. For now, patients should be counseled that risk of chronic injury is small, probably less than 5–10% depending on the severity and type of injury. And the risk of chronic liver failure and death is around 1% or less. Nevertheless, follow-up is critical, especially if enzymes do not return to normal or imaging suggests development of advanced fibrosis or portal hypertension.
Abbreviations:
- AIH
autoimmune hepatitis
- ALF
acute liver failure
- DILI
drug-induced liver injury
- DILIN
Drug-Induced Liver Injury Network
- DRESS
drug reaction with eosinophilia and systemic symptoms
- ERCP
endoscopic retrograde cholangiopancreatography
- HDS
herbal and dietary supplements
- IBD
inflammatory bowel disease
- MRCP
magnetic resonance cholangiopancreatography
- NAFLD
non-alcoholic fatty liver disease
- NRH
nodular regenerative hyperplasia
- QoL
quality of life
- RUCAM
Rousell Uclaf Causality Assessment Method
- SADRAC
Swedish Adverse Drug Reactions Committee
- SF-36
36-Item Short Form Health Survey
- VBDS
vanishing bile duct syndrome
Compliance with Ethical Standards
Conflict of Interest
Paul H. Hayashi and Einar S. Bjornsson declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References:
- 1.Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, et al. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann Intern Med. 2016;164(11):724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *2.Bjornsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatol. 2009;50(3):511–7.Reports rate of chronic DILI in national (Sweden) hospital database.
- 3.Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129(2):512–21. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924–34, 34 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55(3):965–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. Electronic address eee, Clinical practice guidelines p, Wendon J, Panel m, Cordoba J, Dhawan A, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047–81. [DOI] [PubMed] [Google Scholar]
- 7.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Gines P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–47. [DOI] [PubMed] [Google Scholar]
- 8.Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62(1):243–52. [DOI] [PubMed] [Google Scholar]
- 9.Ruhl CE, Everhart JE. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology. 2012;55(2):447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aithal PG, Day CP. The natural history of histologically proved drug induced liver disease. Gut. 1999;44(5):731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toghill PJ, Smith PG, Benton P, Brown RC, Matthews HL. Methyldopa liver damage. Br Med J. 1974;3(5930):545–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein GB, Lam KC, Mistilis SP. Drug-induced active chronic hepatitis. Am J Dig Dis. 1973;18(3):177–84. [DOI] [PubMed] [Google Scholar]
- 13.Seeff LB. Drug-induced chronic liver disease, with emphasis on chronic active hepatitis. Semin Liver Dis. 1981;1(2):104–15. [DOI] [PubMed] [Google Scholar]
- 14.Andrade RJ, Lucena MI, Fernandez MC, Pelaez G, Pachkoria K, Garcia-Ruiz E, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129(2):512–21. [DOI] [PubMed] [Google Scholar]
- *15.Andrade RJ, Lucena MI, Kaplowitz N, Garcia-Munoz B, Borraz Y, Pachkoria K, et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44(6):1581–8.Reports rate of chronic DILI in a registry accruing cases across Spain.
- 16.Fontana RJ, Hayashi PH, Gu J, Reddy KR, Barnhart H, Watkins PB, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014;147(1):96–108 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **17.Fontana RJ, Hayashi PH, Barnhart H, Kleiner DE, Reddy KR, Chalasani N, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450–9.Reports rate of chronic DILI at 12 months with quality of life data in a U.S. DILI registry.
- *18.Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):1419–25, 25 e1–3; quiz e19–20.Only population based study reporting DILI incidence and covering an entire nation (Iceland).
- 19.Zimmerman HJ. Drug-Induce Liver Injury In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed Philadelphia: Lipponcott Williams & Wilkins; 1999:432–3. [Google Scholar]
- 20.Farrell GC. Drug-induced Chronic Active Hepatitis. In, Farrell GC. Drug-Induced Liver Disease Churchill Livingstone. 1994:423–38. [Google Scholar]
- 21.Zimmerman HJ. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver 2nd ed Philadelphia: Lipponcott Williams & Wilkins; 1999(681–7). [Google Scholar]
- 22.Langman G, Hall PM, Todd G. Role of non-alcoholic steatohepatitis in methotrexate-induced liver injury. J Gastroenterol Hepatol. 2001;16(12):1395–401. [DOI] [PubMed] [Google Scholar]
- 23.Saphner T, Triest-Robertson S, Li H, Holzman P. The association of nonalcoholic steatohepatitis and tamoxifen in patients with breast cancer. Cancer. 2009;115(14):3189–95. [DOI] [PubMed] [Google Scholar]
- 24.Mattar W, Juliar B, Gradus-Pizlo I, Kwo PY. Amiodarone hepatotoxicity in the context of the metabolic syndrome and right-sided heart failure. J Gastrointestin Liver Dis. 2009;18(4):419–23. [PubMed] [Google Scholar]
- 25.deLemos AS, Foureau DM, Jacobs C, Ahrens W, Russo MW, Bonkovsky HL. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014;34(2):194–204. [DOI] [PubMed] [Google Scholar]
- 26.Amacher DE, Chalasani N. Drug-induced hepatic steatosis. Semin Liver Dis. 2014;34(2):205–14. [DOI] [PubMed] [Google Scholar]
- 27.Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology. 2006;44(1):7–14. [DOI] [PubMed] [Google Scholar]
- 28.Ghabril M, Vuppalanchi R. Drug-induced nodular regenerative hyperplasia. Semin Liver Dis. 2014;34(2):240–5. [DOI] [PubMed] [Google Scholar]
- 29.Carman N, Mack DR, Benchimol EI. Therapeutic Drug Monitoring in Pediatric Inflammatory Bowel Disease. Curr Gastroenterol Rep. 2018;20(5):18. [DOI] [PubMed] [Google Scholar]
- 30.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193–213. [DOI] [PubMed] [Google Scholar]
- 31.Angitapalli R, Litwin AM, Kumar PR, Nasser E, Lombardo J, Mashtare T, et al. Adjuvant FOLFOX chemotherapy and splenomegaly in patients with stages II-III colorectal cancer. Oncology. 2009;76(5):363–8. [DOI] [PubMed] [Google Scholar]
- 32.Slade JH, Alattar ML, Fogelman DR, Overman MJ, Agarwal A, Maru DM, et al. Portal hypertension associated with oxaliplatin administration: clinical manifestations of hepatic sinusoidal injury. Clin Colorectal Cancer. 2009;8(4):225–30. [DOI] [PubMed] [Google Scholar]
- 33.Degott C, Feldmann G, Larrey D, Durand-Schneider AM, Grange D, Machayekhi JP, et al. Drug-induced prolonged cholestasis in adults: a histological semiquantitative study demonstrating progressive ductopenia. Hepatology. 1992;15(2):244–51. [DOI] [PubMed] [Google Scholar]
- 34.Moradpour D, Altorfer J, Flury R, Greminger P, Meyenberger C, Jost R, et al. Chlorpromazine-induced vanishing bile duct syndrome leading to biliary cirrhosis. Hepatology. 1994;20(6):1437–41. [DOI] [PubMed] [Google Scholar]
- 35.Olsson R, Wiholm BE, Sand C, Zettergren L, Hultcrantz R, Myrhed M. Liver damage from flucloxacillin, cloxacillin and dicloxacillin. J Hepatol. 1992;15(1–2):154–61. [DOI] [PubMed] [Google Scholar]
- **36.Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65(4):1267–77.Large report of DILI induced vanishing bile duct syndrome and poor outcomes.
- 37.Watanabe N, Takashimizu S, Kojima S, Kagawa T, Nishizaki Y, Mine T, et al. Clinical and pathological features of a prolonged type of acute intrahepatic cholestasis. Hepatol Res. 2007;37(8):598–607. [DOI] [PubMed] [Google Scholar]
- 38.Chatterjee S, Richert L, Augustijns P, Annaert P. Hepatocyte-based in vitro model for assessment of drug-induced cholestasis. Toxicol Appl Pharmacol. 2014;274(1):124–36. [DOI] [PubMed] [Google Scholar]
- 39.Bachour-El Azzi P, Sharanek A, Abdel-Razzak Z, Antherieu S, Al-Attrache H, Savary CC, et al. Impact of inflammation on chlorpromazine-induced cytotoxicity and cholestatic features in HepaRG cells. Drug Metab Dispos. 2014;42(9):1556–66. [DOI] [PubMed] [Google Scholar]
- 40.Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148(7):1340–52 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turkish A, Luo JJ, Lefkowitch JH. Ketamine abuse, biliary tract disease, and secondary sclerosing cholangitis. Hepatology. 2013;58(2):825–7. [DOI] [PubMed] [Google Scholar]
- **42.Gudnason HO, Bjornsson HK, Gardarsdottir M, Thorisson HM, Olafsson S, Bergmann OM, et al. Secondary sclerosing cholangitis in patients with drug-induced liver injury. Dig Liver Dis. 2015;47(6):502–7.Series of DILI induced sclerosing cholangitis reported from an Icelandic DILI registry.
- 43.Horsley-Silva JL, Dow EN, Menias CO, Smith ML, Carballido EM, Lindor KD, et al. Docetaxel Induced Sclerosing Cholangitis. Dig Dis Sci. 2015;60(12):3814–6. [DOI] [PubMed] [Google Scholar]
- **44.Ahmad J, Rossi S, Rodgers SK, Ghabril M, Fontana R, Stolz A, et al. Sclerosing Cholangitis Like Changes on Magnetic Resonance Cholangiography in Patients with Drug Induced Liver Injury Clin Gastroenterol Hepatol. (in press).Series of DILI induced sclerosing cholangitis reported from a U.S. DILI registry with blinded and systematic MRCP readings.
- 45.Gossard AA, Angulo P, Lindor KD. Secondary sclerosing cholangitis: a comparison to primary sclerosing cholangitis. Am J Gastroenterol. 2005;100(6):1330–3. [DOI] [PubMed] [Google Scholar]
- 46.Imam MH, Talwalkar JA, Lindor KD. Secondary sclerosing cholangitis: pathogenesis, diagnosis, and management. Clin Liver Dis. 2013;17(2):269–77. [DOI] [PubMed] [Google Scholar]
- 47.Dikengil A, Siskind BN, Morse SS, Swedlund A, Bober-Sorcinelli KE, Burrell MI. Sclerosing cholangitis from intraarterial floxuridine. J Clin Gastroenterol. 1986;8(6):690–3. [DOI] [PubMed] [Google Scholar]
- 48.Ludwig J, Kim CH, Wiesner RH, Krom RA. Floxuridine-induced sclerosing cholangitis: an ischemic cholangiopathy? Hepatology. 1989;9(2):215–8. [DOI] [PubMed] [Google Scholar]
- 49.Schwab GP, Wetscher GJ, Vogl W, Redmond E. Methimazole-induced cholestatic liver injury, mimicking sclerosing cholangitis. Langenbecks Arch Chir. 1996;381(4):225–7. [DOI] [PubMed] [Google Scholar]
- 50.Seto WK, Ng M, Chan P, Ng IO, Cheung SC, Hung IF, et al. Ketamine-induced cholangiopathy: a case report. Am J Gastroenterol. 2011;106(5):1004–5. [DOI] [PubMed] [Google Scholar]
- 51.Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42(2):481–9. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi PH, Fontana RJ. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis. 2014;34(2):134–44. [DOI] [PubMed] [Google Scholar]
- **53.Hayashi PH, Rockey DC, Fontana RJ, Tillmann HL, Kaplowitz N, Barnhart HX, et al. Death and liver transplantation within 2 years of onset of drug-induced liver injury. Hepatology. 2017;66(4):1275–85.Report of long-term mortality from DILI in prospectively followed patients.


