Abstract
Large quantities of thinned young pears, a natural source of bioactive compounds, are abandoned as agricultural by-products in many orchards. Hence, ten thinned young pear varieties were systematically investigated in terms of their chemical composition and antioxidant potential. Through ultra-performance liquid chromatography coupled with electrospray ionization triple quadrupole mass spectrometry (UPLC-Q TRAP-MS/MS), 102 polyphenols and 16 triterpenoids were identified and individually quantified within a short time using multiple reaction monitoring (MRM). Subsequently, the antioxidant capacities of these pears were determined with DPPH assays, and the correlation between total antioxidant activity and each component was analyzed. The results indicated that the bioactive compound content and antioxidant capacity in thinned pears were considerably high. Regarding chemical composition, chlorogenic acid, quinic acid and arbutin were the primary polyphenols and ursolic acid was the predominant triterpenoid, whereas 27 polyphenolic compounds, especially chlorogenic acid and most of the flavan-3-ols, were the main antioxidants in young pears. These findings should provide a scientific basis for the further use of pear fruit by-products.
Keywords: thinned young pear, polyphenol, triterpenoid, identification, quantification, mass spectrometry, antioxidant
1. Introduction
Pear (Pyrus spp.) is one of the most widely produced fruits in the world and is cultivated in more than 50 countries. It is a dietary source of bioactive components such as polyphenols and triterpenic acids [1,2]. In the marketplace, pear fruit size and quality are extremely important for successful commercialization. For this reason, thinning of pear trees is a cultural practice often adopted in all orchards to remove excess and undesirable fruit. Thinning is one of the most effective measures to improve fruit size and quality at harvest and to balance the pear yield in the following year [3]. According to the data published by the United Nations Food and Agriculture Organization, in 2016, the total harvesting area and pear production worldwide was 1584.96 thousand hectares and 27.35 million tonnes, respectively. Hence, many thinned young pears are produced every year and abandoned as agricultural by-products, thereby usually generating large quantities of waste, which may affect the growth of fruit trees by increasing the acidity and disturbing the microbial community of the grove soil [4]. Recent studies have indicated that these agricultural by-products might provide an agricultural and food resource, because the content of bioactive substances in unripe pears in early stages of growth was significantly higher than in later stages [5]. Therefore, collecting and using these young pears rather than discarding them directly in fruit orchards may be both valuable and necessary.
The mature pear fruit is typically eaten fresh and is also an important material for both pharmaceutical and food applications, due to its nutritional and health promoting benefits, such as anti-inflammatory, antioxidant, antitussive, anti-diabetic, antimicrobial and diuretic activities [2,6]. Polyphenols, the major functional ingredients in pears, have already been studied in different pear varieties [7,8], and their health-promoting benefits have been demonstrated to be closely associated with strong antioxidant and anti-inflammatory properties [7]. In addition, triterpenoid compounds—which are well known for their beneficial health properties, such as their anti-inflammatory, antioxidant, antiviral and immunomodulatory activities [9]—have also been reported to be abundant in pears [1,2]. Ursolic, oleanolic and betulinic acids are the commonly reported compounds in mature pears. Nevertheless, the chemical composition characteristics in immature pears still require further study.
In recent years, ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry (UPLC-MS/MS) has been widely and successfully used for characterizing and quantifying many compounds in complex samples [10,11]. Especially, through use of the multiple reaction monitoring (MRM) scan mode, MS/MS detection could achieve high sensitivity and selectivity without establishing baseline separation of the target analytes, and a large array of compounds could be simultaneously determined in a very short time [12]. In addition, precursor and production ion monitoring could not only increase the specificity of detection but also aid in identifying the molecules. Consequently, we reasoned that UPLC-MS/MS might be an ideal strategy for the determination of multiple chemical profiles from pears.
Hence, in the present study, we developed an ultra-performance liquid chromatography coupled with electrospray ionization triple quadrupole mass spectrometry (UPLC-Q TRAP-MS/MS) system to characterize and quantify the bioactive components in ten varieties of thinned young pears. The high resolution of UPLC and the sensitivity of the MRM mode allowed us to simultaneously monitor 102 polyphenols within 15 min and to quantify 16 triterpenoids within another 10 min. The differences in the content of bioactive components among various pear varieties were assessed. Moreover, the antioxidant capacities of young pears in vitro were investigated with 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) assays, and a correlation analysis between total antioxidant activity and the content of individual component was performed to identify potential bioactive compounds. This study might provide a scientific basis for further use of discarded thinned-young pears.
2. Results and Discussion
2.1. Identification of Polyphenol and Triterpenoid Composition
The characteristic phenolic and triterpene profiles in ten varieties of thinned young pears were investigated rapidly with high sensitivity through UPLC-Q TRAP-MS/MS coupled with information-dependent acquisition (IDA) method. The compounds were unambiguously or tentatively identified on the basis of comparison of retention time and mass spectral data with available standards and published data [7,8,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. As presented in Table 1 and Figure S1, 102 polyphenloic compounds were rapidly separated from pear extracts within 15 min. The compounds belonged to different chemical classes: phenolic acids (28), phenolic glycosides (16), flavones (33) and flavan-3-ols (25). Besides, 16 triterpenoids were detected within another 10 min, and some of them were detected and characterized from pear species for the first time.
Table 1.
Characterization of chemical compounds in young pear fruits by ultra-performance liquid chromatography coupled with electrospray ionization triple quadrupole mass spectrometry (UPLC-Q TRAP-MS/MS).
| Peak NO. | Rt (min) | Formula | [M − H]− (m/z) | MS/MS Fragments (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| Polyphenol | |||||
| 1 | 0.81 | C7H12O6 | 191.1 | 172.9[M − H − H2O]− | Quinic acid a |
| 2 | 1.76 | C9H8O2 | 147.0 | 129.0[M − H − H2O]− | Cinnamic acid isomer |
| 3 | 2.06 | C12H16O7 | 317.1 # | 271.2[M − H]−, 160.9[M − H − C6H6O2]−, 109.0[M − H − Glc]− | Arbutin a |
| 4 | 2.77 | C30H24O12 | 575.3 | 449.0[M − H − C6H6O3]−, 285.2[M − H − C15H14O6]− | A-type procyanidin dimer |
| 5 | 3.19 | C16H18O9 | 353.3 | 191.1[M − H − caffeoyl]−, 179.2[M − H − quinoyl]− | 4-O-Caffeoylquinic acid (Cryptochlorogenic acid) a |
| 6 | 3.37 | C15H20O9 | 343.2 | 181.0[M − H − hexose]− | Dihydro-caffeoyl-O-hexoside |
| 7 | 3.48 | C30H26O12 | 577.3 | 425.2[M − H − C8H8O3]−, 288.9[M − H − C15H12O6]− | B-type procyanidin dimer |
| 8 | 3.51 | C15H20O8 | 327.2 | 147.1[M − H − hexose − H2O]− | Hydroxyphenylpropionic acid-O-hexoside |
| 9 | 3.76 | C15H20O10 | 359.3 | 197.1[M − H − hexose]− | Syringic acid-O-hexoside |
| 10 | 3.84 | C12H14O6 | 253.1 | 135.1[M − H − C3H6O2 − CO2]− | Caffeoylglycerol |
| 11 | 3.88 | C15H20O9 | 343.2 | 181.0[M − H − hexose]− | Dihydro-caffeoyl-O-hexoside |
| 12 | 4.11 | C15H14O6 | 289.1 | 245.3[M − H − CO2]−, 202.9[M − H − H2O − C3O2]− | (+)-Catechin a |
| 13 | 4.13 | C16H18O9 | 353.3 | 191.1[M − H − caffeoyl]−, 161.1[M − H − quinoyl − H2O]− | 3-O-Caffeoylquinic acid (Chlorogenic acid) a |
| 14 | 4.31 | C45H38O18 | 865.3 | 577.1[M − H − C15H12O6]−, 407.1[M − H − C15H12O6 − C8H8O3 − H2O]−, 289.0[M − H − 2C15H12O6]− | B-type procyanidin trimer |
| 15 | 4.38 | C16H18O9 | 353.3 | 191.1[M − H − caffeoyl]−, 173.0[M − H − caffeoyl − H2O]− | 5-O-Caffeoylquinic acid (Neochlorogenic acid) a |
| 16 | 4.64 | C22H18O10 | 441.3 | 325.1[M − H − C4H4O4]−, 163.0[M − H − C4H4O4 − caffeoyl]−, 118.9[p-coumaric acid − H − CO2]− | p-Coumaroylcaffeoyl malate |
| 17 | 4.89 | C30H26O12 | 577.3 | 425.1[M − H − C8H8O3]−, 407.0[M − H − C8H8O3 − H2O]−, 339.0[M − H − C8H8O3 − H2O − C3O2]−, 289.1[M − H − C15H12O6]−, 245.0[M − H − C15H12O6 − CO2]− | B-type procyanidin dimer |
| 18 | 4.98 | C45H38O18 | 865.3 | 577.0[M − H − C15H12O6]−, 287.1[M − H − C15H12O6 − C15H14O6]− | B-type procyanidin trimer |
| 19 | 5.07 | C45H36O18 | 863.3 | 573.3[M − H − C15H14O6]−, 289.2[M − H − C15H12O6 − C15H10O6]− | A-type procyanidin trimer |
| 20 | 5.28 | C16H18O9 | 353.3 | 191.1[M − H − caffeoyl]− | 1-O-Caffeoylquinic acid |
| 21 | 5.30 | C15H18O9 | 341.1 | 178.9[M − H − hexose]− | Caffeoyl-O-hexoside |
| 22 | 5.44 | C16H16O8 | 335.3 | 179.1[M − H − shikimoyl]− | Caffeoylshikimic acid |
| 23 | 5.45 | C19H30O8 | 431.2 # | 385.0[M − H]−, 223.1[M − H − Glc]− | Roseoside |
| 24 | 5.48 | C15H14O6 | 289.1 | 245.0[M − H − CO2]−, 202.9[M − H − H2O − C3O2]− | (−)-Epicatechin a |
| 25 | 5.53 | C16H18O8 | 337.3 | 190.9[M − H − p-coumaroyl]−, 163.2 [M − H − quinoyl]− | 4-p-Coumaroylquinic acid |
| 26 | 5.57 | C15H20O10 | 359.3 | 197.0[M − H − hexose]−, 160.9[M − H − hexose − 2H2O]− | Syringic acid-O-hexoside |
| 27 | 5.64 | C45H36O18 | 863.3 | 711.3[M − H − C8H8O3]−, 573.0[M − H − C15H14O6]−, 289.0[M − H − C15H12O6 − C15H10O6]− | A-type procyanidin trimer |
| 28 | 6.05 | C16H16O8 | 335.3 | 179.0[M − H − shikimoyl]−, 135.1 [M − H − shikimoyl − CO2]− | Caffeoylshikimic acid |
| 29 | 6.49 | C45H36O18 | 863.3 | 711.1[M − H − C8H8O3]−, 573.0[M − H − C15H14O6]−, 451.2[M − H − C15H12O6 − C7H8O2]−, 289.2[M − H − C15H12O6 − C15H10O6]− | A-type procyanidin trimer |
| 30 | 6.54 | C30H24O12 | 575.3 | 285.3[M − H − C15H14O6]− | A-type procyanidin dimer |
| 31 | 6.69 | C17H20O9 | 367.4 | 191.0[M − H − feruloyl]−, 193.2[M − H − quinoyl]− | 3-O-Feruloylquinic acid |
| 32 | 6.71 | C19H16O12 | 435.3 | 273.0[M − H − caffeoyl]−, 205.0[M − H − caffeoylmalonyl]− | Caffeoyl-malonyl-methylcitric aci |
| 33 | 7.01 | C45H38O18 | 865.3 | 577.1[M − H − C15H12O6]−, 451.1[M − H − C15H12O6 − C6H6O3]−, 407.1[M − H − C15H12O6 − C8H8O3 − H2O]−, 289.0[M − H − 2C15H12O6]− | B-type procyanidin trimer |
| 34 | 7.24 | C16H18O8 | 337.3 | 190.9[M − H − p-coumaroyl]−, 163.2 [M − H − quinoyl]− | 5-p-Coumaroylquinic acid |
| 35 | 7.35 | C30H26O12 | 577.3 | 289.3[M − H − C15H12O6]− | B-type procyanidin dimer |
| 36 | 7.40 | C17H20O9 | 367.4 | 205.1[M − H − caffeoyl]− | 4-O-Caffeoylquinic acid methyl ester |
| 37 | 7.58 | C30H24O12 | 575.3 | 285.2[M − H − C15H14O6]− | A-type procyanidin dimer |
| 38 | 7.61 | C32H38O20 | 741.4 | 301.1[M − H − Xyl-Rha-Gal]− | Quercetin-3-O-xylosylrhamnosylglucoside |
| 39 | 7.84 | C21H22O10 | 433.3 | 323.0[M − H − C6H6O2]−, 161.1[M − H − arbutin − H2O]− | Caffeoylarbutin |
| 40 | 7.89 | C60H50O24 | 1153.3 | 739.1[M − H − C15H12O6 − C6H6O2]−, 449.2[C30H26O12 − H − H2O − C6H6O2]−, 287.1[C30H26O12 − H − C15H14O6]− | B-type procyanidin tetramer |
| 41 | 8.04 | C24H24O13 | 519.4 | 315.3[M − H − acetyl-hexose]− | Isorhamnetin-acylated-hexoside |
| 42 | 8.05 | C21H22O10 | 433.3 | 323.0[M − H − C6H6O2]−, 178.9[M − H − arbutin]−, 160.9[M − H − arbutin − H2O]−, 133.1[M − H − arbutin − H2O − CO]− | Caffeoylarbutin |
| 43 | 8.11 | C26H28O16 | 595.4 | 301.1[M − H − Ara-Gal]− | Quercetin-3-O-arabinosylgalactoside |
| 44 | 8.13 | C45H36O18 | 863.3 | 573.1[M − H − C15H14O6]−, 451.1[M − H − C15H12O6 − C7H8O2]−, 289.2[M − H − C15H12O6 − C15H10O6]− | A-type procyanidin trimer |
| 45 | 8.19 | C30H24O12 | 575.3 | 448.9[M − H − C6H6O3]−, 285.1[M − H − C15H14O6]− | A-type procyanidin dimer |
| 46 | 8.32 | C15H20O10 | 359.3 | 197.2[M − H − hexose]− | Syringic acid-O-hexoside |
| 47 | 8.41 | C9H8O3 | 163.0 | 118.9[M − H − CO2]− | Hydroxycinnamic acid |
| 48 | 8.43 | C17H20O9 | 367.4 | 205.0[M − H − caffeoyl]− | 3-O-Caffeoylquinic acid methyl ester |
| 49 | 8.63 | C26H28O16 | 595.4 | 301.1[M − H − Ara-Glc]− | Quercetin-3-O-arabinosylglucoside |
| 50 | 8.65 | C27H30O16 | 609.4 | 301.0[M − H − Rha-Gal] | Quercetin-3-O-rhamnosylgalactoside |
| 51 | 8.72 | C21H20O11 | 447.3 | 285.2[M − H − Glc]− | Luteolin-7-O-galactoside |
| 52 | 8.87 | C27H30O16 | 609.4 | 301.0[M − H − Rut]− | Rutin a |
| 53 | 8.91 | C45H38O18 | 865.3 | 577.1[M − H − C15H12O6]−, 451.1[M − H − C15H12O6 − C6H6O3]−, 407.1[M − H − C15H12O6 − C8H8O3 − H2O]−, 289.0[M − H − 2C15H12O6]− | B-type procyanidin trimer |
| 54 | 8.93 | C25H24O11 | 499.4 | 353.1[M − H − p-coumaroyl]−, 337.2[M − H − caffeoyl]−, 191.2[M − H − caffeoyl − p-coumaroyl]−, 163.1[p-coumaroyl − H]− | p-Coumaroylcaffeoylquinic acid |
| 55 | 8.98 | C21H20O12 | 463.2 | 301.2[M − H − Gal]− | Quercetin-3-O-galactoside (Hyperoside) a |
| 56 | 9.01 | C27H30O15 | 593.3 | 285.1[M − H − Rha-Gal]− | Kaempferol-3-O-rhamnosylgalactoside |
| 57 | 9.14 | C21H22O10 | 433.3 | 323.4[M − H − C6H6O2]−, 161.2[M − H − arbutin − H2O]−, 133.2[M − H − arbutin − H2O − CO]− | Caffeoylarbutin |
| 58 | 9.15 | C25H24O11 | 499.4 | 353. 1[M − H − p-coumaroyl]−, 336.9[M − H − caffeoyl]−, 191.1[M − H − caffeoyl − p-coumaroyl]−, 163.0[p-coumaroyl − H]− | p-Coumaroylcaffeoylquinic acid |
| 59 | 9.16 | C21H20O12 | 463.2 | 301.2[M − H − Glc]− | Quercetin-3-O-glucoside |
| 60 | 9.17 | C30H26O12 | 577.3 | 425.1[M − H − C8H8O3]−, 289.0[M − H − C15H12O6]− | B-type procyanidin dimer |
| 61 | 9.25 | C25H24O12 | 515.5 | 353.1[M − H − caffeoyl]−, 191.0[M − H − 2caffeoyl]− | Di-O-caffeoylquinic acid |
| 62 | 9.26 | C21H20O11 | 447.3 | 285.0[M − H − Glc]− | Luteolin-7-O-glucoside (Luteoloside) a |
| 63 | 9.30 | C45H38O18 | 865.3 | 577.2[M − H − C15H12O6]−, 406.9[M − H − C15H12O6 − C8H8O3 − H2O]−, 289.0[M − H − 2C15H12O6]− | B-type procyanidin trimer |
| 64 | 9.38 | C27H30O15 | 593.3 | 285.1[M − H − Rha-Glc]− | Kaempferol-3-O-rhamnosylglucoside |
| 65 | 9.44 | C21H18O11 | 445.3 | 401.0[M − H − CO2]−, 357.2[M − H − 2CO2]−, 313.2[M − H − 3CO2]−, 225.2[M − H − C6H8O6 − CO2]−, 181.1[M − H − C6H8O6 − 2CO2]− | Apigenin-O-glucuronide or isomer |
| 66 | 9.46 | C30H24O12 | 575.3 | 539.0[M − H − H2O − H2O]−, 449.1[M − H − C6H6O3]−, 407.1[M − H − CO2 − C7H8O2]−, 285.1[M − H − C15H14O6]− | A-type procyanidin dimer |
| 67 | 9.51 | C45H34O18 | 861.3 | 735.1[M − H − C6H6O3]−, 693.2[M − H − CO2 − C7H8O2]−, 571.2[M − H − C15H14O6]− | A-type procyanidin trimer |
| 68 | 9.54 | C21H22O9 | 417.2 | 307.3[M − H − C6H6O2]−, 163.1[M − H − arbutin]−, 145.1[M − H − arbutin − H2O]− | p-Coumaroylarbutin |
| 69 | 9.65 | C17H20O9 | 367.4 | 205.0[M − H − caffeoyl]−, 191.1[M − H − caffeoyl − methyl]− | 5-O-Caffeoylquinic acid methyl ester |
| 70 | 9.69 | C45H36O18 | 863.3 | 575.0[M − H − C15H12O6]−, 449.0[M − H − C15H12O6 − C6H6O3]− | A-type procyanidin trimer |
| 71 | 9.70 | C19H16O12 | 435.3 | 273.1[M − H − caffeoyl]−, 205.3[M − H − caffeoylmalonyl]−, 161.1[M − H − caffeoylmalonyl − CO2]− | Caffeoyl-malonyl-methylcitric acid |
| 72 | 9.75 | C21H18O11 | 445.3 | 401.2[M − H − CO2]−, 357.2[M − H − 2CO2]−, 313.1[M − H − 3CO2]−, 225.1[M − H − C6H8O6 − CO2]− | Apigenin-O-glucuronide or isomer |
| 73 | 9.76 | C27H30O15 | 593.3 | 285.1[M − H − Rut]− | Kaempferol-3-O-rutinoside a |
| 74 | 9.78 | C23H22O13 | 505.3 | 445.2[M − H − CH3COOH]−, 301.1[M − H − acetyl-Gal]− | Quercetin-acylated-galactoside |
| 75 | 9.80 | C28H32O16 | 623.5 | 315.0[M − H − Gal-Rha]− | Isorhamnetin-3-O-rhamnosylgalactoside |
| 76 | 9.87 | C25H24O12 | 515.5 | 353.0[M − H − caffeoyl]− | 3,4-O-Dicaffeoylquinic acid (Isochlorogenic acid B) a |
| 77 | 9.92 | C27H30O14 | 577.3 | 269.1[M − H − Rut]− | Apigenin rutinoside |
| 78 | 9.95 | C28H32O16 | 623.5 | 315.0[M − H − Rut]− | Isorhamnetin-3-O-rutinoside a |
| 79 | 10.07 | C21H20O11 | 447.3 | 285.0[M − H − Glc]− | Kaempferol-3-O-galactoside |
| 80 | 10.09 | C23H22O13 | 505.3 | 445.1[M − H − CH3COOH]−, 301.1[M − H − acetyl-Glc]− | Quercetin-acylated-glucoside |
| 81 | 10.10 | C22H22O12 | 477.3 | 315.0[M − H − Gal]− | Isorhamnetin-3-O-galactoside |
| 82 | 10.14 | C28H32O15 | 607.4 | 299.1[M − H − Nhe]− | Chrysoeriol-7-neohesperidoside |
| 83 | 10.21 | C25H24O12 | 515.5 | 353.2[M − H − caffeoyl]−, 191.0[M − H − 2caffeoyl]−, 178.9[M − H − caffeoyl-quinoyl]− | 3,5-O-Dicaffeoylquinic acid (Isochlorogenic acid A) a |
| 84 | 10.22 | C30H24O12 | 575.3 | 406.9[M − H − CO2 − C7H8O2]− | A-type procyanidin dimer |
| 85 | 10.27 | C21H22O9 | 417.2 | 307.3[M − H − C6H6O2]−, 163.0[M − H − arbutin]−, 145.1[M − H − arbutin − H2O]− | p-Coumaroylarbutin |
| 86 | 10.28 | C22H22O12 | 477.3 | 315.0[M − H − Glc]− | Isorhamnetin-3-O-glucoside |
| 87 | 10.31 | C21H20O11 | 447.3 | 285.0[M − H − hexose]− | Kaempferol-3-O-glucoside |
| 88 | 10.34 | C21H20O10 | 431.2 | 269.1[M − H − hexose]− | Apigenin-O-hexoside |
| 89 | 10.38 | C25H24O11 | 499.4 | 353. 0[M − H − p-coumaroyl]−, 337.0[M − H − caffeoyl]−, 191.0[M − H − caffeoyl − p-coumaroyl]− | p-Coumaroylcaffeoylquinic acid |
| 90 | 10.58 | C22H22O11 | 461.4 | 298.9[M − H − Gal]− | Chrysoeriol-7-O-galactoside |
| 91 | 10.60 | C45H34O18 | 861.3 | 735.0[M − H − C6H6O3]−, 693.1[M − H − CO2 − C7H8O2]−, 571.1[M − H − C15H14O6]−, 288.9[M − H − 2C15H10O6]− | A-type procyanidin trimer |
| 92 | 10.71 | C25H24O12 | 515.5 | 353.1[M − H − caffeoyl]−, 191.1[M − H − 2caffeoyl]−, 179.0[M − H − caffeoyl-quinoyl]− | 4,5-O-Dicaffeoylquinic acid (Isochlorogenic acid C) a |
| 93 | 10.75 | C22H22O11 | 461.4 | 299.2[M − H − Glc]− | Chrysoeriol-7-O-glucoside |
| 94 | 10.80 | C23H22O12 | 489.1 | 285.1[M − H − acetyl-Gal]− | Kaempferol-acylated-galactoside |
| 95 | 10.85 | C24H24O13 | 519.4 | 315.3[M − H − acetyl-Gal]−, 299.2[M − H − acetyl-Gal − CH4]− | Isorhamnetin-acylated-galactoside |
| 96 | 11.05 | C24H24O13 | 519.5 | 315.0[M − H − acetyl-Glc]−, 299.2[M − H − acetyl-Glc − CH4]−, 271.2[M − H − acetyl-Glc − CH4 − H2O]− | Isorhamnetin-acylated-glucoside |
| 97 | 11.30 | C25H24O11 | 499.4 | 353. 0[M − H − p-coumaroyl]−, 337.1[M − H − caffeoyl]−, 190.9[M − H − caffeoyl − p-coumaroyl]−, 163.0[p−coumaroyl − H]− | p-Coumaroylcaffeoylquinic acid |
| 98 | 11.78 | C23H22O12 | 489.1 | 285.1[M − H − acetyl-Glc]− | Kaempferol-acylated-glucoside |
| 99 | 11.80 | C17H20O9 | 367.4 | 205.1[M − H − caffeoyl]−, 190.9[M − H − caffeoyl-methyl]−, 147.0[M − H − caffeoyl-methyl − CO2]− | 1-O-Caffeoylquinic acid methyl ester |
| 100 | 12.13 | C21H20O11 | 447.3 | 295.4[M − H − Galloyl]− | Galloyl-coumaric acid pentoside |
| 101 | 12.15 | C22H22O12 | 477.3 | 273.1[M − H − acetyl-hexose]− | Phloretin-acylated-hexoside |
| 102 | 12.38 | C21H20O11 | 447.3 | 295.1[M − H − Galloyl]− | Galloyl-coumaric acid pentoside |
| Triterpenoid | |||||
| 1′ | 1.21 | C30H48O5 | 487.3 | 469.1[M − H − H2O]−, 425.0[M − H − H2O − CO2]− | Euscaphic acid |
| 2′ | 1.40 | C30H48O5 | 487.3 | 469.3[M − H − H2O]−, 425.2[M − H − H2O − CO2]− | Tormentic acid |
| 3′ | 1.59 | C30H46O5 | 485.3 | 467.1[M − H − H2O]−, 423.2[M − H − H2O − CO2]− | Anmurcoic acid |
| 4′ | 1.95 | C30H48O4 | 471.3 | 453.2[M − H − H2O]−, 407.0[M − H − H2O − CH2O]− | Pomolic acid isomer |
| 5′ | 2.47 | C30H46O4 | 469.3 | 425.1[M − H − CO2]−, 407.0[M − H − H2O − CO2]− | 1-Hydroxy-3-oxours-12-en-28-oic acid or isomer |
| 6′ | 2.63 | C30H48O4 | 471.3 | 453.3[M − H − H2O]−, 407.1[M − H − H2O − CH2O]− | Pomolic acid a |
| 7′ | 2.89 | - | 701.5 | 641.2, 555.1, 540.1, 481.1 | Unknown |
| 8′ | 3.11 | C30H48O4 | 471.3 | 453.2[M − H − H2O]−, 407.1[M − H − H2O − CH2O]− | Alphitolic acid a |
| 9′ | 3.45 | C30H48O4 | 471.3 | 453.0[M − H − H2O]−, 407.1[M − H − H2O − CH2O]− | Maslinic acid a |
| 10′ | 3.79 | C30H48O4 | 471.3 | 453.2[M − H − H2O]−, 407.3[M − H − H2O − CH2O]− | Corosolic acid |
| 11′ | 4.38 | C30H46O4 | 469.3 | 425.2[M − H − CO2]−, 407.1[M − H − CO2 − H2O]− | 1-Hydroxy-3-oxours-12-en-28-oic acid or isomer |
| 12′ | 4.79 | C30H46O4 | 469.3 | 425.1[M − H − CO2]−, 407.3[M − H − CO2 − H2O]− | 1-Hydroxy-3-oxours-12-en-28-oic acid or isomer |
| 13′ | 6.89 | C30H48O3 | 455.3 | 407.4[M − H − H2O − CH2O]− | Betulinic acid a |
| 14′ | 7.44 | C30H48O3 | 455.3 | 407.4[M − H − H2O − CH2O]− | Oleanolic acid a |
| 15′ | 7.95 | C30H48O3 | 455.3 | 407.2[M − H − H2O − CH2O]− | Ursolic acid a |
| 16′ | 8.37 | - | 687.4 | 627.1, 541.2, 526.2, 467.0 | Unknown |
Note: # the proposal ion was [M − H + HCOOH]−; a identification confirmed by authentic standards.
The [M − H]− nominal mass and the characteristic fragment ion in negative mode (Table 1) were used for component identification. In accordance with previous studies, neutral loss of H2O molecules (18 Da) was frequently found for phenolic acids, such as quinic acid (peak 1) [13,14]. Direct loss of the sugar moiety was characteristically detected from the phenolic glycosides and flavones, such as arbutin (peak 3), roseoside (peak 23), rutin (peak 52), hyperoside (peak 55) and luteoloside (peak 62), and the fragmentation of all these compounds yielded aglycone ions [8,15,16]. The esterified compounds derived from two or three phenolic acids were easily distinguished on the basis of their typical fragmentation patterns, owing to the loss of phenolic acid residues; these compounds included caffeoylquinic acid (peaks 5, 13, 15 and 20), dicaffeoylquinic acid (peaks 61, 76, 83 and 92), p-coumaroylquinic acid (peaks 25 and 34) and feruloylquinic acid (peak 31) [13,14,16,17]. In addition, characteristic fragment ions of five arbutin conjugates, including two p-coumaroylarbutins (peaks 68 and 85) and three caffeoylarbutins (peaks 39, 42 and 57), were obtained by loss of hydroquinone (110 Da) and the arbutin moiety (254 Da) [18]. Regarding different polymeric procyanidins compounds (peaks 30, 37, 40, 44, etc.) formed from two to four catechin and epicatechin molecules, their identical fragmentation pathways may have occurred through loss of (epi)catechin residues or the retro-Diels-Alder fragment [13]. In particular, apart from the most common triterpenic compounds in pears, such as betulinic, oleanolic and ursolic acid (peaks 13ʹ, 14ʹ and 15ʹ), 13 other triterpenic acids were identified from young pear extracts, and their typical resulting product ions corresponded to the loss of H2O (18 Da), CO2 (44 Da) and CH2O (30 Da) [23,24,25,26,27].
2.2. Quantification of Polyphenols and Triterpenoids
Most compounds obtained showed a symmetrical peak shape and good resolution under the present chromatographic conditions (Figure 1 and Figure S2). Before sample analysis, the performance of the method was validated through a series of tests. The linearity, regression and linear ranges of 23 available compounds were obtained by using the external standard method and are listed in Table 2. All of the correlation coefficient values (R2 > 0.9995) indicated appropriate correlations between the concentrations of investigated compounds and their peak areas within the test ranges. The limits of detection (LODs) and limits of quantitation (LOQs) were also estimated on the basis of 3:1 and 10:1 signal-to-noise (S/N) ratios obtained with mixed standards containing the compounds at low concentration. The relative standard deviation (RSD) values for intra- and inter-day precision ranged from 0.43% to 1.12% and 1.47% to 3.26%, respectively, thus indicating acceptable precision of the method (Table S1). The RSD values of experimental repeatability were below 7.65% for the contents of all peaks, thus indicating that the method was repeatable (Tables S2 and S3). In addition, the sample stability RSD values were below 6.74% in 72 h, thus indicating that the analytes were stable (Tables S2 and S3). All results demonstrated that the developed method could provide a reliable, sensitive and stable means for the quantitative analysis of various pear varieties.
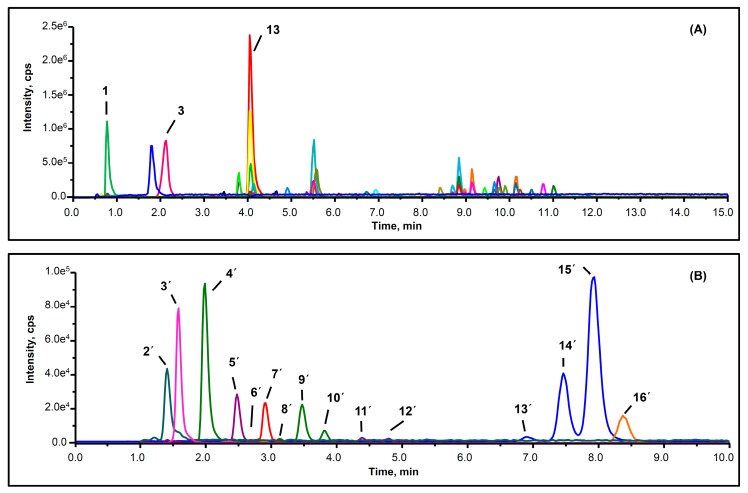
Figure 1.
Representative UPLC-MS/MS chromatograms of thinned young pears in the multiple reaction monitoring (MRM) mode. (A) the polyphenolic profiles of ‘Hongqieli’ extract, (B) the triterpenoid profiles of ‘Yali’ extract.
Table 2.
Calibration curves, LODs and LOQs data used for UPLC-MS/MS quantification of polyphenols and triterpenoids.
| Analyte | Ion Transition | Calibration Curve | R 2 | Linear Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|---|
| Quinic acid | 191 > 93 | y = 10.2 x + 0.06 | 0.9996 | 7843.1–784,313.6 | 13.1 | 78.4 |
| Arbutin | 317 > 161 | y = 19.9 x + 0.6 | 0.9999 | 3919.2–391,921.6 | 16.3 | 98.0 |
| (+)-Catechin | 289 > 289 | y = 270.9 x − 0.7 | 0.9997 | 73.5–14,703.9 | 2.5 | 7.4 |
| (−)-Epicatechin | 289 > 289 | y = 246.8 x + 1.7 | 0.9998 | 4.9–49,166.6 | 1.6 | 4.9 |
| Chlorogenic acid | 353 > 179 | y = 1.3 x − 1.1 | 0.9996 | 3923.1–196,156.8 | 65.4 | 392.3 |
| Cryptochlorogenic acid | 353 > 179 | y = 101.1 x + 30.0 | 0.9998 | 32.6–1302.6 | 10.9 | 32.6 |
| Neochlorogenic acid | 353 > 173 | y = 128.7 x + 4.1 | 0.9999 | 25.7–1029.8 | 8.6 | 25.7 |
| Isochlorogenic acid A | 515 > 353 | y = 82.1 x − 0.9 | 0.9998 | 66.9–33,427.8 | 5.6 | 33.4 |
| Isochlorogenic acid B | 515 > 353 | y = 287.7 x + 69.3 | 0.9998 | 10.5–524.3 | 3.5 | 10.5 |
| Isochlorogenic acid C | 515 > 353 | y = 267.2 x + 43.2 | 0.9997 | 17.3–690.3 | 2.9 | 17.3 |
| p-Coumaric acid | 163 > 163 | y = 1270.4 x + 90.3 | 0.9998 | 2.0–9817.6 | 0.2 | 2.0 |
| Caffeic acid | 179 > 179 | y = 165.7 x + 15.4 | 0.9998 | 9.8–98,352.8 | 1.6 | 9.8 |
| Rutin | 609 > 300 | y = 365.1 x + 2.1 | 0.9999 | 19.7–4912.7 | 1.6 | 9.8 |
| 609 > 609 | y = 478.3 x + 53.1 | 0.9998 | 4.9–9825.5 | 1.6 | 4.9 | |
| Kaempferol-3-O-rutinoside | 593 > 285 | y = 36.2 x + 0.7 | 0.9997 | 49.4–4941.2 | 19.8 | 49.4 |
| 593 > 593 | y = 90.2 x + 2.3 | 0.9997 | 19.8–4941.2 | 9.9 | 19.8 | |
| Isorhamnetin-3-O-rutinoside | 623 > 315 | y = 136.3 x + 5.3 | 0.9998 | 20.0–9982.3 | 5.0 | 20.0 |
| 623 > 623 | y = 342.7 x − 12.9 | 0.9999 | 10.0–9982.3 | 5.0 | 10.0 | |
| Luteoloside | 447 > 285 | y = 646.2 x + 95.2 | 0.9997 | 5.0–981.2 | 1.6 | 4.9 |
| 447 > 447 | y = 504.6 x − 11.0 | 0.9998 | 5.0–981.2 | 1.6 | 4.9 | |
| Hyperoside | 463 > 300 | y = 518.6 x + 20.7 | 0.9995 | 9.8–978.6 | 3.3 | 9.8 |
| Oleanic acid | 455 > 455 | y = 2546.2 x − 63.3 | 0.9999 | 9.8–982.2 | 0.8 | 2.0 |
| Ursolic acid | 455 > 455 | y = 2329.5 x + 44.9 | 0.9999 | 0.8–987.6 | 0.3 | 0.8 |
| Betulinic acid | 455 > 455 | y = 4256.9 x − 540.1 | 1.0000 | 0.8–489.7 | 0.3 | 0.8 |
| Pomolic acid | 471 > 471 | y = 1302.8 x − 22.8 | 0.9999 | 2.0–198.3 | 0.8 | 2.0 |
| Maslinic acid | 471 > 471 | y = 1255.5 x − 2.9 | 0.9999 | 2.0–493.1 | 0.8 | 2.0 |
| Corosolic acid | 471 > 471 | y = 1196.7 x − 8.6 | 0.9999 | 2.0–493.6 | 0.8 | 2.0 |
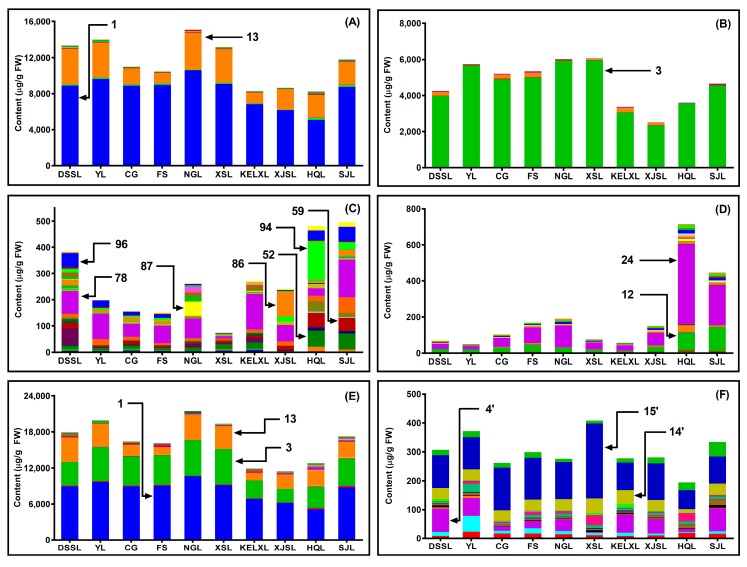
Using the proposed UPLC-MS/MS method, the quantitative analysis of 10 pear varieties was performed through the external standard methods. The thinned young pears were found to be a valuable source of bioactive molecules (Figure 2 and Table 3). The total polyphenolic content ranged from 11.4 mg/g fresh weight (FW) in ‘Xinjiangsuanli’ to 21.4 mg/g FW in ‘Nanguoli’ sample, with an average of 16.4 mg/g FW, while the total triterpenoid content ranged from 193.0 μg/g FW in ‘Hongqieli’ to 403.1 μg/g FW in ‘Xiangshuili’, with an average of 298.7 μg/g FW. These concentrations in immature pears were clearly higher than those in many mature pears previously reported by other authors [13,28]. The chemical composition showed similar characteristics among different pear varieties (Figure 2). Quinic acid, arbutin and chlorogenic acid (peaks 1, 3 and 13) were the major polyphenolic constituents in various pear fruitlets, and pomolic acid analogue, oleanolic acid and ursolic acid (peaks 4′, 14′ and 15′) were the predominant triterpenic compounds. However, there were still many significant discrepancies among different pear varieties.
Figure 2.
The content of chemical constituents in thinned young pears. (A) 28 phenolic acids, (B) 16 phenolic glycosides, (C) 33 flavones, (D) 25 flavan-3-ols and procyanidins, (E) total polyphenloic compounds, (F) 16 triterpenoids. (1) quinic acid; (3) arbutin; (12) (+)-catechin; (13) chlorogenic acid; (24) (−)-epicatechin; (52) rutin; (59) quercetin-3-O-glucoside; (78) isorhamnetin-3-O-rutinoside; (86) isorhamnetin-3-O-glucoside; (87) kaempferol-3-O-glucoside; (94) kaempferol-acylated-galactoside; (96) isorhamnetin-acylated-glucoside; (4′) pomolic acid isomer; (14′) oleanolic acid; (15′) ursolic acid.
Table 3.
The content of chemical constituents in thinned young pears of ten different varieties (mean ± SD, n = 3).
| Peak NO. | Compound | The Compound Content in Different Pear Varieties (μg/g FW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DSSL | YL | CG | FS | NGL | XSL | KELXL | XJSL | HQL | SJL | Mean | ||
| Phenolic acid | ||||||||||||
| 1 | Quinic acid | 8798.1 ± 122.4 | 9532.7 ± 74.3 | 8788.1 ± 74.6 | 8892.3 ± 85.8 | 10,500.5 ± 93.4 | 8984.8 ± 68.8 | 6725.8 ± 44.4 | 6064.2 ± 49.4 | 4991.7 ± 49.5 | 8666.34 ± 103.37 | 8194.5 |
| 2 | Cinnamic acid isomer | 120.0 ± 1.7 | 153.2 ± 2.6 | 130.7 ± 1.9 | 144.4 ± 2.1 | 60.4 ± 0.6 | 90.4 ± 0.8 | 56.3 ± 0.8 | 30.9 ± 0.6 | 217.4 ± 4.2 | 203.8 ± 1.9 | 120.8 |
| 5 | Cryptochlorogenic acid | 5.3 ± 0.1 | 10.5 ± 0.2 | 5.7 ± 0.1 | 4.0 ± 0.1 | 2.6 ± 0.1 | 4.5 ± 0.1 | 6.7 ± 0.1 | 6.8 ± 0.1 | 8.3 ± 0.2 | 6.3 ± 0.2 | 6.1 |
| 10 | Caffeoylglycerol | 4.6 ± 0.1 | N.Q. | 19.9 ± 0.4 | 14.4 ± 0.2 | N.Q. | 5.4 ± 0.1 | 9.12 ± 0.1 | 4.2 ± 0.1 | 25.6 ± 0.2 | 31.2 ± 0.3 | 11.5 |
| 13 | Chlorogenic acid | 3988.5 ± 33.9 | 3816.4 ± 50.6 | 1783.6 ± 16.2 | 1162.7 ± 9.1 | 4139.0 ± 39.9 | 3760.7 ± 28.2 | 1207.7 ± 14.3 | 2311.6 ± 17.1 | 2564.1 ± 28.1 | 2485.3 ± 23.5 | 2722.0 |
| 15 | Neochlorogenic acid | 2.8 ± 0.1 | 3.4 ± 0.1 | 1.6 ± 0.1 | 1.0 ± 0.0 | 2.1 ± 0.0 | 3.2 ± 0.1 | 1.7 ± 0.0 | 2.8 ± 0.0 | 1.8 ± 0.1 | 2.0 ± 0.0 | 2.2 |
| 16 | p-Coumaroylcaffeoyl malate | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.Q. | 5.6 ± 0.1 | 10.8 ± 0.1 | 8.7 ± 0.2 | 2.5 |
| 20 | 1-O-Caffeoylquinic acid | 26.2 ± 0.3 | 22.4 ± 0.3 | 12.2 ± 0.2 | 14.8 ± 0.2 | 20.5 ± 0.2 | 28.2 ± 0.3 | 10.9 ± 0.1 | 24.0 ± 0.3 | 23.2 ± 0.2 | 24.3 ± 0.2 | 20.7 |
| 22 | Caffeoylshikimic acid | 1.0 ± 0.0 | N.Q. | 0.9 ± 0.0 | 1.0 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 2.0 ± 0.0 | 0.8 ± 0.0 | 1.8 ± 0.1 | 1.4 ± 0.0 | 1.0 |
| 25 | 4-p-Coumaroylquinic acid | 28.1 ± 0.2 | 32.4 ± 0.4 | 5.2 ± 0.1 | 3.0 ± 0.1 | 22.5 ± 0.5 | 17.2 ± 0.2 | 4.8 ± 0.1 | 8.6 ± 0.1 | 34.3 ± 0.5 | 22.4 ± 0.2 | 17.8 |
| 28 | Caffeoylshikimic acid | 25.7 ± 0.2 | 19.0 ± 0.2 | 32.8 ± 0.5 | 20.4 ± 0.2 | 13.5 ± 0.2 | 27.6 ± 0.3 | 7.4 ± 0.2 | 5.0 ± 0.1 | 8.3 ± 0.1 | 12.5 ± 0.1 | 17.2 |
| 31 | 3-O-Feruloylquinic acid | 49.9 ± 0.6 | 41.2 ± 0.5 | 12.8 ± 0.2 | 5.0 ± 0.1 | 49.6 ± 0.8 | 51.8 ± 0.9 | 6.5 ± 0.1 | 9.8 ± 0.2 | 19.9 ± 0.3 | 53.2 ± 0.9 | 30.0 |
| 32 | Caffeoyl-malonyl-methylcitric acid | 4.9 ± 0.1 | 5.3 ± 0.1 | 1.7 ± 0.0 | 0.5 ± 0.0 | 7.2 ± 0.1 | 6.4 ± 0.1 | 0.7 ± 0.0 | 1.2 ± 0.0 | 2.3 ± 0.0 | 6.3 ± 0.1 | 3.7 |
| 34 | 5-p-Coumaroylquinic acid | 1.0 ± 0.0 | 1.1 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.8 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 3.1 ± 0.0 | 2.3 ± 0.1 | 1.1 |
| 36 | 4-O-Caffeoylquinic acid methyl ester | 1.9 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 1.4 ± 0.0 | 2.4 ± 0.0 | 2.3 ± 0.0 | 1.4 ± 0.1 | 1.4 ± 0.0 | 6.4 ± 0.2 | 7.7 ± 0.2 | 2.7 |
| 47 | Hydroxycinnamic acid | 1.1 ± 0.0 | N.D. | 1.4 ± 0.0 | 1.7 ± 0.0 | 1.2 ± 0.0 | 1.5 ± 0.0 | 0.6 ± 0.0 | 5.7 ± 0.1 | 3.4 ± 0.0 | 1.9 ± 0.0 | 1.8 |
| 48 | 3-O-Caffeoylquinic acid methyl ester | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.Q. | 5.0 ± 0.1 | 5.1 ± 0.1 | 1.0 |
| 54 | p-Coumaroylcaffeoylquinic acid | N.D. | N.D. | N.D. | N.D. | 1.3 ± 0.0 | N.Q. | 0.6 ± 0.0 | N.D. | N.D. | N.D. | 0.2 |
| 58 | p-Coumaroylcaffeoylquinic acid | N.D. | N.D. | N.D. | N.D. | 4.5 ± 0.1 | N.Q. | 2.1 ± 0.0 | N.Q. | N.D. | N.D. | 0.7 |
| 61 | Di-O-caffeoylquinic acid | 3.1 ± 0.1 | N.D. | N.D. | N.D. | 1.7 ± 0.0 | N.Q. | 3.0 ± 0.0 | N.Q. | N.D. | N.D. | 0.8 |
| 69 | 5-O-Caffeoylquinic acid methyl ester | 6.2 ± 0.2 | N.Q. | 1.7 ± 0.0 | 9.6 ± 0.2 | 44.1 ± 0.7 | N.Q. | 12.6 ± 0.2 | N.Q. | 17.5 ± 0.2 | 13.3 ± 0.2 | 10.5 |
| 71 | Caffeoyl-malonyl-methylcitric acid | 11.6 ± 0.2 | N.Q. | 3.9 ± 0.0 | 18.6 ± 0.2 | 87.7 ± 0.9 | 1.3 ± 0.0 | 23.1 ± 0.2 | N.Q. | 34.7 ± 0.3 | 26.2 ± 0.3 | 20.7 |
| 76 | Isochlorogenic acid B | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.8 ± 0.0 | N.D. | N.Q. | 0.5 ± 0.0 | N.Q. | 0.5 ± 0.0 | N.Q. | 0.4 |
| 83 | Isochlorogenic acid A | 139.2 ± 2.2 | 240.8 ± 2.6 | 52.9 ± 1.3 | 34.8 ± 0.3 | 21.1 ± 0.3 | 60.4 ± 0.7 | 74.3 ± 0.8 | 52.8 ± 0.5 | 116.0 ± 1.4 | 89.2 ± 2.0 | 88.2 |
| 89 | p-Coumaroylcaffeoylquinic acid | 0.8 ± 0.0 | 0.6 ± 0.0 | N.D. | N.D. | 1.5 ± 0.0 | N.Q. | N.Q. | N.Q. | 1.1 ± 0.1 | 0.6 ± 0.0 | 0.5 |
| 92 | Isochlorogenic acid C | 3.2 ± 0.1 | 4.2 ± 0.1 | 2.8 ± 0.0 | 1.8 ± 0.1 | 2.0 ± 0.0 | 1.6 ± 0.0 | 2.4 ± 0.1 | 1.6 ± 0.0 | 1.6 ± 0.0 | 1.3 ± 0.0 | 2.3 |
| 97 | p-Coumaroylcaffeoylquinic acid | 17.2 ± 0.2 | 21.3 ± 0.2 | 4.8 ± 0.1 | 3.1 ± 0.1 | 2.3 ± 0.0 | 3.9 ± 0.1 | 8.1 ± 0.1 | 3.8 ± 0.0 | 18.2 ± 0.2 | 11.2 ± 0.2 | 9.4 |
| 99 | 1-O-Caffeoylquinic acid methyl ester | 1.2 ± 0.0 | N.Q. | N.Q. | 1.6 ± 0.0 | 5.4 ± 0.1 | N.Q. | 3.4 ± 0.1 | N.Q. | 6.8 ± 0.2 | 5.4 ± 0.1 | 2.4 |
| Total phenolic acids | 13,242.2 ± 117.8 | 13,905.9 ± 96.8 | 10,864.4 ± 86.4 | 10,337.2 ± 95.9 | 14,994.6 ± 99.2 | 13,052.6 ± 72.1 | 8172.6 ± 52.8 | 8541.3 ± 61.4 | 8123.9 ± 50.4 | 11,688.1 ± 89.6 | 11,292.3 | |
| Phenolic glycoside | ||||||||||||
| 3 | Arbutin | 3925.2 ± 70.1 | 5605.5 ± 53.9 | 4843.8 ± 80.4 | 4945.5 ± 78.1 | 5899.1 ± 71.3 | 5905.9 ± 60.1 | 3007.4 ± 49.4 | 2285.6 ± 22.3 | 3511.6 ± 57.3 | 4510.3 ± 31.6 | 4444.0 |
| 6 | Dihydro-caffeoyl-O-hexoside | 9.1 ± 0.2 | 47.6 ± 0.5 | 25.9 ± 0.2 | 3.8 ± 0.1 | 48.4 ± 0.6 | 1.9 ± 0.1 | 1.4 ± 0.0 | 3.3 ± 0.1 | 19.2 ± 0.4 | 12.2 ± 0.1 | 17.3 |
| 8 | Hydroxyphenylpropionic acid-O-hexoside | 2.5 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 1.3 ± 0.1 | 0.5 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.6 |
| 9 | Syringic acid-O-hexoside | 3.8 ± 0.1 | N.Q. | 17.7 ± 0.2 | 12.7 ± 0.3 | N.Q. | 3.5 ± 0.1 | 7.6 ± 0.2 | 3.4 ± 0.1 | 21.7 ± 0.6 | 27.7 ± 0.5 | 9.8 |
| 11 | Dihydro-caffeoyl-O-hexoside | 8.8 ± 0.1 | 7.9 ± 0.1 | 4.9 ± 0.1 | 4.2 ± 0.1 | 8.7 ± 0.1 | 3.6 ± 0.1 | 10.1 ± 0.1 | 4.6 ± 0.1 | 2.8 ± 0.1 | 5.2 ± 0.1 | 6.1 |
| 21 | Caffeoyl-O-hexoside | 8.9 ± 0.1 | 12.9 ± 0.1 | 4.8 ± 0.1 | 4.8 ± 0.1 | 5.1 ± 0.1 | 5.6 ± 0.1 | 2.2 ± 0.0 | N.Q. | 3.8 ± 0.1 | 5.0 ± 0.1 | 5.3 |
| 23 | Roseoside | 0.3 ± 0.0 | 0.7 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.9 ± 0.0 | 1.3 ± 0.0 | 0.5 |
| 26 | Syringic acid-O-hexoside | 1.4 ± 0.0 | 1.6 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 1.1 ± 0.0 | 0.8 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 | 1.8 ± 0.1 | 1.0 ± 0.0 | 0.9 |
| 39 | Caffeoylarbutin | N.D. | N.D. | N.D. | N.D. | N.Q. | N.D. | N.D. | 7.0 ± 0.1 | N.D. | 44.0 ± 0.5 | 5.1 |
| 42 | Caffeoylarbutin | 193.7 ± 2.7 | N.Q. | 201.1 ± 1.5 | 239.1 ± 2.5 | N.Q. | 74.1 ± 0.7 | 225.7 ± 1.7 | 126.9 ± 2.1 | N.Q. | 2.6 ± 0.1 | 106.3 |
| 46 | Syringic acid-O-hexoside | 0.4 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.2 ± 0.0 | N.Q. | N.Q. | 0.2 ± 0.0 | 0.4 |
| 57 | Caffeoylarbutin | 9.9 ± 0.1 | N.Q. | 13.9 ± 0.2 | 14.9 ± 0.4 | N.Q. | 5.2 ± 0.1 | 13.5 ± 0.2 | 8.9 ± 0.2 | N.Q. | 2.8 ± 0.1 | 6.9 |
| 68 | p-Coumaroylarbutin | 33.9 ± 0.4 | 0.4 ± 0.0 | 34.8 ± 0.5 | 50.9 ± 0.8 | 1.0 ± 0.0 | 12.1 ± 0.2 | 39.7 ± 0.7 | 20.0 ± 0.2 | 0.4 ± 0.0 | 0.3 ± 0.0 | 19.3 |
| 85 | p-Coumaroylarbutin | 11.1 ± 0.2 | N.Q. | 14.0 ± 0.2 | 18.7 ± 0.4 | 0.3 ± 0.0 | 7.0 ± 0.1 | 17.4 ± 0.2 | 9.8 ± 0.1 | 1.3 ± 0.0 | 2.1 ± 0.1 | 8.2 |
| 100 | Galloyl-coumaric acid pentoside | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.6± 0.0 | 0.3 |
| 102 | Galloyl-coumaric acid pentoside | 0.1 ± 0.0 | 0.1 ± 0.00 | 0.1 ± 0.0 | N.Q. | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 |
| Total phenolic glycosides | 4209.2 ± 72.9 | 5678.1 ± 53.6 | 5162.8 ± 83.5 | 5296.0 ± 77.7 | 5966.6 ± 71.3 | 6021.3 ± 59.5 | 3326.3 ± 50.8 | 2471.0 ± 23.4 | 3564.4 ± 56.8 | 4616.0 ± 32.1 | 4631.2 | |
| Flavone | ||||||||||||
| 38 | Quercetin-3-O-xylosylrhamnosylglucoside | 1.7 ± 0.0 | 1.4 ± 0.0 | 2.6 ± 0.1 | 1.5 ± 0.0 | 1.1 ± 0.0 | 2.7 ± 0.0 | 4.8 ± 0.1 | N.Q. | N.D. | N.D. | 1.6 |
| 41 | Isorhamnetin-acylated-hexoside | 1.1 ± 0.0 | N.Q. | 1.1 ± 0.1 | 1.3 ± 0.0 | N.Q. | 0.4 ± 0.0 | 1.2 ± 0.0 | 0.7 ± 0.0 | N.D. | N.D. | 0.6 |
| 43 | Quercetin-3-O-arabinosylgalactoside | 5.9 ± 0.1 | 2.0 ± 0.1 | 5.1 ± 0.1 | 2.6 ± 0.1 | 1.0 ± 0.0 | 2.7 ± 0.1 | 3.4 ± 0.1 | N.Q. | N.D. | N.D. | 2.3 |
| 49 | Quercetin-3-O-arabinosylglucoside | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.1 |
| 50 | Quercetin-3-O-rhamnosylgalactoside | 0.3 ± 0.0 | 0.5 ± 0.0 | 1.0 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 2.2 ± 0.1 | 0.5 ± 0.0 | N.Q. | 16.9 ± 0.5 | 6.9 ± 0.1 | 2.9 |
| 51 | Luteolin-7-O-galactoside | N.D. | N.D. | N.D. | N.D. | 0.8 ± 0.0 | N.Q. | N.D. | N.D. | N.D. | N.D. | 0.1 |
| 52 | Rutin | 12.6 ± 0.1 | 10.1 ± 0.2 | 13.3 ± 0.2 | 8.3 ± 0.2 | 12.3 ± 0.2 | 19.7 ± 0.2 | 24.5 ± 0.4 | 1.6 ± 0.0 | 61.9 ± 1.1 | 61.2 ± 0.3 | 22.5 |
| 55 | Hyperoside | 2.7 ± 0.0 | 1.4 ± 0.0 | 2.0 ± 0.1 | 1.0 ± 0.0 | 0.4 ± 0.0 | 1.5 ± 0.0 | 0.4 ± 0.0 | 3.0 ± 0.1 | 11.4 ± 0.2 | 8.8 ± 0.1 | 3.2 |
| 56 | Kaempferol-3-O-rhamnosylgalactoside | 63.9 ± 1.7 | N.Q. | N.D. | N.D. | 12.1 ± 0.1 | N.Q. | 17.7 ± 0.3 | N.Q. | N.Q. | N.Q. | 9.4 |
| 59 | Quercetin-3-O-glucoside | 20.6 ± 0.2 | 7.9 ± 0.2 | 16.5 ± 0.3 | 7.7 ± 0.2 | 5.3 ± 0.1 | 12.3 ± 0.2 | 6.5 ± 0.1 | 16.1 ± 0.3 | 56.1 ± 0.7 | 50.4 ± 0.5 | 19.9 |
| 62 | Luteoloside | 14.1 ± 0.1 | N.Q. | N.D. | N.D. | 5.8 ± 0.1 | N.Q. | 11.5 ± 0.1 | N.Q. | N.D. | N.D. | 3.2 |
| 64 | Kaempferol-3-O-rhamnosylglucoside | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 1.95 ± 0.1 | 1.3 ± 0.1 | 0.3 |
| 65 | Apigenin-O-glucuronide or isomer | N.D. | N.D. | N.D. | N.D. | 0.2 ± 0.0 | N.D. | N.D. | N.D. | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.1 |
| 72 | Apigenin-O-glucuronide or isomer | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | N.D. | 0.5 ± 0.0 | N.D. | N.D. | 3.3 ± 0.1 | 1.5 ± 0.0 | 0.7 |
| 73 | Kaempferol-3-O-rutinoside | 2.3 ± 0.1 | 1.7 ± 0.1 | 2.8 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 1.2 ± 0.0 | 4.5 ± 0.2 | 4.0 ± 0.1 | 17.4 ± 0.5 | 15.9 ± 0.4 | 5.5 |
| 74 | Quercetin-acylated-galactoside | 5.98 ± 0.1 | 2.1 ± 0.0 | 3.3 ± 0.0 | 1.6 ± 0.0 | 0.9 ± 0.0 | 4.8 ± 0.1 | N.D. | N.D. | 39.4 ± 0.9 | 16.1 ± 0.2 | 7.4 |
| 75 | Isorhamnetin-3-O-rhamnosylgalactoside | 10.8 ± 0.2 | 21.3 ± 0.4 | 10.2 ± 0.2 | 7.3 ± 0.1 | 8.7 ± 0.1 | 2.3 ± 0.1 | 12.6 ± 0.4 | 15.8 ± 0.2 | 20.2 ± 0.2 | 58.8 ± 0.7 | 16.8 |
| 77 | Apigenin rutinoside | 3.8 ± 0.1 | N.Q. | N.D. | N.D. | 1.2 ± 0.0 | N.Q. | N.D. | N.D. | N.Q. | 1.0 ± 0.0 | 0.6 |
| 78 | Isorhamnetin-3-O-rutinoside | 86.9 ± 1.2 | 98.1 ± 1.1 | 49.9 ± 0.7 | 66.4 ± 1.0 | 75.3 ± 1.7 | 9.1 ± 0.1 | 136.6 ± 1.6 | 62.9 ± 1.4 | 28.5 ± 0.5 | 143.8 ± 1.3 | 75.8 |
| 79 | Kaempferol-3-O-galactoside | 4.3 ± 0.1 | 1.7 ± 0.0 | 8.2 ± 0.1 | 8.2 ± 0.2 | 1.6 ± 0.1 | 3.5 ± 0.1 | 2.1 ± 0.1 | 12.8 ± 0.3 | 16.8 ± 0.5 | 8.1 ± 0.2 | 6.7 |
| 80 | Quercetin-acylated-glucoside | 0.5 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.6 ± 0.0 | N.Q. | N.Q. | 3.1 ± 0.0 | 2.1 ± 0.1 | 0.8 |
| 81 | Isorhamnetin-3-O-galactoside | 5.7 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.1 | 1.7 ± 0.0 | 0.6 ± 0.1 | 0.9 ± 0.1 | 0.4 ± 0.0 | 19.8 ± 0.2 | 1.4 ± 0.0 | 4.6 ± 0.1 | 4.0 |
| 82 | Chrysoeriol-7-neohesperidoside | 9.3 ± 0.2 | N.Q. | N.Q. | N.Q. | 2.7 ± 0.1 | N.Q. | 2.0 ± 0.0 | N.Q. | N.Q. | N.Q. | 1.4 |
| 86 | Isorhamnetin-3-O-glucoside | 21.3 ± 0.4 | 8.8 ± 0.2 | 13.0 ± 0.3 | 9.2 ± 0.2 | 4.6 ± 0.1 | 2.4 ± 0.0 | 3.7 ± 0.1 | 95.1 ± 0.9 | 7.4 ± 0.2 | 22.1 ± 0.4 | 18.8 |
| 87 | Kaempferol-3-O-glucoside | 2.4 ± 0.1 | N.Q. | N.D. | 1.1 ± 0.1 | 55.1 ± 0.9 | N.Q. | 2.6 ± 0.1 | N.Q. | N.Q. | N.Q. | 6.1 |
| 88 | Apigenin-O-hexoside | 9.8 ± 0.2 | N.Q. | N.D. | N.D. | 19.6 ± 0.4 | N.Q. | 3.5 ± 0.1 | N.Q. | 0.6 ± 0.1 | 0.7 ± 0.0 | 3.4 |
| 90 | Chrysoeriol-7-O-galactoside | 15.7 ± 0.2 | N.Q. | N.D. | N.D. | 14.7 ± 0.2 | N.Q. | 19.9 ± 0.1 | N.Q. | N.Q. | N.Q. | 5.0 |
| 93 | Chrysoeriol-7-O-glucoside | 0.5 ± 0.0 | N.D. | N.D. | N.D. | 22.9 ± 0.6 | N.Q. | N.D. | N.D. | 0.2 ± 0.0 | 0.3 ± 0.0 | 2.4 |
| 94 | Kaempferol-acylated-galactoside | 14.5 ± 0.4 | 7.1 ± 0.2 | 6.3 ± 0.1 | 8.3 ± 0.2 | 4.0 ± 0.1 | 2.4 ± 0.1 | N.Q. | 2.0 ± 0.1 | 150.5 ± 2.1 | 28.0 ± 0.3 | 22.3 |
| 95 | Isorhamnetin-acylated-galactoside | 6.2 ± 0.1 | 2.95 ± 0.1 | 1.3 ± 0.0 | 1.1 ± 0.0 | N.D. | N.D. | N.D. | N.D. | 3.8 ± 0.1 | 3.3 ± 0.1 | 1.9 |
| 96 | Isorhamnetin-acylated-glucoside | 53.7 ± 1.1 | 26.1 ± 0.2 | 15.1 ± 0.2 | 14.6 ± 0.3 | 4.8 ± 0.1 | 2.0 ± 0.1 | N.Q. | 3.1 ± 0.0 | 36.5 ± 0.7 | 55.6 ± 0.9 | 21.2 |
| 98 | Kaempferol-acylated-glucoside | 2.8 ± 0.1 | N.Q. | N.D. | N.D. | N.D. | N.D. | 5.4 ± 0.1 | 1.3 ± 0.0 | N.Q. | 1.2 ± 0.1 | 1.1 |
| 101 | Phloretin-acylated-hexoside | 1.1 ± 0.0 | N.Q. | N.D. | 2.1 ± 0.1 | N.D. | N.D. | 3.3 ± 0.1 | N.D. | N.D. | N.D. | 0.6 |
| Total flavones | 380.9 ± 4.7 | 196.1 ± 0.3 | 154.7 ± 1.1 | 147.3 ± 1.5 | 259.0 ± 3.1 | 71.1 ± 1.4 | 266.9 ± 2.0 | 238.3 ± 3.6 | 478.1 ± 3.7 | 492.2 ± 1.6 | 268.5 | |
| Flavan-3-ol | ||||||||||||
| 4 | A-type procyanidin dimer | N.D. | N.D. | N.Q. | 0.7 ± 0.0 | N.D. | N.D. | N.Q. | 0.7 ± 0.0 | 3.3 ± 0.1 | 1.3 ± 0.0 | 0.6 |
| 7 | B-type procyanidin dimer | N.D. | N.D. | 0.9 ± 0.0 | 1.7 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 2.7 ± 0.1 | 6.8 ± 0.2 | 4.3 ± 0.1 | 1.8 |
| 12 | (+) -Catechin | 17.6 ± 0.3 | 16.5 ± 0.2 | 26.1 ± 0.4 | 40.1 ± 0.7 | 25.5 ± 0.4 | 12.9 ± 0.2 | 7.0 ± 0.1 | 26.6 ± 0.5 | 99.3 ± 1.3 | 134.3 ± 1.2 | 40.6 |
| 14 | B-type procyanidin trimer | N.D. | N.D. | 0.5 ± 0.0 | 1.6 ± 0.0 | N.D. | N.D. | N.Q. | 0.8 ± 0.0 | 3.2 ± 0.1 | 1.4 ± 0.0 | 0.7 |
| 17 | B-type procyanidin dimer | N.D. | N.D. | 2.2 ± 0.0 | 6.2 ± 0.1 | 1.1 ± 0.0 | 1.2 ± 0.0 | 1.9 ± 0.0 | 5.98 ± 0.1 | 37.8 ± 0.7 | 8.3 ± 0.2 | 6.5 |
| 18 | B-type procyanidin trimer | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 1.9 ± 0.0 | N.Q. | 0.2 |
| 19 | A-type procyanidin trimer | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.Q. | 0.6 ± 0.0 | 1.6 ± 0.1 | 0.8 ± 0.0 | 0.3 |
| 24 | (-)-Epicatechin | 29.2 ± 0.5 | 18.3 ± 0.2 | 48.7 ± 0.9 | 85.7 ± 0.9 | 120.4 ± 1.7 | 40.0 ± 0.8 | 29.7 ± 0.4 | 69.8 ± 1.3 | 445.7 ± 7.2 | 219.6 ± 2.6 | 110.7 |
| 27 | A-type procyanidin trimer | 0.5 ± 0.0 | N.D. | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | N.Q. | N.Q. | 0.9 ± 0.1 | 1.7 ± 0.0 | 2.3 ± 0.1 | 0.7 |
| 29 | A-type procyanidin trimer | 0.7 ± 0.0 | N.D. | 1.7 ± 0.0 | 2.0 ± 0.0 | 2.9 ± 0.1 | 1.5 ± 0.1 | 1.1 ± 0.0 | 3.4 ± 0.1 | 10.0 ± 0.1 | 5.5 ± 0.1 | 2.9 |
| 30 | A-type procyanidin dimer | N.D. | N.D. | N.Q. | 0.6 ± 0.0 | N.D. | N.D. | N.Q. | 0.9 ± 0.0 | 3.2 ± 0.1 | 1.2 ± 0.0 | 0.6 |
| 33 | B-type procyanidin trimer | N.D. | N.D. | 0.6 ± 0.0 | 3.0 ± 0.0 | N.D. | N.D. | 0.6 ± 0.0 | 2.0 ± 0.0 | 15.2 ± 0.1 | 2.3 ± 0.0 | 2.4 |
| 35 | B-type procyanidin dimer | N.D. | N.D. | N.Q. | 0.9 ± 0.0 | N.D. | N.D. | N.Q. | 0.6 ± 0.0 | 2.1 ± 0.1 | 1.9 ± 0.1 | 0.6 |
| 37 | A-type procyanidin dimer | 0.5 ± 0.0 | N.D. | N.Q. | 1.0 ± 0.0 | 1.5 ± 0.0 | N.Q. | 1.2 ± 0.0 | 0.7 ± 0.0 | 4.0 ± 0.1 | 2.0 ± 0.1 | 1.1 |
| 40 | B-type procyanidin tetramer | N.D. | N.D. | N.D. | 0.7 ± 0.0 | N.D. | N.D. | N.D. | 0.4 ± 0.0 | 2.7 ± 0.0 | 0.7 ± 0.0 | 0.5 |
| 44 | A-type procyanidin trimer | N.D. | N.D. | 0.7 ± 0.0 | 1.0 ± 0.0 | N.D. | N.D. | N.Q. | 1.1 ± 0.0 | 2.9 ± 0.1 | 2.1 ± 0.1 | 0.8 |
| 45 | A-type procyanidin dimer | 2.7 ± 0.1 | 2.5 ± 0.1 | 2.1 ± 0.0 | 1.7 ± 0.0 | 3.0 ± 0.1 | 1.7 ± 0.0 | 1.1 ± 0.0 | 4.5 ± 0.1 | 3.6 ± 0.1 | 7.4 ± 0.1 | 3.0 |
| 53 | B-type procyanidin trimer | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 1.1 ± 0.0 | N.Q. | 0.1 |
| 60 | B-type procyanidin dimer | N.D. | N.D. | 0.9 ± 0.1 | 2.3 ± 0.0 | 1.0 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.0 | 1.7 ± 0.0 | 10.2 ± 0.3 | 3.2 ± 0.0 | 2.1 |
| 63 | B-type procyanidin trimer | N.D. | N.D. | N.D. | 0.6 ± 0.0 | N.D. | N.D. | N.D. | N.D. | 2.1 ± 0.1 | 0.5 ± 0.0 | 0.3 |
| 66 | A-type procyanidin dimer | 3.6 ± 0.1 | 2.1 ± 0.0 | 4.5 ± 0.0 | 5.2 ± 0.1 | 10.6 ± 0.2 | 4.8 ± 0.1 | 3.6 ± 0.1 | 8.6 ± 0.2 | 20.1 ± 0.2 | 14.4 ± 0.3 | 7.8 |
| 67 | A-type procyanidin trimer | 2.0 ± 0.0 | 1.7 ± 0.0 | 1.6 ± 0.1 | 0.8 ± 0.0 | 1.9 ± 0.0 | 1.0 ± 0.0 | N.Q. | 1.5 ± 0.0 | 1.4 ± 0.0 | 5.2 ± 0.1 | 1.7 |
| 70 | A-type procyanidin trimer | N.D. | N.D. | 1.0 ± 0.0 | 1.8 ± 0.0 | 1.3 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0 | 2.6 ± 0.0 | 8.9 ± 0.1 | 4.3 ± 0.0 | 2.2 |
| 84 | A-type procyanidin dimer | 2.9 ± 0.1 | 2.8 ± 0.1 | 3.1 ± 0.1 | 3.7 ± 0.0 | 5.7 ± 0.1 | 3.2 ± 0.0 | 2.3 ± 0.0 | 5.6 ± 0.1 | 16.1 ± 0.2 | 10.8 ± 0.1 | 5.6 |
| 91 | A-type procyanidin trimer | 3.6 ± 0.1 | 2.2 ± 0.1 | 2.9 ± 0.1 | 1.7 ± 0.0 | 10.6 ± 0.3 | 3.9 ± 0.1 | 1.9 ± 0.1 | 4.4 ± 0.1 | 6.7 ± 0.2 | 7.8 ± 0.2 | 4.6 |
| Total flavan-3-ols | 63.5 ± 0.1 | 46.1 ± 0.5 | 98.0 ± 0.4 | 163.8 ± 0.9 | 186.3 ± 2.3 | 72.1 ± 0.8 | 52.3 ± 0.6 | 146.1 ± 1.6 | 711.7 ± 8.5 | 441.2 ± 3.8 | 198.1 | |
| Total polyphenols (mg/g FW) | 17.9 ± 0.1 | 19.8 ± 0.1 | 16.3 ± 0.1 | 15.9 ± 0.2 | 21.4 ± 0.1 | 19.2 ± 0.1 | 11.8 ± 0.1 | 11.4 ± 0.1 | 12.9 ± 0.1 | 17.2 ± 0.1 | 16.4 | |
| Triterpenoid | ||||||||||||
| 1′ | Euscaphic acid | N.D. | N.D. | N.D. | 0.4 ± 0.0 | 0.3 ± 0.0 | N.D. | 0.5 ± 0.0 | N.D. | N.D. | N.D. | 0.1 |
| 2′ | Tormentic acid | 6.0 ± 0.1 | 20.5 ± 0.5 | 14.5 ± 0.4 | 13.4 ± 0.3 | 11.6 ± 0.3 | 8.2 ± 0.2 | 5.3 ± 0.1 | 7.6 ± 0.1 | 15.8 ± 0.2 | 13.1 ± 0.2 | 11.6 |
| 3′ | Anmurcoic acid | 13.9 ± 0.2 | 55.3 ± 0.7 | 9.6 ± 0.1 | 18.0 ± 0.4 | 11.1 ± 0.1 | 8.7 ± 0.2 | 11.0 ± 0.2 | 6.7 ± 0.1 | 3.8 ± 0.1 | 9.3 ± 0.1 | 14.8 |
| 4′ | Pomolic acid isomer | 82.2 ± 1.2 | 60.7 ± 1.0 | 15.0 ± 0.3 | 25.5 ± 0.3 | 42.1 ± 0.8 | 2.9 ± 0.1 | 64.5 ± 1.2 | 50.0 ± 0.9 | 9.5 ± 0.2 | 81.1 ± 1.1 | 43.4 |
| 5′ | 1-Hydroxy-3-oxours-12-en-28-oic acid/isomer | 4.6 ± 0.1 | 7.3 ± 0.1 | N.D. | 0.5 ± 0.0 | 0.4 ± 0.0 | N.D. | 1.5 ± 0.0 | 2.3 ± 0.0 | N.D. | 1.2 ± 0.0 | 1.8 |
| 6′ | Pomolic acid | 6.6 ± 0.1 | 2.4 ± 0.0 | 1.8 ± 0.1 | 2.6 ± 0.1 | 1.9 ± 0.1 | 5.9 ± 0.1 | 4.5 ± 0.1 | 1.9 ± 0.0 | 2.7 ± 0.1 | 9.7 ± 0.2 | 4.0 |
| 7′ | 701 m/z [M − H]- | 6.1 ± 0.1 | 8.3 ± 0.2 | 5.2 ± 0.1 | 6.1 ± 0.2 | 5.3 ± 0.1 | 4.6 ± 0.1 | 4.9 ± 0.0 | 7.5 ± 0.2 | 13.4 ± 0.1 | 19.1 ± 0.3 | 8.1 |
| 8′ | Alphitolic acid | 1.4 ± 0.0 | 1.6 ± 0.0 | 0.4 ± 0.0 | 1.1 ± 0.0 | 0.9 ± 0.0 | 2.3 ± 0.0 | 2.7 ± 0.1 | 0.4 ± 0.0 | 0.9 ± 0.0 | 0.5 ± 0.0 | 1.2 |
| 9′ | Maslinic acid | 4.0 ± 0.1 | 28.5 ± 0.4 | 4.5 ± 0.1 | 10.9 ± 0.3 | 10.7 ± 0.2 | 11.1 ± 0.2 | 6.7 ± 0.1 | 7.8 ± 0.1 | 9.7 ± 0.1 | 8.7 ± 0.2 | 10.2 |
| 10′ | Corosolic acid | 2.3 ± 0.1 | 9.8 ± 0.2 | 5.2 ± 0.1 | 11.9 ± 0.2 | 8.8 ± 0.2 | 35.7 ± 0.5 | 2.8 ± 0.1 | 5.2 ± 0.1 | 28.4 ± 0.5 | 5.2 ± 0.1 | 11.5 |
| 11′ | 1-Hydroxy-3-oxours-12-en-28-oic acid/isomer | N.D. | 0.9 ± 0.0 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 0.1 |
| 12′ | 1-Hydroxy-3-oxours-12-en-28-oic acid/isomer | N.D. | 0.9 ± 0.0 | 1.3 ± 0.0 | 2.1 ± 0.0 | N.D. | 0.9 ± 0.0 | N.D. | N.D. | 0.3 ± 0.0 | N.D. | 0.5 |
| 13′ | Betulinic acid | 8.1 ± 0.1 | 1.4 ± 0.0 | 1.2 ± 0.0 | 2.3 ± 0.0 | 2.6 ± 0.0 | 6.2 ± 0.1 | 13.0 ± 0.1 | 1.8 ± 0.0 | 0.6 ± 0.0 | 1.6 ± 0.0 | 3.9 |
| 14′ | Oleanolic acid | 38.5 ± 0.6 | 37.1 ± 0.4 | 35.7 ± 0.4 | 37.2 ± 0.5 | 38.5 ± 0.4 | 49.9 ± 0.6 | 49.0 ± 0.7 | 37.8 ± 0.6 | 13.5 ± 0.3 | 39.6 ± 0.5 | 37.7 |
| 15′ | Ursolic acid | 113.1 ± 1.7 | 112.8 ± 1.1 | 149.7 ± 1.6 | 146.5 ± 1.8 | 129.3 ± 1.3 | 257.1 ± 2.3 | 97.1 ± 1.98 | 125.5 ± 1.8 | 66.7 ± 1.1 | 94.9 ± 1.2 | 129.3 |
| 16′ | 687 m/z [M − H]- | 18.9 ± 0.3 | 20.1 ± 0.4 | 16.1 ± 0.2 | 19.0 ± 0.2 | 11.0 ± 0.2 | 9.8 ± 0.2 | 14.2 ± 0.2 | 19.8 ± 0.2 | 28.0 ± 0.5 | 49.3 ± 0.6 | 20.6 |
| Total triterpenoids | 305.7 ± 1.12 | 367.5 ± 1.4 | 260.3 ± 1.1 | 297.4 ± 1.4 | 274.6 ± 2.6 | 403.1 ± 2.2 | 277.5 ± 3.4 | 274.3 ± 3.2 | 193.0 ± 1.2 | 333.3 ± 2.6 | 298.7 | |
Note: N.D., not detectable; N.Q., not quantifiable; DSSL, ‘Dangshansuli’; YL, ‘Yali’; CG, ‘Cuiguan’; FS, ‘Fengshui’; NGL, ‘Nanguoli’; XSL, ‘Xiangshuili’; KELXL, ‘Kuerlexiangli’; XJSL, ‘Xinjiangsuanli’; HQL, ‘Hongqieli’; SJL, ‘Sanjili’.
Phenolic acids are the most representative polyphenols in pears and other fruits [7,29], accounting for >64% of all the polyphenolic compounds in the investigated young pears (Table 3). Relatively higher total phenolic acid content was found in thinned ‘Nanguoli’, ‘Yali’, ‘Dangshansuli’ and ‘Xiangshuili’ (14,994.6, 13,905.9, 13,242.2 and 13,052.6 μg/g FW, respectively), whereas lower content was observed in ‘Hongqieli’, ‘Kuerlexiangli’ and ‘Xinjiangsuanli’ varieties (8123.9, 8172.6 and 8541.3 μg/g FW, respectively). The primary phenolic acid, quinic acid (peak 1), accounted for ~ 73% of the total phenolic acid content and ranged from 10,500.5 μg/g FW in ‘Nanguoli’ to 4991.7 μg/g FW in thinned ‘Hongqieli’. These values were in marked contrast to previously reported ranges of mature pears [1,7]. In published literature, little quinic acid (≤0.2 mg/100 g of dry matter) could be found in different anatomical parts of ripe pears [1]. These results suggest that, as the raw material of many secondary metabolic products, quinic acid would be largely exhausted during the process of pear fruit growth and development. The second predominant phenolic acid, chlorogenic acid (peak 13), accounted for approximately 24% of the total phenolic acid and varied from 4139.0 μg/g FW in ‘Nanguoli’ to 1162.7 μg/g FW in ‘Fengshui’. Although chlorogenic acid remains abundant in phenolic compounds during pear fruit development, the average concentration in thinned pears was ~34 times higher than that in fully ripe pears reported by others [13].
Arbutin (peak 3) was the most abundant phenolic glycoside compound, accounting for >90% of the total phenolic glycosides in young pears and ranging from 5905.9 μg/g FW in ‘Xiangshuili’ to 2285.6 μg/g FW in ‘Xinjiangsuanli’. The levels were clearly higher than those in ripe pears [1,2]. Accordingly, the abundant arbutin in young pears would be gradually metabolized to other compounds as pear fruits grow and mature. Besides, the content of arbutin and total phenolic glycoside in Pyrus sinkiangensis (‘Kuerlexiangli’ and ‘Xinjiangsuanli’) and Pyrus communis (‘Hongqieli’ and ‘Sanjili’) varieties were much lower than those of other species. This result was similar to the findings for phenolic acid and total polyphenol content, thus implying a possible relationship in the biosynthetic regulation of phenolic acids and phenolic glycosides in pears.
The total content of flavones in the investigated pear varieties (Figure 2C and Table 3) was clearly much lower than that of phenolic acids and phenolic glycosides, and it accounted for only ~ 1.6% of the total polyphenol content, because most of the flavone compounds are located in the pear peel but not the flesh [7,28]. Nonetheless, the samples differed distinctly in terms of flavone composition and content. As P. communis cultivars, the young fruits of ‘Sanjili’ and ‘Hongqieli’ obviously showed the highest total flavone content (492.2 and 478.1 μg/g FW, respectively), attributing to the abundance of rutin, quercetin-3-O-glucoside, isorhamnetin-3-O-rutinoside, kaempferol-acylated-galactoside and isorhamnetin-acylated-glucoside (peaks 52, 59, 78, 94 and 96). In contrast, the Pyrus ussuriensis sample obtained from ‘Xiangshuili’ showed the lowest flavone content, with a total value of 71.1 μg/g FW. Of the 33 flavones monitored in this study, only nine compounds were found in all the fruits tested, including rutin, quercetin-3-O-galactoside, quercetin-3-O-glucoside, Kaempferol-3-O-rutinoside, isorhamnetin-3-O-rhamnosylgalactoside, isorhamnetin-3-O-rutinoside, kaempferol-3-O-galactoside and isorhamnetin-3-O-glucoside (peaks 52, 55, 59, 73, 75, 78, 79 and 86). Those compounds are also the major flavonols in the peels of ripe pears, as reported by others [2,28]. As the distinguished flavone in pears [30], the highest content of isorhamnetin-3-O-rutinoside (peak 78) was 143.8 μg/g FW in ‘Sanjili’, whereas the lowest was only 9.1 μg/g FW in ‘Xiangshuili’. Meanwhile, some other distinguished flavones were also found in different varieties. The ‘Nanguoli’ sample was characterized by kaempferol-3-O-glucoside (peak 87) with an exceptionally high content value of 55.1 μg/g FW, while the ‘Xinjiangsuanli’ sample, which showed lower total flavone content, contained markedly higher amounts of isorhamnetin-3-O-glucoside (peak 86) with a value of 95.1 μg/g FW.
The analysis also revealed statistically significant discrepancies in the concentrations of flavan-3-ols and procyanidins among different pear varieties (Figure 2D and Table 3). Reflecting a species characteristic of P. communis, the total content of flavan-3-ols in immature fruits of ‘Hongqieli’ and ‘Sanjili’ (711.7 and 441.2 μg/g FW) was not only markedly higher than those in other investigated pear species but also obviously higher than those in mature fruits of P. communis cultivars reported by others [13]. This result was similar to the comparison of the flavone amounts. Among 25 flavan-3-ols in the 10 young pear varieties, (−)-epicatechin (peak 24) was predominant (ranging from 445.7 μg/g FW in ‘Hongqieli’ to 18.3 μg/g FW in ‘Yali’) and was followed by (+)-catechin (peak 12) (ranging from 134.3 μg/g FW in ‘Sanjili’ to 7.0 μg/g FW in ‘Kuerlexiangli’).
Besides polyphenols, triterpenes are also important biologically active constituents of pears. Therefore, the content of ursolic, oleanolic and betulinic acid has often been used in quality evaluation indexes for pears and other-related products [2]. In this paper, besides these three compounds, 13 other triterpenes were investigated in pear fruits. Among 16 triterpene compounds in the tested pears, ursolic acid was predominant (peak 15′, ranging from 257.1 μg/g FW in ‘Xiangshuili’ to 66.7 μg/g FW in ‘Hongqieli’) and was followed by the pomolic acid isomer (peak 4′, ranging from 82.2 μg/g FW in ‘Dangshansuli’ to 2.9 μg/g FW in ‘Xiangshuili’) and oleanolic acid (peak 14′, ranging from 49.9 μg/g FW in ‘Xiangshuili’ to 13.5 μg/g FW in ‘Hongqieli’) (Figure 2F and Table 3). This result might aid in further study of the triterpene composition in mature pears and related products.
2.3. Antioxidant Capacity of Thinned Pears
To obtain an overall view of the potent health benefits of thinned pears, the antioxidant capacities of 10 varieties were measured through DPPH analysis and expressed as micromolar Trolox equivalent per gram fresh weight (μmol TE/g FW) (Table 4). Among these samples, ‘Hongqieli’ variety showed the strongest antioxidant potential (30.4 μmol TE/g FW) and was followed by ‘Xinjiangsuanli’, ‘Xiangshuili’ and ‘Nanguoli’ (22.5, 22.4 and 22.2 μmol TE/g FW, respectively). In contrast, ‘Kuerlexiangli’, ‘Cuiguan’ and ‘Fengshui’ (10.1, 12.4 and 15.6 μmol TE/g FW, respectively) had the lowest antioxidant activity. Owing to the diversity in the content of chemical compounds contained in different varieties, the coefficient of variance for total antioxidant activity was 29.4%. Nevertheless, the antioxidant capacity of the thinned young pears was significantly higher than that reported by other authors for mature pears [1], in agreement with previous literature [5]. Recently, some studies proved a highly significant linear correlation between antioxidant capacity and polyphenol content in fruits and vegetables [31]. Therefore, similar strong antioxidant activity of ‘Xiangshuili’ and ‘Nanguoli’ might be attributed to their abundant polyphenols, whereas the moderate antioxidant potential of ‘Kuerlexiangli’ might be also influenced by its low polyphenol content. However, the fruitlets of ‘Hongqieli’ and ‘Sanjili’, which were poor in total polyphenols but rich in flavan-3-ols, still had high antioxidant capacity, thus indicating that the free radical scavenging activity of flavan-3-ols might be much stronger than those of other polyphenols. In addition, of interest was the finding that ‘Xinjiangsuanli’, in which the content of most polyphenolic compounds was lower than those in other varieties except isorhamnetin-3-O-glucoside (peak 86), also showed strong antioxidant potential. One of the reasons for this result might be that some other biologically active compounds undetected by the current method might contribute to the free radical scavenging activity of the ‘Xinjiangsuanli’ sample.
Table 4.
Antioxidant capacities of thinned young pears based on DPPH analysis (mean ± SD, n = 3).
| Pear Variety | Antioxidant Capacity (μmol TE/g FW) | Pear Variety | Antioxidant Capacity (μmol TE/g FW) |
|---|---|---|---|
| DSSL | 20.7 ± 0.2 c | KELXL | 10.1 ± 0.2 g |
| YL | 20.6 ± 0.2 c | XJSL | 22.5 ± 0.3 b |
| CG | 12.4 ± 0.2 f | HQL | 30.4 ± 0.3 a |
| FS | 15.6 ± 0.1 e | SJL | 19.9 ± 0.2 d |
| NGL | 22.2 ± 0.2 b | mean | 19.7 |
| XSL | 22.4 ± 0.2 b | C.V. (%) | 29.4 |
Note: different letters indicate significantly different at p < 0.05.
2.4. Hierarchical Clustering Analysis
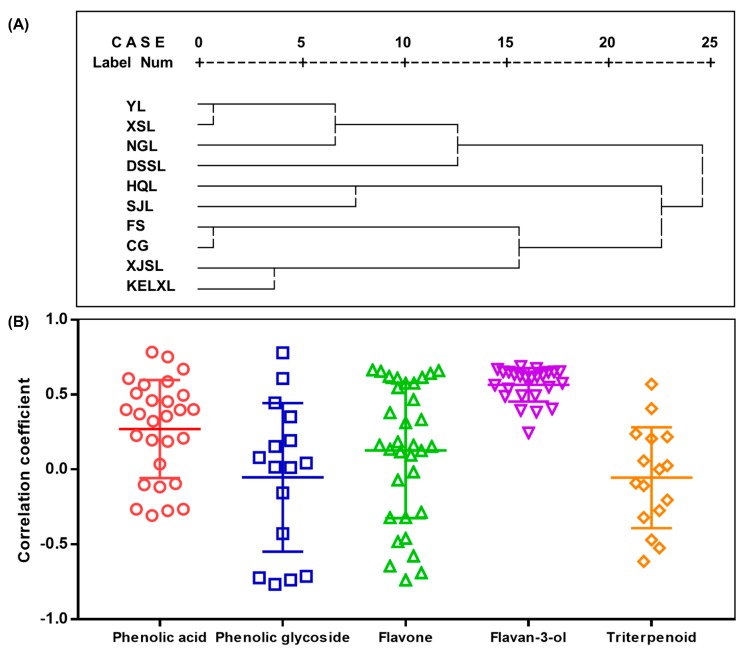
Hierarchical clustering analysis (HCA) was performed to discriminate different pear samples based on the chemical constituents and antioxidant activity. The result of HCA is shown in Figure 3A. It was obvious that the samples could be classified into three clusters: two Pyrus bretschneideri Rehd. (‘Yali’ and ‘Dangshansuli’) and two P. ussuriensis Maxim. (‘Xiangshuili’ and ‘Nanguoli’) in cluster 1, two P. communis L. (‘Hongqieli’ and ‘Sanjili’) in cluster 2, two Pyrus pyrifolia Nakai (‘Fengshui’ and ‘Cuiguan’) and two P. sinkiangensis Yu (‘Kuerlexiangli’ and ‘Xinjiangsuanli’) in cluster 3. In cluster 3, P. pyrifolia Nakai and P. sinkiangensis Yu samples were distinctly divided into two groups. There were relatively small differences among the samples from the same pear species. The results were broadly consistent with the cultivar classification system of pear fruit [32] and showed that different pear species could be clearly distinguished according to their chemical and antioxidant characteristics.
Figure 3.
Hierarchical clustering analysis of pear samples (A) and the spectrum–effect relationship between total antioxidant activity and individual chemical compound (B).
2.5. Correlation Analysis
A correlation analysis between total antioxidant activity and each component content was carried out (Figure 3B and Table S4), which further demonstrated that thinned pears containing higher levels of bioactive compounds had more potent health benefits. The results indicated that predominant quinic acid and arbutin had a weak influence on the total activity, whereas chlorogenic acid, the other primary phenolic compound, exhibited a noticeable correlation (R = 0.566). This result was quite similar to those reported by other authors [33,34]. Consequently, the strong antioxidant activity of ‘Dangshansuli’, ‘Yali’, ‘Xiangshuili’ and ‘Nanguoli’ might be attributed to the higher content of chlorogenic acid, whereas the unsatisfactory activity of ‘Kuerlexiangli’, ‘Cuiguan’ and ‘Fengshui’ might also be ascribed to the lower content of this compound. In addition, there were 27 polyphenolic compounds, including four phenolic acids (peaks 16, 20, 25 and 34), two phenolic glycosides (peaks 26 and 102), seven flavones (peaks 50, 55, 65, 73, 74, 80 and 94) and 14 flavan-3-ols (peaks 4, 17, 18, 19, 24, 29, 30, 33, 40, 53, 60, 66, 70 and 84), that showed a marked positive correlation (R > 0.6) with the antioxidant activity of the pear extracts, and these compounds might be the principal antioxidant contributors. These results provide further evidence that young pears may be a natural source of biological antioxidants in the pharmaceutical, cosmetic and food industries. In particular, all of flavan-3-ol compounds abundant in P. communis cultivars showed a significant positive correlation with the total antioxidant activity, thus indicating that the young fruits of P. communis might have advantages in antioxidant related industries. However, there were still eight compounds, including four phenolic glycosides (peaks 42, 57, 68 and 85), four flavones (peaks 38, 41, 43 and 101) and two triterpenic acids (peaks 1ʹ and 14ʹ), showing an obvious negative correlation (R < −0.5) with the total activity. All of the four phenolic glycosides were arbutin derivatives according to their aglycon. In published literature, it was reported that the DPPH scavenging capacity of oleanolic acid was significantly lower than phenolic compounds, such as chlorogenic acid and procyanidin compound [35]. In pear extracts, most triterpenoid constituents except corosolic acid showed a weak contribution to the total antioxidant activity, perhaps due to a similar molecular skeleton. Nevertheless, other health benefits of triterpenoids have been demonstrated by many researchers [9,26,36].
3. Materials and Methods
3.1. Reagents and Standards
HPLC-grade acetonitrile was purchased from Merck (Darmstadt, Germany). HPLC-grade formic acid, DPPH and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (Steinheim, Germany). HPLC-grade water was purified with a Millipore Water Purification System (Bedford, MA, USA). The standards arbutin, (+)-catechin, (−)-epicatechin, neochlorogenic acid, cryptochlorogenic acid, chlorogenic acid, rutin, oleanolic, ursolic, p-coumaric, caffeic and quinic acid were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Luteoloside, kaempferol-3-O-rutinoside, isorhamnetin-3-O-rutinoside, isochlorogenic acid A, isochlorogenic acid B, isochlorogenic acid C and hyperoside were bought from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). Betulinic, pomolic, corosolic and maslinic acid were purchased from Wuhan ChemFaces Biochemical Co., Ltd. (Wuhan, China). The purity of each reference standard was determined to be higher than 98% by HPLC.
3.2. Materials
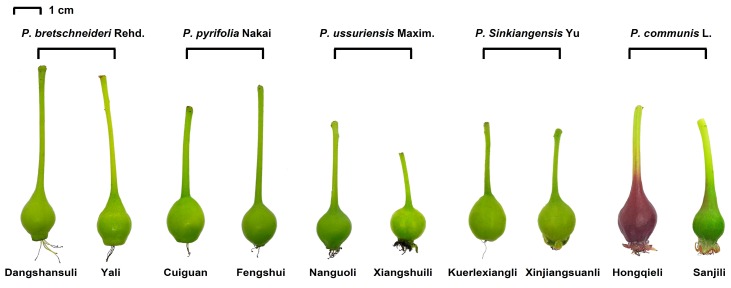
According to the classification system of pears from previous literature [32], 10 pear varieties from five typical Pyrus species were selected and their fruitlets were hand-thinned about 20 days after full bloom at pear orchards in various regions of China (Figure 4). For two P. pyrifolia Nakai, thinned fruits of ‘Fengshui’ (FS) and ‘Cuiguan’ (CG) were obtained individually from the pear orchard of Nanjing Agricultural University (Jiangsu, China). For two P. bretschneideri Rehd., thinned fruits of ‘Dangshansuli’ (DSSL) were obtained from a commercial orchard in Gaoyou (Jiangsu, China), and those of ‘Yali’ (YL) were obtained from the Shijiazhuang Fruit Institute (Hebei, China). For two P. ussuriensis Maxim., thinned fruits of ‘Nanguoli’ (NGL) and ‘Xiangshuili’ (XSL) were obtained individually from the Liaoning Fruit Institute (Liaoning, China). For two P. communis L., thinned fruits of ‘Starkrimson’ (Hongqieli, HQL) and ‘Docteur Jules Guyot’ (Sanjili, SJL) were obtained individually from the Yantai Agricultural Research Academy (Shandong, China). For two P. sinkiangensis Yu, thinned fruits of ‘Kuerlexiangli’ (KELXL) and ‘Xinjiangsuanli’ (XJSL) were obtained individually from the Xiangli Fruit Institute (Xinjiang, China).
Figure 4.
Visual appearance of thinned young fruits from ten pear varieties at 20 days after full bloom.
In the course of the measurements, five trees for each variety, which were randomly located and had comparable growth vigor and tree age, were selected to ensure that the samples were representative. For each variety, 150 fruitlets (30 samples per tree) of similar size and with an absence of defects were randomly and manually picked. After collection, the fruits were immediately transferred to the laboratory, washed with water, cut and directly frozen in liquid nitrogen, then crushed to a homogeneous powder with a laboratory mill in liquid nitrogen (Jingxin Industrial Development Co., Shanghai, China). The powders were kept at −80 °C until analysis.
3.3. Extract Preparation
Some studies have proved that an aqueous alcohol system and sonication could improve the extraction of polyphenolics [37]. The pear extracts were prepared as described in previous literature [38], with some modifications. Briefly, the powdered frozen pear samples were extracted with mixed methanol/water (80:20, v/v) and vortexed for 30 s, then sonicated (300 W, 50 kHz) for 30 min at 30 °C. The ratio of frozen powders and solvents was 1 g per 25 mL. The sample tube was shaken at 5-min intervals during sonication to resuspend the sample. This method has been demonstrated to be adequate for complete extraction. Next, the slurry was centrifuged at 20,000× g (Microfuge 20, Beckman Coulter Inc., CA, USA) for 15 min, and the supernatant was filtered through a 0.22 µm membrane and kept at 4 °C prior to analysis.
3.4. UPLC-Q TRAP-MS/MS Analysis of Polyphenols and Triterpenoids
A Shimadzu UPLC system (Shimadzu, Kyoto, Japan), interfaced with an AB Sciex 4500 Q trap mass spectrometer (AB Sciex, Foster City, CA, USA), installed with an electrospray ionization interface and a six-port/two-channel valve, was used for UPLC-Q TRAP-MS/MS analysis. The Shimadzu UPLC system consisted of two LC-20ADXR solvent delivery units, a Shimadzu LC-20AD pump, a SIL-20ACXR autosampler, a CTO-20AC column oven, a DGU-20A3R degasser, and a CBM-20A controller. AB Sciex Analyst Software Package Version 1.6.3 was applied to control the entire system, data acquisition and processing.
3.4.1. UPLC Analysis
Chromatographic separation of bioactive components was accomplished with a Waters Acquity UPLC HSS T3 C18 column (2.1 mm × 100 mm, 1.8 μm, Milford, MA, USA) [11]. The temperature of the sample was set to 4°C, and the column temperature was maintained at 30 °C. The injection volume of the auto-sampler was set at 1 μL. For polyphenol composition analysis, the mobile phase consisted of 0.1% (v/v) aqueous formic acid (A) and acetonitrile (B) at a flow rate of 0.4 mL/min. The gradient elution program was as follows: 0–3 min, 1%–15% B; 3–11 min, 15%–40% B; 11–13 min, 40% B; 13–13.1 min, 40%–1% B; 13.1–15 min, 1% B. Before triterpenoid analysis, most pear extract solutions mentioned above were diluted 10 times with mixed methanol/water (80:20, v/v), whereas the extracted sample was diluted four times for ‘Hongqieli’ and 20 times for ‘Xiangshuili’ individually. Chromatographic analysis was then carried out through isocratic elution with methanol/water/ammonium acetate (81:19:0.1, v/v) at a flow rate of 0.4 mL/min.
3.4.2. Identification of Bioactive Compounds in IDA Mode
The mass spectrometer was operated using enhanced MS scanning and enhanced product ion scanning in negative ion mode to identify polyphenolic and triterpenoid compounds in the pears. Enhanced product ion scans were triggered from the IDA method. The criterion for IDA was set for the two most intense ions after each dynamic survey scan spectrum with an intensity threshold of 5000 cps, and former target ions were never excluded during scanning. The parameters for enhanced product ion scanning were as follows: declustering potential (DP), 100 V; collision energy (CE), 35 eV; and collision energy spread (CES), 15 V. Nitrogen was used as the nebulizer, curtain, heater and collision gas. The ion source temperature (TEM) was raised to 500 °C, and the ion-spray voltages (IS) were maintained at 4500 V in negative ion mode. The gas settings were as follows: nebulizer gas (GS1) at 50 psi, heater (GS2) at 50 psi, curtain gas (CUR) at 30 psi and collision gas (CAD) at high level. The dwell time was fixed at 20 ms for each ion transition. Fragments formed in the enhanced product ion scans were detected in the mass range of m/z 100–1500 by using dynamic fill and a scan rate of 4000 Da/s.
3.4.3. Quantification of Bioactive Compounds in MRM Mode
For quantification of bioactive components, mass spectrometric analysis was performed in negative mode by using electrospray ionization. The peak areas of all analytes were acquired in MRM mode for quantification. Optimization of MS parameters for each selected mass transition was carried out through direct infusion of a mixed standard solution and is summarized in Table S1. The other operating parameters were as follows: TEM, 500 °C; IS, 4500 V; GS1, 50 psi; GS2, 50 psi; and CUR, 30 psi. Then, calibration curves were determined experimentally for available standards. Because of a lack of standards for some tentatively identified compounds, their Q1 and Q3 were both set as quasi-molecular ions in MRM mode, and the DP values were optimized at 70, 100, 135, and 160 V. Their calibration curves were determined with some other available standards. Caffeic acid derivatives were expressed as caffeic acid, p-coumaric acid derivatives were expressed as p-coumaric acid, procyanidins were expressed as (−)-epicatechin, luteolin derivatives were expressed as luteoloside, isorhamnetin derivatives were expressed as isorhamnetin-3-O-rutinoside, kaempferol derivatives were expressed as kaempferol-3-O-rutinoside, quercetin derivatives and other flavonoid glycosides were expressed as rutin, and triterpenic acids were expressed as ursolic acid. All determinations were performed in triplicate.
3.4.4. Validation of Methodology
The analytical performance of the methodology used for the quantification of bioactive compounds was investigated, including linearity, LOQs, LODs, stability, precision and repeatability. Serial calibration samples were prepared in methanol/water (80:20, v/v). The linearity was investigated with more than six concentration levels for each analyte, and each concentration was measured in triplicate. LOQs and LODs corresponded to the concentrations at S/N ratios of ~10 and 3, respectively.
To confirm instrument precision, the intra- and inter-day variations on the basis of relative standard deviations were assessed by using the calibration sample at a moderate concentration. For intra-day variability assays, the sample was measured in six replicates within one day, whereas the sample was examined in triplicate per day for three consecutive days to conduct inter-day assays.
Method repeatability was evaluated with injections of six extracts prepared independently from four selected samples (‘Dangshansuli’, ‘Nanguoli’, ‘Hongqieli’ and ‘Sanjili’) for polyphenol analysis and from two selected samples (‘Yali’ and ‘Nanguoli’) for triterpenoid analysis. Moreover, the tested sample solution was deposited at 4 °C, and the stability study of the pear extract was assessed at different time intervals (0, 12, 24, 48, and 72 h).
3.5. Analysis of Antioxidant Capacity
The antioxidant capacities of thinned young pears were measured through bleaching of the DPPH radical, as reported previously [2], with some modifications. Briefly, fresh DPPH stock solution was prepared by dissolving 19.7 mg DPPH in 100 mL of methanol and was then sealed and stored at 4 °C. One milliliter of different concentrations of pear extracts was mixed with 1 mL of DPPH solution. The mixture was shaken vigorously and incubated in the dark for 30 min at 37 °C, and the absorbance was then measured at 517 nm. All determinations were performed in triplicate.
3.6. Statistical Analysis
Data are shown as the means of three independent determinations ± standard deviation. Hierarchical clustering analysis of ten pears was performed using the Statistical Package for the Social Science (SPSS) software version 22.0 (SPSS, Chicago, IL, USA) and the data was standardized before analysis. Multivariate correlation analysis was carried out in SPSS statistics software for evaluation of the spectrum–effect relationships between the content of each component and the total antioxidant capacity in thinned pears.
4. Conclusions
The aim of this study was to investigate the chemical composition and antioxidant potential of thinned pears. From five typical Pyrus species, ten pear varieties were selected. Then, 102 polyphenols and 16 triterpenoids were identified from these thinned young fruits through UPLC-Q TRAP-MS/MS in IDA mode, and detailed quantification of these compounds was accomplished within a short time by UPLC-MS/MS in MRM mode. To the best of our knowledge, this is the first report on simultaneous qualitative and quantitative analysis of 16 triterpenoid compounds in pears. According to the identified components, the most abundant polyphenolic constituents present were chlorogenic acid, quinic acid and arbutin, and ursolic acid was the dominant triterpenoid in young pears. Subsequently, the antioxidant capacities of these young pears were determined with DPPH assays, and a correlation analysis between total antioxidant activity and each component was performed. Twenty-seven polyphenolic compounds, especially chlorogenic acid and most of the flavan-3-ols, were found to be the principal antioxidant contributors. In addition, the bioactive compound content and antioxidant capacity of thinned pears were observed to be significantly higher in young pears than mature pears. These results may draw special attention to the potential health benefits of discarded thinned young pears as a natural source of bioactive components such as polyphenolics and triterpenoids. The health benefits of these fruit by-products are also promising and should be investigated further.
Acknowledgments
The authors sincerely thank Yingtao wang in the Shijiazhuang Fruit Institute (Hebei, China), Shoufeng Sha in the Liaoning Fruit Institute (Liaoning, China), and Zishun He in the Xiangli Fruit Institute (Xinjiang, China), for their kind assistance with sample collection.
Supplementary Materials
The following are available online, Figure S1: Representative UPLC-Q TRAP-MS/MS total ion chromatograms of thinned young pears in negative ion mode. Figure S2: Representative MRM chromatograms for 102 polyphenols and 16 triterpenoids in thinned pears. Table S1: The optimized MS parameters at MRM mode and summary of the intra- and inter-day precision for available analytes. Table S2: Repeatability and stability for 102 polyphenols in representative pear extracts. Table S3: Repeatability and stability for 16 triterpenoids in representative pear extracts. Table S4: The correlation coefficients between antioxidant activity and chemical compounds in thinned young pears.
Author Contributions
Conceptualization, S.Z.; Data curation, S.T.; Formal analysis, L.S.; Funding acquisition, L.S. and S.Z.; Investigation, L.S.; Methodology, L.S.; Project administration, S.Z.; Resources, S.T.; Supervision, S.T.; Validation, L.S.; Writing—original draft, L.S.; Writing—review and editing, S.T. All the authors read and approved the final manuscript.
Funding
This research work was funded by the National Natural Science Foundation of China (31830081, 31801825, 31772276, 31672105), the Fundamental Research Funds for the Central Universities (KJQN201924), the China Postdoctoral Science Foundation (2018M632316), the Earmarked Fund for China Agriculture Research System (CARS-28), the National High-tech R&D Program of China (863 Program: 2011AA10020602) and the Independent Innovation Program of Jiangsu Province (CX(18)3029).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the thinned young pears reported in this study are available from the authors.
References
- 1.Kolniak-Ostek J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.) Food Chem. 2016;203:491–497. doi: 10.1016/j.foodchem.2016.02.103. [DOI] [PubMed] [Google Scholar]
- 2.Li X., Wang T., Zhou B., Gao W., Cao J., Huang L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.) Food Chem. 2014;152:531–538. doi: 10.1016/j.foodchem.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Wouters N., De Ketelaere B., Deckers T., De Baerdemaeker J., Saeys W. Multispectral detection of floral buds for automated thinning of pear. Comput. Electron. Agric. 2015;113:93–103. doi: 10.1016/j.compag.2015.01.015. [DOI] [Google Scholar]
- 4.Mazzola M. Elucidation of the microbial complex having a causal role in the development of apple replant disease in Washington. Phytopathology. 1998;88:930–938. doi: 10.1094/PHYTO.1998.88.9.930. [DOI] [PubMed] [Google Scholar]
- 5.Cho J.Y., Lee S.H., Kim E.H., Yun H.R., Jeong H.Y., Lee Y.G., Kim W.S., Moon J.H. Change in chemical constituents and free radical-scavenging activity during Pear (Pyrus pyrifolia) cultivar fruit development. Biosci. Biotechnol. Biochem. 2015;79:260–270. doi: 10.1080/09168451.2014.973362. [DOI] [PubMed] [Google Scholar]
- 6.Wang T., Li X., Zhou B., Li H., Zeng J., Gao W. Anti-diabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.) J. Funct. Foods. 2015;13:276–288. doi: 10.1016/j.jff.2014.12.049. [DOI] [Google Scholar]
- 7.Brahem M., Renard C.M.G.C., Eder S., Loonis M., Ouni R., Mars M., Le Bourvellec C. Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res. Int. 2017;95:125–133. doi: 10.1016/j.foodres.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Simirgiotis M., Quispe C., Bórquez J., Areche C., Sepúlveda B. Fast detection of phenolic compounds in extracts of easter pears (Pyrus communis) from the atacama desert by ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC–Q/Orbitrap/MS/MS) Molecules. 2016;21:92. doi: 10.3390/molecules21010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muffler K., Leipold D., Scheller M.C., Haas C., Steingroewer J., Bley T., Neuhaus H.E., Mirata M.A., Schrader J., Ulber R. Biotransformation of triterpenes. Process Biochem. 2011;46:1–15. doi: 10.1016/j.procbio.2010.07.015. [DOI] [Google Scholar]
- 10.Lu Z., Wang Q., Wang M., Fu S., Zhang Q., Zhang Z., Zhao H., Liu Y., Huang Z., Xie Z., et al. Using UHPLC Q-Trap/MS as a complementary technique to in-depth mine UPLC Q-TOF/MS data for identifying modified nucleosides in urine. J. Chromatogr. B. 2017;1051:108–117. doi: 10.1016/j.jchromb.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Zuo Y. High-Performance Liquid Chromatography (HPLC): Principles, Procedures and Practices. Nova Science Publishers; New York, NY, USA: 2014. [Google Scholar]
- 12.Kasote D.M., Ghosh R., Chung J.Y., Kim J., Bae I., Bae H. Multiple reaction monitoring mode based liquid chromatography-mass spectrometry method for simultaneous quantification of brassinolide and other plant hormones involved in abiotic stresses. Int. J. Anal. Chem. 2016;2016 doi: 10.1155/2016/7214087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolniak-Ostek J. Identification and quantification of polyphenolic compounds in ten pear cultivars by UPLC-PDA-Q/TOF-MS. J. Food. Compost. Anal. 2016;49:65–77. doi: 10.1016/j.jfca.2016.04.004. [DOI] [Google Scholar]
- 14.Kolniak-Ostek J., Oszmiański J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS) Int. J. Mass Spectrom. 2015;392:154–163. doi: 10.1016/j.ijms.2015.10.004. [DOI] [Google Scholar]
- 15.Kang J., Price W.E., Ashton J., Tapsell L.C., Johnson S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016;211:215–226. doi: 10.1016/j.foodchem.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 16.Spínola V., Pinto J., Castilho P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015;173:14–30. doi: 10.1016/j.foodchem.2014.09.163. [DOI] [PubMed] [Google Scholar]
- 17.Fotirić Akšić M.M., Dabić D.Č., Gašić U.M., Zec G.N., Vulić T.B., Tešić Ž.L., Natić M.M. Polyphenolic profile of pear leaves with different resistance to pear psylla (Cacopsylla pyri) J. Agric. Food Chem. 2015;63:7476–7486. doi: 10.1021/acs.jafc.5b03394. [DOI] [PubMed] [Google Scholar]
- 18.Kalisz S., Oszmianski J., Wojdylo A. Increased content of phenolic compounds in pear leaves after infection by the pear rust pathogen. Physiol. Mol. Plant Pathol. 2015;91:113–119. doi: 10.1016/j.pmpp.2015.07.001. [DOI] [Google Scholar]
- 19.Lin L.Z., Harnly J.M. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.) J. Agric. Food Chem. 2008;56:9094–9101. doi: 10.1021/jf8013487. [DOI] [PubMed] [Google Scholar]
- 20.Boudjelal A., Henchiri C., Siracusa L., Sari M., Ruberto G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia. 2012;83:286–292. doi: 10.1016/j.fitote.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Gattuso G., Caristi C., Gargiulli C., Bellocco E., Toscano G., Leuzzi U. Flavonoid glycosides in bergamot juice (Citrus bergamia Risso) J. Agric. Food Chem. 2006;54:3929–3935. doi: 10.1021/jf060348z. [DOI] [PubMed] [Google Scholar]
- 22.Benayad Z., Gomez-Cordoves C., Es-Safi N.E. Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seeds by HPLC-DAD-ESI/MS analysis. Int. J. Mol. Sci. 2014;15:20668–20685. doi: 10.3390/ijms151120668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao J., Peng L.Q., Xu J.J. Microcrystalline cellulose based matrix solid phase dispersion microextration for isomeric triterpenoid acids in loquat leaves by ultrahigh-performance liquid chromatography and quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2016;1472:16–26. doi: 10.1016/j.chroma.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 24.Jóźwiak A., Jóźwiak G., Waksmundzka-Hajnos M. Simultaneous HPLC determination of pomolic, ursolic and euscaphic/tormentic acids in roots and rhizomes of various Potentilla species. Acta Chromatogr. 2014;26:97–110. doi: 10.1556/AChrom.26.2014.1.14. [DOI] [Google Scholar]
- 25.Verardo G., Gorassini A., Ricci D., Fraternale D. High triterpenic acids production in callus cultures from fruit pulp of two apple varieties. Phytochem. Analysis. 2017;28:5–15. doi: 10.1002/pca.2638. [DOI] [PubMed] [Google Scholar]
- 26.Chen B., Long P., Sun Y., Meng Q., Liu X., Cui H., Lv Q., Zhang L. The chemical profiling of loquat leaf extract by HPLC-DAD-ESI-MS and its effects on hyperlipidemia and hyperglycemia in rats induced by a high-fat and fructose diet. Food Funct. 2017;8:687–694. doi: 10.1039/C6FO01578F. [DOI] [PubMed] [Google Scholar]
- 27.Grigoras C.G., Destandau E., Fougère L., Elfakir C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind. Crop. Prod. 2013;49:794–804. doi: 10.1016/j.indcrop.2013.06.026. [DOI] [Google Scholar]
- 28.Galvis Sánchez A.C., Gil-Izquierdo A., Gil M.I. Comparative study of six pear cultivars in terms of their phenolic and vitamin C contents and antioxidant capacity. J. Sci. Food Agric. 2003;83:995–1003. doi: 10.1002/jsfa.1436. [DOI] [Google Scholar]
- 29.Ahmad N., Zuo Y., Lu X., Anwar F., Hameed S. Characterization of free and conjugated phenolic compound in fruits of selected wild plants. Food Chem. 2016;190:80–89. doi: 10.1016/j.foodchem.2015.05.077. [DOI] [PubMed] [Google Scholar]
- 30.Willems J.L., Low N.H. Structural identification of compounds for use in the detection of juice-to-juice debasing between apple and pear juices. Food Chem. 2018;241:346–352. doi: 10.1016/j.foodchem.2017.08.104. [DOI] [PubMed] [Google Scholar]
- 31.Du G.R., Li M.J., Ma F.W., Liang D. Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. 2009;113:557–562. doi: 10.1016/j.foodchem.2008.08.025. [DOI] [Google Scholar]
- 32.Zhang S. Pears. China Agriculture Press; Beijing, China: 2013. (In Chinese) [Google Scholar]
- 33.Cinkilic N., Cetintas S.K., Zorlu T., Vatan O., Yilmaz D., Cavas T., Tunc S., Ozkan L., Bilaloglu R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013;53:359–363. doi: 10.1016/j.fct.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Hong Y., Qiao Y., Lin S., Jiang Y., Chen F. Characterization of antioxidant compounds in Eriobotrya fragrans Champ leaf. Sci. Hortic. 2008;118:288–292. doi: 10.1016/j.scienta.2008.06.018. [DOI] [Google Scholar]
- 35.Bai X., Lai T., Zhou T., Li Y., Li X., Zhang H. In vitro antioxidant activities of phenols and oleanolic acid from mango peel and their cytotoxic effect on A549 cell line. Molecules. 2018;23:1395. doi: 10.3390/molecules23061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lia Z.J., Wan C.P., Cai L., Li S.Q., Zheng X., Qi Y., Dong J.W., Yin T.P., Zhou Z.X., Tan N.H., et al. Terpenoids with cytotoxic activity from the branches and leaves of Pyrus pashia. Phytochem. Lett. 2015;13:246–251. doi: 10.1016/j.phytol.2015.06.015. [DOI] [Google Scholar]
- 37.Wang C., Zuo Y. Ultrasound-assisted hydrolysis and gas chromatography-mass spectrometric determination of phenolic compounds in cranberry products. Food Chem. 2011;128:562–568. doi: 10.1016/j.foodchem.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 38.Li X., Zhang J.-Y., Gao W.-Y., Wang Y., Wang H.-Y., Cao J.-G., Huang L.-Q. Chemical composition and anti-inflammatory and antioxidant activities of eight pear cultivars. J. Agric. Food Chem. 2012;60:8738–8744. doi: 10.1021/jf303235h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.