Abstract
Nuclear factor erythroid 2-related factor 2 (NRF2) regulates cytoprotective antioxidant processes. In this study, the prognostic potential of NRF2 and its interactions with the estrogen receptor α (ERα) in ovarian cancer cells was investigated. NRF2 and ERα protein expression in ovarian cancer tissue was analyzed as well as mRNA expression of NRF2 (NFE2L2) and ERα (ESR1) in four ovarian cancer and one benign cell line. NFE2L2 silencing was carried out to evaluate a potential interplay between NRF2 and ERα. Cytoplasmic NRF2 expression as inactive form had significantly higher expression in patients with low-grade histology (p = 0.03). In the serous cancer subtype, high cytoplasmic NRF2 expression (overall survival (OS), median 50.6 vs. 29.3 months; p = 0.04) and high ERα expression (OS, median 74.5 vs. 27.1 months; p = 0.002) was associated with longer overall survival as well as combined expression of both inactive cytoplasmic NRF2 and ERα in the whole cohort (median 74.5 vs. 37.7 months; p = 0.04). Cytoplasmic NRF2 expression showed a positive correlation with ERα expression (p = 0.004). NFE2L2 was found to be highly expressed in the ovarian cancer cell lines OVCAR3, UWB1.289, and TOV112D. Compared with the benign cell line HOSEpiC, ESR1 expression was reduced in all ovary cancer cell lines (all p < 0.001). Silencing of NFE2L2 induced a higher mRNA expression of ESR1 in the NFE2L2 downregulated cancer cell lines OVCAR3 (p = 0.003) and ES2 (p < 0.001), confirming genetic interactions of NRF2 and ERα. In this study, both inactive cytoplasmic NRF2 and high ERα expression were demonstrated to be associated with improved survival in ovarian cancer patients. Further understanding of interactions within the estradiol–ERα–NRF2 pathway could better predict the impact of endocrine therapy in ovarian cancer.
Keywords: estrogen receptor alpha, nuclear factor erythroid 2-related factor 2, ovarian cancer, immunohistochemistry
1. Introduction
Ovarian cancer is the eighth most frequent cause of cancer death among women and the most lethal gynecological malignancy [1]. Relative five-year survival is less than 50% for patients with epithelial ovarian carcinoma (EOC) [2]. Main reasons for poor prognosis are insufficient screening methods, late stage detection, and resistance to chemotherapy later in the clinical course. As most patients have advanced stage disease, recommended therapy consists of cytoreductive surgery and platinum-based chemotherapy which might be combined with antiangiogenic bevacizumab. Residual disease after initial debulking surgery is the most important prognostic factor being influenced by treating physicians, while further clinical and pathological prognostic factors include the degree of differentiation, the International Federation of Gynecology and Obstetrics (FIGO) stage, and histological subtype [3,4,5,6]. With serous, mucinous, endometrioid, and clear cell histology, invasive EOC exhibits several histopathological subtypes that are phenotypically, molecularly, and etiologically distinct [7]. The association between tumor biomarker expression and survival varies substantially between subtypes and can be distinguished in overall analyses of all EOCs [8,9].
According to current investigations, the occurrence of EOC seems to be related to oxidative stress [9]. By activating the nuclear factor erythroid-2-related factor 2 (NRF2), a relevant regulator of antioxidant and cytoprotective genes, both healthy and tumor cells can cope with oxidative stress. NRF2 is ubiquitously expressed at low levels in all human organs. As NRF2 regulates a major cellular defense mechanism, tight regulation is crucial to maintain cellular homeostasis. High constitutive levels of NRF2 have been described in different tumors or cancer cell lines [10,11,12,13,14]. Overexpression of NRF2 might protect cancer cells from the cytotoxic effects of anticancer therapies, resulting in resistance for chemo- or radiotherapy [15,16].
So far, the role of estrogen in EOC is still debated [17]. While application of exogenous hormones for menopause-related symptoms could be associated with an increased risk of EOC [18], a protective effect of oral contraceptives has been described. The estrogen receptor (ER) is expressed in two isoforms, the ERα and ERß [19]. ERα mediates the effects of female steroid hormones on proliferation and apoptosis of EOC cells, and immunohistochemical assessment of ER status is routinely done for the clinical management of breast cancer [19]. Molecular and cell biological interactions between NRF2 and ERα have been reported so far [16,20]. The aryl hydrocarbon receptor and ERα differentially modulate NRF2 transactivation in MCF-7 breast cancer cells [16]. Furthermore, studies show an important crosstalk between NRF2 and ERα in neurophysiological processes [16,20].
To better understand these effects in EOC, we first assessed the prognostic influence of NRF2 and ERα in various subtypes of EOC. To understand the interaction of NRF2 and Erα on a molecular level, we investigated the expression and their correlation in vitro.
2. Results
2.1. NRF2/ERα Expression Correlates with Cinical and Pathological Data
Nuclear staining of NRF2 was technically successful in 145 of 156 cases (93%) with positive staining in 144 of 145 cases (99%). Cytoplasmic staining of NRF2 was evaluable with technically adequate staining in 139 of 156 cases (89%) (Figure 1 and Figures S1 and S2) and NRF2 expression was observed in all these 139 specimens (100%). Median (range) immunoreactivity scores (IRS) for NRF2 in nuclei and cytoplasm were 8 (2,12) and 8 (4,12), respectively.
Figure 1.
Detection of nuclear factor erythroid-2-related factor 2 (NRF2) (A1, B1) and estrogen receptor (ER)α (A2, B2) with immunohistochemistry. High (A1) and low (B1) cytoplasmic NRF2 stains in serous subtype correspond with high (A2) and low (B2) ERα stains, respectively. NRF2 shows faint staining in the nucleus in both cases (A1, B1).
NRF2 expression displayed correlations to clinical and pathological data (Table 1). NRF2 staining in both cytoplasm and nucleus was different between the histological subtypes (p = 0.001 and p = 0.02, respectively) with low nuclear NRF2 expression in serous, clear cell, and endometrioid histology and high expression in the mucinous subtype. In comparison, the strongest and weakest cytoplasmic NRF2 staining was found in the serous and clear cell subtypes, respectively. Cytoplasmic NRF2 expression had significantly higher expression in patients with low-grade histology (p = 0.03), and low nuclear NRF2 expression was associated with age (p = 0.045) (Table 1).
Table 1.
Expression profile of NRF2 staining regarding clinical and pathological characteristics.
| Parameters | N | Nuclear NRF2 Expression | p | N | Cytoplasmic NRF2 Expression | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Low | High | Negative | Low | High | |||||
| Histology | ||||||||||
| Serous | 103 | 0 | 87 | 16 | 0.02 | 98 | 0 | 54 | 44 | 0.001 |
| Clear cell | 11 | 1 | 7 | 3 | 11 | 0 | 11 | 0 | ||
| Endometrioid | 20 | 0 | 18 | 2 | 19 | 0 | 12 | 7 | ||
| Mucinous | 11 | 0 | 3 | 8 | 11 | 0 | 7 | 4 | ||
| Lymph node | ||||||||||
| pN0/X | 96 | 0 | 76 | 20 | NS | 93 | 0 | 59 | 34 | NS |
| pN1 | 49 | 1 | 39 | 9 | 46 | 0 | 25 | 21 | ||
| Distant Metastasis | ||||||||||
| pM0/X | 141 | 1 | 112 | 28 | NS | 135 | 0 | 83 | 52 | NS |
| pM1 | 4 | 0 | 3 | 1 | 4 | 0 | 1 | 3 | ||
| Grading | ||||||||||
| Low | 33 | 0 | 25 | 8 | NS | 33 | 0 | 16 | 17 | 0.03 |
| High | 100 | 1 | 83 | 16 | 95 | 0 | 64 | 31 | ||
| FIGO | ||||||||||
| I/II | 41 | 0 | 31 | 10 | NS | 40 | 0 | 24 | 16 | NS |
| III/IV | 99 | 0 | 81 | 18 | 94 | 0 | 56 | 38 | ||
| Age | ||||||||||
| ≤60 years | 77 | 1 | 56 | 20 | 0.045 | 75 | 0 | 43 | 32 | NS |
| >60 years | 68 | 0 | 59 | 9 | 64 | 0 | 41 | 23 | ||
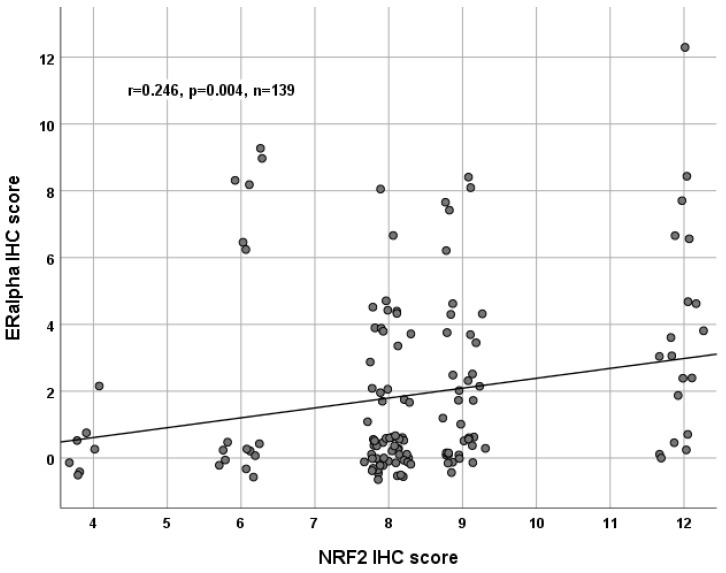
ERα staining was successfully performed in all 156 cases (100%), and ERα expression was observed in 70 of 156 (45%) specimens with a median (range) IRS of 4 (1,12) (Figure 1 and Figure S1). There was no significant difference in the ERα expression comparing all histological subtypes (p = 0.21). Analysis of clear cell and endometrioid ovarian cancer subtypes revealed nearly significant upregulation (p = 0.05). Analyzing the grading, there were no significant differences in general, and low-graded patients showed significantly higher ERα expression compared to high-graded patients (p = 0.028). All other parameters, such as FIGO, lymph node involvement (pN), and distant metastasis (pM), showed no significant differences in the ERα expression. NRF2 cytoplasmic expression correlated with ERα expression (p = 0.004, Table 2 and Figure 1 and Figure 2).
Table 2.
Correlation analysis.
| Staining | NRF2 Nucleus | NRF2 Cytoplasm | ERα |
|---|---|---|---|
| NRF2 Nucleus | |||
| cc | 1.000 | 0.013 | −0.019 |
| p | 0.88 | 0.82 | |
| n | 146 | 138 | 146 |
| NRF2 Cytoplasm | |||
| cc | 0.013 | 1.000 | 0.246 |
| p | 0.88 | 0.004 | |
| n | 138 | 139 | 139 |
| ERα | |||
| cc | −0.019 | 0.246 | 1.000 |
| p | 0.82 | 0.004 | |
| n | 146 | 139 | 156 |
Immunoreactivity scores (IRS) of NRF2 and ERα staining in different compartments was correlated to each other using Spearman’s correlation analysis. cc = correlation coefficient, p = two-tailed significance, n = number of patients.
Figure 2.
Correlation analysis of NRF2 and ERα in ovarian cancer tissue (n = 139). A significant correlation of cytoplasmic NRF2 expression with ERα expression was noted. For better visualization, dots have been jittered.
2.2. High NRF2/ERα Expression is Associated with Improved Overall Survival
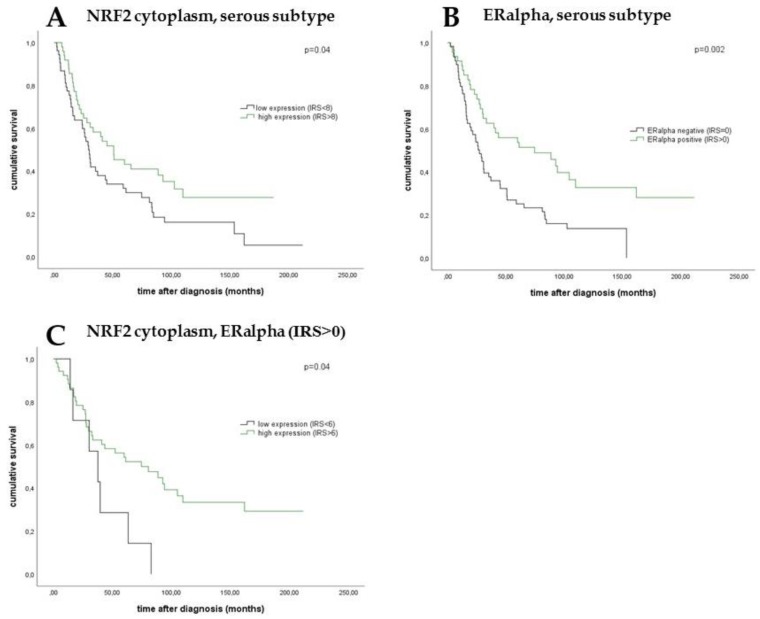
The median age of the patients was 58.7 (standard deviation (SD) of 31.4) years with a range of 31–88 years. Median overall survival of the EOC patients was 34.4 (SD 57.8) months. Cytoplasmic NRF2 expression in the serous cancer subtype was associated with longer overall survival (Figure 3, median 50.6 vs. 29.3 months; p = 0.04) as it was noted for ERα expression (Figure 3, median 74.5 vs. 27.1 months; p = 0.002). Improved OS was also seen for patients with combined and high expression of both NRF2 and ERα in the cytoplasm comparing all histological subtypes (Figure 3, median 74.5 vs. 37.7 months; p = 0.04).
Figure 3.
Kaplan–Meier estimates of NRF2 expression, ERα expression, and combined NRF2 and ERα expression were analyzed. In the serous subtype, patients with a high cytoplasmic expression of NRF2 showed a significantly increased overall survival compared with patients with a low cytoplasmic expression (A). In addition, high ERα expression was associated with significantly better overall survival in serous ovarian cancer compared with patients with a low ERα expression (B). Patients with combined high NRF2 expression in the cytoplasm and ERα expression in epithelial ovarian carcinoma (EOC) had significantly increased overall survival compared with those with low cytoplasmic expression and ERα expression (C).
2.3. Clinical and Pathological Parameters are Independent Prognostic Factors
Cancer grading, the FIGO classification, and patients’ age were independent prognostic factors in the present cohort (Table 3). In contrast, prognostic impact of histological subtype, NRF2, and/or ERα staining/expression was not significant.
Table 3.
Multivariate analysis.
| Covariate | Coefficient (bi) | [HR Exp(bi)] | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Histology (serous vs. other) | −0.108 | 0.898 | 0.678 | 1.188 | 0.45 |
| Grade (low vs. high) | 0.519 | 1.680 | 1.211 | 2.332 | 0.002 |
| FIGO (I, II vs. III, IV) | 0.722 | 2.058 | 1.421 | 2.979 | 0.000 |
| Patients’ age (≤60 vs. >60 years) | 0.000 | 1.000 | 1.000 | 1.000 | 0.001 |
| NRF2 cytoplasmic/ ERα | −0.166 | 0.847 | 0.531 | 1.351 | 0.49 |
2.4. Downregulation of NFE2L2 Increases ESR1 Expression, Confirming Their Genetic Interaction
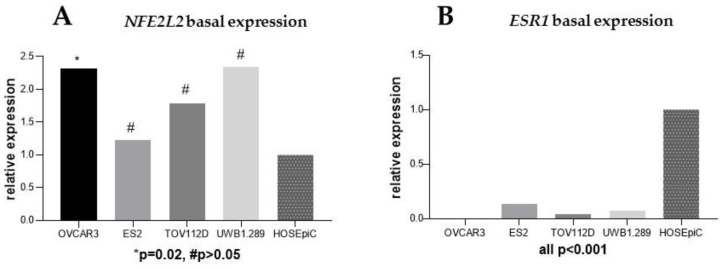
Basal expressions of both NFE2L2 and ESR1 were analyzed by qPCR in all four EOC cell lines and compared with a benign ovarian cell line (HOSEpiC). As shown in Figure 4 and compared to HOSEpiC, NFE2L2 expression increased 2-fold in both OVCAR3 (p = 0.02) and UWB1.289 (p = 0.08) and was 1.5-fold elevated in the TOV112D (p = 0.30) cell lines. In comparison, ESR1 expression was markedly reduced in all EOC cell lines compared to the benign ovarian cells (all p < 0.001).
Figure 4.
Basal gene expression of NFE2L2 (A) and ESR1 (B) in four ovarian cancer cell lines was compared to the expression in the benign ovarian cell line (HOSEpiC).
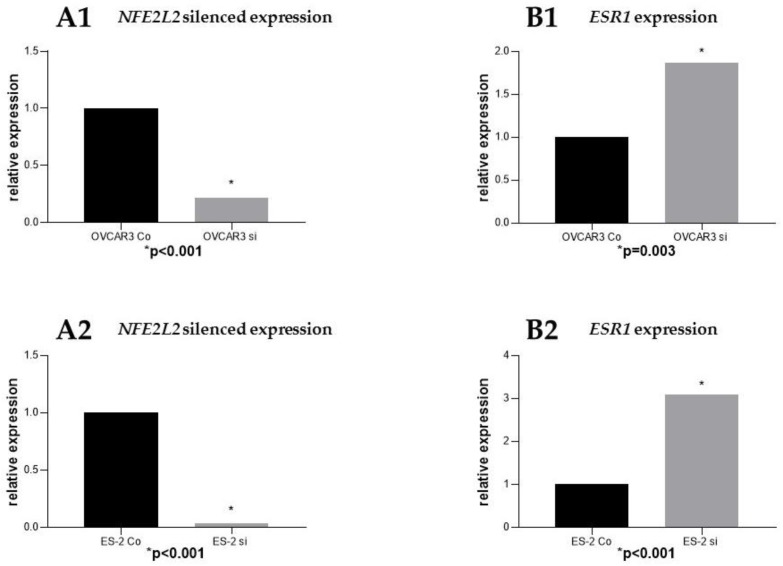
Following effective silencing of NFE2L2 with siRNA to evaluate the impact on ESR1 expression (Figure 5), an elevated expression of ESR1 in the NFE2L2 downregulated cancer cell lines OVCAR3 (p = 0.003) and ES2 was noted (p < 0.001).
Figure 5.
siRNA downregulation of NFE2L2 in the ovarian cancer cell lines OVCAR3 (A1) and ES2 (A2). ESR1 expression following NFE2L2 downregulation in both cell lines (B1, B2).
3. Discussion
This cell and molecular biological experimental study reveals that NRF2 expression differs in histologic subtypes of EOC, with the strongest cytoplasmic expression in the serous subtype. Cytoplasmic NRF2 expression had significantly higher expression in patients with low-grade histology. Patients with higher cytoplasmic NRF2 expression in the serous type confirmed to have a significantly improved OS. Moreover, we could reveal that the combination of cytoplasmic NRF2 and ERα expression was associated with significantly longer OS. Molecular testing in cell lines exhibited that the ESR1 gene was lower expressed in all four EOC cell lines, which could be upregulated by NFE2L2 silencing in the subsequently NFE2L2-downregulated cancer cell lines.
NRF2 has been traditionally considered as a tumor suppressor because its cytoprotective functions are deemed to be the main cellular defense mechanism against exogenous and endogenous insults, including xenobiotic and oxidative stress [21,22]. Under homeostatic conditions, NRF2 activation prevents excessive cellular damage produced by metabolic, xenobiotic, and oxidative stress [22]. NRF2 activation is thus important in cancer chemoprevention. Cancer chemoprevention mechanisms seem to be mediated through the Keap1–NRF2 pathway, and in experimental models, NRF2/Keap1 mutations are present at preneoplastic stages [23]. Further, NRF2-null mice are more prone to develop cancer in response to chemical and physical stimuli (nitrosamine, ultraviolet light, and aflatoxin) [17]. On the other hand, recent studies demonstrated that NRF2 hyperactivation may also create an environment favoring survival of normal as well as malignant cells, protecting them from apoptosis and senescence and against oxidative stress, chemotherapeutic agents, and radiotherapy [24,25]. Hence, the potential dual role of NRF2 in cancer may explain the described results below.
Our findings are in line with previous reports showing that nuclear or activated NRF2 expression is associated with upregulation of NRF2 target genes and poorer OS and disease-free survival (DFS), whereas patients with high cytoplasmic or inactive NRF2 expression displayed better OS and DFS [26]. Our evaluation of ER expression in the EOC tissue samples confirmed previous reports. In patients with EOC, the ER, especially ERα, is significantly associated with improved OS [8], grading, progression-free survival, and cause-specific survival, respectively [27]. There is a strong relationship between circulating sex hormones and female reproductive cancers (e.g., ovarian, breast, and endometrial cancers) [28]. Interestingly, estradiol may play a dual role in modulating NRF2 activity. On the one hand, its metabolites activate NRF2 via the generation of reactive oxygen species (ROS) (independent of the ER) [29]. While recent studies demonstrated that estradiol leads to an activation of NRF2 in a wide range of cell types [30,31], the estradiol effect was only noted on protein and not on mRNA levels, suggesting that the main effect of estradiol is based on NRF2 protein stabilization [32]. However, binding to ERα (dependent of ER) appears to be the mechanism for estradiol itself to inhibit the NRF2 downstream genes [9]. ERα, but not ERβ, interacts with NRF2 in an estradiol-dependent way and thereby represses NRF2-mediated transcription [33]. Thus, EOC patients with high tumor expression of ERα show a strong influence of the estradiol–ERα-dependent pathway, resulting in inactivated NRF2 and better survival rates. Otherwise, low ERα expression causes a dysbalance in favor of the estradiol–ERα-independent pathway with an activation of NRF2 (Figure 6). Studies show that other NRF2-associated factors also could play a crucial role in the above-described interaction. Glutathione S-transferase (GST), an NRF2 target gene, is modulated by miR-186 overexpression in OVCAR3 cells with consecutively increased sensitivity of ovarian cancer cells to paclitaxel [34]. Furthermore, it was described that the KEAP1–NRF2 pathway is important in ovarian cancer cell reaction to cigarette-smoke-induced ROS [35].
Figure 6.
Summary of the hypothesized interaction within the estradiol–ERα–NRF2 pathway: High expression of ERα leads to an induction of the estradiol–ERα-dependent pathway, resulting in transcriptionally inactive NRF2 (low nuclear, high cytoplasmic expression) and consecutively less impact on tumor growth. In contrast, low ERα expression favors the estradiol–ERα-independent pathway, with activation of NRF2 (high nuclear, low cytoplasmic expression) causing tumor progression.
Endocrine therapy in EOC has been considered as a potential approach in subgroups of patients with a specific tumor biology that responds to this therapy [36]. Hereby, the rationale for endocrine treatment is based on the high ER/PR IHC expression as a predictive marker [37]. A present prospective study demonstrated evidence for the usefulness of letrozole as an aromatase inhibitor in serous EOC [38]. Under the conditions described above, treatment with aromatase inhibitors could cause a prognostically beneficial predominance of the ERα–NRF2-dependent pathway. As revealed in the present investigation, a putative functional association of endocrine therapy and NRF2 underlines the relationship of NRF2/ERα, as confirmed by significant correlation of expression. In addition to the mentioned approach, further therapeutic strategies as interference of DNA repair mechanisms are of great interest to overcome treatment burden [39,40,41,42].
The retrospective design, the relatively small number of tissue samples evaluated, and the semiquantitative scoring method may critically be regarded as limitations of the submitted work. The data are hypothesis generating and further prospective studies with a larger patient collective and standardized immunohistochemical and molecular methods are warranted to gain more detailed and better insight into this research field.
However, despite these drawbacks, our analysis indicates for the first time a putative molecular role of the estradiol–ERα–NRF2 pathway as a basis for a better understanding of endocrine therapy in EOC.
4. Materials and Methods
4.1. Ethical Approval
The current study was approved by the Ethics Committee of the Ludwig-Maximilians-University, Munich, Germany (approval number 227-09) on 30 September 2009. All tissue samples used for this study were obtained from material from the archives of LMU Munich, Department Gynecology and Obstetrics, Ludwig-Maximilians-University, Munich, Germany, initially used for pathological diagnostics. The diagnostic procedures were completed before the current study was performed. During the analysis, the observers were fully blinded to patients’ data. The study was approved by the Ethics Committee of LMU Munich. All experiments were performed according to the standards of the Declaration of Helsinki (1975).
4.2. Patients and Specimens
Tissue samples of 156 patients who underwent surgery for EOC at the Department of Obstetrics and Gynecology, Ludwig-Maximillian’s-University Munich from 1990 to 2002 were analyzed in this study. Clinical data was obtained from the patients’ charts and follow up data from the Munich Cancer Registry. All samples had been formalin-fixated and paraffin-embedded (FFPE). Patients with benign or borderline tumors were excluded and no patients had adjuvant chemotherapy. Specialized pathologists for EOC examined and classified the samples for tumor grading—low (n = 38), high (n = 117)—and histological subtypes—serous (n = 110), endometrioid (n = 21), clear cell (n = 12), and mucinous (n = 13). Staging was performed using TNM and FIGO (WHO) classification: I (n = 35), II (n = 10,) III (n = 103), and IV (n = 3). Data on primary tumor extension were available in 155 cases—T1 (n = 40), T2 (n = 18), T3 (n = 93), and T4 (n = 4)—as well as data on lymph node involvement in 95 cases—N0 (n = 43), N1 (n = 52). Data on distant metastasis were available in nine cases—M0 (n = 3), M1 (n = 6).
4.3. Immunohistochemistry
Immunohistochemistry was performed as previously described by our lab [43]. For NRF2 staining, FFPE EOC samples were incubated with anti-NRF2 (Abcam, Cambridge, UK, rabbit, monoclonal, clone EP1808) at a final concentration of 5.93 µg/ml (1:100 dilution) for 1 h at room temperature. Afterwards, slides were incubated with isotype-matching MACH 3 Rabbit AP Polymer Detection (Biocare Medical, Pacheco, CA, USA, catalogue-number M3R533). The Permanent AP Red Kit (Zytomed Systems GmbH, Berlin, Germany, catalogue-number ZUC-001) was used as a chromogen. Slides were then counterstained with Gill´s hematoxylin (Vector Laboratories, Burlingame, CA, USA). System controls were included.
For the detection of ERα, resected EOC tissue samples were fixed in formalin and embedded in paraffin after surgery. ERα staining was performed by blocking slides with goat serum (1:100 dilution, Vectastain® ABC-Elite-Kit, Linaris, Dossenheim, Germany, catalogue-number PK-6101) for 30 min at room temperature. Subsequently, slides were incubated with anti-ERα primary antibody (1:400 dilutions, Abcam, Cambridge, UK, rabbit, monoclonal, clone EPR703(2)) for 16 h at 4 °C. Afterwards, slides were incubated with isotype-matching anti-rabbit IgG secondary antibody and avidin–biotin–peroxidase complex both for 30 min at room temperature, according to the Vectastain® ABC-Elite-Kit (Linaris, Dossenheim, Germany, catalogue-number PK-6101). All slides were washed twice in PBS for 2 min after every incubation step. 3,3’-Diaminobenzidine chromogen (DAB; Dako, Glostrup, Denmark, catalogue-number K3468) was used for visualization reaction. Slides were then counterstained with Mayer’s acidic hematoxylin (Waldeck-Chroma, Münster, Germany, catalogue number 2E-038) and dehydrated in an ascending series of alcohol followed by xylol. System controls were included.
4.4. Staining Evaluation and Statistical Analysis
All EOC specimens were examined with a Leitz (Wetzlar, Germany) photomicroscope and specific NRF2 and ERα immunohistochemical staining reaction was observed in the nuclei and cytoplasm of the cells. The intensity and distribution pattern of NRF2 and ERα staining was rated using the semiquantitative immunoreactivity score (IRS, Remmele’s score). To obtain the IRS result, the optional staining intensity (0 = no, 1 = weak, 2 = moderate, and 3 = strong staining) and the percentage of positive stained cells (0 = no staining, 1 = <10% of the cells, 2 = 11%–50% of the cells, 3 = 51%–80% of the cells, and 4 = >81%) were multiplied. Nuclear and cytoplasmic NRF2 staining was successfully performed in 145 (93%) and 139 (89%) of 156 EOC tissue specimens, respectively. Cut-off points for the IRSs were selected for cytoplasmic and nuclear NRF2 staining considering the distribution pattern of IRSs in the collective. Nuclear and cytoplasmic NRF2 staining were regarded as negative with an IRS of 0–2, as low with IRS of 4–8, and as high with IRS of ≥9. ERα staining was successfully performed in all 156 (100%) EOC specimens. Cellular ERα staining was considered as negative with an IRS of 0 and as positive with an IRS of >0.
Statistical analysis was performed using SPSS 25.0 (v25, IBM, Armonk, New York). Distribution of clinical pathological variables was evaluated with the chi-squared test. The Mann–Whitney U test was used to compare IRSs of NRF2 between different clinical and pathological subgroups. Correlations between findings of immunohistochemical staining were calculated using Spearman’s analysis. Survival times were analyzed by Kaplan–Meier (log-rank) estimates. To identify an appropriate cut-off, the ROC curve was drawn, which is considered as one of the most reliable methods for cut-off point selection. In this context, the ROC curve was a plot representing sensitivity on the y-axis and (1-specificity) on x-axis [44]. Consecutively, Youden’s index, defined as the maximum (sensitivity+specificity-1) [45], was used to find the optimal cut-off maximizing the sum of sensitivity and specificity [46,47]. For multivariate analyses, a Cox regression model was applied, with p-values less than 0.05 considered to be significant. Ct values of each gene were obtained with qPCR and the relative expressions were calculated using the 2−ΔΔCt formula. Statistical data was acquired using Graph Pad Prism 7.03 (v7, La Jolla, CA, USA).
4.5. Cell Lines
The human ovarian cancer cell lines OVCAR3 (serous), ES-2 (clear cell), TOV112D (endometrioid), and UWB1.289 (serous, BRCA1 negative) were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were maintained in culture in RPMI 1640 medium (ThermoFisher Scientific, Waltham, MA, USA) supplemented with 10% FBS in a humidified incubator at 37 °C under 5% CO2. The benign ovarian cell line HOSEpiC was purchased from ScienCell (Carlsbad, CA, USA). HOSEpiC cells were maintained in culture in Ovarian Epithelial Cell Medium (OEpiCM) (ScienCell, Carlsbad, CA, USA, catalogue-number 7311) in a humidified incubator at 37 °C under 5% CO2.
4.6. PCR
RNA isolation was performed using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands) and 1 µg of RNA was converted into first-strand cDNA using the MMLV Reverse Transcriptase 1st-Strand cDNA Synthesis Kit (Epicentre, Madison, WI, USA) according to the instructions of the manufacturer. The basal mRNA expressions of NFE2L2 and ESR1 were quantified by qPCR applying FastStart Essential DNA Probes Master and gene-specific primers (Roche, Basel, Switzerland). For normalization of expressions the housekeeping genes, β-Actin and GAPDH were used as reference controls. Basal expressions of NFE2L2 and ESR1 in the ovarian cancer cell lines were compared with their expressions in the benign ovarian cell lines.
4.7. siRNA
The specific siRNA for NFE2L2 (Silencer Select Pre-designed and Custom Designed siRNA, Ambion, Carlsbad, CA, USA) was kindly provided by Beate Niesler (Department of Human Molecular Genetics, University of Heidelberg). Cells were transfected with siRNA using Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA, USA) to silence the expression of NFE2L2 in the cell lines. RNA isolation and mRNA quantification by qPCR was repeated as outlined above. mRNA expression levels of NFE2L2 and ESR1 in NFE2L2-downregulated cells were compared with NFE2L2-containing cells.
5. Conclusions
Here, ESR1 expression was reduced in different ovarian cancer cells vs. benign cells in vitro (all p < 0.001). NFE2L2 silencing showed a higher expression of ESR1 in the NFE2L2-downregulated cancer cell lines OVCAR3 (p = 0.003) and ES2 (p < 0.001). In the serous cancer subtype, high cytoplasmic NRF2 expression (OS, median 50.6 vs. 29.3 months; p = 0.04) and high ERα expression (OS, median 74.5 vs. 27.1 months; p = 0.002) was associated with longer overall survival as well as combined expression of both inactive cytoplasmic NRF2 and ERα in the whole cohort (median 74.5 vs. 37.7 months; p = 0.04). Thus, interactions of NRF2 and ERα impact survival in ovarian cancer patients and may be important factors for the response to endocrine treatment strategies.
Acknowledgments
The authors are grateful to Martina Rahmeh and Christina Kuhn for excellent technical assistance.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/20/1/112/s1.
Author Contributions
B.C. and U.J. conceived and designed the experiments; M.K. performed the experiments; M.K. and U.J. analyzed the data; D.M., E.S., and B.N. contributed reagents/materials/analysis tools; B.C., M.K., and F.T. wrote the paper. D.M., E.S., B.N., T.K., A.B., S.M., and U.J. critically reviewed the paper.
Funding
This work has been funded by the “Monika Kutzner” foundation and the “Brigitte & Dr. Konstanze Wegener” foundation.
Conflicts of Interest
Bastian Czogalla has received a research grant from the “Monika Kutzner” foundation and “Brigitte & Dr. Konstanze Wegener” foundation, all other authors declare no conflict of interest.
References
- 1.Prat J., Franceschi S. Cancers of the female reproductive organs. In: Stewart B.W., Wild C.P., editors. World Cancer Report. IARC; Lyon, France: 2014. pp. 465–481. [Google Scholar]
- 2.Baldwin L.A., Huang B., Miller R.W., Tucker T., Goodrich S.T., Podzielinski I., DeSimone C.P., Ueland F.R., van Nagell J.R., Seamon L.G. Ten-year relative survival for epithelial ovarian cancer. Obstet. Gynecol. 2012;120:612–618. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 3.du Bois A., Reuss A., Pujade-Lauraine E., Harter P., Ray-Coquard I., Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 4.Aletti G.D., Gostout B.S., Podratz K.C., Cliby W.A. Ovarian cancer surgical resectability: RELATIVE impact of disease, patient status, and surgeon. Gynecol. Oncol. 2006;100:33–37. doi: 10.1016/j.ygyno.2005.07.123. [DOI] [PubMed] [Google Scholar]
- 5.Vergote I., De Brabanter J., Fyles A., Bertelsen K., Einhorn N., Sevelda P., Gore M.E., Kærn J., Verrelst H., Sjövall K., et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176–182. doi: 10.1016/S0140-6736(00)03590-X. [DOI] [PubMed] [Google Scholar]
- 6.Dembo A.J., Davy M., Stenwig A.E., Berle E.J., Bush R.S., Kjorstad K. Prognostic factors in patients with stage I epithelial ovarian cancer. Obstet. Gynecol. 1990;75:263–273. [PubMed] [Google Scholar]
- 7.Kossai M., Leary A., Scoazec J.Y., Genestie C. Ovarian Cancer: A Heterogeneous Disease. Pathobiol. J. Immunopathol. Mol. Cell. Boil. 2018;85:41–49. doi: 10.1159/000479006. [DOI] [PubMed] [Google Scholar]
- 8.Sieh W., Kobel M., Longacre T.A., Bowtell D.D., deFazio A., Goodman M.T., Hogdall E., Deen S., Wentzensen N., Moysich K.B., et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013;14:853–862. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Wijst M.G., Brown R., Rots M.G. Nrf2, the master redox switch: The Achilles’ heel of ovarian cancer? Biochim. Biophys. Acta. 2014;1846:494–509. doi: 10.1016/j.bbcan.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Namani A., Matiur Rahaman M., Chen M., Tang X. Gene-expression signature regulated by the KEAP1-NRF2-CUL3 axis is associated with a poor prognosis in head and neck squamous cell cancer. BMC Cancer. 2018;18:46. doi: 10.1186/s12885-017-3907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boustani M.R., Khoshnood R.J., Nikpasand F., Taleshi Z., Ahmadi K., Yahaghi E., Goudarzi P.K. Overexpression of ubiquitin-specific protease 2a (USP2a) and nuclear factor erythroid 2-related factor 2 (Nrf2) in human gliomas. J. Neurol. Sci. 2016;363:249–252. doi: 10.1016/j.jns.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Ji L., Wei Y., Jiang T., Wang S. Correlation of Nrf2, NQO1, MRP1, cmyc and p53 in colorectal cancer and their relationships to clinicopathologic features and survival. Int. J. Clin. Exp. Pathol. 2014;7:1124–1131. [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang T., Chen N., Zhao F., Wang X.J., Kong B., Zheng W., Zhang D.D. High levels of Nrf2 determine chemoresistance in type II endometrial cancer. Cancer Res. 2010;70:5486–5496. doi: 10.1158/0008-5472.CAN-10-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryoo I.G., Choi B.H., Kwak M.K. Activation of NRF2 by p62 and proteasome reduction in sphere-forming breast carcinoma cells. Oncotarget. 2015;6:8167–8184. doi: 10.18632/oncotarget.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D.D. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid. Redox Signal. 2010;13:1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 16.Lo R., Matthews J. The aryl hydrocarbon receptor and estrogen receptor alpha differentially modulate nuclear factor erythroid-2-related factor 2 transactivation in MCF-7 breast cancer cells. Toxicol. Appl. Pharmacol. 2013;270:139–148. doi: 10.1016/j.taap.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Lenhard M., Tereza L., Heublein S., Ditsch N., Himsl I., Mayr D., Friese K., Jeschke U. Steroid hormone receptor expression in ovarian cancer: Progesterone receptor B as prognostic marker for patient survival. BMC Cancer. 2012;12:553. doi: 10.1186/1471-2407-12-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukanova A., Kaaks R. Endogenous hormones and ovarian cancer: Epidemiology and current hypotheses. Cancer Epidemiol. Biomark. Prev. 2005;14:98–107. [PubMed] [Google Scholar]
- 19.Cunat S., Hoffmann P., Pujol P. Estrogens and epithelial ovarian cancer. Gynecol. Oncol. 2004;94:25–32. doi: 10.1016/j.ygyno.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Zhu C., Wang S., Wang B., Du F., Hu C., Li H., Feng Y., Zhu R., Mo M., Cao Y., et al. 17beta-Estradiol up-regulates Nrf2 via PI3K/AKT and estrogen receptor signaling pathways to suppress light-induced degeneration in rat retina. Neuroscience. 2015;304:328–339. doi: 10.1016/j.neuroscience.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 21.Villeneuve N.F., Lau A., Zhang D.D. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: AN insight into cullin-ring ubiquitin ligases. Antioxid. Redox Signal. 2010;13:1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakabayashi N., Slocum S.L., Skoko J.J., Shin S., Kensler T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes J.D., McMahon M., Chowdhry S., Dinkova-Kostova A.T. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid. Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 24.Menegon S., Columbano A., Giordano S. The Dual Roles of NRF2 in Cancer. Trends Mol. Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi K., Yamamoto M. The KEAP1-NRF2 System in Cancer. Front. Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H.-Y., Kim K., Kim Y.-B., Kim H., No J.H. Expression Patterns of Nrf2 and Keap1 in Ovarian Cancer Cells and their Prognostic Role in Disease Recurrence and Patient Survival. Int. J. Gynecol. Cancer. 2017;27:412–419. doi: 10.1097/IGC.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 27.Burges A., Bruning A., Dannenmann C., Blankenstein T., Jeschke U., Shabani N., Friese K., Mylonas I. Prognostic significance of estrogen receptor alpha and beta expression in human serous carcinomas of the ovary. Arch. Gynecol. Obstet. 2010;281:511–517. doi: 10.1007/s00404-009-1185-y. [DOI] [PubMed] [Google Scholar]
- 28.Chuffa L.G., Lupi-Junior L.A., Costa A.B., Amorim J.P., Seiva F.R. The role of sex hormones and steroid receptors on female reproductive cancers. Steroids. 2017;118:93–108. doi: 10.1016/j.steroids.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Liao H., Zhou Q., Zhang Z., Wang Q., Sun Y., Yi X., Feng Y. NRF2 is overexpressed in ovarian epithelial carcinoma and is regulated by gonadotrophin and sex-steroid hormones. Oncol. Rep. 2012;27:1918–1924. doi: 10.3892/or.2012.1700. [DOI] [PubMed] [Google Scholar]
- 30.Gorrini C., Baniasadi P.S., Harris I.S., Silvester J., Inoue S., Snow B., Joshi P.A., Wakeham A., Molyneux S.D., Martin B., et al. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J. Exp. Med. 2013;210:1529–1544. doi: 10.1084/jem.20121337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorrini C., Gang B.P., Bassi C., Wakeham A., Baniasadi S.P., Hao Z., Li W.Y., Cescon D.W., Li Y.T., Molyneux S., et al. Estrogen controls the survival of BRCA1-deficient cells via a PI3K-NRF2-regulated pathway. Proc. Natl. Acad. Sci. USA. 2014;111:4472–4477. doi: 10.1073/pnas.1324136111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Symonds D.A., Merchenthaler I., Flaws J.A. Methoxychlor and estradiol induce oxidative stress DNA damage in the mouse ovarian surface epithelium. Toxicol. Sci. 2008;105:182–187. doi: 10.1093/toxsci/kfn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansell P.J., Lo S.C., Newton L.G., Espinosa-Nicholas C., Zhang D.D., Liu J.H., Hannink M., Lubahn D.B. Repression of cancer protective genes by 17beta-estradiol: Ligand-dependent interaction between human Nrf2 and estrogen receptor alpha. Mol. Cell. Endocrinol. 2005;243:27–34. doi: 10.1016/j.mce.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Sun K.X., Jiao J.W., Chen S., Liu B.L., Zhao Y. MicroRNA-186 induces sensitivity of ovarian cancer cells to paclitaxel and cisplatin by targeting ABCB1. J. Ovarian Res. 2015;8:80. doi: 10.1186/s13048-015-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim C.W., Go R.E., Hwang K.A., Bae O.N., Lee K., Choi K.C. Effects of cigarette smoke extracts on apoptosis and oxidative stress in two models of ovarian cancer in vitro. Toxicol. In Vitro. 2018;52:161–169. doi: 10.1016/j.tiv.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Langdon S.P., Gourley C., Gabra H., Stanley B. Endocrine therapy in epithelial ovarian cancer. Expert Rev. Anticancer Ther. 2017;17:109–117. doi: 10.1080/14737140.2017.1272414. [DOI] [PubMed] [Google Scholar]
- 37.Bjornstrom L., Sjoberg M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 38.Heinzelmann-Schwarz V., Knipprath Meszaros A., Stadlmann S., Jacob F., Schoetzau A., Russell K., Friedlander M., Singer G., Vetter M. Letrozole may be a valuable maintenance treatment in high-grade serous ovarian cancer patients. Gynecol. Oncol. 2018;148:79–85. doi: 10.1016/j.ygyno.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharjee S., Nandi S. Synthetic lethality in DNA repair network: A novel avenue in targeted cancer therapy and combination therapeutics. IUBMB Life. 2017;69:929–937. doi: 10.1002/iub.1696. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharjee S., Nandi S. Choices have consequences: The nexus between DNA repair pathways and genomic instability in cancer. Clin. Transl. Med. 2016;5:45. doi: 10.1186/s40169-016-0128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhattacharjee S., Nandi S. DNA damage response and cancer therapeutics through the lens of the Fanconi Anemia DNA repair pathway. Cell Commun. Signal. 2017;15:41. doi: 10.1186/s12964-017-0195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharjee S., Nandi S. Rare Genetic Diseases with Defects in DNA Repair: Opportunities and Challenges in Orphan Drug Development for Targeted Cancer Therapy. Cancers. 2018;10:298. doi: 10.3390/cancers10090298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholz C., Heublein S., Lenhard M., Friese K., Mayr D., Jeschke U. Glycodelin A is a prognostic marker to predict poor outcome in advanced stage ovarian cancer patients. BMC Res. Notes. 2012;5:551. doi: 10.1186/1756-0500-5-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakas C.T., Alonzo T.A., Yiannoutsos C.T. Accuracy and cut-off point selection in three-class classification problems using a generalization of the Youden index. Stat. Med. 2010;29:2946–2955. doi: 10.1002/sim.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Perkins N.J., Schisterman E.F. The Inconsistency of “Optimal” Cut-points Using Two ROC Based Criteria. Am. J. Epidemiol. 2006;163:670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fluss R., Faraggi D., Reiser B. Estimation of the Youden Index and its Associated Cutoff Point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.