Abstract
Background
Herpes simplex virus (HSV) has various presentations, depending on the patient’s immune status, age, and the route of transmission. In adults, HSV type 1 is found predominantly in the oral area, and HSV type 2 (HSV-2) is commonly found in the genital area. HSV-2 infection without genital lesions is uncommon. Herein we report a unique case of pharyngotonsillitis as an initial manifestation of disseminated HSV-2 infection without genital involvement.
Case presentation
A 46-year-old male was admitted to our hospital with a 1-week history of fever and sore throat. His past medical history included hypereosinophilic syndrome diagnosed at age 45 years. Physical examination revealed throat congestion, bilaterally enlarged tonsils with exudates, tender cervical lymphadenopathy in the left posterior triangle, and mild epigastric tenderness. The laboratory data demonstrated bicytopenia, elevated liver enzyme levels, and hyperferritinemia. A bone marrow smear showed hypocellular marrow with histiocytes and hemophagocytosis. The diagnosis of HLH was confirmed, and the patient was treated with methylprednisolone pulse therapy on days 1–3. On day 5, despite initial improvement of the fever and sore throat, multiple, new, small bullae developed on the patient’s face, trunk, and extremities. Additional testing showed that he was positive for HSV-specific immunoglobulin M and immunoglobulin G. Disseminated HSV infection was suspected, and intravenous acyclovir (10 mg/kg every 8 h) was begun. A subsequent direct antigen test of a bulla sample was positive for HSV-2. Moreover, tonsillar and esophageal biopsies revealed viral inclusion bodies. Immunohistochemical staining and a quantitative real-time polymerase chain reaction (PCR) assay confirmed the presence of HSV-2. Disseminated HSV-2 infection with multiple bullae, tonsillitis, esophagitis, and suspected hepatic involvement was diagnosed. After a 2-week course of intravenous acyclovir, his hematological status and liver function normalized, and his cutaneous skin lesions resolved. He was discharged on day 22 in good general health and continued taking oral valacyclovir for viral suppression due to his immunosuppressed status.
Conclusion
Disseminated HSV-2 infection should be considered as one of the differential diagnoses in patients with pharyngotonsillitis and impaired liver function of unknown etiology even if there are no genital lesions.
Keywords: Herpes simplex virus type 2, Disseminated infection, Hemophagocytic lymphohistiocytosis
Background
Herpes simplex virus (HSV) is a large, double-stranded DNA virus belonging to the ubiquitous Herpesviridae family of viruses, which includes the herpes simplex virus-1 (HSV-1), herpes simplex virus-2 (HSV-2), varicella zoster virus, cytomegalovirus, Epstein-Barr virus, human herpes viruses 6 and 7, and Kaposi’s sarcoma-associated herpesvirus (type 8) [1]. HSV has various presentations, depending on the immune status and age of the host and the route of transmission [2]. Although the typical clinical manifestations are gingivostomatitis and orolabial, ocular, and genital disease, this clinical picture may be complicated by disseminated infection in immunocompromised hosts and neonates [3].
HSV-1 infection usually involves the face and skin “above the waist.” On the other hand, HSV-2 infection usually involves the genitalia and skin “below the waist” in sexually active adolescents and adults [1]. Thus, HSV-2 infection “above the waist” without genital lesions is uncommon. Herein we report a unique case of disseminated HSV-2 infection complicated by hemophagocytic lymphohistiocytosis (HLH), which developed during immunosuppressive therapy for hypereosinophilic syndrome.
Case presentation
A 46-year-old male was admitted to our hospital with a 1-week history of fever and sore throat. His past medical history included hypereosinophilic syndrome diagnosed at age 45 years. An extensive workup failed to disclose any underlying diseases. The patient had been receiving oral prednisolone (8 mg/day) and azathioprine (100 mg/day) regularly for approximately nine months, and his hematological status was stable. He denied any recent travel, illicit drug use or exposure to arthropods. He is married and reported monogamous sexual activity with his wife.
On initial presentation, his temperature was 39 °C, his blood pressure was 145/103 mmHg, his pulse rate was 111 beats per minute and regular, his respiratory rate was 20 breaths per minute, and his percutaneous oxygen saturation was 99% on ambient air. Physical examination revealed throat congestion, bilaterally enlarged tonsils with exudates, tender cervical lymphadenopathy in the left posterior triangle, and mild epigastric tenderness. An examination of the genital area revealed no abnormal findings such as an ulcer or blisters, and the remainder of the examination was unremarkable.
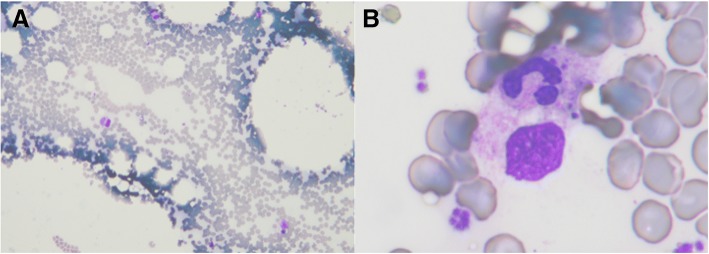
The laboratory data at admission demonstrated bicytopenia (white blood cell count: 1400 /μL; platelet count: 13.4 × 104 /μL), elevated liver enzyme levels (aspartate aminotransferase (AST): 1558 U/L; alanine aminotransferase (ALT): 1007 U/L; lactate dehydrogenase (LDH): 2688 U/L; alkaline phosphatase: 265 U/L; total bilirubin: 0.9 mg/dL), and hyperferritinemia (11,480 ng/ml; normal range: 3.6–114.0 ng/ml). Serologic tests for hepatitis A, B, and C viruses and human immunodeficiency virus (HIV) were negative. Serum antibodies confirmed past infection by the Epstein-Barr virus, cytomegalovirus, and varicella zoster virus. Computed tomography demonstrated prominent hepatosplenomegaly and multiple low-density areas in the liver. An upper gastrointestinal endoscopy revealed multiple vitiligo lesions in the esophagus. A bone marrow smear showed hypocellular marrow with histiocytes (3.0%) and hemophagocytosis (Fig. 1). HLH was diagnosed based on the diagnostic criteria for HLH [4]. Given that the presenting symptom of pharyngotonsillitis is an initial manifestation, we suspected that the HLH was caused by an infectious etiology. There was no evidence of either bacterial or fungal infection in the blood and urine cultures or serological examinations. The patient received meropenem (1 g every 8 h) and intravenous methylprednisolone pulse therapy (1 g/day) on hospital days 1–3.
Fig. 1.
Bone marrow smear on admission showing hemophagocytosis with a Giemsa stain, which demonstrates many histiocytes with hemophagocytosis. a: Low power image. b: High power image
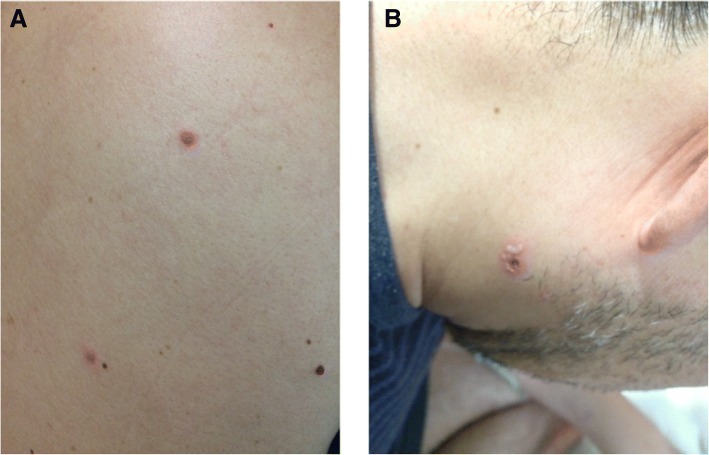
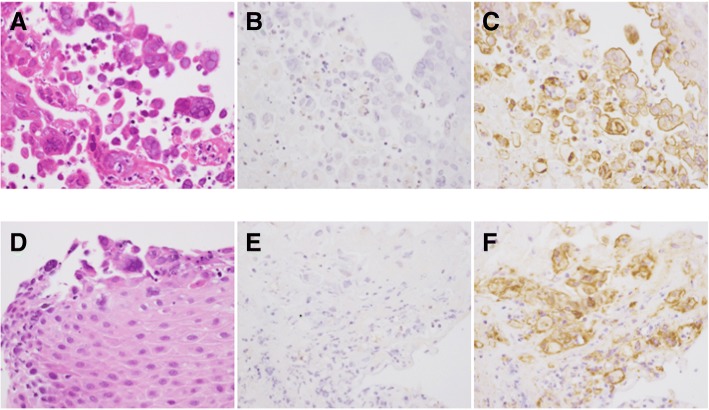
On day 5, despite initial improvement of the fever and sore throat, multiple, new, small bullae developed on the patient’s face, trunk, and extremities (Fig. 2), and the liver enzyme level remained elevated (AST: 935 U/L; ALT: 959 U/L; LDH: 2140 U/L). Additional testing showed positivity for HSV-specific immunoglobulin M (IgM) and immunoglobulin G. Disseminated HSV infection was suspected, and intravenous acyclovir (10 mg/kg every 8 h) and methylprednisolone pulse therapy were administered again on days 5–7. A subsequent direct antigen test of a bullae sample was positive for HSV-2, and a polymerase chain reaction (PCR) assay of the peripheral blood mononuclear cells (PBSCs) detected HSV-2 DNA. Moreover, tonsillar and esophageal biopsies revealed viral inclusion bodies. Immunohistochemical staining and quantitative real-time PCR from the formalin-fixed paraffin-embedded tissues confirmed the presence of HSV-2 but not HSV-1 (Fig. 3). The methods for immunohistochemical staining [5] and quantitative real-time PCR to test for HSV-1, − 2 [6], and β-actin [7] were described previously. Based on these results, disseminated HSV-2 infection with multiple bullae, tonsillitis, and esophagitis was diagnosed. Hepatic involvement was also suspected. It is worth noting that the patient denied any past history of oral or genital HSV infection. A lumbar puncture was not performed due to the lack of neurological deficits or symptoms.
Fig. 2.
Multiple bullae 5 days after admission. a: trunk, b: face
Fig. 3.
Histopathological findings of tonsillar (a-c) and esophageal (d-f) biopsies. a, d: Hematoxylin and eosin staining showing discohesive superficial squamous epithelial cells containing Cowdry type a or type b intranuclear inclusions. b, e: Negative immunohistochemical staining for HSV-1. c, f: Positive immunohistochemical staining for HSV-2
The patient improved steadily on a two-week course of intravenous acyclovir. His hematological status and liver function normalized, and his cutaneous skin lesions resolved. He was discharged on day 22 in good general health and continued to receive oral valacyclovir for viral suppression due to his immunosuppressed status. Prednisolone was tapered gradually to 12.5 mg on discharge. No recurrence has been observed 7 months after the treatment.
Discussion and conclusions
In the present case, pharyngotonsillitis developed as an initial manifestation of disseminated HSV-2 infection without genital involvement. To the best of our knowledge, no previous case with such a clinical course has been reported. In general, viral causes of tonsillitis include rhinovirus, respiratory syncytial virus, Epstein-Barr virus, parainfluenza virus, influenza virus, coxsackievirus, adenovirus, etc [8] The majority of herpetic pharyngotonsillitis cases in adults are due to HSV-1, but a few reports have shown that HSV-2 can also produce the same symptoms in sexually active patients [9, 10]. However, the present patient denied any oral sexual activity during a period of several months and had no sign of genital involvement.
In our search of the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) using the search terms, “disseminated,” “herpes simplex virus,” and “type 2,” we identified 15 case reports of adult disseminated HSV-2 infection in the English-language literature since 2000 [11–21]. Case reports not published in English, pediatric cases (patients younger than 15 years old), and review articles were excluded. We defined disseminated HSV-2 infection as any case with positive HSV-2 IgM antibody test results, the presence of HSV-2 detected by DNA-PCR in the PBSCs, or the concurrent involvement of two or more noncontiguous sites. Our case and 15 other reported cases are summarized in Table 1. The patients’ mean age was 28 (range: 18–67) years. Eight patients (50.0%) were male and eight (50.0%) were female. Six patients (37.5%) were treated with immunosuppressive agents at the onset of HSV-2 infection. Regarding the site of infection, 11 patients (68.8%) presented with liver involvement and six (37.5%) presented with a skin infection. Interestingly, including the present case, genital symptoms were not observed in nine patients (56.3%). The patients denied any past history of sexually transmitted infection. The etiology of the HSV-2 infection was obscure in most cases. Fourteen patients (87.5%) received intravenous acyclovir and ten (71.4%) responded to treatment.
Table 1.
Adult disseminated HSV-2 infection: 15 reported cases and the presented case
| Year, author [reference] | Age (y)/Sex | Underlying condition or disease | Immunosuppressive therapy at the onset | Infected organs | Complications | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 2005, Yamaguchi K et al. [11] | N/A / F | Pregnancy | None | Genital, skin | HLH | ACV | Survived |
| 2006, Czartoski T et al. [12] | 28 / F | Immunocompetent | None | Liver, skin | Candidemia | ACV | Died |
| 2006, Ramasamy K et al. [13] | 33 / M | Bone marrow failure of unknown etiology | Oxymetholone, prednisolone | Skin | HLH, rhabdomyolysis | ACV | Died |
| 2007, Abbo L et al. [14] | 51 / M | Immunocompetent | None | Liver, colon | FHF | ACV | Died |
| 2010, Peppercorn A et al. [15] | 58 / M | Burn | None | Skin, liver, lung | Septic shock | BSC | Dead |
| 2012, Lee L et al. [16] | 24 / F | AIDS | None | Pharynx, genital | None | ACV | Survived |
| 2015, Rimawi BH et al. [17] | 25 / F | Immunocompetent | None | Liver, skin, genital | None | ACV | Survived |
| 2015, Rimawi BH et al. [17] | 24 / F | Immunocompetent | None | Liver, skin | None | ACV | Survived |
| 2015, Rimawi BH et al. [17] | 27 / F | Pregnancy | None | Liver, genital | None | ACV | Survived |
| 2015, Erdmann N et al. [18] | 22 / M | Atopic dermatitis | Systemic corticosteroids | Skin, genital | None | ACV | Survived |
| 2016, Down C et al. [19] | 67 / M | Prostate cancer | None | Liver, genital | Ireus | ACV | Survived |
| 2016, Ikumi K et al. [20] | 56 / M | Muscle sclerosis | Methylprednisolone pulse therapy | Liver, rectum, adrenal grand etc. | HLH | None | Died |
| 2017, Natu A et al. [21] | 23 / M | Crohn’s disease | Vedolizumab, prednisolone | Liver, mouth, genital | Crohn’s flare | ACV | Survived |
| 2017, Natu A et al. [21] | 18 / F | Pregnancy | None | Liver | Septic shock, FHF | ACV | Died |
| 2017, Natu A et al. [21] | 65 / F | Liver cirrhosis | Liver transplantation | Liver | None | ACV | Survived |
| Presented case | 46 / M | Hypereosinophilic syndrome | Azathioprine, prednisolone | Tonsil, esophagus,skin | HLH | ACV | Survived |
Abbreviations: ACV acyclovir, AIDS acquired immune deficiency syndrome, BSC best supportive care, M male, F female, FHF fulminant hepatic failure, HLH hemophagocytic lymphohistiocytosis, y year
One plausible explanation of the pathogenesis of the present case is a reactivation of HSV-2 infection. Tobian et al. reported a case in which the initiation of antiretroviral therapy (ART) for HIV infection reactivated HSV-2, possibly via immune reconstitution inflammatory syndrome (IRIS) [22]. IRIS describes a collection of inflammatory disorders associated with an apparently paradoxical worsening of preexisting infectious processes following the initiation of ART in HIV-positive individuals [23]. Similar illnesses also occur in some HIV-negative patients after tapering immunosuppressive drugs [24–26]. In our case, we didn’t assess serological data using pre-serum specimens to confirm the past infection of HSV-2. However, the prednisolone dosage was gradually tapered due to the favorable clinical course of the hypereosinophilic syndrome, which suggests the possibility of HSV-2 reactivation accompanied by IRIS-like phenomenon. Physicians should consider the possibility of viral reactivation in patients receiving immunosuppressive agents if they encounter an infection of unknown etiology.
In the present case, the disseminated HSV-2 infection triggered the development of HLH. Ramos-Casals M et al. reported that Herpesviridae account for 62% of cases of virus-associated HLH including Epstein-Barr virus (43%), cytomegalovirus (9%), and other herpes viruses (10%) [27]. There are very few reports of HLH caused by HSV-2 [11, 13, 20]. Thus, no optimal treatment strategy for HLH caused by HSV-2 has yet been established. Several previous studies reported that patients recovered from infection-associated HLH through the specific treatment of the underlying infection and supportive care alone [28]. On the other hand, when an HSV-2 infection is disseminated, HLH can progress to multiple organ failure with an extremely poor prognosis [13, 20]. Our patient was treated successfully with intravenous acyclovir and methylprednisolone pulse therapy due to early diagnosis. The treatment of HLH might require an individual approach depending on the underlying trigger, disease severity, and clinical course.
The present study reported the first case of disseminated HSV-2 infection associated with pharyngotonsilitis, esophagitis, and HLH without genital involvement in an immunocompromised host. The patient recovered after receiving intravenous acyclovir and methylprednisolone pulse therapy. HSV-2 pharyngotonsillitis is rare and difficult to diagnose, especially if genital lesions are not present. This report emphasizes and promotes awareness of the importance of the early recognition and appropriate clinical management of HSV-2 infection. Additional data from similar cases and further studies to characterize HSV-2 infection and HLH in adults, particularly those who are immunocompromised, are needed.
Acknowledgements
The authors would like to thank the patient described in this case for allowing us to share it with the scientific community. The authors are also grateful to the staff at Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital for their excellent patient care.
Funding
None.
Availability of data and materials
Not applicable.
Abbreviations
- ALT
Alanine aminotransferase
- ART
Antiretroviral therapy
- AST
Aspartate aminotransferase
- HIV
Human immunodeficiency virus
- HLH
Hemophagocytic lymphohistiocytosis
- HSV
Herpes simplex virus
- IgM
Immunoglobulin M
- IRIS
Immune reconstitution inflammatory syndrome
- LDH
Lactate dehydrogenase
- PBSCs
Peripheral blood mononuclear cells
- PCR
Polymerase chain reaction
Authors’ contributions
SK, KF, and NS: conception, design, clinical assessments, acquisition and analysis of data, interpretation of results, and manuscript writing. KI, AF, HT, FC, KS: clinical assessments, acquisition of the data, interpretation of results, and revision of the manuscript. HH, HK, TH: acquisition of the histopathological data, interpretation of results, and revision of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication was obtained from the patient.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuhei Kurosawa, Email: 1a.4ftnn6@gmail.com.
Noritaka Sekiya, Phone: +81-3-3823-2101, Email: qnmnk410@ybb.ne.jp, Email: n-sekiya@cick.jp.
Kazuaki Fukushima, Email: f.a019141@gmail.com.
Kazuhiko Ikeuchi, Email: kikeuchi004@gmail.com.
Akito Fukuda, Email: a.fukuda0730@gmail.com.
Hideyuki Takahashi, Email: hideyutakahashi-tky@umin.ac.jp.
Fangyi Chen, Email: pychen@cick.jp.
Hideki Hasegawa, Email: hasegawa@nih.go.jp.
Harutaka Katano, Email: katano@nih.go.jp.
Tsunekazu Hishima, Email: hishima@cick.jp.
Keigo Setoguchi, Email: setoguch@cick.jp.
References
- 1.Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57:737–763. doi: 10.1016/j.jaad.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Waggoner-Fountain LA, Grossman LB. Herpes simplex virus. Pediatr Rev. 2004;25:86–93. doi: 10.1542/pir.25-3-86. [DOI] [PubMed] [Google Scholar]
- 3.Brady RC, Bemstein DI. Treatment of herpes simplex virus infections. Antivir Res. 2004;61:73–81. doi: 10.1016/j.antiviral.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 5.Kashiwase M, Sata T, Yamauchi Y, Minoda H, Usui N, Iwasaki T, et al. Progressive outer retinal necrosis caused by herpes simplex virus type 1 in a patient with acquired immunodeficiency syndrome. Ophthalmology. 2000;107:790–794. doi: 10.1016/S0161-6420(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 6.Weidmann M, Meyer-König U, Hufert FT. Rapid detection of herpes simplex virus and varicella-zoster virus infections by real-time PCR. J Clin Microbiol. 2003;41:1565–1568. doi: 10.1128/JCM.41.4.1565-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuramochi H, Hayashi K, Uchida K, Miyakura S, Shimizu D, Vallböhmer D, et al. Vascular endothelial growth factor messenger RNA expression level is preserved in liver metastases compared with corresponding primary colorectal cancer. Clin Cancer Res. 2006;12:29–33. doi: 10.1158/1078-0432.CCR-05-1275. [DOI] [PubMed] [Google Scholar]
- 8.Putto A. Febrile exudative tonsillitis: viral or streptococcal? Pediatrics. 1987;80:6–12. [PubMed] [Google Scholar]
- 9.Tustin AW, Kaiser AB. Life-threatening pharyngitis caused by herpes simplex virus, type 2. Sex Transm Dis. 1979;6:23–24. doi: 10.1097/00007435-197901000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Rosain J, Froissart A, Estrangin E, Rozenberg F. Severe acute paharyngotonsillitis due to herpes simplex virus type 2 in a young woman. J Clin Virol. 2015;63:63–65. doi: 10.1016/j.jcv.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi K, Yamamoto A, Hisano M, natori M, Murashima A. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105:1241–1244. doi: 10.1097/01.AOG.0000157757.54948.9b. [DOI] [PubMed] [Google Scholar]
- 12.Czartoski T, Liu C, Koelle DM, Schmechel S, Kalus A, Wald A. Fulminant, acyclovir-resistant, herpes simplex virus type 2 hepatitis in an immunocompetent woman. J Clin Microbiol. 2006;44:1584–1586. doi: 10.1128/JCM.44.4.1584-1586.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramasamy K, Lim ZY, Savvas M, Salisbury JR, Dokal I, Mufti GJ, et al. Disseminated herpes simplex virus (HSV-2) infection with rhabdomyolysis and hemophagocytic lymphohistiocytosis in patient with bone marrow failure syndrome. Ann Heamtol. 2006;85:629–630. doi: 10.1007/s00277-006-0126-0. [DOI] [PubMed] [Google Scholar]
- 14.Abbo L, Alcaide ML, Pano JR, Robinson PG, Campo RE. Fulminant hepatitis from herpes simplex virus type 2 in an immunocompetent adult. Transpl Infect Dis. 2007;9:323–326. doi: 10.1111/j.1399-3062.2007.00207.x. [DOI] [PubMed] [Google Scholar]
- 15.Peppercorn A, Veit L, Sigei C, Weber DJ, Jones S, Cairns BA. Overwhelming disseminated herpes simplex virus type 2 infection in a patient with severe burn injury: case report and literature review. J Burn Care Res. 2010;31:492–498. doi: 10.1097/BCR.0b013e3181db51cb. [DOI] [PubMed] [Google Scholar]
- 16.Lee L, Agwu A, Hutton N. Sever primary HSV-2 in a perinatal HIV-infected woman with advanced Immunosuppresion. Case Rep Med. 2012;2012:346039. doi: 10.1155/2012/346039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rimawi BH, Meserve J, Rimawi RH, Min Z, Gnann JW., Jr Disseminated herpes simplex virus with fulminant hepatitis. Case Reports Hepatol. 2015;2015:463825. doi: 10.1155/2015/463825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdmann N, Hewitt BA, Atkinson TP, Wagoner V. Disseminated Primary Herpes Simplex Virus Type 2 Infection in a 32-Year-Old male. Open Forum Infect Dis. 2015;2:ofv092. doi: 10.1093/ofid/ofv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Down C, Mehta A, Salama G, Hissong E, Rosenblatt R, Cantor M, et al. Herpes simplex virus hepatitis in an immunocompetent host resembling hepatic pyogenic abscesses. Case Reports Hepatol. 2016;2016:8348172. doi: 10.1155/2016/8348172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikumi K, Ando T, Katano H, Katsuno M, Sakai Y, Yoshida M, et al. HSV-2-related hemophagocytic lymphohistiocytosis in a fingolimond-treated patient with MS. Neurol Neuroimmunol Neuroinflamm. 2016;3:e247. doi: 10.1212/NXI.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natu A, Iuppa G, Packer CD. Herpes simplex virus hepatitis: presentation of multi-institutional cases to promote early diagnosis and Management of the Disease. Case Reports Hepatol. 2017;2017:3180984. doi: 10.1155/2017/3180984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobian AA, Grabowski MK, Serwadda D, Newell K, Ssebbowa P, Franco V, et al. Reactivation of herpes virus type 2 after initiation of antiretroviral therapy. J Infect Dis. 2013;208:839–846. doi: 10.1093/infdis/jit252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shelburne SA, Montes M, Hamill RJ. Immune reconstitution inflammatory syndrome: more answers, more questions. J Antimicrob Chemother. 2006;57:167–170. doi: 10.1093/jac/dki444. [DOI] [PubMed] [Google Scholar]
- 24.Chenq VC, Yuen KY, Chan WM, Wong SS, Ma SS, Chan RM. Immunorestitution disease involving the innate and adaptive response. Clin Infect Dis. 2000;30:882–892. doi: 10.1086/313809. [DOI] [PubMed] [Google Scholar]
- 25.Shiohara T, Kurata M, Mizukawa Y, Kano Y. Recognition of immune reconstitution syndrome necessary for better management of patients with severe drug eruptions and those immunosuppressive therapy. Allergol Int. 2010;59:333–343. doi: 10.2332/allergolint.10-RAI-0260. [DOI] [PubMed] [Google Scholar]
- 26.Scemia A, Gerber S, Duquesne A, et al. Dramatic improvement of severe cryptococcosis-induced immune reconstitution syndrome with adalimumab in a renal transplant recipient. Am J Transplant. 2015;15:560–564. doi: 10.1111/ajt.13002. [DOI] [PubMed] [Google Scholar]
- 27.Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 28.Koubâa M, Mâaloul I, Marrakchi C, Mdhaffar M, Lahiani D, Hammami B, et al. Hemophagocytic syndrome associated with visceral leishmaniasis in an immunocompetent adult-case report and review of literature. Ann Hematol. 2012;91:1143–1145. doi: 10.1007/s00277-011-1367-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.