Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
HBV DNA monitoring–guided preemptive nucleos(t)ide therapy can prevent HBV hepatitis during anti-CD20 immunochemotherapy in B-cell NHL.
Prophylactic nucleos(t)ide therapy can prevent HBV reactivation and may be appropriate for high-risk patients.
Abstract
Risk of hepatitis B virus (HBV) reactivation was assessed in B-cell non-Hodgkin lymphoma (NHL) patients with resolved HBV infection (hepatitis B surface antigen negative, hepatitis B core antibody positive) who received obinutuzumab- or rituximab-containing immunochemotherapy in the phase 3 GOYA and GALLIUM studies. HBV DNA monitoring was undertaken monthly to 1 year after the last dose of study drug. In case of HBV reactivation (confirmed, HBV DNA ≥29 IU/mL), immunochemotherapy was withheld and nucleos(t)ide analog treatment (preemptive NAT) started. Immunochemotherapy was restarted if HBV DNA became undetectable or reactivation was not confirmed, and discontinued if HBV DNA exceeded 100 IU/mL on NAT. Prophylactic NAT was allowed by investigator discretion. Among 326 patients with resolved HBV infection, 27 (8.2%) had HBV reactivation, occurring a median of 125 days (interquartile range, 85-331 days) after the first dose. In 232 patients without prophylactic NAT, 25 (10.8%) had HBV reactivation; all received preemptive NAT. Ninety-four patients received prophylactic NAT; 2 (2.1%) had HBV reactivation. No patients developed HBV-related hepatitis. On multivariate Cox analysis, detectable HBV DNA at baseline was strongly associated with an increased risk of reactivation (adjusted hazard ratio [HR], 18.22; 95% confidence interval [CI], 6.04-54.93; P < .0001). Prophylactic NAT was strongly associated with a reduced risk (adjusted HR, 0.09; 95% CI, 0.02-0.41; P = .0018). HBV DNA monitoring–guided preemptive NAT was effective in preventing HBV-related hepatitis during anti–CD20-containing immunochemotherapy in B-cell NHL patients with resolved HBV infection. Antiviral prophylaxis was also effective and may be appropriate for high-risk patients. These trials were registered at www.clinicaltrials.gov as NCT01287741 (GOYA) and NCT01332968 (GALLIUM).
Visual Abstract
Introduction
Hepatitis B virus (HBV) reactivation is an identified risk associated with treatment of non-Hodgkin lymphoma (NHL) in patients with resolved/occult HBV infection, particularly for immunocompromised patients and patients with preexisting liver disease, who are at increased risk of developing hepatitis-related liver failure.1-3 While HBV reactivation has been reported with some cytotoxic chemotherapies (eg, anthracyclines) and high-dose corticosteroids,4-10 the highest rates of reactivation are typically observed during immunochemotherapy with the anti-CD20 monoclonal antibody rituximab, especially when it is combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy.11-19 Reports of reactivation are lacking for other common chemotherapy partners, such as bendamustine, with only a few cases described.20,21 Among patients with resolved HBV infection, the rate of HBV reactivation during rituximab-based immunochemotherapy (by various definitions, without antiviral prophylaxis) varies from 3% to 41.5%.6,18,22 A recent pooled risk estimate from a meta-analysis of a randomized trial and several well-designed observational studies suggests a reactivation rate of 16.9% during rituximab-based treatment.6
Prophylactic anti-HBV nucleos(t)ide therapy (NAT), started before any HBV reactivation, can be an effective strategy to prevent HBV reactivation and HBV-related hepatitis.23-29 However, such an approach may result in overexposure to antiviral medication, possibly leading to an increase in antiviral-related side effects and drug resistance.30-32 Delayed HBV reactivation may also occur after stopping prophylactic NAT.29 Additionally, it may not be economically viable to provide long-term prophylactic NAT for all patients.30 An alternative effective approach to prevent HBV reactivation-related hepatitis is the use of HBV DNA monitoring-guided preemptive NAT.18,22,33 With this strategy, regular HBV DNA monitoring is used to identify patients in the initial stages of HBV reactivation who are then treated preemptively with NAT. Such an approach reduces exposure to antiviral treatment but requires access to specialized monitoring and testing facilities.
Despite the risk of HBV reactivation, rituximab remains a key component of immunochemotherapy regimens used to treat newly diagnosed and relapsed/refractory B-cell NHLs, such as diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).34-37 However, a glycoengineered type II anti-CD20 monoclonal antibody, obinutuzumab, is being developed as a treatment for B-cell malignancies.38,39 This novel antibody demonstrates greater direct cell-death induction and antibody-dependent cellular cytotoxicity and phagocytosis than rituximab.40,41 To date, 2 phase 3 trials have compared the efficacy and safety of obinutuzumab with rituximab, when given in combination with chemotherapy, in previously untreated patients with DLBCL (GOYA) or indolent B-cell NHL (predominantly FL; GALLIUM).42,43 In GALLIUM, obinutuzumab-based immunochemotherapy and maintenance significantly improved progression-free survival compared with rituximab-based therapy and maintenance in FL patients; however, no differences in outcomes were observed between obinutuzumab- and rituximab-containing immunochemotherapy in DLBCL patients in GOYA. Across both studies, obinutuzumab had a safety profile comparable to that of rituximab. While there were numerically more treatment-related adverse events with obinutuzumab, the safety profile was generally acceptable and manageable. Given the association between anti-CD20 antibody treatment and HBV reactivation, HBV DNA levels were monitored prospectively in both trials.
The aims of the current post hoc analysis were to quantify the risk of HBV reactivation and HBV-related hepatitis, which were prospectively defined adverse events of particular interest in the GOYA and GALLIUM studies, and explore risk factors for HBV reactivation in B-cell NHL (DLBCL or FL) patients with resolved/occult HBV infection who received obinutuzumab- or rituximab-based immunochemotherapy.
Patients and methods
Clinical studies
The design and conduct of the open-label, multicenter, randomized, phase 3 GOYA (NCT01287741) and GALLIUM (NCT01332968) trials have been described previously.42,43 Brief details of their study designs are provided in the supplemental Methods (available on the Blood Web site). The trials were conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice. Study protocols were approved by the ethics committees of participating centers. All patients provided written informed consent.
Eligibility criteria related to HBV infection
A positive test result for chronic HBV infection (defined as positive hepatitis B surface antigen [HBsAg] serology) was an exclusion criterion in both trials. Patients with resolved HBV infection (defined as HBsAg negative but positive for antibody against hepatitis B core antigen [anti-HBc]) were included if HBV DNA was undetectable. However, patients with detectable HBV DNA that could not be quantified were also eligible for enrollment and were not excluded from this analysis. Patients were required to undergo regular HBV DNA monitoring.
HBV assessments and monitoring to prevent HBV-related hepatitis
Baseline screening was carried out locally for HBsAg, anti-HBc, and hepatitis B surface antibody (anti-HBs). To prevent HBV reactivation-related hepatitis in patients with resolved HBV infection, HBV DNA monitoring was performed prospectively in both trials every 3 to 4 weeks (ie, with each cycle of immunochemotherapy) until the end of induction. During maintenance and follow-up in GALLIUM and during follow-up in GOYA, HBV DNA monitoring was undertaken approximately every 4 weeks (monthly) until at least 1 year after the last dose of any study drug (anti-CD20 antibody or chemotherapy, as induction or maintenance). HBV DNA was measured in serum using a quantitative, real-time polymerase chain reaction (PCR) assay (COBAS TaqMan HBV PCR assay; Roche Molecular Systems Inc.) with a lower limit of quantitation of 29 IU/mL. Blood samples collected for assessment of HBV DNA were analyzed at a central laboratory.
HBV reactivation was defined as confirmed, quantifiable HBV DNA ≥29 IU/mL (reflecting the lower limit of quantitation) as follows: 1 result of HBV DNA ≥100 IU/mL, 2 consecutive assessments of ≥29 to <100 IU/mL, or a single assessment between 29 and 100 IU/mL with initiation of NAT prior to confirmation. HBV DNA levels of <29 IU/mL were classified as either HBV DNA detectable, but not quantifiable, or HBV DNA not detectable.
The approaches used for monitoring and managing HBV reactivation are shown in supplemental Table 1. Despite minor differences between the trials, confirmed HBV DNA levels of ≥29 IU/mL were managed by withholding immunochemotherapy and administering preemptive NAT (entecavir, lamivudine, tenofovir, or adefovir). NAT was initiated immediately if HBV DNA levels were ≥29 IU/mL in GALLIUM; if reactivation was not confirmed on retest, NAT was stopped and retesting was undertaken every 3 to 4 weeks. In GOYA, NAT was initiated following confirmation of HBV reactivation, which was within 2 weeks of first detection. Immunochemotherapy was resumed once HBV DNA returned to undetectable levels or if reactivation was not confirmed by HBV DNA retesting. If a patient’s HBV DNA level exceeded 100 IU/mL while receiving NAT, immunochemotherapy was discontinued permanently. The study protocols recommended that NAT was to be continued for at least 1 year after the last dose of obinutuzumab or rituximab, which reflects the period in which most cases of delayed HBV reactivation were observed.13,18 While use of NAT was mandated in GOYA and GALLIUM, the exact choice of drug was not prespecified in either study protocol. Prophylactic NAT, started before any HBV reactivation, was also allowed (but not mandated) to prevent HBV-related hepatitis in both studies based on the discretion of the participating investigator.
Analyses
The primary objectives of the analysis were to evaluate the incidence of confirmed, quantifiable HBV reactivation (as defined in “Patients and methods”) and HBV-related hepatitis (defined as an exacerbation/development of clinical hepatitis with increased HBV DNA) in anti–HBc-positive B-cell lymphoma patients who had received obinutuzumab- or rituximab-based immunochemotherapy in GOYA and GALLIUM. Additional objectives were to assess time to HBV reactivation and factors related to the risk of HBV reactivation. The analysis population included all patients who were seronegative for HBsAg but seropositive for anti-HBc at baseline. The clinical cutoff date for the analyses was 29 April, 2016 for the GOYA data and 31 January, 2016 for the GALLIUM data.
Time to HBV reactivation was defined as the time from first dose of immunochemotherapy to the first occurrence of HBV reactivation. Patients without HBV reactivation were censored at the time of their last HBV DNA assessment. Survival parameters for time to HBV reactivation were estimated using Kaplan-Meier methodology and analyzed according to the use of prophylactic NAT.
Cox proportional hazard models (univariate and multivariate) were used to identify independent factors (covariates) related to the risk of HBV reactivation. For each analysis, treatment effects were calculated and adjusted for both the covariate and interaction. Statistical significance was determined using the Wald test. Factors with a significance of <.2 by univariate analysis were included in the multivariate analyses, along with baseline age and sex. A significance level of <.05 was used for the multivariate analysis. All analyses were performed using SAS version 9.2.
Qualified researchers may request access to individual patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies (https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx), as well as Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm), are available online.
Results
Analysis population
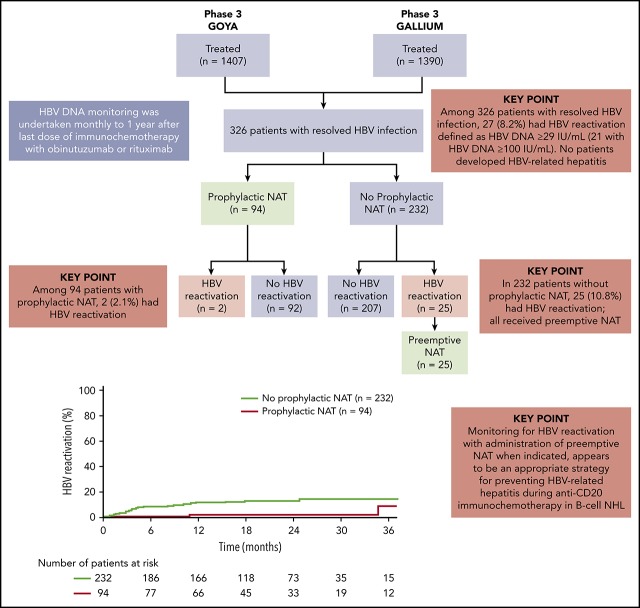
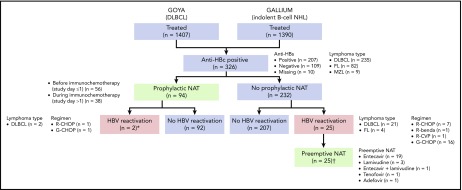
Of the 2797 patients evaluable for safety across the 2 trials (GALLIUM, n = 1390; GOYA, n = 1407), 326 (11.7%; DLBCL, n = 235 [72.1%]; FL, n = 82 [25.2%]; other indolent B-cell NHL, n = 9 [2.8%]) were seronegative for HBsAg but seropositive for anti-HBc at baseline (analysis population; Figure 1). Among these patients, the anti-HBs result was positive in 207 patients and negative in 109 patients; 10 patients had missing values. Eleven patients had detectable but nonquantifiable HBV DNA (<29 IU/mL) at baseline.
Figure 1.
Analysis population and patient flow. benda, bendamustine; CVP, cyclophosphamide, vincristine, prednisone; G, obinutuzumab; MZL, marginal zone lymphoma; R, rituximab. *The 2 patients with HBV reactivation who had received prophylactic NAT had their study treatment withheld until their HBV DNA returned to undetectable levels; neither patient developed HBV-related hepatitis. †None of 25 patients with HBV reactivation who were treated with preemptive NAT developed HBV-related hepatitis.
Baseline demographic and disease characteristics for the analysis population are shown by treatment arm (ie, obinutuzumab-based chemotherapy [G-Chemo; n = 155] vs rituximab-based chemotherapy [R-Chemo; n = 171]) in Table 1. Some imbalances that could potentially influence HBV reactivation were observed between the 2 arms for sex, HBV serology, detectable HBV DNA levels, and use of prophylactic NAT. The median interval between HBV DNA measurements among the 326 patients who were seropositive for anti-HBc was 28.0 (interquartile range [IQR], 21.0-32.0) days.
Table 1.
Patient demographics and disease characteristics at baseline by treatment arm in patients with positive anti-HBc serology (analysis population, n = 326)
| Characteristic | G-Chemo (n = 155) | R-Chemo (n = 171) |
|---|---|---|
| Median age, y (range) | 61 (25-81) | 59 (19-83) |
| Female, n (%) | 60 (38.7) | 81 (47.4) |
| Race, n (%) | ||
| White | 49 (31.6) | 64 (37.4) |
| Black | 3 (1.9) | 0 |
| Asian | 103 (66.5) | 105 (61.4) |
| Other | 0 | 2 (1.2) |
| Region, n (%) | ||
| East Asia* | 86 (55.5) | 89 (52.0) |
| Europe† | 48 (31.0) | 57 (33.3) |
| Other‡ | 21 (13.5) | 25 (14.6) |
| Lymphoma type, n (%) | ||
| DLBCL (GOYA) | 121 (78.1) | 114 (66.7) |
| FL (GALLIUM) | 29 (18.7) | 53 (31.0) |
| MZL (GALLIUM) | 5 (3.2) | 4 (2.3) |
| HBV serology, n (%) | ||
| Anti-HBc+, anti-HBs+ | 94 (60.6) | 113 (66.1) |
| Anti-HBc+, anti-HBs- | 57 (36.8) | 52 (30.4) |
| Missing anti-HBs | 4 (2.6) | 6 (3.5) |
| HBV DNA <29 IU/mL at baseline, n (%) | ||
| Not detectable | 143 (92.3) | 157 (91.8) |
| Detectable, but not quantifiable | 7 (4.5) | 4 (2.3) |
| Missing | 5 (3.2) | 10 (5.8) |
| ECOG performance status, n (%) | ||
| 0 | 76 (49.0) | 81 (47.4) |
| 1 | 68 (43.9) | 69 (40.4) |
| 2 | 11 (7.1) | 21 (12.3) |
| IPI risk category (GOYA), n (%) | n = 121 | n = 114 |
| High | 20 (16.5) | 18 (15.8) |
| High-intermediate | 41 (33.9) | 31 (27.2) |
| Low-intermediate | 38 (31.4) | 42 (36.8) |
| Low | 22 (18.2) | 23 (20.2) |
| FLIPI-1 risk category (GALLIUM, FL), n (%) | n = 29 | n = 53 |
| High | 11 (37.9) | 21 (39.6) |
| Intermediate | 10 (34.5) | 19 (35.8) |
| Low | 8 (27.6) | 13 (24.5) |
| IPI risk category (GALLIUM, non-FL), n (%) | n = 5 | n = 4 |
| High | 1 (20.0) | 0 |
| High-intermediate | 2 (40.0) | 2 (50.0) |
| Low-intermediate | 1 (20.0) | 1 (25.0) |
| Low | 1 (20.0) | 1 (25.0) |
| Prophylactic NAT, n (%) | 40 (25.8) | 54 (31.6) |
| Lamivudine | 33 (21.3) | 46 (26.9) |
| Entecavir | 7 (4.5) | 8 (4.7) |
| Treatment, n (%) | ||
| CHOP | 141 (91.0)§ | 148 (86.5)‖ |
| Benda | 13 (8.4) | 19 (11.1) |
| CVP | 1 (0.6) | 4 (2.3) |
Benda, bendamustine; Chemo, chemotherapy; CVP, cyclophosphamide, vincristine, and prednisone; ECOG, Eastern Cooperative Oncology Group; FLIPI, Follicular Lymphoma International Prognostic Index; G, obinutuzumab; IPI, International Prognostic Index; MZL, marginal zone lymphoma; R, rituximab.
East Asia includes China, Japan, South Korea, and Taiwan.
Europe includes Belgium, Czech Republic, Germany, Hungary, Italy, Russia, Spain, Switzerland, and the United Kingdom.
Other includes Australia, Canada, and Thailand.
G-CHOP: GOYA, n = 121; GALLIUM, n = 20.
R-CHOP: GOYA, n = 114; GALLIUM, n = 34.
HBV reactivation and antiviral treatment (prophylactic or preemptive therapy)
Of the 326 patients with resolved HBV infection, 119 (36.5%) received NAT, including entecavir, lamivudine, adefovir, and tenofovir (Figure 1). Of these patients, 94 (79.0%) received prophylactic NAT (at the discretion of the investigator) for a median duration of 482.5 days (IQR, 173-625); 56 patients (59.6%) started prophylactic NAT before immunochemotherapy commenced (study day ≤1), and 38 patients (40.4%) started prophylactic NAT afterward (study day >1, in the absence of HBV reactivation; median time to start of NAT, 8 days [IQR, 3-65]). Baseline characteristics are shown by use of prophylactic NAT in supplemental Table 2. Use of prophylactic NAT was most common in white European patients who were seronegative for anti-HBs. Median time from last dose of immunochemotherapy to the end of prophylactic NAT was 243 days (IQR, 15-390).
Twenty-seven patients in total (8.2%; DLBCL, n = 23 [85.2%]; FL, n = 4 [14.8%]) had HBV reactivation (supplemental Figure 1), occurring at a median time of 125 days (IQR, 85-331) after the first dose of immunochemotherapy; 21 of these patients had HBV DNA levels ≥100 IU/mL. Nine of the 27 patients were seropositive for anti-HBs, 17 were seronegative for anti-HBs, and information was missing for 1 patient. The characteristics and clinical course of the 27 patients with HBV reactivation are summarized in supplemental Table 3.
Among the 232 patients who did not receive prophylactic NAT, 25 (10.8%; including 7 of 8 patients with detectable, but not quantifiable, HBV DNA at baseline) had HBV reactivation with a median HBV DNA peak of 342 IU/mL (IQR, 101-1230) (Figure 1; supplemental Table 3; patients 1-25). Nine of these patients (36.0%) had HBV reactivation after completion of immunochemotherapy, occurring at a median of 196 days (IQR, 153-310) after the last dose of immunochemotherapy. All 25 patients received preemptive NAT following HBV reactivation; 19 received entecavir, 3 lamivudine, 1 entecavir and lamivudine, 1 tenofovir, and 1 adefovir. Among the 94 patients who received prophylactic NAT, 2 (2.1%) had HBV reactivation, 1 at 18 months after stopping NAT (18 months of lamivudine) and 1 who was still on NAT (11 months of lamivudine) (Figure 1; supplemental Table 3; patients 26 and 27).
The distribution of the 27 patients with HBV reactivation by study treatment was 16 out of 121 (13.2%) and 7 out of 114 (6.1%) patients treated with G-CHOP and R-CHOP, respectively, in GOYA (all DLBCL patients by enrollment); 1 out of 34 (2.9%) and 3 out of 57 (5.3%) patients treated with obinutuzumab- (1 G-CHOP) and rituximab-based chemotherapy (1 R-CHOP, 1 R-CVP, and 1 R-bendamustine) in GALLIUM (all FL patients) (supplemental Figure 1). Among 16 patients with HBV reactivation during immunochemotherapy, 12 withheld study treatment of a median of 22 days. No patients discontinued immunochemotherapy due to HBV reactivation. HBV-related hepatitis was not observed in any of 27 patients with HBV reactivation.
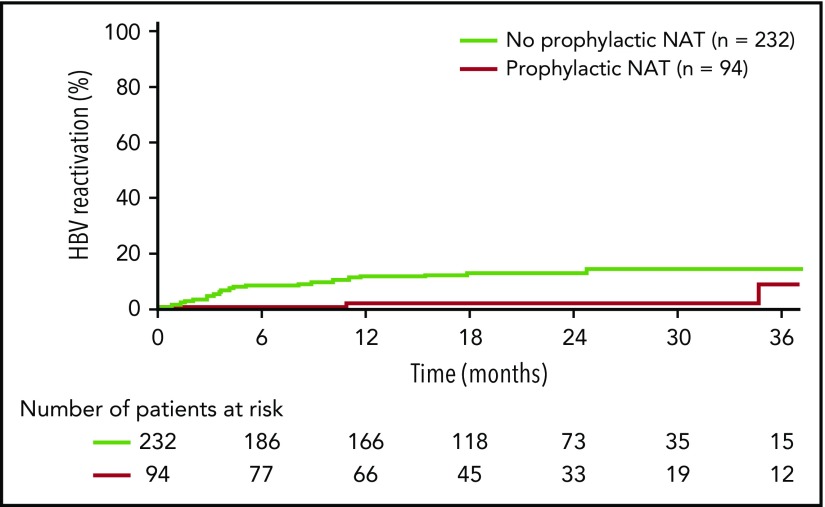
Kaplan-Meier analysis revealed a longer time to HBV reactivation in patients who received prophylactic NAT compared with those who did not (Figure 2). Estimated HBV reactivation rates (95% confidence intervals [CIs]) were 1.5% (0% to 4.3%) vs 10.6% (6.4% to 14.9%) at 1 year and 1.5% (0% to 4.3%) vs 11.9% (7.4% to 16.5%) at 2 years.
Figure 2.
Time to HBV reactivation in anti–HBc-positive patients by receipt of prophylactic NAT (analysis population, n = 326).
Cox regression analysis of risk factors for reactivation
Univariate Cox regression analysis indicated an association (P < .2) between HBV reactivation risk and patients who were seronegative for anti-HBs at baseline, patients with a diagnosis of DLBCL, and patients with detectable baseline HBV DNA levels (Table 2). The analysis also supported a reduction in the risk of HBV reactivation in patients who received prophylactic NAT.
Table 2.
Cox regression analysis for time to HBV reactivation (analysis population, n = 326)
| Effect/covariate* | Time to HBV reactivation (univariate)† | Time to HBV reactivation (multivariate) | ||||
|---|---|---|---|---|---|---|
| Crude HR | 95% CI | P | Adjusted HR | 95% CI | P | |
| Age at baseline (continuous, by 10 y) | 1.24 | 0.90-1.79 | .2260 | 1.63 | 1.00-2.37 | .0351 |
| Sex (male vs female) | 1.51 | 0.68-3.37 | .3110 | 1.19 | 0.50-2.86 | .6923 |
| ECOG performance status at baseline (2 vs 0 or 1) | 1.04 | 0.25-4.42 | .9531 | NA | NA | NA |
| Lymphoma type‡ (DLBCL [GOYA] vs non-DLBCL [GALLIUM]) | 3.06 | 1.04-9.04 | .0430 | 3.76 | 1.09-12.96 | .0356 |
| IPI, FLIPI score (high-intermediate/high vs low/intermediate/low-intermediate) | 1.51 | 0.71-3.21 | .2877 | NA | NA | NA |
| Anti-HBs at baseline (negative vs positive) | 4.35 | 1.93-9.78 | .0004 | 4.00 | 1.71-9.37 | .0014 |
| HBV DNA level at baseline, IU/mL (detectable vs not detectable) | 12.42 | 4.96-31.07 | <.0001 | 18.22 | 6.04-54.93 | <.0001 |
| Prophylactic NAT (yes vs no) | 0.19 | 0.04-0.79 | .0226 | 0.09 | 0.02-0.41 | .0018 |
| Antibody treatment group (G-Chemo vs R-Chemo) | 1.94 | 0.89-4.24 | .0963 | 1.79 | 0.78-4.16 | .1748 |
| Chemotherapy group§ (CHOP vs non-CHOP) | 1.86 | 0.44-7.94 | .3990 | NA | NA | NA |
NA, not applicable.
Reference groups for each factor are shown in bold. Race/ethnicity was not included in the model.
Factors with P < .2 by univariate analysis, baseline age, and sex were included in the multivariate analysis; 95% Wald confidence interval and P value for Wald test.
Includes DLBCL patients from GOYA and non-DLBCL patients from GALLIUM.
Includes patients from both GOYA and GALLIUM.
On multivariate analysis, the following independent factors were associated with the risk of HBV reactivation: older age (by decade), type of lymphoma (DLBCL), seronegativity for anti-HBs, detectable HBV DNA at baseline, and prophylactic NAT (Table 2). Detectable HBV DNA at baseline was strongly associated with an increased risk of reactivation (adjusted hazard ratio [HR]; [95% CI], 18.22 [6.04-54.93]). Conversely, prophylactic NAT was strongly associated with a reduced risk of HBV reactivation (adjusted HR [95% CI], 0.09 [0.02-0.41]).
Multivariate analysis using the DLBCL (GOYA) cohort also showed the following independent risk factors for HBV reactivation: seronegativity for anti-HBs (HR [95% CI], 4.21 [1.60-11.07]); detectable baseline HBV DNA levels (HR [95% CI], 36.36 [10.44-126.71]); and prophylactic NAT (HR [95% CI], 0.08 [0.02-0.37]) (supplemental Table 4).
Discussion
The association between rituximab use and HBV reactivation in patients receiving immunochemotherapy for lymphoma is well established.6,11,19,22 However, little is known about the risk of HBV reactivation with the type II anti-CD20 antibody obinutuzumab. This analysis of data collected during the phase 3 GOYA and GALLIUM studies42,43 provides valuable information on the incidence and risk management of HBV reactivation and HBV-related clinical hepatitis in B-cell NHL (DLBCL or FL) patients with resolved HBV infection receiving frontline obinutuzumab- or rituximab-based immunochemotherapy.
The overall incidence of HBV reactivation in this analysis was 8.2%. In the 232 patients who did not receive prophylactic NAT, the incidence of HBV reactivation was 10.8%, which equated to 25 patients. Importantly, HBV-related hepatitis was not observed among these 25 patients, although relatively high-peak HBV DNA levels exceeding 20 000 IU/mL were seen in 3 patients. Two large-scale prospective studies have demonstrated that monthly monitoring of HBV DNA can help identify patients in the initial stages of HBV reactivation who may benefit from prompt antiviral treatment.18,22 In 2 of the 3 patients with high-peak HBV DNA levels, the interval between HBV DNA measurements was ≥3 months, which incurred protocol violations and meant that instigation of preemptive NAT was delayed. In the third patient, preemptive NAT was not started immediately after the confirmed HBV reactivation.
In accordance with the study protocols, 12 of the 25 patients who had HBV reactivation without prophylactic NAT had their immunochemotherapy delayed (for a median of approximately 3 weeks) because of HBV reactivation; this action has the potential to negatively impact clinical outcomes. In GOYA and GALLIUM, nonprophylaxis patients with HBV reactivation were managed not only by administrating preemptive NAT but also by withholding immunochemotherapy, based on the scarce evidence available regarding HBV reactivation at the start of the trials. Current evidence now shows that if HBV reactivation is identified at an early stage by HBV DNA monitoring, it is possible to start preemptive NAT immediately without withholding immunochemotherapy.18,22
Delayed HBV reactivation (ie, 6-12 months after completion of lymphoma treatment) is an important concern in patients receiving anti-CD20 immunochemotherapy.4,6,44-48 In the present study, of the 25 patients with HBV reactivation who did not receive prophylactic NAT, 9 (36%) had HBV reactivation after completion of immunochemotherapy, occurring at a median of 196 days (IQR, 153-310) after the last dose. The observation of HBV reactivation after cessation of immunochemotherapy suggests that HBV DNA monitoring is necessary for at least 1 year after completion of lymphoma treatment.
Among the 94 patients who received prophylactic NAT, 2 (2.1%) had HBV reactivation. Reactivation occurred during NAT (lamivudine) in 1 patient and after stopping NAT (lamivudine) in the other. Although HBV genotypic data were not collected in GOYA and GALLIUM, long-term use of prophylactic lamivudine has been associated with drug resistance in other studies of patients with resolved HBV infection receiving immunochemotherapy.31,32 Prior studies suggest that entecavir, the most widely used preemptive NAT in GOYA and GALLIUM (used in 80% of patients who received preemptive NAT), is more effective than lamivudine in preventing HBV reactivation-related hepatitis; this is believed to be due to its higher barrier to resistance.2,3,6,12,23,49 For patients with lamivudine-resistance–associated HBV reactivation, entecavir could therefore be a salvage option. However, there is emerging evidence to suggest that the risk of entecavir resistance may be increased in patients with lamivudine resistance or prior exposure to lamivudine.50,51 Irrespective of which drug is used, HBV DNA monitoring (if available) is always recommended to evaluate the efficacy of prophylactic NAT because of the potential for suboptimal adherence and/or drug resistance. In addition to the potential for the development of resistance, there are concerns about the economic burden of prolonged antiviral treatment and the risk of delayed HBV reactivation after stopping NAT. As prophylactic NAT cannot be continued indefinitely in patients with resolved HBV infection, HBV DNA monitoring is necessary after stopping prophylactic NAT to diagnose delayed HBV reactivation. Furthermore, HBV DNA monitoring can be used to identify patients with previously unknown host and viral risk factors for HBV reactivation. Considering all these points, preemptive NAT guided by HBV DNA monitoring appears to be a reasonable strategy for most patients with resolved HBV infection. Prophylactic NAT, on the other hand, may be most appropriate for patients with clearly defined risk factors for HBV reactivation, such as anti-HBs seronegativity or detectable HBV DNA. In this regard, some institutions have already introduced computerized alert systems to notify health care providers of patients with known risk factors for HBV reactivation when prescribing immunochemotherapy.52,53
On multivariate regression analysis, both seronegativity for anti-HBs and detectable HBV DNA levels at baseline appeared to be associated with an increased risk of HBV reactivation, as were older age and lymphoma subtype (DLBCL). The finding that seronegativity for anti-HBs was a risk factor for HBV reactivation was consistent with the results of a recent large meta-analysis demonstrating a significant association between anti-HBs seropositivity and a decreased risk of reactivation in patients with resolved HBV receiving chemotherapy for hematologic malignancies without antiviral prophylaxis.54 Lymphoma and detectable thresholds of HBV DNA have both been identified previously as risk factors for HBV reactivation.2,4,8,15,17,18,55,56 With respect to lymphoma type as a risk factor, a prospective observational study of regular HBV DNA monitoring in 269 B-cell lymphoma patients treated with rituximab plus corticosteroid-containing chemotherapy showed that DLBCL was associated with HBV reactivation in a univariate analysis, but it was not found to be an independent risk factor in a subsequent multivariate analysis.18 In contrast, using data collected in 2 large-scale prospective trials, the present analysis demonstrated that DLBCL was one of several independent risk factors for HBV reactivation. However, as all DLBCL patients in our analysis received CHOP chemotherapy, a known risk factor for HBV reactivation,11-19 we cannot conclusively determine whether the association was with lymphoma type or the chemotherapy regimen. Further basic research and clinical trials are now warranted to improve our understanding of the pathogenesis of HBV reactivation in DLBCL patients who receive anti-CD20 antibodies with chemotherapy and to determine if DLBCL truly increases the risk of reactivation compared with other lymphoma types. Use of prophylactic NAT, which was not mandated in either study protocol but was allowed at the discretion of the investigator, was associated with a reduced risk of HBV reactivation, as demonstrated in other studies of NAT in lymphoma patients with resolved HBV infection treated with R-Chemo.12,23,29
There was no significant difference in the risk of HBV reactivation between obinutuzumab- and rituximab-based immunochemotherapy on multivariate analysis (Table 2; supplemental Table 4). However, confounding factors prevent conclusions on whether there is a higher risk of HBV reactivation with obinutuzumab- vs rituximab-based regimens, due to the imbalance of baseline risk factors between the 2 treatment groups. The higher rate of HBV reactivation in GOYA compared with GALLIUM probably results from the higher number of patients enrolled from regions with a high endemic prevalence of HBV infection (data not shown). It is not clear whether there are any differences in background HBV prevalence among B-cell lymphoma subtypes due to conflicting reports.57,58
Our findings indicate that HBV DNA monitoring–guided preemptive NAT may be effective for preventing HBV-related hepatitis in patients treated with obinutuzumab- and rituximab-based immunochemotherapy (including maintenance) for B-cell lymphomas. Thus, while preemptive NAT is associated with a higher risk of HBV reactivation compared with prophylaxis, this approach does not appear to increase the risk of clinical hepatitis. Currently, most guidelines recommend that anti–HBc-positive patients should undergo regular HBV DNA monitoring unless they are receiving prophylactic NAT, although the optimal frequency of assessment has not been determined.2,26-28,59-62 Although further investigations are required, our findings suggest that monthly HBV DNA monitoring may be adequate. Preemptive NAT avoids unnecessary antiviral treatment, thereby reducing drug costs.29,30 However, for patients with resolved HBV infection who are at elevated risk for HBV reactivation (eg, those with reduced liver function, anti-HBs seronegativity, or detectable HBV DNA), prophylactic NAT appears to be an appropriate strategy, and this is reflected in guideline recommendations.2,26-28,59-62 Among patients not receiving prophylactic NAT in this analysis, 7 of 8 patients with detectable, but not quantifiable, HBV DNA at baseline had HBV reactivation, suggesting that these “high-risk” patients could be candidates for prophylactic NAT. Prophylactic NAT (preferably using an antiviral drug with a high barrier to resistance) may also be beneficial in situations where regular HBV DNA monitoring with an appropriately sensitive test is not feasible for logistical reasons or if a physician suspects that the patient will not adhere to the monitoring schedule. However, as discussed above, such a blanket approach to antiviral treatment has the potential to increase the risk of drug resistance, especially if an antiviral with a low barrier to resistance (eg, lamivudine) is used.8 In regions or countries where only NATs with a low barrier to resistance are available, preemptive treatment may be preferable, but only if adequate HBV DNA monitoring tests are available. In near future, a new ultra-high-sensitivity HBsAg assay is likely to become available as an alternative method to monitor HBV reactivation.63 This assay is likely to be cheaper and more accessible and provide more rapid results than conventional HBV DNA monitoring and is therefore likely to be favored in regions or countries with limited resources.
Health economic analyses comparing prophylactic NAT with HBV DNA monitoring–guided preemptive NAT are lacking. This may be related to the complexity of conducting an economic analysis and providing a meaningful comparison due to vast differences in NAT and HBV DNA monitoring availability, practices, and costs between countries or centers .
It is difficult to compare the rate of HBV reactivation observed in the current analysis with other analyses due to the heterogeneous definitions of reactivation and various assay methodologies used in previous studies.6,8,9 The definition selected for this analysis was based on the HBV reactivation management protocols and sensitivity of the PCR assays used in GOYA and GALLIUM and may be regarded as more conservative than many of the definitions used in the literature. If historic definitions used in retrospective studies were to be applied to our data (eg, an HBV DNA cutoff ≥100 IU/mL with increasing serum aminotransferase levels, at least a 10-fold rise in HBV DNA, or reverse seroconversion to HBsAg-positive status),3,6,9 the reported rate of HBV reactivation would likely be lower. This discrepancy highlights the need for a standardized approach for both measuring and defining HBV reactivation. However, extension of preemptive NAT to clinical practice based on this trial would necessitate use of the same definition of HBV reactivation and a similarly sensitive HBV DNA assay used at monthly intervals.
This analysis was limited by imbalances in relevant baseline characteristics between the 2 treatment arms, which was unsurprising given that HBV reactivation was not a major end point in either trial. In particular, a selection bias was noted, whereby white Europeans were more likely to receive prophylactic NAT than patients of other races and regions. This bias was likely due to fact that the decision to use prophylactic NAT was made by physicians, based presumably on their assessment of risk, national or local guidelines, personal preference, availability of resources, reimbursement considerations, and availability of data regarding HBV DNA monitoring–guided preemptive NAT. The studies were also limited by the fact that slightly different approaches were used in GOYA and GALLIUM to manage HBV reactivation, including minor differences in the criteria for initiating preemptive NAT.
In conclusion, HBV DNA monitoring–guided preemptive NAT appeared to be effective in preventing HBV-related hepatitis during treatment with obinutuzumab- or rituximab-containing immunochemotherapy in patients with B-cell NHL and resolved HBV infection. Prophylactic NAT was also effective in preventing HBV reactivation (91% risk reduction) and may be an appropriate option for high-risk patients with anti-HBs seronegativity or detectable HBV DNA at baseline.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors would like to acknowledge the important contributions made by the investigators and the clinical and safety teams for the GALLIUM and GOYA trials.
The current studies were sponsored by F. Hoffmann-La Roche Ltd. Third-party medical writing assistance, under the direction of S.K., was provided by Louise Profit, Cheryl Wright, and Scott Malkin of Gardiner-Caldwell Communications and was funded by F. Hoffmann-La Roche Ltd.
Footnotes
Presented in poster form at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 10 December 2017.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.D.Z. contributed to the conception and design of the study, the collection, assembly, analysis, and interpretation of data, and manuscript writing; E.U., H.K., and T.N. contributed to the conception and design of the study and the collection, assembly, analysis, and interpretation of data; G.F.-R. and S.K. contributed to the design and conduct of the study, analysis and interpretation of data, and manuscript writing; G.S. and J.J. contributed to the collection, assembly, analysis, and interpretation of data; K.T. contributed to the design and conduct of the study, collection, analysis, and interpretation of data, and manuscript writing; L.A. contributed to the collection and assembly of data and manuscript writing; M.G.P. and H.P.-L. contributed to the analysis and interpretation of data and manuscript writing; W.S.K. contributed to the conception and design of the study and the collection and assembly of data; Y.L.K. contributed to the treatment of patients and interpretation of data; Y.T. and X.H. contributed to the analysis and interpretation of data; and all authors reviewed the manuscript and provided final approval.
Conflict-of-interest disclosure: A.D.Z. reports consulting or advisory roles for Adaptive Biotechnologies, Amgen, Celgene, Genentech, Gilead Sciences, and Dr. Reddy's Laboratories and research funding from Bristol-Myers Squibb, Genentech, Gilead Sciences, and Roche. E.U. reports employment and stock ownership from Chugai Pharmaceutical. G.F.-R. reports employment and stock ownership from Roche. G.S. reports employment from Roche. H.K. reports employment and stock ownership from Chugai Pharmaceutical. H.P.-L. reports employment from Roche. K.T. reports consulting fees from HUYA Bioscience International and Zenyaku Kogyo and grants, honoraria, and consulting fees from Celgene; grants and honoraria from Chugai Pharmaceutical, Eisai, Janssen, Kyowa Hakko Kirin, Mundipharma, Ono Pharmaceutical, and Takeda; and grants from AbbVie, GlaxoSmithKline, and Servier. L.A. reports consulting or advisory roles for Bayer, Celgene, Gilead Sciences, Roche, and Sandoz, research funding from Gilead Sciences, and participation in a speakers bureau for Celgene. M.G.P. reports that her spouse is an employee of Roche. S.K. reports honoraria and research funding from Bristol-Myers Squibb, Chugai Pharmaceutical, Roche, and Kyowa Hakko Kirin. T.N. reports employment and stock ownership from Roche. Y.L.K reports advisory roles for Abbvie, Amgen, Bristol-Myers Squibb, Janssen, Novartis, Roche, and Takeda. Y.T. reports honoraria and research funding from Bristol-Myers Squibb, Chugai Pharmaceutical and Gilead Sciences. The remaining authors declare no competing financial interests.
Correspondence: Shigeru Kusumoto, Department of Hematology and Oncology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-chou, Mizuho-ku, Nagoya, Aichi 467-8601, Japan; e-mail: skusumot@med.nagoya-cu.ac.jp.
REFERENCES
- 1.Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis. 2013;33(2):167-177. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. March 2015. http://www.worldhepatitisalliance.org/sites/default/files/resources/documents/Hep%20B%20Guidelines.pdf. Accessed 2 March 2018. [PubMed]
- 3.Ozoya OO, Sokol L, Dalia S. Hepatitis B reactivation with novel agents in non-Hodgkin’s lymphoma and prevention strategies. J Clin Transl Hepatol. 2016;4(2):143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HY, Kim W. Chemotherapy-related reactivation of hepatitis B infection: updates in 2013. World J Gastroenterol. 2014;20(40):14581-14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang JP, Barbo AG, Perrillo RP. Hepatitis B reactivation during cancer chemotherapy: an international survey of the membership of the American Association for the Study of Liver Diseases. J Viral Hepat. 2015;22(3):346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):221-244.e3. [DOI] [PubMed] [Google Scholar]
- 7.Hui CK, Cheung WW, Zhang HY, et al. . Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131(1):59-68. [DOI] [PubMed] [Google Scholar]
- 8.Tavakolpour S, Alavian SM, Sali S. Hepatitis B reactivation during immunosuppressive therapy or cancer chemotherapy, management, and prevention: a comprehensive review-screened. Hepat Mon. 2016;16(4):e35810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bessone F, Dirchwolf M. Management of hepatitis B reactivation in immunosuppressed patients: An update on current recommendations. World J Hepatol. 2016;8(8):385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umemura T, Tanaka E, Kiyosawa K, Kumada H; Japan de novo Hepatitis B Research Group. Mortality secondary to fulminant hepatic failure in patients with prior resolution of hepatitis B virus infection in Japan. Clin Infect Dis. 2008;47(5):e52-e56. [DOI] [PubMed] [Google Scholar]
- 11.Dong HJ, Ni LN, Sheng GF, Song HL, Xu JZ, Ling Y. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol. 2013;57(3):209-214. [DOI] [PubMed] [Google Scholar]
- 12.Kim SJ, Hsu C, Song Y-Q, et al. . Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: analysis from the Asia Lymphoma Study Group. Eur J Cancer. 2013;49(16):3486-3496. [DOI] [PubMed] [Google Scholar]
- 13.Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol. 2009;90(1):13-23. [DOI] [PubMed] [Google Scholar]
- 14.Kusumoto S, Tobinai K. Screening for and management of hepatitis B virus reactivation in patients treated with anti-B-cell therapy. Hematology Am Soc Hematol Educ Program. 2014;2014:576-583. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsumi Y, Yamamoto Y, Ito S, et al. . Hepatitis B virus reactivation with a rituximab-containing regimen. World J Hepatol. 2015;7(21):2344-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozessohn L, Chan KK, Feld JJ, Hicks LK. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for lymphoma: a meta-analysis. J Viral Hepat. 2015;22(10):842-849. [DOI] [PubMed] [Google Scholar]
- 17.Yeo W, Chan TC, Leung NW, et al. . Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27(4):605-611. [DOI] [PubMed] [Google Scholar]
- 18.Kusumoto S, Tanaka Y, Suzuki R, et al. . Monitoring of hepatitis B virus (HBV) DNA and risk of HBV reactivation in B-cell lymphoma: a prospective observational study. Clin Infect Dis. 2015;61(5):719-729. [DOI] [PubMed] [Google Scholar]
- 19.Evens AM, Jovanovic BD, Su YC, et al. . Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22(5):1170-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiraki Y, Kawano A, Shigematsu H, Miki K, Nomura H, Shimoda S. Two cases of malignant lymphoma with reactivation of resolved hepatitis B virus infection after bendamustine hydrochloride monotherapy. Nippon Shokakibyo Gakkai Zasshi. 2016;113(9):1582-1587. [DOI] [PubMed] [Google Scholar]
- 21.Tsutsumi Y, Ogasawara R, Miyashita N, Tanaka J, Asaka M, Imamura M. HBV reactivation in malignant lymphoma patients treated with rituximab and bendamustine. Int J Hematol. 2012;95(5):588-591. [DOI] [PubMed] [Google Scholar]
- 22.Seto WK, Chan TS, Hwang YY, et al. . Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32(33):3736-3743. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Li X, Zhu J, et al. . Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312(23):2521-2530. [DOI] [PubMed] [Google Scholar]
- 24.Abramson JS, Chung RT. Optimal antiviral prophylaxis against hepatitis B reactivation in patients receiving rituximab-based chemotherapy for lymphoma. JAMA. 2014;312(23):2505-2507. [DOI] [PubMed] [Google Scholar]
- 25.Buensalido JA, Chandrasekar PH. Prophylaxis against hepatitis B reactivation among patients with lymphoma receiving rituximab. Expert Rev Anti Infect Ther. 2014;12(2):151-154. [DOI] [PubMed] [Google Scholar]
- 26.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662. [DOI] [PubMed] [Google Scholar]
- 27.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.euEuropeanAssociation for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370-398. [DOI] [PubMed] [Google Scholar]
- 28.Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy [published correction appears in Gastroenterology. 2015;148(2):455]. Gastroenterology. 2015;148(1):215-219. [DOI] [PubMed] [Google Scholar]
- 29.Huang YH, Hsiao LT, Hong YC, et al. . Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31(22):2765-2772. [DOI] [PubMed] [Google Scholar]
- 30.Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Is antiviral prophylaxis necessary to prevent hepatitis B virus (HBV) reactivation in patients with HBV-resolved infection receiving rituximab-containing chemotherapy? J Clin Oncol. 2013;31(35):4480. [DOI] [PubMed] [Google Scholar]
- 31.Castelli R, Ferraris L, Pantaleo G, Lambertenghi Deliliers G, Cicardi M. High rate of hepatitis B viral breakthrough in elderly non-Hodgkin lymphomas patients treated with Rituximab based chemotherapy. Dig Liver Dis. 2016;48(11):1394-1397. [DOI] [PubMed] [Google Scholar]
- 32.Grossi G, Viganò M, Facchetti F, et al. . Failure of long-term lamivudine prophylaxis in patients with resolved hepatitis B infection undergoing chemotherapy and allogenic hematopoietic stem cell transplantation for hematological malignancies: two case reports. Haematologica. 2017;102(10):e423-e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu C, Tsou HH, Lin SJ, et al. ; Taiwan Cooperative Oncology Group. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014;59(6):2092-2100. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz SM, Zelenetz AD, Gordon LI, et al. . NCCN guidelines insights: non-Hodgkin’s lymphomas, version 3.2016. J Natl Compr Canc Netw. 2016;14(9):1067-1079. [DOI] [PubMed] [Google Scholar]
- 35.Zelenetz AD, Gordon LI, Wierda WG, et al. . Diffuse large B-cell lymphoma version 1.2016. J Natl Compr Canc Netw. 2016;14(2):196-231. [DOI] [PubMed] [Google Scholar]
- 36.Dreyling M, Ghielmini M, Rule S, Salles G, Vitolo U, Ladetto M; ESMO Guidelines Committee. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v83-v90. [DOI] [PubMed] [Google Scholar]
- 37.Tilly H, Gomes da Silva M, Vitolo U, et al. ; ESMO Guidelines Committee. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v116-v125. [DOI] [PubMed] [Google Scholar]
- 38.Gazyva (obinutuzumab) injection, for intravenous infusion [full prescribing information; revised February 2016]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125486s013lbl.pdf. Accessed 14 March 2018.
- 39.Gazyvaro 1,000 mg concentrate for solution for infusion [summary of product characteristics; last updated 28 July, 2016]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002799/WC500171594.pdf. Accessed 14 March 2018.
- 40.Herter S, Herting F, Mundigl O, et al. . Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12(10):2031-2042. [DOI] [PubMed] [Google Scholar]
- 41.Mössner E, Brünker P, Moser S, et al. . Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitolo U, Trněný M, Belada D, et al. . Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3529-3537. [DOI] [PubMed] [Google Scholar]
- 43.Marcus R, Davies A, Ando K, et al. . Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331-1344. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Rodriguez MJ, Canales MA, Hernandez-Maraver D, Hernandez-Navarro F. Late reactivation of resolved hepatitis B virus infection: an increasing complication post rituximab-based regimens treatment? Am J Hematol. 2008;83(8):673-675. [DOI] [PubMed] [Google Scholar]
- 45.Dominguez N, Manzano ML, Muñoz R, Martin A, Fernandez I, Castellano G. Late reactivation of occult hepatitis B virus infection in a patient with chronic lymphocytic leukemia after rituximab and fludarabine-based regimen. Leuk Lymphoma. 2015;56(4):1160-1163. [DOI] [PubMed] [Google Scholar]
- 46.Chew E, Thursky K, Seymour JF. Very late onset hepatitis-B virus reactivation following rituximab despite lamivudine prophylaxis: the need for continued vigilance. Leuk Lymphoma. 2014;55(4):938-939. [DOI] [PubMed] [Google Scholar]
- 47.Nakaya A, Fujita S, Satake A, et al. . Delayed HBV reactivation in rituximab-containing chemotherapy: How long should we continue anti-virus prophylaxis or monitoring HBV-DNA? Leuk Res. 2016;50:46-49. [DOI] [PubMed] [Google Scholar]
- 48.Dai MS, Chao TY, Kao WY, Shyu RY, Liu TM. Delayed hepatitis B virus reactivation after cessation of preemptive lamivudine in lymphoma patients treated with rituximab plus CHOP. Ann Hematol. 2004;83(12):769-774. [DOI] [PubMed] [Google Scholar]
- 49.Li HR, Huang JJ, Guo HQ, et al. . Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat. 2011;18(12):877-883. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Cho Y, Lee DH, et al. . Prior exposure to lamivudine increases entecavir resistance risk in chronic hepatitis B Patients without detectable lamivudine resistance. Antimicrob Agents Chemother. 2014;58(3):1730-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee GH, Inoue M, Toh JK, et al. . Two-step evolution of the hepatitis B drug-resistant mutations in a patient who developed primary entecavir resistance. Liver Int. 2013;33(4):642-646. [DOI] [PubMed] [Google Scholar]
- 52.Sampedro B, Hernández-López C, Ferrandiz JR, et al. . Computerized physician order entry-based system to prevent HBV reactivation in patients treated with biologic agents: the PRESCRIB project. Hepatology. 2014;60(1):106-113. [DOI] [PubMed] [Google Scholar]
- 53.Notsumata K, Kumai T, Ueda T, et al. . Automated risk management of HBV reactivation by encoding patients’ medical records. Kanzo. 2014;55(2):91-99. [Google Scholar]
- 54.Paul S, Dickstein A, Saxena A, et al. . Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis. Hepatology. 2017;66(2):379-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi K, Ishigami M, Ishizu Y, et al. . Clinical characteristics and molecular analysis of hepatitis B virus reactivation in hepatitis B surface antigen-negative patients during or after immunosuppressive or cytotoxic chemotherapy. J Gastroenterol. 2016;51(11):1081-1089. [DOI] [PubMed] [Google Scholar]
- 56.Yeo W, Zee B, Zhong S, et al. . Comprehensive analysis of risk factors associating with Hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90(7):1306-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcucci F, Spada E, Mele A, Caserta CA, Pulsoni A. The association of hepatitis B virus infection with B-cell non-Hodgkin lymphoma - a review. Am J Blood Res. 2012;2(1):18-28. [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong W, Lv R, Li H, et al. . Prevalence of hepatitis B and hepatitis C viral infections in various subtypes of B-cell non-Hodgkin lymphoma: confirmation of the association with splenic marginal zone lymphoma. Blood Cancer J. 2017;7(3):e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coffin CS, Fung SK, Ma MM; Canadian Association for the Study of the Liver. Management of chronic hepatitis B: Canadian Association for the Study of the Liver consensus guidelines. Can J Gastroenterol. 2012;26(12):917-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin P, Lau DT, Nguyen MH, et al. . A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2015 update. Clin Gastroenterol Hepatol. 2015;13(12):2071-87.e16. [DOI] [PubMed] [Google Scholar]
- 61.Sarin SK, Kumar M, Lau GK, et al. . Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shinkai N, Kusumoto S, Murakami S, et al. . Novel monitoring of hepatitis B reactivation based on ultra-high sensitive hepatitis B surface antigen assay. Liver Int. 2017;37(8):1138-1147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.