Abstract
Background
The prophylactic application of antimicrobials that are active against Staphylococcus aureus can prevent infections. However, implementation in clinical practice is limited. We have reviewed antimicrobial approaches for the prevention of S. aureus infections.
Methods
We searched the Cochrane Central Register of Controlled Trials, PubMed/MEDLINE and EMBASE databases and trial registries using synonyms for S. aureus, infections and prevention as search terms. We included randomized controlled trials and systematic reviews only.
Results
Most studies were conducted with mupirocin. Mupirocin is effective in preventing S. aureus infections in patients receiving dialysis treatment and in surgical patients, particularly if the patients are carriers of S. aureus. The combination of mupirocin and chlorhexidine, but not chlorhexidine alone, is also effective against S. aureus infections. So far, vaccines have not proven successful in protecting against S. aureus infections. Regarding prophylactic povidone–iodine and systemic antibiotics, there is limited evidence supporting their effectiveness against S. aureus infections. Antimicrobial honey has not been proven to be more effective or non-inferior to mupirocin in protecting against S. aureus infections.
Conclusions
The current evidence supports the use of mupirocin as prophylaxis for preventing infections with S. aureus, particularly in carriers and in the surgical setting or in patients receiving dialysis treatment. Other antimicrobial agents have not been sufficiently proven to be effective so far, or have been proven ineffective. New trials with vaccines and anti-staphylococcal peptides are currently underway and may lead to new preventive strategies in the future.
Introduction
Staphylococcus aureus is a Gram-positive, aerobic bacterium that is an important cause of infections in humans. Approximately 30% of the healthy human population are carriers of S. aureus. Common carriage sites are the anterior nares, pharynx, perineum and skin.1,2S. aureus can cause invasive infections in the community and healthcare setting and has a broad spectrum of clinical syndromes, ranging from rather benign infections (e.g. folliculitis) to potentially life-threatening infections (e.g. bloodstream infection).3,4 Common healthcare-associated infections (HAIs) caused by S. aureus include surgical site infections (SSIs), hospital-acquired bloodstream infections (HA-BSIs) and pneumonia.4 These are important causes of morbidity, mortality and increased healthcare expenditure.5,6
Between 1998 and 2003, ∼1% of hospitalized patients developed an S. aureus infection in the USA. These infections accounted for an annual economic burden of ∼US$4.5 billion.7 Moreover, the disease burden has been increasing over time.8,9 Similarly, studies from Europe10,11 and Asia12 show an increased burden of disease associated with nosocomial S. aureus infections. The increased burden of disease is partly caused by the emergence of infections caused by MRSA,13 which adds to, rather than replaces, the burden of disease caused by MSSA.10,14 Taken together, these studies emphasize the significance of this pathogen in a global context. Because of the far-reaching consequences of S. aureus infections, prevention of these infections has been the subject of many investigations.
To establish effective preventive interventions, it is important to have knowledge of the characteristics and epidemiology of the causative pathogen, the pathogenesis of disease and the risk factors that predispose to S. aureus infections. Over the past few decades, several patient groups at high risk of staphylococcal disease have been identified, including patients with diabetes mellitus, end-stage renal disease or HIV infection.1,15 Interestingly, several studies have demonstrated that (nasal) carriers of S. aureus have an increased risk of developing S. aureus infections1,2 and that the vast majority of S. aureus infections arise from the patient’s own bacterial flora.16,17 This has been established in several populations, including surgical patients and patients receiving dialysis treatment.1,2 Besides these so-called endogenous infections, S. aureus infections may also develop after exogenous acquisition from healthcare workers, the environment and other patients.18 Differentiating between an endogenous infection and infection due to cross-transmission is important when assessing the effectiveness of different types of preventive interventions, as most of these interventions primarily target one route of infection. For instance, decolonization treatments are primarily aimed at preventing endogenous S. aureus infection, whereas interventions aimed at improving hygiene measures (e.g. hand hygiene) are mainly aimed at limiting cross-transmission.2,19
There is a vast amount of literature available on preventive interventions against S. aureus infections, but the evidence is heterogeneous regarding study designs, study populations, the epidemiological setting and the type of intervention. This has hampered translation of study results to clinical practice. Therefore, an overview of the available evidence concerning the use of preventive antimicrobial approaches against S. aureus infections is warranted. As such, the aim of this review is to assess the literature for evidence that explores the antimicrobial approaches that have been studied in humans for the prevention of S. aureus infections.
Methods
We performed a narrative review of randomized controlled trials (RCTs), systematic reviews and meta-analyses that investigated a (combination of) topical, oral or intravenous antimicrobial(s) given prophylactically (and preoperatively) to prevent S. aureus infections in carriers, non-carriers and unknown carriers of S. aureus in healthcare and non-healthcare settings. The term ‘prophylactic’ was defined as the administration of the antimicrobial(s) prior to the onset of symptoms that indicated the presence of an infection. RCTs that compared a preventive intervention to either placebo, alternative treatment, or standard treatment (no prophylaxis) and that reported, as an outcome, the number of acquired infections caused by S. aureus were eligible for inclusion. Systematic reviews and meta-analyses of RCTs with a similar objective as the current review were also assessed for inclusion. We excluded studies with other study designs (e.g. non-randomized studies, case series, case reports); studies that did not assess S. aureus infection as an outcome; studies that investigated the impact of care bundles that encompassed more interventions than only administration of prophylactic antimicrobials; studies that only included paediatric patients (i.e. patients below the age of 18 years); and studies that investigated perioperative, systemic, antibiotic prophylaxis, which we defined as the administration of antibiotics prior to or during surgery.
We identified relevant studies by searching the electronic databases PubMED/MEDLINE and EMBASE, and by scanning the reference lists of identified articles. The following search terms, along with their synonyms, were used to develop the literature search strategies: ‘Staphylococcus aureus’, ‘prophylaxis’, ‘preventive’, ‘randomized trial’, ‘review’ and ‘meta-analysis’. For the complete list of search terms, including synonyms, see the Supplementary data, available at JAC Online. Only studies in Dutch, English, Spanish, or studies in other languages that could easily be translated into English were eligible for inclusion. The Cochrane Central Register of Controlled Trials, Clinicaltrials.gov and WHO International Clinical Trial Registry Platform databases were also reviewed for identification of relevant trials. No restrictions on publication date were applied. Both title and abstract screening and screening of the full text of the retrieved studies were performed independently by two reviewers (D. P. R T. and D. H.) using a web-based software application. Disagreements on the eligibility of studies were resolved by reaching consensus through discussion. Data extraction was performed by one reviewer (D. P. R. T.). The data we abstracted included demographic information, information about setting and methodology, intervention details and reported outcomes. Because of the heterogeneity in interventions, results are presented by preventive antimicrobial approach.
Results
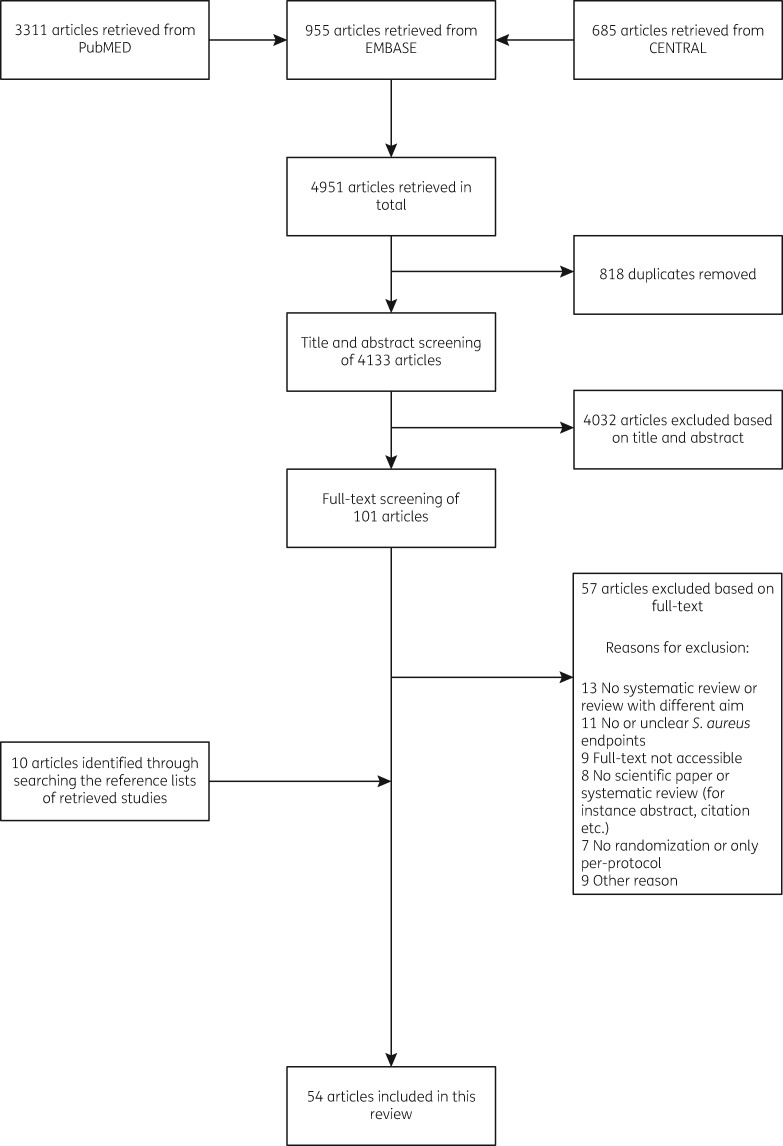
The literature search was conducted at the end of November 2017 and yielded 4951 articles. After removal of duplicates, 4133 articles were screened for inclusion. Based on the title and abstract screening, 4032 articles were excluded. The full text of the remaining 101 articles was assessed, and 57 additional articles were excluded based on a variety of reasons (e.g. no S. aureus infection as outcome or no comparator). After scanning the reference lists of the remaining articles and searching the trial databases, we identified 10 additional articles. Finally, 54 articles were included in the analysis. Figure 1 depicts the flowchart of included studies, including the reasons for excluding studies.
Figure 1.
Flowchart of included studies.
Mupirocin
Our search identified several studies that assessed the effect of prophylactic mupirocin on the occurrence of S. aureus infection in different patient groups. Here we report the findings according to the patient groups in which they were found.
Dialysis patients
Two strategies of mupirocin application were investigated in studies that included dialysis patients: (i) application of mupirocin to the nares (intranasal application); or (ii) application of mupirocin to the catheter exit-site. As the location of mupirocin application may modify the effect of mupirocin on the development of S. aureus infections, we present the data according to location of mupirocin application.
Intranasal application
The studies by the Mupirocin Study Group,20 Sit et al.21 and Boelaert et al.22 assessed the effect of prophylactic intranasal mupirocin on the occurrence of S. aureus infections in patients receiving dialysis treatment. The Mupirocin Study Group20 and Sit et al.21 included patients on continuous ambulatory peritoneal dialysis (CAPD), whereas Boelaert et al.22 included haemodialysis patients. The Mupirocin Study Group20 and Boelaert et al.22 included nasal carriers of S. aureus only, whereas Sit et al.21 also included non-carriers. Both Boelaert et al.22 and the Mupirocin Study Group20 demonstrated that intranasal mupirocin significantly reduced the number of infections caused by S. aureus compared with placebo. Sit et al.,21 however, found no statistically significant differences in the occurrence of S. aureus infection between the mupirocin and control group. In conclusion, intranasal mupirocin prophylaxis is effective in protecting patients on dialysis who are carriers of S. aureus from developing infections with this microorganism.
Catheter exit-site application
Several studies investigated the effect of prophylactic mupirocin at the catheter exit-site on the occurrence of S. aureus infections. Johnson et al.,23 Sesso et al.24 and Wong et al.25 compared mupirocin with no prophylaxis, Bernardini et al. (2005)26 compared mupirocin with gentamicin cream at the catheter exit-site, and Bernardini et al. (1996)27 had oral rifampicin as a comparator. Johnson et al.23 and Sesso et al.24 included haemodialysis patients, whereas the other three studies included CAPD patients.25–27 All studies included both carriers and non-carriers of S. aureus. When mupirocin was compared with no prophylaxis, mupirocin use led to a significant reduction in the number of exit-site infections by Gram-positive bacteria. This reduction was primarily the result of a lower incidence of infection caused by S. aureus.23–25 In two studies, the use of mupirocin led to a lower incidence of S. aureus bacteraemia.23,24S. aureus peritonitis was assessed in the study by Wong et al.25 only and occurred infrequently, resulting in insufficient power to draw conclusions. Based on these studies it is concluded that mupirocin at the catheter exit-site prevents S. aureus exit-site infection and bacteraemia. This effect was irrespective of nasal S. aureus carriage. In contrast, the studies that compared mupirocin with gentamicin cream at the catheter exit-site26 or oral rifampicin27 found no statistically significant differences in S. aureus exit-site or peritonitis rates between the mupirocin and the gentamicin or rifampicin groups. The authors therefore concluded that gentamicin and rifampicin were as effective as mupirocin in preventing S. aureus catheter-related infections. However, these studies were probably not adequately powered to show non-inferiority for this outcome because both studies were designed as superiority and not as non-inferiority trials. The study that compared mupirocin with gentamicin was only powered to show a statistically significant difference in the incidence of Pseudomonas aeruginosa exit-site infections between the groups, and the other study that compared mupirocin with rifampicin did not do a power calculation at all. Interestingly, patients who received gentamicin were less likely to develop infections with Gram-negative bacteria (including P. aeruginosa).26 Patients who received oral rifampicin, on the other hand, were more likely to stop treatment prematurely due to side effects.
In addition to the original studies, our search also retrieved three systematic reviews with meta-analyses.28–30 However, only Xu et al.28 and Grothe et al.29 performed a separate meta-analysis of RCTs. These meta-analyses included a selection of the previous studies. According to these two meta-analyses, mupirocin is superior to placebo or no prophylaxis in reducing the risk of S. aureus exit-site infections (reduction of 73%–87%)28,29 and S. aureus peritonitis in CAPD patients (reduction of 40%),28 and the risk of S. aureus bacteraemia in haemodialysis patients (reduction of 82%).29 The meta-analysis done by Tacconelli et al.,30 which also included observational studies, confirms these findings. In conclusion, application of mupirocin at the catheter exit site leads to fewer S. aureus catheter-related and bloodstream infections in patients receiving dialysis treatment.
Surgical patients
The effect of prophylactic mupirocin on postsurgical S. aureus infection has been studied repeatedly. In the studies by Perl et al.,31 Kalmeijer et al.,32 Konvalinka et al.,33 Garcia et al.34 and Suzuki et al.,35 intranasal mupirocin was compared with placebo31–33 or no prophylaxis.34,35 In comparison, Shuman et al.,36 Tai et al.37 and Bode et al.38 combined intranasal mupirocin with chlorhexidine body wash and compared this with no prophylaxis36,37 or placebo for both the nasal ointment and body wash.38 Both Konvalinka et al.33 and Bode et al.38 included S. aureus carriers only. Tai et al.37 included both S. aureus carriers and non-carriers, but only randomized carriers to either the mupirocin or control group and included a separate group of non-carriers. Finally, Perl et al.31 and Garcia et al.34 performed a separate subgroup analysis for S. aureus carriers. The study populations of all the studies described above combined included elective patients undergoing cardiothoracic, general, oncologic, neurologic, orthopaedic, and/or head and neck surgical procedures. The study by Bode et al.38 also included a small fraction of patients who did not undergo surgery.
When the effect was assessed in carriers and non-carriers as one group, mupirocin did not significantly reduce the risk of nosocomial S. aureus infection when compared with placebo or no prophylaxis.31,32,34–36 In contrast, when the effect of mupirocin was assessed in S. aureus carriers only, Perl et al.31 showed that mupirocin was more effective than placebo in protecting against nosocomial S. aureus infection (OR: 0.49; 95% CI: 0.25–0.92). Bode et al.38 also observed a lower incidence of S. aureus infection among carriers treated with mupirocin and chlorhexidine compared with carriers who received placebo (relative risk: 0.42: 95% CI: 0.23–0.75 for all nosocomial S. aureus infections and relative risk: 0.21; 95% CI 0.07–0.62 for deep SSIs caused by S. aureus). Similar results were found by Tai et al.37 (4% versus 11% S. aureus infection in respectively the mupirocin and chlorhexidine gluconate and the no-prophylaxis group, relative risk: 0.3; 95% CI: 0.1–1.0). The study by Konvalinka et al.33 was the only one using just S. aureus carriers that did not find a statistically significant effect of intranasal mupirocin on infections caused by S. aureus.
We also found two systematic reviews and meta-analyses of RCTs that investigated the pooled effect of prophylactic intranasal mupirocin on the occurrence of S. aureus infection in surgical populations. These included a selection of the previous studies. A Cochrane review found that mupirocin protected against the development of postoperative S. aureus infections when compared with placebo in S. aureus nasal carriers (relative risk: 0.55; 95% CI: 0.34–0.89).39 This protective effect was also demonstrated in another systematic review (some studies were included in both reviews).40
In summary, there is convincing evidence that intranasal mupirocin protects surgical patients who are carriers of S. aureus from acquiring postsurgical S. aureus infections. Studies in which preoperative mupirocin prophylaxis was given universally, on the other hand, failed to show a statistically significant reduction in the postoperative S. aureus infection rate. Thus, existing evidence supports screening and targeted decolonization with preoperative mupirocin prophylaxis for the prevention of postoperative S. aureus infections.
Other populations
Non-surgical patients. Wertheim et al.41 studied the efficacy of prophylactic intranasal mupirocin against S. aureus infections in nasal carriers of S. aureus admitted to non-surgical hospital departments. In this study, mupirocin did not lead to statistically significant reductions in nosocomial S. aureus infections compared with placebo. Based on these findings, the authors concluded that targeted decolonization of nonsurgical patients who are nasal carriers of S. aureus does not provide protection against nosocomial S. aureus infections.41 Similarly, Harbarth et al.42 could not show a statistically significant reduction in MRSA infections in hospitalized patients >16 years of age who were carriers of MRSA and who received prophylactic intranasal mupirocin compared with those who received placebo. This was probably due to a lack of power (1.48 versus 2.82 infections per 1000 patient-days, respectively; relative risk: 0.53; 95% CI: 0.14–2.02).42
ICU patients. Camus et al.43 investigated whether one or two courses of intranasal mupirocin and chlorhexidine gluconate body wash would be effective in preventing S. aureus infections in intubated adult patients in the ICU (who did or did not receive polymyxin and tobramycin). They found that the overall incidence of S. aureus (MRSA and MSSA combined) infections and MRSA infections alone were significantly lower in patients who received the intervention compared with the placebo group, with ORs of 0.39 (95% CI: 0.16–0.96) and 0.41 (95% CI: 0.20–0.85), respectively. They did not find a significant effect of the intervention on MSSA infections alone, though the effect estimate was similar (OR: 0.43; 95% CI: 0.13–1.40).43 In another cluster-randomized study, adult ICU patients were randomized to one of three strategies for preventing MRSA clinical isolates and infections: (i) nasal screening for MRSA colonization and contact precautions for patients with a positive history of MRSA colonization or infection or MRSA test; (ii) same as the first strategy, but with the addition of a 5 day decolonization regimen with intranasal mupirocin and chlorhexidine gluconate bathing; and (iii) no screening, but all patients received the 5 day decolonization regimen with intranasal mupirocin and chlorhexidine gluconate. In strategy 3, contact precautions were comparable to strategy 1. Both strategies 2 and 3 led to statistically significant reductions in the hazard of MRSA-positive clinical cultures compared with their baseline, whereas this effect was not seen for strategy 1. Also, the effect of strategy 2 (HR: 0.75; 95% CI: 0.63–0.89) was intermediate between the effects of strategies 1 (HR: 0.92; 95% CI: 0.77–1.10) and 3 (HR: 0.63; 95% CI: 0.52–0.75). However, when strategy 2 was compared with 1, no statistically significant difference in hazard could be demonstrated (P = 0.09). Strategy 3, on the other hand, was superior to strategy 1 in reducing the hazard of MRSA-positive clinical cultures (P = 0.003). The effects of the strategies on ICU-attributable MRSA bloodstream infection were also assessed, but no statistically significant differences were found across the study groups. However, the study was not powered to detect a significant difference in bloodstream infections. Thus, decolonization with mupirocin and chlorhexidine gluconate, particularly universal decolonization, may be superior to no decolonization in preventing MRSA infection in the ICU.44 Nardi et al.45 also conducted a study with ICU patients, in which they evaluated the effect of adding intranasal mupirocin to a selective decontamination of the digestive tract (SDD) regimen to decrease ICU-acquired infections caused by, among others, Gram-positive bacteria. The SSD regimen consisted of oral administration of tobramycin, polymyxin and amphotericin.45 The primary aim of SDD is to eliminate and prevent colonization with aerobic Gram-negative bacteria and yeast from the gastrointestinal tract, leaving the anaerobic flora unaffected.46 Nardi et al.45 showed that adding intranasal mupirocin to the SDD regimen resulted in a significant reduction in the incidence of S. aureus pneumonia cases, although no significant differences were observed for the incidence of S. aureus bacteraemia or catheter-related bacteraemia. Based on these results, the authors concluded that adding mupirocin to the SDD regimen would probably lead to a reduction in lung infections caused by S. aureus, which may be an important step in protecting intubated patients against S. aureus pneumonia.45
Long-term care facility residents. Mody et al.47 randomly assigned 127 residents of two long-term care facilities who were persistent carriers of S. aureus to receive either intranasal mupirocin (64 residents) or placebo (63 residents). One-hundred and two residents (55 residents in the mupirocin group and 47 in the placebo group) completed the therapy and were evaluated for treatment efficacy. Although mupirocin was effective in eradicating S. aureus colonization, only a trend towards reduction of newly confirmed or probable infections with S. aureus was observed in this long-term care setting (3 of 55 patients in the mupirocin group and 7 of 47 residents in the placebo group, P = 0.10).47 Thus, this study could not show any benefit of using mupirocin to prevent S. aureus infections in this population. However, if we assume that mupirocin is truly effective in preventing S. aureus infections in this population, and that the observed difference in S. aureus infection occurrence between the two study groups is true, then a post-hoc sample size calculation (with an α of 5% and power level of 80%) reveals that this study was underpowered to show a statistically significant result.
General population. Ellis et al.48 conducted a cluster-randomized placebo-controlled trial to assess the effect of targeted intranasal mupirocin versus placebo for the prevention of community-associated MRSA (CA-MRSA) soft-tissue infection. They randomly assigned clusters of soldiers to a mupirocin or placebo group and treated only the CA-MRSA carriers within the clusters with either intranasal mupirocin or placebo. In this study, no statistically significant difference in the CA-MRSA infection rate was found between carriers treated with mupirocin or placebo (7.7% of carriers in the placebo group and 10.6% in the mupirocin group developed infections; a difference of −2.9%, 95% CI: −7.5% to 1.7%), nor in the overall CA-MRSA infection rate, the CA-MRSA infection rate in individuals not initially colonized with CA-MRSA or the CA-MRSA infection rate in individuals who during the study became colonized with CA-MRSA. Based on these results, the authors concluded that a mupirocin-based CA-MRSA eradication strategy in nasal carriers of CA-MRSA would not lead to a decrease of soft-tissue infections in treated individuals or their milieu. Not all infections were cultured in this study, and it is therefore possible that the authors included infections in the analysis that were not caused by (MR)SA.48 If this occurred more often in the placebo than in the mupirocin group, then this could explain why no statistically significant result was found (if the therapy is truly efficacious). Another possible explanation for these findings is that CA-MRSA exhibits a distinct colonization pattern compared with other non-CA-MRSA strains. For instance, a study by Yang et al.49 showed that CA-MRSA also has a preference for gastrointestinal colonization compared with other non-(CA-)MRSA. If this is indeed the case, then applying mupirocin to the nostrils will lead to nasal decolonization, but not to decolonization of the gastrointestinal tract.
In another study, Weintrob et al.50 assessed the combined effect of prophylactic intranasal mupirocin and hexachlorophene body wash for the prevention of MRSA infections among community-dwelling MRSA-colonized HIV-infected persons. This population has an increased risk of MRSA infection.51 In this study, the prophylactic treatment was not superior to placebo in preventing MRSA skin and soft-tissue infections. However, this study was not sufficiently powered to show superiority.50
In conclusion, most of the studies described above failed to show a benefit of prophylactic intranasal mupirocin for the prevention of S. aureus infection in the non-surgical and non-dialysis setting. However, we found one meta-analysis that assessed the effectiveness of mupirocin in preventing S. aureus infections among colonized and non-colonized patients in the non-surgical setting (including ICU, dialysis, long-term care facility, etc.). This meta-analysis included data from both trials and non-experimental studies and from several original studies included in this review. In contrast to what the results of the separate studies suggest, this meta-analysis does show that mupirocin use leads to a reduction of 49% (relative risk: 0.51; 95% CI: 0.40–0.65) in the risk of S. aureus infections compared with no mupirocin. However, there was a significant degree of heterogeneity between the studies (I2 = 66%).52 Therefore, it remains unclear in non-surgical patients who will benefit from a decolonization treatment.
Chlorhexidine gluconate
Our search yielded few RCTs that investigated the effect of chlorhexidine gluconate, with or without other agents (excluding mupirocin), on the occurrence of S. aureus infections. In a study by Hayek et al.,53 no significant differences in postsurgical S. aureus infections were observed between elective surgery patients who received two preoperative baths with chlorhexidine gluconate and patients who received preoperative baths with placebo.53 This suggests that chlorhexidine gluconate alone does not effectively protect against postoperative S. aureus infections. However, as the original placebo had to be replaced with another placebo during the study because of antimicrobial properties exhibited by the former placebo and due to uncertainty about how the authors dealt with this in their analysis, caution is advised in interpreting the results of this study.53 A later study, which compared chlorhexidine gluconate body wash with a non-antimicrobial agent for the prevention of acquisition of MDR organisms (MDROs) and HA-BSIs, found that chlorhexidine gluconate use led to a statistically significant reduction in the overall incidence of HA-BSIs compared with control (respectively 4.8 and 6.6 HA-BSI/1000 patient-days, P = 0.007). This reduction was primarily the result of a lower incidence of primary HA-BSI caused by coagulase-negative staphylococci and fungi. Chlorhexidine gluconate use was not associated with a statistically significant reduction of primary S. aureus HA-BSIs (respectively 0.36 and 0.32 HA-BSI/1000 patient-days, P = 0.80).54 Noto et al.,55 who performed a non-blinded cluster-randomized study to compare once-daily bathing of ICU patients with chlorhexidine-gluconate-impregnated cloths or with non-antimicrobial cloths, also did not find a statistically significant difference in the rate of HA-BSI from any pathogen or from S. aureus between the chlorhexidine gluconate and the control group (any pathogen: rate difference of 0.38 HA-BSI/1000 patient-days, 95% CI: −0.81 to 1.57, P = 0.53; S. aureus HA-BSI: rate difference of −0.11 HA-BSI/1000 patient-days, 95% CI: −0.44 to 0.66, P = 0.95). Thus, daily baths with chlorhexidine gluconate did not reduce the rate of other common HAIs in this study.55 Another cluster-randomized trial performed by Ellis et al.,56 which compared different hygiene strategies among military trainees at high risk of skin- and soft-tissue infections (SSTIs), found no statistically significant differences in the rate of overall SSTIs or the rate of MRSA SSTIs (overall SSTI rate ratio of 1.13, 95% CI: 0.90–1.41; MRSA SSTI rate ratio of 0.75, 95% CI: 0.49–1.16) between military trainees exposed to enhanced hygiene education and weekly showers with plain soap (strategy 1), and trainees exposed to enhanced hygiene education and weekly showers with chlorhexidine gluconate body wash (strategy 2). Thus, chlorhexidine gluconate body wash was not superior to plain soap in preventing overall and MRSA SSTIs in this population.56
Although most studies were conducted with chlorhexidine gluconate body wash for body decolonization, we found one study that investigated chlorhexidine gluconate for oropharyngeal and nasal decolonization. In the study by Segers et al.,57 adult patients undergoing sternotomy for cardiothoracic surgery were randomly assigned to receive prophylaxis with either an oropharyngeal rinse and nasal ointment containing chlorhexidine gluconate, or a rinse and ointment containing placebo. The prophylaxis was administered from the day of hospitalization until removal of the nasogastric tube, usually the day after surgery. In addition, all patients received antibiotic treatment according to local protocol. These patients were followed to assess the incidence of S. aureus nosocomial chlorhexidine gluconate infection after surgery. A total of 485 and 469 patients were randomly assigned to the chlorhexidine gluconate and the placebo group, respectively. Randomization occurred independently of S. aureus colonization status. The authors found that chlorhexidine gluconate was more effective than placebo in preventing nosocomial infections overall (absolute risk reduction: 6.4%; 95% CI 1.1%–11.7%), lower respiratory tract infections overall (absolute risk reduction: 6.5%; 95% CI: 2.3%–10.7%), and deep SSIs (absolute risk reduction: 3.2%; 95% CI: 0.9%–5.5%). However, there was no statistically significant difference between the chlorhexidine gluconate and placebo group regarding nosocomial infections caused by S. aureus, although fewer S. aureus isolates were recovered from patients developing nosocomial infections in the chlorhexidine gluconate group than from the placebo group. In conclusion, prophylaxis with chlorhexidine gluconate nasal ointment and nasopharyngeal rinse may decrease the risk of acquiring a nosocomial infection of any cause after cardiothoracic surgery but may not decrease the risk of acquiring a S. aureus nosocomial infection.57
We also found two systematic reviews and one meta-analysis that investigated the impact of topical chlorhexidine gluconate on infections with MDROs in patients admitted to the ICU. The reviews by Derde et al.58 and Karki et al.59 included trials and observational studies. Although both reviews reported that acquisition of MRSA colonization in the ICU was significantly reduced in patients exposed to chlorhexidine gluconate, chlorhexidine gluconate use did not lead to a significant reduction in MRSA infection rates in most studies,58 not even after pooling the results of individual studies (pooled incidence rate ratio: 0.82, 95% CI: 0.51–1.31).59
In conclusion, there is insufficient evidence that topical chlorhexidine gluconate prevents hospital-acquired infections caused by S. aureus (including MRSA). A possible explanation for the lack of efficacy against S. aureus infections could be that chlorhexidine gluconate is less effective against S. aureus or MRSA than against other pathogens. This could theoretically explain why most studies show a decrease in overall infection rates following chlorhexidine gluconate use, but not a decrease in S. aureus infection rates. However, this is not backed by in vitro studies, which show that S. aureus has reasonable susceptibility to chlorhexidine gluconate.60 Another plausible explanation for these findings, at least regarding CA-MRSA infections, is that CA-MRSA may have other preferential colonization body sites than non-CA-MRSA, e.g. the gastrointestinal tract.49 As chlorhexidine gluconate is administered topically, this could explain why its application does not lead to gastrointestinal decolonization and to a decrease in MRSA infection.
Povidone–iodine
Wilson et al.61 conducted a study among patients treated with CAPD, who were randomly assigned to prophylaxis with either dry powder spray containing povidone–iodine or no spray at the catheter exit-site at the moment of routine exit-site care. They found that the number of exit-site infection events caused by S. aureus was significantly higher in the no-spray group than in the povidone–iodine spray group (22 events in the no-spray group compared with 9 events in the povidone–iodine spray group, P < 0.01, although more than one event occurred in several patients). On the other hand, no statistically significant difference in the incidence of peritonitis was observed between the groups. Despite these partially positive results, the authors did not recommend the use of povidone–iodine spray in routine exit-site care because of reported side effects (mild cases of rash and pruritus) and a perceived increase in infections with Pseudomonas spp. in the povidone–iodine-exposed patients.61
In another open-label study, Phillips et al.62 compared a one-time application of intranasal povidone–iodine solution prior to surgery with intranasal mupirocin twice daily for 5 days prior to surgery in patients who were undergoing spinal and arthroplasty surgery. The authors found no significant differences between the povidone–iodine and mupirocin group (ITT analysis) in the rate of deep SSI of any cause or deep S. aureus SSI, although more S. aureus infections were observed in the mupirocin group. Despite the negative results, the authors concluded that povidone–iodine could be an alternative to mupirocin because of its lower market price, lower rates of treatment-related symptoms and the better compliance with treatment.62 The authors did not expect a difference in SSI rate between the two groups, so the results were according to their expectation. However, they calculated the study sample size based on an assumed doubling of the SSI rate in the povidone–iodine group (n = 1500 patients per treatment group), which they were unable to reach. Also, the study was not designed to show equivalence or non-inferiority of povidone–iodine compared with mupirocin, nor were data provided that supported the conclusion of the study. Because of this, caution is advised with interpreting the results of this study.
To sum up, there is some evidence that povidone–iodine may be effective against S. aureus infection. If we consider the methodology of the studies conducted so far, we can conclude that more robust and adequately powered studies are needed to be able to draw firm conclusions regarding the effectiveness of this intervention for S. aureus infection prophylaxis. It should then also be assessed whether topical or intranasal application is better suited for the preventive approach.
Prophylactic antibiotics
We found several studies and one meta-analysis that investigated the use of different types of antibiotics as prophylactic agents for the prevention of S. aureus infection. We excluded studies that investigated perioperative systemic antibiotic prophylaxis because we considered this type of prophylaxis a separate category that is not specifically targeted at prevention of S. aureus infections. Also, it has been studied extensively and is part of several leading, international guidelines.
Selective oropharyngeal decontamination (SOD)
In the study by Abele-Horn et al.,63 patients admitted to an ICU were randomly assigned to a study group (58 patients) treated with SOD (containing 2% amphotericin B, 2% tobramycin and 2% polymyxin) and cefotaxime, or to a control group (30 patients) that did not receive these antibiotics. Control patients could have received antibiotics for other reasons however, for instance if they underwent surgery or if infection was suspected. Whereas the intervention proved effective in preventing pneumonia caused by primarily Gram-negative micro-organisms, it did not lead to a statistically significant decrease in S. aureus pneumonia [a similar proportion of patients in the SOD group (±16%) and control group (±17%) developed S. aureus pneumonia]. However, this study was probably underpowered to show a statistically significant result for this outcome (based on the results of this study and a post-hoc sample size analysis with an α of 5% and power of 80). In conclusion, there is insufficient evidence to draw conclusions regarding the effect of SOD on S. aureus pneumonia acquired in the ICU.63
Selective decontamination of the digestive tract
Hammond et al.64 conducted a randomized study in which trauma patients admitted to an ICU department were randomly allocated to receive either SDD (containing amphotericin B, polymyxin E and tobramycin for oropharyngeal and enteral decolonization) or placebo at ICU admission. S. aureus carriage was not assessed at randomization. Both groups received parenteral antibiotic treatment with cefotaxime. After randomization, the patients were followed to assess the occurrence of secondary infections. Thirty-nine and 33 patients were enrolled in the SDD and placebo group, respectively. Despite the expectations, SDD was not able to prevent the occurrence of secondary infections overall (SDD group: 17 infections; placebo group: 16 infections) or secondary infections caused by S. aureus, which were largely infections of the respiratory tract (4 secondary infections with S. aureus occurred in both groups). They also observed a statistically significant increase in colonization by several micro-organisms, including MRSA, in the SDD group compared with the placebo group, suggesting that prolonged use of SDD may even increase the risk of secondary infection by certain micro-organisms that are part of the endogenous flora. Based on these results, SDD does not prevent overall secondary infection or secondary infection caused by S. aureus in this patient population.64 However, this study was probably not adequately powered to show a statistically significant result for this outcome.
In contrast, Quinio et al.,65 who conducted a double-blind placebo-controlled trial in which they randomly assigned patients with multiple trauma admitted to an ICU to receive either SDD treatment (containing polymyxin E, gentamicin and amphotericin B) (76 patients) or placebo (72 patients), found that the overall nosocomial infection rate was significantly reduced in the SDD group (42 of 76 patients) compared with the placebo group (66 of 72 patients) (P = 0.01). This reduction was mainly due to a lower rate of bronchopneumonia in the SDD group. However, when they assessed lower airway infections (bronchopneumonia and tracheobronchitis) caused by S. aureus only, they found no statistically significant difference in the rate of S. aureus lower airway infections between the SDD group (25 infections in 50 patients) and placebo group (12 infections of 28 patients).65
In the study by Silvestri et al.,66 mechanically ventilated ICU patients were randomly assigned to receive either an SDD regimen with oropharyngeal gel containing vancomycin or SDD alone and were followed to record ICU-acquired infections. Randomization was performed independently of S. aureus carriage status. They found that patients who received oropharyngeal vancomycin and SDD were at lower risk of developing ICU-acquired (i.e. secondary and exogenous) lower respiratory infections with MRSA than those who received SDD alone (OR: 0.26; 95% CI: 0.08–0.88). In addition, no emergence of vancomycin resistance was observed. Based on this, oropharyngeal vancomycin seems to be effective against ICU-acquired lower respiratory infections with MRSA in this patient group.66 More studies are needed to confirm these findings. These should also address the effects on resistance to vancomycin as this is one of the most important agents left for treatment of MRSA.
In conclusion, SDD use probably protects mechanically ventilated patients against acquisition of infections overall. However, there is lack of evidence supporting a protective effect of SDD on S. aureus infections specifically.
Trimethoprim/sulfamethoxazole
Swartz et al.67 demonstrated that giving trimethoprim/sulfamethoxazole prophylactically to patients on chronic peritoneal dialysis treatment significantly reduces the number of S. aureus peritonitis episodes (2 of 28 episodes) compared with not giving trimethoprim/sulfamethoxazole (11 of 37 episodes). However, the number of tunnel-tract infections caused by S. aureus was similar between the groups. From the 13 S. aureus peritonitis episodes that were observed, only two (both in the non-treated group) occurred in non-carriers of S. aureus. Based on this study, trimethoprim/sulfamethoxazole seems to be effective against S. aureus peritonitis among carriers of S. aureus.67
In another study, which was conducted in Japan by Kimura et al.,68 adult patients with severe burns (≥20% of body surface area) and requiring ventilatory support were randomly assigned to receive either a 10 day prophylactic treatment with trimethoprim/sulfamethoxazole or placebo 4–6 days after the burn injury. These patients were followed to assess the occurrence of MRSA pneumonia. A total of 21 patients were enrolled in the trimethoprim/sulfamethoxazole group and 19 patients in the placebo group. The authors found that the patients in the trimethoprim/sulfamethoxazole group had a significantly lower risk of developing ventilator-associated pneumonia and MRSA pneumonia compared with patients in the control group; the incidence of MRSA pneumonia was 4.8% (1 of 21 patients) in the trimethoprim/sulfamethoxazole group compared with 36.8% (7 of 19 patients) in the placebo group (P = 0.017). The intervention was well tolerated, and no resistance development was observed during the study. Based on this, the authors concluded that trimethoprim/sulfamethoxazole prophylaxis effectively reduces the incidence of MRSA pneumonia in severely burned patients requiring ventilatory support.68 It should be noted that the incidence of infections in the untreated groups was remarkably high, raising questions about the generalizability of these findings.
Rifampicin
A meta-analysis described four rather small RCTs that assessed the effect of oral rifampicin prophylaxis (with or without intranasal bacitracin) on S. aureus exit-site infections in patients receiving peritoneal dialysis or haemodialysis. The comparison in all studies was with no prophylaxis. Two of the four studies included S. aureus carriers only. The meta-analysis showed that oral rifampicin prophylaxis, with or without bacitracin, led to a statistically significant reduction in S. aureus exit-site infections compared with no prophylaxis (OR: 0.16; 95% CI: 0.06–0.44). In addition, one of the RCTs reported that oral rifampicin prophylaxis could be as effective as mupirocin (for catheter exit-site application) prophylaxis against S. aureus infection, though this study was not designed to show equivalence. Despite these favourable results, the authors discouraged the use of oral rifampicin prophylaxis due to concerns about drug toxicity and emergence of antibiotic resistance, and preferred the use of topical antimicrobials. Therefore, despite the apparent effectiveness, it remains a matter of discussion whether rifampicin prophylaxis should be used for prevention of S. aureus infections.69
Polyhexanide
In one small single-centre, open-label, randomized study, the efficacy of a polyhexanide solution was assessed in the prevention of exit-site infections in peritoneal dialysis patients. In this study, 60 patients undergoing peritoneal dialysis treatment were randomly allocated to either daily care of the exit-site wounds with polyhexanide solution (30 patients) or 0.9% saline plus povidone–iodine solution (30 patients) and were followed for 12 months to assess the occurrence of exit-site infections. Only 46 patients completed the follow-up period (22 in the polyhexanide and 24 in the povidone–iodine group). Although this study was underpowered due to the small sample size (by ∼26 patients/group), more S. aureus exit-site infections developed in the povidone–iodine group (three of nine exit-site infections in six patients) than in the polyhexanide group (zero of three exit-site infections in two patients). This result was not statistically significant. Overall fewer exit-site infections were observed in the polyhexanide group than in the povidone–iodine group, and this result was statistically significant (P = 0.03). In conclusion, more robust and adequately powered studies are needed to be able to draw conclusive statements about the effectiveness of polyhexanide as a prophylactic agent against S. aureus infections.70
Summary
Prophylactic use of common (systemic) antibiotics may be a potential preventive approach against nosocomial infections caused by S. aureus. However, the evidence is scarce, and well-designed trials are needed to further explore the usefulness of these antibiotics as prophylactic agents against S. aureus infection. When performing these trials, more attention should be paid to long-term adverse effects, including the development of antimicrobial resistance and the occurrence of other adverse events that may outweigh the potential benefits of this approach.
Honey
Honey is a safe and relatively cheap natural resource that has been shown to have several antimicrobial properties. In addition, antimicrobial resistance against honey has not been reported.71 Honey has been used since ancient times to treat wounds,72 yet only in recent years have randomized studies been conducted to assess its efficacy for the prevention of infections. Our search retrieved two randomized studies that assessed honey as prophylaxis against S. aureus infections.
In the study by Johnson et al.,73 patients receiving haemodialysis treatment were randomized to receive either Medihoney (antibacterial honey derived from Leptospermum spp.) or mupirocin at the catheter exit-site in addition to standard exit-site care for the prevention of catheter-associated infections. Nasal S. aureus carriage was assessed, but carriers were not treated with intranasal mupirocin. In this study, no statistically significant difference in the incidence of catheter-associated bacteraemia (of any cause) was observed between the groups (0.97 versus 0.85 episodes/1000 catheter-days in the honey and mupirocin group, respectively), nor did any of the study participants develop an exit-site infection. Also, no mupirocin-resistant staphylococcal strains were isolated during the study. As both interventions were well tolerated, the authors concluded that Medihoney had a similar efficacy to mupirocin for preventing catheter-associated infections in haemodialysis patients.73 However, this trial was not adequately powered to demonstrate non-inferiority.
In the HONEYPOT trial,74 patients receiving peritoneal dialysis treatment were randomized to receive either antibacterial honey at the catheter exit-site or intranasal mupirocin, in addition to standard exit-site care, for the prevention of peritoneal-dialysis-related infections. Intranasal mupirocin was only given to the patients randomly assigned to the intervention group and who were S. aureus nasal carriers at the start of the trial or during follow-up. This study found no statistically significant difference in the rate of peritoneal-dialysis-related infections caused by S. aureus (incidence rate ratio: 1.58; 95% CI: 0.95–2.62) or MRSA (incidence rate ratio: 0.42; 95% CI: 0.04–4.02) between the groups, though patients in the honey group with diabetes were at higher risk of catheter-associated infections (HR: 1.85; 95% CI: 1.05–3.24), especially peritonitis (HR: 2.25; 95% CI: 1.16–4.36). Based on these results, honey could not be recommended for the prevention of peritoneal-dialysis-related infections.74 A sub-study of the HONEYPOT trial also failed to show a statistically significant difference in the rate of S. aureus-specific peritonitis (incidence rate ratio: 2.04; 95% CI: 0.85–4.92) or S. aureus-specific exit-site infection (incidence rate ratio: 1.05; 95% CI: 0.59–1.88) between the groups, though more S. aureus infections occurred in the honey group compared with the mupirocin group.75
In summary, the evidence so far does not support the use of honey as a prophylactic anti-staphylococcal agent.
Antimicrobial peptides
In recent years, antimicrobial peptides have shown promise as a novel strategy to prevent and treat infections with S. aureus.76 Most trials so far have tested these compounds for the treatment of infections, including diabetic foot ulcer,77 acute bacterial skin infections (ClinicalTrials.gov Identifier: NCT01211470) and Gram-positive skin infections (ClinicalTrials.gov Identifier: NCT01223222). However, we only found one trial that fulfilled the inclusion criteria of this review. In this randomized study, mechanically ventilated patients were randomly assigned to receive either an oral solution of iseganan, a synthetic antimicrobial peptide, or a placebo for the prevention of ventilator-associated pneumonia (VAP). However, before the last patient could be enrolled, the study was terminated because of a presumed, albeit non-significant, higher rate of VAP and mortality in the iseganan group compared with the placebo group. At study closure, no significant differences in the incidence or distribution of bacterial causes (including S. aureus) of VAP were observed between the two groups.78 Thus, prophylaxis with iseganan was unsuccessful in preventing VAP caused by several micro-organisms, including S. aureus.
Vaccines
Vaccination can be a powerful intervention against infections, including nosocomial infections. Several attempts have been made to develop a safe and efficacious vaccine to prevent S. aureus infections. Two investigational anti-S. aureus vaccines showed promise and reached advanced clinical trial stages.
The first one is the StaphVAX vaccine (Nabi Biopharmaceuticals). This vaccine consisted of the S. aureus type 5 and 8 capsular polysaccharides conjugated to an equal weight of non-toxic recombinant P. aeruginosa exotoxin A. The efficacy of this vaccine was investigated in two studies. The aim of the first study was to investigate whether a single dose of StaphVAX was more efficacious than placebo in preventing S. aureus bacteraemia in end-stage renal disease (ESRD) patients receiving haemodialysis. These included both S. aureus carriers and non-carriers. It was found that StaphVAX was not more efficacious than placebo in the a priori defined follow-up period of 3–54 weeks after vaccination, although it led to a statistically significant reduction in the number of S. aureus bacteraemia in the follow-up period of 3–40 weeks after vaccination (vaccine efficacy 57%; 95% CI: 10%–81%). In addition, the authors noticed a steep decline in antibody levels during the study. Based on this, they concluded that the vaccine might induce partial immunity against S. aureus bacteraemia for ∼40 weeks after vaccination.79 Following this study, Fattom et al.80 conducted a similar study with the same main endpoint and patient population, but with the aim of assessing the efficacy of StaphVAX versus placebo up to 35 weeks after a single dose or up to 60 weeks after one or two vaccine doses. This study was unsuccessful in showing efficacy of the vaccine in protecting against S. aureus bacteraemia.80 The reasons proposed to explain the lack of vaccine efficacy included: suboptimal quality of the vaccine itself, which in turn may have led to induction of antibodies of lower quality and functionality; immunological impairment associated with ESRD and haemodialysis; and the target of only one virulence factor by the vaccine.79,80
The other investigational vaccine that has been studied is V710 (Merck Sharp & Dohme Corp.). This vaccine consisted of the highly conserved S. aureus 0657nl iron-regulated surface determinant B. The efficacy and safety of a single dose of the vaccine was investigated in patients who were undergoing cardiothoracic surgery. Both carriers and non-carriers of S. aureus were included. The vaccine was administered 14–60 days prior to surgery, and the patients were followed for 90 days after surgery to assess development of postoperative S. aureus bacteraemia and/or S. aureus deep sternal wound infections. The study was prematurely terminated due to safety concerns (the mortality rate was statistically significantly higher in S. aureus-infected vaccine recipients; in some cases the mortality was related to multiple organ failure) and the low probability of demonstrating vaccine efficacy. It is not precisely known why the vaccine failed, but the authors reported that perhaps the opsonophagocytic activity of the vaccine may have failed to induce bacterial killing, thereby allowing intracellular survival of S. aureus, which may have potentiated morbidity and mortality.81 Interestingly, when Paling et al.82 conducted a post-hoc analysis using this study’s data to develop a risk prediction model to quantify the risk of acquiring a S. aureus SSI or BSI after cardiothoracic surgery while including the possible effect of competing events, they found, in contrast to the results from the main study, that V710 did protect against postoperative S. aureus infection both in the univariate and multivariate analysis (OR: 0.67; 95% CI: 0.48–0.91).
To summarize, at present no vaccine has proven to be effective in human beings. However, much knowledge has been gained from these unsuccessful trials. This has resulted in novel concepts and ideas83–85 that may increase the probability of developing efficacious vaccine therapies against S. aureus infections in the future.
Discussion
This review presents an overview of the antimicrobial agents that have been studied specifically as possible preventive therapies against S. aureus infections. To our knowledge, this is the first review of its kind. The search strategy was developed to be broad and inclusive, but also manageable. Also, the eligibility criteria of studies were developed such that the findings would be based on a high level of evidence, be specific for S. aureus infection prevention and be generalizable to the adult patient population. Most research of good quality has been conducted with mupirocin. From the existing studies we can conclude that there is compelling evidence that topical application of mupirocin to the nares is effective in preventing infections with S. aureus. More specifically, patients undergoing dialysis treatment have a lower probability of developing S. aureus bloodstream infections and catheter-related infections (between 40% and 80% reduction). Similarly, in surgical patients the risk of acquiring any S. aureus infection after surgery is approximately halved. Patients on mechanical ventilation are probably also at lower risk of developing pneumonia with S. aureus after a prophylactic course of mupirocin (∼60% reduction). However, the latter is based on limited evidence and further randomized studies are needed to corroborate the protective effect of mupirocin in this patient group. The literature clearly shows that the protective effect of mupirocin is more pronounced in known carriers of S. aureus as opposed to non-carriers. It is questionable if the latter group benefits at all from this strategy. In surgical patients, the current evidence shows that screening and decolonization is a cost-effective strategy, saving an estimated 1900 euros per treated carrier.86 In addition, it significantly reduces the 1 year mortality following clean surgery.87 Recently, the WHO issued the first global guidelines on SSI prevention, in which it recommends decolonization of carriers undergoing cardiothoracic or orthopaedic surgery. A conditional recommendation is made for other surgical procedures as there are fewer studies in these groups.88 The question is whether these recommendations will be widely implemented, as screening is often logistically demanding and not always feasible in all settings. If this is the case, then universal decolonization may be a better option, although there are concerns that this strategy carries a higher risk for the development of resistance.89 Yet, most studies to date in which only a short and defined course of mupirocin was used did not find that the treatment selected for significant levels of mupirocin-resistant S. aureus strains.31–33 Development of mupirocin resistance has been mainly reported in studies in which patients were exposed to the drug for a prolonged time.90,91 Thus, it is unlikely that mupirocin resistance will be a major issue when used as a single, short course for (preoperative) antibiotic prophylaxis. In contrast, when prolonged use is warranted, we recommend restricting the use to only carriers of S. aureus who are at increased risk of S. aureus infection and screening for development of mupirocin resistance.
Concerning other antimicrobial agents, the evidence is scarce or lacking. Although we expected that there would be limited evidence, we were somewhat surprised how little proof there actually is, especially when considering that some of these antimicrobial approaches are widely used as preventive anti-staphylococcal agents. Chlorhexidine gluconate on its own is not proven effective in preventing healthcare-associated infections caused by S. aureus, though it may reduce the burden of healthcare-associated infections caused by other pathogens. However, prolonged use may lead to antimicrobial resistance.60 For povidone–iodine and honey there is also a lack of evidence showing efficacy of these treatments against healthcare-associated S. aureus infections. Studies that assessed the antibiotics co-trimoxazole (trimethoprim/sulfamethoxazole), vancomycin and rifampicin have shown some positive results. However, as these antibiotics are primarily used to treat rather than to prevent infections, and since a broader use of antibiotics may lead to the development of antibiotic resistance and to a decreasing availability of effective antimicrobial treatments,92 it is unclear whether these antibiotics should be used for prophylaxis. Also, these antimicrobials carry a higher risk of causing adverse effects. Because of this, wide implementation of these antimicrobials as prophylactic agents against S. aureus has not been realized in clinical practice; only vancomycin is sometimes used as part of the preoperative antibiotic prophylactic regimen.93 Concerning antimicrobial peptides, thus far, limited studies have assessed the efficacy of these compounds as prophylactic anti-infective drugs. The only study that reported the effect of an antimicrobial peptide on the incidence of S. aureus infections failed to show any significant results, and there were concerns about the safety for the participants. Consequently, the usefulness of these compounds as prophylactic agents against S. aureus infections remains to be demonstrated. However, this technology is still evolving, and several antimicrobial peptides have been successfully tested against S. aureus in preclinical studies.76 It is likely that one or more of these compounds may eventually be tested in human beings and that this endeavour may lead to novel antimicrobial approaches in the future. Regarding anti-staphylococcal vaccines, all attempts so far to develop an effective vaccine for human use have failed, despite the theoretical promise of this intervention. Currently, only one Phase 2b vaccine trial is ongoing. The trial, entitled ‘STaphylococcus aureus SuRgical Inpatient Vaccine Efficacy’ (STRIVE), is assessing a multi-antigen S. aureus vaccine (PF-06290510) for the prevention of postoperative invasive infections caused by S. aureus in adult patients undergoing elective spinal fusion surgery (ClinicalTrials.gov Identifier: NCT02388165). The vaccine under study is composed of four surface antigens of S. aureus that are conserved and globally represented in many S. aureus strains. These antigens are CP5, CP8, ClfA and MntC, and they represent three major S. aureus virulence mechanisms that are active in the early stages of infection.94,95 In this trial, a single dose of the vaccine administered 10–60 days prior to the elective surgical procedure will be compared with a placebo for the prevention of postoperative invasive S. aureus infections. The trial started in 2015 and is expected to complete enrolment in the second half of 2018. It remains to be seen whether this vaccine candidate will be the first vaccine that is proven efficacious in humans. Besides this trial, there are also other trials investigating antimicrobial regimens for S. aureus decolonization and prevention of infections caused by S. aureus. There are several ongoing trials comparing mupirocin monotherapy or the combination of mupirocin and chlorhexidine gluconate with some other antimicrobial agent (or combination of agents) in several populations, including ICU patients (ClinicalTrials.gov Identifier: NCT03140423), surgical patients (EudraCT Number: 2010-022701-17) and patients requiring dialysis treatment (EudraCT Number: 2015-002223-25). As the effectiveness of mupirocin has already been shown in several studies, these trials may lead to new antimicrobial preventive approaches only if the comparison drug(s) are cheaper, non-inferior or superior to mupirocin (with or without chlorhexidine gluconate), easier to apply (increase compliance) and/or lead to a comparable or a reduction in the rate of adverse effects. Another promising antimicrobial approach that is currently being explored in clinical research is the use of anti-staphylococcal monoclonal antibodies as prophylactic agents against S. aureus infection in high-risk patient groups. A potential advantage of this approach is that it will not induce bacterial resistance to the same extent as conventional antibiotics. In addition, studies suggest that this approach may also increase the effectiveness of the conventional antibiotic treatment, making this approach particularly appealing in settings with a relatively high degree of antimicrobial resistance.96 Currently, a Phase 2 trial entitled ‘Human Monoclonal Antibody Against Staphylococcus Aureus Alpha Toxin In Mechanically Ventilated Adult Subjects’ (SAATELLITE) is ongoing, investigating the efficacy and safety of a single dose of human monoclonal antibody against the S. aureus α toxin (MEDI4893) in mechanically ventilated patients (ClinicalTrials.gov Identifier: NCT02296320). This study completed patient recruitment in the second half of 2018. It remains to be seen whether this therapy can be added to our current antimicrobial arsenal against S. aureus infection. Lastly, there are also approaches besides preventive antimicrobials that have been studied as potential decolonization agents against S. aureus, including: (i) application of probiotics to minimize gastrointestinal colonization of S. aureus;97 (ii) the use of bacteriophages for S. aureus biofilm eradication;98 or (iii) faecal microbiota transplantation to repair dysbiosis caused by certain MRSA infections.99 Although these S. aureus eradication approaches may lead to a reduction in S. aureus infections, this has yet to be demonstrated in adequately designed and powered randomized trials.
Conclusions
S. aureus is a leading cause of infections and the risk of infection is strongly related to carriage of the micro-organism. There is compelling evidence that mupirocin is effective in preventing infections caused by S. aureus, particularly in patients who are carrying S. aureus in the surgical setting and who are undergoing dialysis treatment. Other antimicrobial agents and vaccines have been unsuccessful so far, or the current evidence is insufficient to recommend them for clinical practice. Trials with alternative approaches to mupirocin are currently underway and may lead to new antimicrobial preventive therapies in the future.
Funding
This study was carried out as part of our routine work for the COMBACTE consortium. For further information please refer to www.COMBACTE.com. This research project received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115523 | 115620 | 115737, resources of which are composed of financial contribution from the European Union Seventh Framework Programme (FP7/2007-2013) and EFPIA companies in-kind contribution.
Transparency declarations
None to declare.
Supplementary Material
References
- 1. Kluytmans J, van Belkum A, Verbrugh H.. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997; 10: 505–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wertheim HF, Verveer J, Boelens HA. et al. Effect of mupirocin treatment on nasal, pharyngeal, and perineal carriage of Staphylococcus aureus in healthy adults. Antimicrob Agents Chemother 2005; 49: 1465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Hal SJ, Jensen SO, Vaska VL. et al. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25: 362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tong SY, Davis JS, Eichenberger E. et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28: 603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klevens RM, Morrison MA, Nadle J. et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298: 1763–71. [DOI] [PubMed] [Google Scholar]
- 6. European Centre for Disease Prevention and Control/European Medicines Agency (ECDC/EMEA). The Bacterial Challenge: Time to React 2009. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf.
- 7. Noskin GA, Rubin RJ, Schentag JJ. et al. National trends in Staphylococcus aureus infection rates: impact on economic burden and mortality over a 6-year period (1998-2003). Clin Infect Dis 2007; 45: 1132–40. [DOI] [PubMed] [Google Scholar]
- 8. Suaya JA, Mera RM, Cassidy A. et al. Incidence and cost of hospitalizations associated with Staphylococcus aureus skin and soft tissue infections in the United States from 2001 through 2009. BMC Infect Dis 2014; 14: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kyaw MH, Kern DM, Zhou S. et al. Healthcare utilization and costs associated with S. aureus and P. aeruginosa pneumonia in the intensive care unit: a retrospective observational cohort study in a US claims database. BMC Health Serv Res 2015; 15: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Kraker ME, Wolkewitz M, Davey PG. et al. Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 2011; 55: 1598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewardson AJ, Allignol A, Beyersmann J. et al. The health and economic burden of bloodstream infections caused by antimicrobial-susceptible and non-susceptible Enterobacteriaceae and Staphylococcus aureus in European hospitals, 2010 and 2011: a multicentre retrospective cohort study. Euro Surveill 2016; 21: doi:10.2807/1560,7917.ES.2016.21.33.30319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim CJ, Kim HB, Oh MD. et al. The burden of nosocomial Staphylococcus aureus bloodstream infection in South Korea: a prospective hospital-based nationwide study. BMC Infect Dis 2014; 14: 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance 2014 http://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf;jsessionid=899CBE62F2122D0DBD723CBBA60788D6?sequence=1.
- 14. Ammerlaan HS, Harbarth S, Buiting AG. et al. Secular trends in nosocomial bloodstream infections: antibiotic-resistant bacteria increase the total burden of infection. Clin Infect Dis 2013; 56: 798–805. [DOI] [PubMed] [Google Scholar]
- 15. Laupland KB, Ross T, Gregson DB.. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000-2006. J Infect Dis 2008; 198: 336–43. [DOI] [PubMed] [Google Scholar]
- 16. von Eiff C, Becker K, Machka K. et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001; 344: 11–16. [DOI] [PubMed] [Google Scholar]
- 17. Kao KC, Chen CB, Hu HC. et al. Risk factors of methicillin-resistant Staphylococcus aureus infection and correlation with nasal colonization based on molecular genotyping in medical intensive care units: a prospective observational study. Medicine (Baltimore) 2015; 94: e1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Price JR, Cole K, Bexley A. et al. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect Dis 2017; 17: 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allegranzi B, Pittet D.. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect 2009; 73: 305–15. [DOI] [PubMed] [Google Scholar]
- 20. Mupirocin Study Group. Nasal mupirocin prevents Staphylococcus aureus exit-site infection during peritoneal dialysis. J Am Soc Nephrol 1996; 7: 2403–8. [DOI] [PubMed] [Google Scholar]
- 21. Sit D, Kadiroglu AK, Kayabasi H. et al. Prophylactic intranasal mupirocin ointment in the treatment of peritonitis in continuous ambulatory peritoneal dialysis patients. Adv Ther 2007; 24: 387–93. [DOI] [PubMed] [Google Scholar]
- 22. Boelaert JR, De Smedt RA, De Baere YA. et al. The influence of calcium mupirocin nasal ointment on the incidence of Staphylococcus aureus infections in haemodialysis patients. Nephrol Dial Transplant 1989; 4: 278–81. [DOI] [PubMed] [Google Scholar]
- 23. Johnson DW, MacGinley R, Kay TD. et al. A randomized controlled trial of topical exit site mupirocin application in patients with tunnelled, cuffed haemodialysis catheters. Nephrol Dial Transplant 2002; 17: 1802–7. [DOI] [PubMed] [Google Scholar]
- 24. Sesso R, Barbosa D, Leme IL. et al. Staphylococcus aureus prophylaxis in hemodialysis patients using central venous catheter: effect of mupirocin ointment. J Am Soc Nephrol 1998; 9: 1085–92. [DOI] [PubMed] [Google Scholar]
- 25. Wong SS, Chu KH, Cheuk A. et al. Prophylaxis against Gram-positive organisms causing exit-site infection and peritonitis in continuous ambulatory peritoneal dialysis patients by applying mupirocin ointment at the catheter exit site. Perit Dial Int 2003; 23: S153–8. [PubMed] [Google Scholar]
- 26. Bernardini J, Bender F, Florio T. et al. Randomized, double-blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J Am Soc Nephrol 2005; 16: 539–45. [DOI] [PubMed] [Google Scholar]
- 27. Bernardini J, Piraino B, Holley J. et al. A randomized trial of Staphylococcus aureus prophylaxis in peritoneal dialysis patients: mupirocin calcium ointment 2% applied to the exit site versus cyclic oral rifampin. Am J Kidney Dis 1996; 27: 695–700. [DOI] [PubMed] [Google Scholar]
- 28. Xu G, Tu W, Xu C.. Mupirocin for preventing exit-site infection and peritonitis in patients undergoing peritoneal dialysis. Nephrol Dial Transplant 2010; 25: 587–92. [DOI] [PubMed] [Google Scholar]
- 29. Grothe C, Taminato M, Belasco A. et al. Screening and treatment for Staphylococcus aureus in patients undergoing hemodialysis: a systematic review and meta-analysis. BMC Nephrol 2014; 15: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tacconelli E, Carmeli Y, Aizer A. et al. Mupirocin prophylaxis to prevent Staphylococcus aureus infection in patients undergoing dialysis: a meta-analysis. Clin Infect Dis 2003; 37: 1629–38. [DOI] [PubMed] [Google Scholar]
- 31. Perl TM, Cullen JJ, Wenzel RP. et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 2002; 346: 1871–7. [DOI] [PubMed] [Google Scholar]
- 32. Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM. et al. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis 2002; 35: 353–8. [DOI] [PubMed] [Google Scholar]
- 33. Konvalinka A, Errett L, Fong IW.. Impact of treating Staphylococcus aureus nasal carriers on wound infections in cardiac surgery. J Hosp Infect 2006; 64: 162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garcia AM, Villa MV, Escudero ME. et al. Use of nasal mupirocin for Staphylococcus aureus: effect on nasal carriers and nosocomial infections. Biomedica 2003; 23: 173–9. [PubMed] [Google Scholar]
- 35. Suzuki Y, Kamigaki T, Fujino Y. et al. Randomized clinical trial of preoperative intranasal mupirocin to reduce surgical-site infection after digestive surgery. Br J Surg 2003; 90: 1072–5. [DOI] [PubMed] [Google Scholar]
- 36. Shuman AG, Shuman EK, Hauff SJ. et al. Preoperative topical antimicrobial decolonization in head and neck surgery. Laryngoscope 2012; 122: 2454–60. [DOI] [PubMed] [Google Scholar]
- 37. Tai YJ, Borchard KL, Gunson TH. et al. Nasal carriage of Staphylococcus aureus in patients undergoing Mohs micrographic surgery is an important risk factor for postoperative surgical site infection: a prospective randomised study. Australas J Dermatol 2013; 54: 109–14. [DOI] [PubMed] [Google Scholar]
- 38. Bode LG, Kluytmans JA, Wertheim HF. et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362: 9–17. [DOI] [PubMed] [Google Scholar]
- 39. van Rijen M, Bonten M, Wenzel R. et al. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev 2008; issue 4: CD006216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Rijen MM, Bonten M, Wenzel RP. et al. Intranasal mupirocin for reduction of Staphylococcus aureus infections in surgical patients with nasal carriage: a systematic review. J Antimicrob Chemother 2008; 61: 254–61. [DOI] [PubMed] [Google Scholar]
- 41. Wertheim HF, Vos MC, Ott A. et al. Mupirocin prophylaxis against nosocomial Staphylococcus aureus infections in nonsurgical patients. Ann Intern Med 2004; 140: 419–25. [DOI] [PubMed] [Google Scholar]
- 42. Harbarth S, Dharan S, Liassine N. et al. Randomized, placebo-controlled, double-blind trial to evaluate the efficacy of mupirocin for eradicating carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 1999; 43: 1412–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Camus C, Sebille V, Legras A. et al. Mupirocin/chlorhexidine to prevent methicillin-resistant Staphylococcus aureus infections: post hoc analysis of a placebo-controlled, randomized trial using mupirocin/chlorhexidine and polymyxin/tobramycin for the prevention of acquired infections in intubated patients. Infection 2014; 42: 493–502. [DOI] [PubMed] [Google Scholar]
- 44. Huang SS, Septimus E, Kleinman K. et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013; 368: 2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nardi G, Di Silvestre AD, De Monte A. et al. Reduction in Gram-positive pneumonia and antibiotic consumption following the use of a SDD protocol including nasal and oral mupirocin. Eur J Emerg Med 2001; 8: 203–14. [DOI] [PubMed] [Google Scholar]
- 46. Stoutenbeek CP, van Saene HK, Miranda DR. et al. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med 1984; 10: 185–92. [DOI] [PubMed] [Google Scholar]
- 47. Mody L, Kauffman CA, McNeil SA. et al. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003; 37: 1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ellis MW, Griffith ME, Dooley DP. et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother 2007; 51: 3591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang ES, Tan J, Eells S. et al. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect 2010; 16: 425–31. [DOI] [PubMed] [Google Scholar]
- 50. Weintrob A, Bebu I, Agan B. et al. Randomized, double-blind, placebo-controlled study on decolonization procedures for methicillin-resistant Staphylococcus aureus (MRSA) among HIV-infected adults. PLoS One 2015; 10: e0128071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crum-Cianflone NF, Burgi AA, Hale BR.. Increasing rates of community-acquired methicillin-resistant Staphylococcus aureus infections among HIV-infected persons. Int J STD AIDS 2007; 18: 521–6. [DOI] [PubMed] [Google Scholar]
- 52. Nair R, Perencevich EN, Blevins AE. et al. Clinical effectiveness of mupirocin for preventing Staphylococcus aureus infections in nonsurgical settings: a meta-analysis. Clin Infect Dis 2016; 62: 618–30. [DOI] [PubMed] [Google Scholar]
- 53. Hayek LJ, Emerson JM, Gardner AM.. A placebo-controlled trial of the effect of two preoperative baths or showers with chlorhexidine detergent on postoperative wound infection rates. J Hosp Infect 1987; 10: 165–72. [DOI] [PubMed] [Google Scholar]
- 54. Climo MW, Yokoe DS, Warren DK. et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med 2013; 368: 533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Noto MJ, Domenico HJ, Byrne DW. et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA 2015; 313: 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ellis MW, Schlett CD, Millar EV. et al. Hygiene strategies to prevent methicillin-resistant Staphylococcus aureus skin and soft tissue infections: a cluster-randomized controlled trial among high-risk military trainees. Clin Infect Dis 2014; 58: 1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Segers P, Speekenbrink RG, Ubbink DT. et al. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA 2006; 296: 2460–6. [DOI] [PubMed] [Google Scholar]
- 58. Derde LP, Dautzenberg MJ, Bonten MJ.. Chlorhexidine body washing to control antimicrobial-resistant bacteria in intensive care units: a systematic review. Intensive Care Med 2012; 38: 931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Karki S, Cheng AC.. Impact of non-rinse skin cleansing with chlorhexidine gluconate on prevention of healthcare-associated infections and colonization with multi-resistant organisms: a systematic review. J Hosp Infect 2012; 82: 71–84. [DOI] [PubMed] [Google Scholar]
- 60. Kampf G. Acquired resistance to chlorhexidine—is it time to establish an ‘antiseptic stewardship’ initiative? J Hosp Infect 2016; 94: 213–27. [DOI] [PubMed] [Google Scholar]
- 61. Wilson AP, Lewis C, O’Sullivan H. et al. The use of povidone iodine in exit site care for patients undergoing continuous peritoneal dialysis (CAPD). J Hosp Infect 1997; 35: 287–93. [DOI] [PubMed] [Google Scholar]
- 62. Phillips M, Rosenberg A, Shopsin B. et al. Preventing surgical site infections: a randomized, open-label trial of nasal mupirocin ointment and nasal povidone-iodine solution. Infect Control Hosp Epidemiol 2014; 35: 826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Abele-Horn M, Dauber A, Bauernfeind A. et al. Decrease in nosocomial pneumonia in ventilated patients by selective oropharyngeal decontamination (SOD). Intensive Care Med 1997; 23: 187–95. [DOI] [PubMed] [Google Scholar]
- 64. Hammond JM, Potgieter PD, Saunders GL.. Selective decontamination of the digestive tract in multiple trauma patients—is there a role? Results of a prospective, double-blind, randomized trial. Crit Care Med 1994; 22: 33–9. [DOI] [PubMed] [Google Scholar]
- 65. Quinio B, Albanese J, Bues-Charbit M. et al. Selective decontamination of the digestive tract in multiple trauma patients. A prospective double-blind, randomized, placebo-controlled study. Chest 1996; 109: 765–72. [DOI] [PubMed] [Google Scholar]
- 66. Silvestri L, van Saene HK, Milanese M. et al. Prevention of MRSA pneumonia by oral vancomycin decontamination: a randomised trial. Eur Respir J 2004; 23: 921.. [DOI] [PubMed] [Google Scholar]
- 67. Swartz R, Messana J, Starmann B. et al. Preventing Staphylococcus aureus infection during chronic peritoneal dialysis. J Am Soc Nephrol 1991; 2: 1085–91. [DOI] [PubMed] [Google Scholar]
- 68. Kimura A, Mochizuki T, Nishizawa K. et al. Trimethoprim-sulfamethoxazole for the prevention of methicillin-resistant Staphylococcus aureus pneumonia in severely burned patients. J Trauma 1998; 45: 383–7. [DOI] [PubMed] [Google Scholar]
- 69. Falagas ME, Fragoulis KN, Bliziotis IA.. Oral rifampin for prevention of S. aureus carriage-related infections in patients with renal failure—a meta-analysis of randomized controlled trials. Nephrol Dial Transplant 2006; 21: 2536–42. [DOI] [PubMed] [Google Scholar]
- 70. Nunez-Moral M, Sanchez-Alvarez E, Gonzalez-Diaz I. et al. Exit-site infection of peritoneal catheter is reduced by the use of polyhexanide. Results of a prospective randomized trial. Perit Dial Int 2014; 34: 271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maddocks SE, Jenkins RE.. Honey: a sweet solution to the growing problem of antimicrobial resistance? Future Microbiol 2013; 8: 1419–29. [DOI] [PubMed] [Google Scholar]
- 72. Zumla A, Lulat A.. Honey—a remedy rediscovered. J R Soc Med 1989; 82: 384–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Johnson DW, van Eps C, Mudge DW. et al. Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J Am Soc Nephrol 2005; 16: 1456–62. [DOI] [PubMed] [Google Scholar]
- 74. Johnson DW, Badve SV, Pascoe EM. et al. Antibacterial honey for the prevention of peritoneal-dialysis-related infections (HONEYPOT): a randomised trial. Lancet Infect Dis 2014; 14: 23–30. [DOI] [PubMed] [Google Scholar]
- 75. Zhang L, Badve SV, Pascoe EM. et al. The effect of exit-site antibacterial honey versus nasal mupirocin prophylaxis on the microbiology and outcomes of peritoneal dialysis-associated peritonitis and exit-site infections: a sub-study of the Honeypot trial. Perit Dial Int 2015; 35: 712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mohammad H, Thangamani S, Seleem MN.. Antimicrobial peptides and peptidomimetics—potent therapeutic allies for staphylococcal infections. Curr Pharm Des 2015; 21: 2073–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lipsky BA, Holroyd KJ, Zasloff M.. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 2008; 47: 1537–45. [DOI] [PubMed] [Google Scholar]
- 78. Kollef M, Pittet D, Sanchez GM. et al. A randomized double-blind trial of iseganan in prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med 2006; 173: 91–7. [DOI] [PubMed] [Google Scholar]
- 79. Shinefield H, Black S, Fattom A. et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 2002; 346: 491–6. [DOI] [PubMed] [Google Scholar]
- 80. Fattom A, Matalon A, Buerkert J. et al. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: phase III randomized study. Hum Vaccin Immunother 2015; 11: 632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fowler VG, Allen KB, Moreira ED. et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 2013; 309: 1368–78. [DOI] [PubMed] [Google Scholar]
- 82. Paling FP, Olsen K, Ohneberg K. et al. Risk prediction for Staphylococcus aureus surgical site infection following cardiothoracic surgery; a secondary analysis of the V710-P003 trial. PLoS One 2018; 13: e0193445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jansen KU, Girgenti DQ, Scully IL. et al. Vaccine review: ‘Staphylococcus aureus vaccines: problems and prospects’. Vaccine 2013; 31: 2723–30. [DOI] [PubMed] [Google Scholar]
- 84. Fowler VG Jr, Proctor RA.. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect 2014; 20: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mohamed N, Wang MY, Le Huec JC. et al. Vaccine development to prevent Staphylococcus aureus surgical-site infections. Br J Surg 2017; 104: e41–54. [DOI] [PubMed] [Google Scholar]
- 86. Rijen MM, Bode LG, Baak DA. et al. Reduced costs for Staphylococcus aureus carriers treated prophylactically with mupirocin and chlorhexidine in cardiothoracic and orthopaedic surgery. PLoS One 2012; 7: e43065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bode LG, Rijen M, Wertheim HF. et al. Long-term mortality after rapid screening and decolonization of Staphylococcus aureus carriers: observational follow-up study of a randomized, placebo-controlled trial. Ann Surg 2016; 263: 511–15. [DOI] [PubMed] [Google Scholar]
- 88. Allegranzi B, Bischoff P, de Jonge S. et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016; 16: e276–87. [DOI] [PubMed] [Google Scholar]
- 89. Humphreys H, Becker K, Dohmen PM. et al. Staphylococcus aureus and surgical site infections: benefits of screening and decolonization before surgery. J Hosp Infect 2016; 94: 295–304. [DOI] [PubMed] [Google Scholar]
- 90. Perez-Fontan M, Rosales M, Rodriguez-Carmona A. et al. Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis 2002; 39: 337–41. [DOI] [PubMed] [Google Scholar]
- 91. Cookson BD. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother 1998; 41: 11–18. [DOI] [PubMed] [Google Scholar]
- 92. Barbosa TM, Levy SB.. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat 2000; 3: 303–11. [DOI] [PubMed] [Google Scholar]
- 93. Bratzler DW, Dellinger EP, Olsen KM. et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013; 70: 195–283. [DOI] [PubMed] [Google Scholar]
- 94. Cheng AG, DeDent AC, Schneewind O. et al. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol 2011; 19: 225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Speziale P, Pietrocola G, Foster TJ. et al. Protein-based biofilm matrices in staphylococci. Front Cell Infect Microbiol 2014; 4: 171.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cheung GY, Otto M.. The potential use of toxin antibodies as a strategy for controlling acute Staphylococcus aureus infections. Expert Opin Ther Targets 2012; 16: 601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Eggers S, Barker AK, Valentine S. et al. Effect of Lactobacillus rhamnosus HN001 on carriage of Staphylococcus aureus: results of the impact of probiotics for reducing infections in veterans (IMPROVE) study. BMC Infect Dis 2018; 18: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Drilling A, Morales S, Jardeleza C. et al. Bacteriophage reduces biofilm of Staphylococcus aureus ex vivo isolates from chronic rhinosinusitis patients. Am J Rhinol Allergy 2014; 28: 3–11. [DOI] [PubMed] [Google Scholar]
- 99. Wei Y, Gong J, Zhu W. et al. Fecal microbiota transplantation restores dysbiosis in patients with methicillin resistant Staphylococcus aureus enterocolitis. BMC Infect Dis 2015; 15: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.