Abstract
Objectives
To describe the pharmacokinetic/pharmacodynamic (PK/PD) modelling and microbiological data that were used to support the recent European approval of ceftaroline fosamil 600 mg q8h by 2 h intravenous (iv) infusion for patients with complicated skin and soft tissue infections (cSSTIs) caused by Staphylococcus aureus with ceftaroline MICs of 2 or 4 mg/L, and the associated EUCAST MIC breakpoint update for q8h dosing (intermediate = 2 mg/L and resistant >2 mg/L).
Methods
A population PK model for ceftaroline and ceftaroline fosamil was developed using PK data from 21 clinical studies. The final model was used to simulate PTA in patients with cSSTI receiving ceftaroline fosamil 600 mg q12h by 1 h iv infusion or 600 mg q8h by 2 h iv infusion. PTA was calculated by MIC for S. aureus PK/PD targets derived from preclinical studies (27% fT>MIC for stasis, 31% fT>MIC for 1 log10 kill and 35% fT>MIC for 2 log10 kill) and compared with S. aureus ceftaroline MIC distributions from a 2013 global surveillance study.
Results
The final population PK model based on 951 subjects adequately described ceftaroline and ceftaroline fosamil PK. High PTA (>90%) was predicted for the ceftaroline fosamil 600 mg q12h dosage regimen against S. aureus isolates with ceftaroline MICs ≤2 mg/L. Greater than 90% PTA was predicted for the ceftaroline fosamil 600 mg q8h dosage regimen against S. aureus with ceftaroline MICs ≤4 mg/L.
Conclusions
The approved ceftaroline fosamil dosage regimens for adults and adolescents with cSSTI achieve high PTA against S. aureus at the associated EUCAST breakpoints.
Introduction
Staphylococcus aureus is the most common cause of complicated skin and soft tissue infections (cSSTIs) worldwide.1 Ceftaroline, the active metabolite of the prodrug ceftaroline fosamil, demonstrates in vitro activity against many pathogens commonly implicated in cSSTI, including MSSA and MRSA, Streptococcus pyogenes and non-ESBL-producing Gram-negative species.2,3
Ceftaroline fosamil is approved in Europe for the treatment of cSSTI and community-acquired pneumonia (CAP), with similar indications in other regions.2,3 Ceftaroline fosamil is not approved for patients with CAP caused by MRSA,2,3 as these patients were excluded from the pivotal Phase 3 trials in CAP.4,5 For adults with cSSTI and normal renal function, ceftaroline fosamil was initially approved at a single dosage of 600 mg q12h by 1 h iv infusion based on data from the Phase 3 CANVAS 1 and 2 studies (NCT00424190 and NCT00423657), which demonstrated the non-inferiority of ceftaroline fosamil 600 mg q12h to vancomycin plus aztreonam.6,7 Ceftaroline MIC breakpoints for S. aureus of susceptible ≤1 mg/L and resistant >1 mg/L were established by EUCAST for the q12h dosage regimen.
Surveillance data in some regions such as Latin America and the Asia Pacific have reported ceftaroline MIC90 values of 2 mg/L for S. aureus8,9 and rare S. aureus isolates with ceftaroline MICs of 4 mg/L have also been identified.10 Therefore, an increased dose of ceftaroline fosamil may be of clinical benefit to treat infections caused by these strains. In the Phase 3 COVERS trial (NCT01499277), an increased total daily ceftaroline fosamil dose and infusion duration (600 mg q8h by 2 h iv infusion) was evaluated in patients with cSSTI and evidence of systemic inflammation and/or underlying comorbidities.11 The higher q8h dose was evaluated to ensure adequate exposure of ceftaroline in severe infections that may be associated with increased clearance of ceftaroline and for infections caused by S. aureus with ceftaroline MICs >2 mg/L. Despite various efforts during COVERS to maximize the number of S. aureus clinical isolates with ceftaroline MICs of ≥2 mg/L, including an MRSA-focused expansion period following the main trial, only one such isolate was identified.11
In the absence of clinical data for the treatment of pathogens with higher MICs, pharmacokinetic/pharmacodynamic (PK/PD) analyses using population PK models in Monte Carlo simulations to determine PTA are important to support dose recommendations and determine clinical breakpoints. The European label for ceftaroline fosamil was recently expanded to recommend an increased dose of 600 mg q8h by 2 h iv infusion for the treatment of patients with cSSTI caused by S. aureus with ceftaroline MICs of 2 or 4 mg/L.2 The approval of the q8h dose led EUCAST to introduce an intermediate category of MIC = 2 mg/L for ceftaroline against S. aureus, per EUCAST current practice,12 and defined the resistant category for the q8h dose as MIC >2 mg/L,13 with the breakpoints for the q12h dose remaining unchanged.13 This paper describes the PK/PD modelling and microbiological data that were used together with clinical data from COVERS to support updates to the European product labelling for ceftaroline fosamil and associated EUCAST breakpoints.
Methods
Ceftaroline PK/PD targets for S. aureus
The duration of time that free drug plasma concentration exceeds the MIC of the target organism (%fT>MIC) is an established PK/PD index for ceftaroline.14,15 Ceftaroline S. aureus PK/PD targets were derived from three preclinical models: an in vivo neutropenic murine thigh infection model,14 an in vitro single-compartment dilutional PK model16 and an in vitro hollow-fibre model.17 In total, 24 S. aureus isolates with ceftaroline MICs ranging from 0.12 to 4 mg/L and a wide range of genotypes, including different MLST and staphylococcal cassette chromosome mec (SCCmec) types, and isolates with genetic variations in the PBP2a domain, were assessed in these studies to: (i) include representative clinical isolates with molecular characteristics most widely prevalent in different geographic regions; and (ii) compensate for limited efficacy data on clinical trial isolates with ceftaroline MIC values ≥2 mg/L. The mean %fT>MIC values across the 24 isolates were used to derive S. aureus PK/PD targets for the PTA analysis of 27% fT>MIC for stasis, 31% fT>MIC for 1 log10 kill and 35% fT>MIC for 2 log10 kill (Table 1).
Table 1.
Summary of preclinical studies used to derive ceftaroline S. aureus PK/PD targets for PTA analysis
| Study | PK/PD model | No. of isolates | MIC range (mg/L) | PK/PD target [mean (SD) %fT>MIC at 24 h] |
||
|---|---|---|---|---|---|---|
| stasis | 1 log10 kill | 2 log10 kill | ||||
| Andes and Craig (2006)14 | neutropenic murine thigh infection model | 4a | 0.12–1 | 26 (8) | 33 (9) | 45 (13) |
| MacGowan et al. (2013)16 | in vitro single-compartment dilutional PK model | 8b | 0.125–2 | 24.5 (8.9) | 27.8 (9.5) | 27.7 (5.7) |
| Singh et al. (2017)17 | in vitro hollow-fibre model | 12c | 2–4 | 28 (7) | 31 (6) | 35 (6) |
| Total | MIC range | Meand (SD) | ||||
| 24 | 0.12–4 | 26.8 (7.7) | 30.7 (8.0) | 34.7 (9.4) | ||
Included two MSSA and two MRSA strains.
Included four MSSA and four MRSA strains.
Included 12 molecularly characterized MRSA strains: 10 isolates with one to four mutations in the non-penicillin binding domain (nPBD) or WT characteristics and two isolates with one mutation each in nPBD and PBD. SCCmec types included nine isolates of Type I–II and three isolates of Type III.
Overall mean of individual isolates from the three data sources.
Population PK model development
Population PK models of ceftaroline and ceftaroline fosamil were developed and updated with clinical trial data throughout the clinical development programme (see footnote to Table S1, available as Supplementary data at JAC Online). In this analysis, the population PK model dataset comprised data from 21 clinical studies [14 Phase 1 studies in healthy subjects and patients with end-stage renal disease (ESRD), 1 Phase 2 and 3 Phase 3 studies in patients with cSSTI and 3 Phase 3 studies in CAP; Table S1].4–7,11,18–26 Ceftaroline fosamil was given as an iv infusion in these studies, apart from in one study in which ceftaroline fosamil was administered intramuscularly.20
First-order conditional estimation with interaction method in NONMEM version 7.2.0 was used to develop the population PK model. Full details of the covariate analysis are provided in the Supplementary Methods. In brief, the previous model (unpublished data; J. Li, S. Das, D. Zhou and N. Al-Huniti) was adapted as the basis to re-evaluate the significance of previously identified significant covariates: age, patient status (healthy volunteers versus patients) and creatinine clearance normalized by body surface area (NCLCR) on ceftaroline CL and patient status on the central volume of distribution of ceftaroline (Vcc). These covariates were re-evaluated with the pooled dataset by a backward elimination procedure to obtain a full model. The full model was subsequently used as a base model to evaluate the effect of the race and gender covariate on ceftaroline CL and Vcc using a forward selection and backward elimination procedure to obtain the final model. The final model was evaluated by standard goodness-of-fit plots and visual predictive check.
PTA analysis
The final model was used to simulate ceftaroline plasma concentration–time courses in 5000 patients with cSSTI receiving ceftaroline fosamil 600 mg q12h by 1 h iv infusion or 600 mg q8h by 2 h iv infusion by renal function category (doses adjusted for renal function as described below). Covariates for the PTA simulation (including age, sex, body weight and NCLCR) were based on patients with cSSTI in one Phase 2 and three Phase 3 studies,6,7,11,19 and subjects from four renal impairment studies.18,25 Individual PK parameters were simulated from the population mean PK parameters, associated individual covariates from the multivariate covariate distribution and inter-subject variability from the final model. The residual error was fixed to zero in the simulation of individual ceftaroline time courses. For subjects with normal renal function (CLCR >80 mL/min), individual covariates of 5000 subjects were sampled with replacement from the baseline covariates in patients included in the final model. For simulations of subjects in renal impairment categories, covariates were simulated from the multivariate covariate distribution and bounded by the appropriate upper and lower limits observed in the dataset. CLCR values were assumed to follow a uniform distribution within the designated range for each category.
The %fT>MIC at steady-state was calculated for subjects with normal renal function or mild renal impairment (CLCR 50–80 mL/min) receiving ceftaroline fosamil 600 mg q12h by 1 h iv infusion or q8h by 2 h iv infusion. Doses were adjusted to 400 mg, 300 mg or 200 mg for simulations of patients with moderate renal impairment (CLCR 30–50 mL/min), severe renal impairment (CLCR 15–30 mL/min) and ESRD (CLCR 5–15 mL/min), respectively.2,3 An 80% unbound fraction of plasma concentration was applied for calculating %fT>MIC.2,3 PTA was calculated as the percentage of 5000 simulated subjects with cSSTI who met the S. aureus PK/PD targets described above at ceftaroline MICs of 0.015, 0.03, 0.06, 0.125, 0.25, 0.5, 1, 2, 4 and 8 mg/L. PTA was also calculated by MIC for non-species-specific %fT>MIC targets ranging from 10%–100%.
To evaluate whether the doses provided adequate coverage for susceptibility profiles of S. aureus isolates encountered in clinical practice, PTAs were compared with ceftaroline MIC frequency distributions of S. aureus clinical isolates collected during 2013 as part of the Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE) surveillance study in medical centres in Europe and the Asia Pacific region. The methodology used in the AWARE surveillance programme has been published elsewhere; in brief, isolates were tested at a central laboratory using reference CLSI broth microdilution methods and susceptibility was interpreted according to CLSI/EUCAST/FDA breakpoints.9,27,28
Results
Final population PK model
The final population PK model dataset included 2575 ceftaroline fosamil concentrations and 8174 ceftaroline concentrations from 951 subjects. Demographic covariate data for the final modelling dataset are summarized in Table S2. The dataset comprised 501 male and 450 female subjects with a median age of 50 years (range 12–93 years) and a median body weight of 73 kg (range 40–134 kg). Of the 951 subjects, 267 (28%) were healthy volunteers and 684 (72%) were patients, including 214 patients with CAP, 463 patients with cSSTI and 7 patients with suspected infection. The ceftaroline fosamil and ceftaroline concentration–time courses were simultaneously modelled as two-compartment disposition PK models with an assumption of 100% conversion of ceftaroline fosamil into ceftaroline. As one study included intramuscular delivery of ceftaroline fosamil, a zero-order absorption component of ceftaroline fosamil after intramuscular administration of ceftaroline fosamil was included in the model. The impact of body weight on CL and V of both ceftaroline and ceftaroline fosamil were modelled allometrically.
Age, participant status (patients versus healthy volunteers) and NCLCR were significant covariates on CL of ceftaroline; participant status was a significant covariate on Vcc. The overall CL of ceftaroline increased as CLCR increased, and decreased as age increased. Race and gender were not considered significant covariates of ceftaroline CL and Vcc. The final population PK model adequately described observed concentrations of both ceftaroline and ceftaroline fosamil and the covariate relationships in patients with cSSTI, judging from standard goodness-of-fit plots and visual predictive checks showing that simulations from the final model were consistent with the observed data (data not shown). All parameter estimates of the final model exhibited small relative standard errors (Table S3).
Ceftaroline in vitro activity against S. aureus isolates collected in the AWARE 2013 surveillance study
A total of 5380 S. aureus clinical isolates (2815 MRSA) were collected in Europe and 2595 S. aureus isolates (1574 MRSA) were collected in the Asia Pacific region. In Europe, the overall ceftaroline MIC90 against all S. aureus isolates including MRSA was 1 mg/L; in the individual countries, ceftaroline MIC90 values were ≤1 mg/L, except in Italy, Poland, Romania, Russia and Turkey, where a ceftaroline MIC90 of 2 mg/L was reported. In the Asia Pacific region, the ceftaroline MIC90 against all S. aureus isolates including MRSA was 2 mg/L. The ceftaroline MIC90 was ≤2 mg/L in all individual countries, except in Thailand where an MIC90 of 4 mg/L was reported. Isolates with ceftaroline MICs >2 mg/L comprised 9 MRSA isolates collected from Thailand with MICs of 8 mg/L and 33 isolates collected from South Korea (4 isolates) and Thailand (29 isolates) with MICs of 4 mg/L.
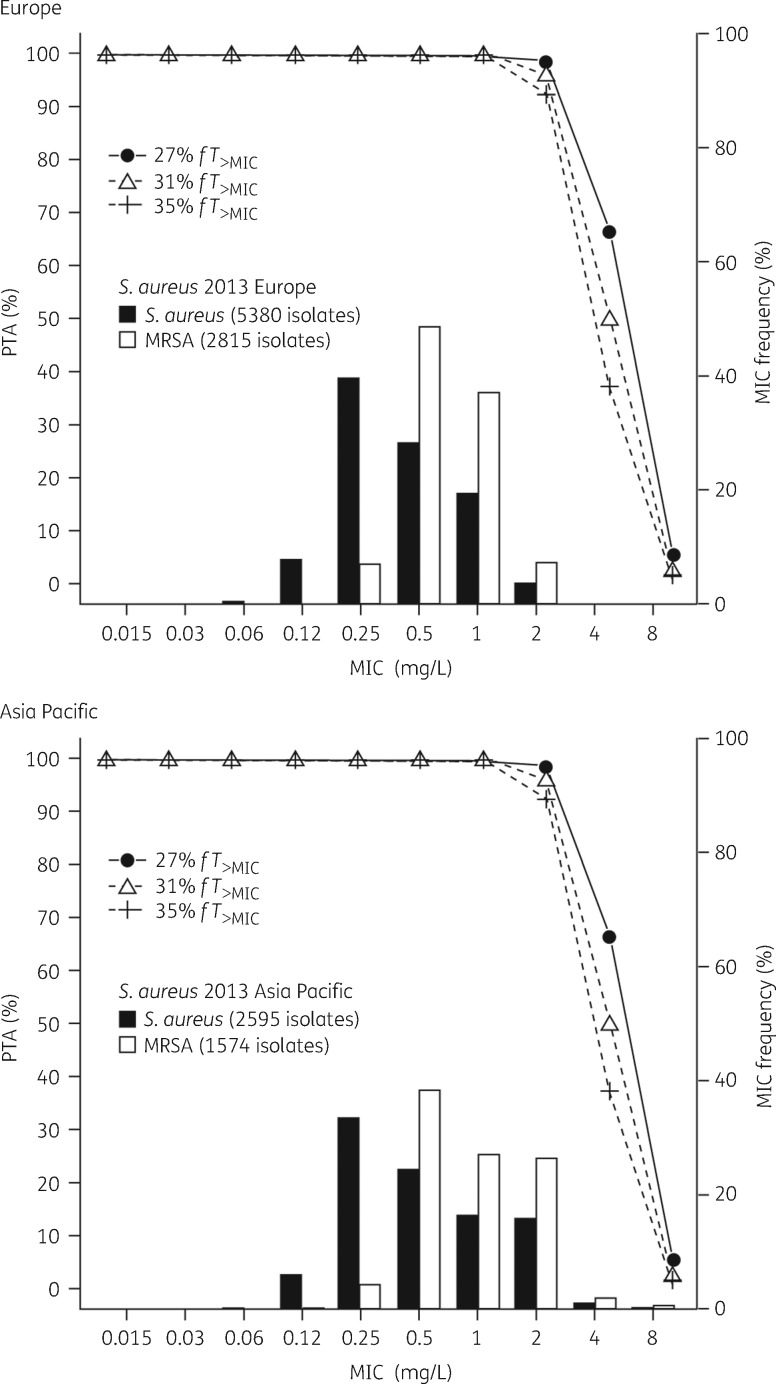
PTA by MIC for patients with normal renal function given ceftaroline fosamil 600 mg q12h by 1 h iv infusion
PTAs by MIC for the S. aureus PK/PD targets in 5000 simulated cSSTI patients with normal renal function given ceftaroline fosamil 600 mg q12h by 1 h iv infusion are shown in Table 2. Ceftaroline fosamil 600 mg q12h is predicted to achieve >95% PTA for the 1 log10 kill target (31% fT>MIC) and >90% PTA for the 2 log10 kill target (35% fT>MIC) against S. aureus testing with ceftaroline MICs ≤2 mg/L. PTA by MIC overlaid with the AWARE 2013 ceftaroline MIC frequency distribution for S. aureus shows that ceftaroline fosamil 600 mg q12h provides >90% PTA for the MIC distributions of S. aureus isolates with a ceftaroline MIC ≤2 mg/L in both Europe and the Asia Pacific region (Figure 1). For PK/PD targets up to and including 50% fT>MIC (beyond the 35% fT>MIC required for 2 log10 kill), >90% PTA was achieved for an MIC of 1 mg/L (Table S4). At a target of 60% fT>MIC, PTA was 78.7% for an MIC of 1 mg/L.
Table 2.
PTA for 5000 simulated cSSTI patients with normal renal function achieving PK/PD targets for S. aureus by MIC following administration of ceftaroline fosamil 600 mg q12h 1 h iv infusion
| Ceftaroline MIC (mg/L) | PTA (%) |
||
|---|---|---|---|
| stasis (27% fT>MIC) | 1 log10 kill (31% fT>MIC) | 2 log10 kill (35% fT>MIC) | |
| 0.015 | 100 | 100 | 100 |
| 0.03 | 100 | 100 | 100 |
| 0.06 | 100 | 100 | 100 |
| 0.125 | 100 | 100 | 100 |
| 0.25 | 100 | 100 | 100 |
| 0.5 | 100 | 100 | 100 |
| 1 | 100 | 100 | 100 |
| 2 | 98.9 | 96.1 | 92.5 |
| 4 | 66.5 | 49.5 | 37.1 |
| 8 | 4.84 | 2.14 | 1.04 |
| 16 | 0.04 | 0 | 0 |
Figure 1.
PTA for 5000 simulated cSSTI patients with normal renal function achieving PK/PD targets for S. aureus by MIC following administration of ceftaroline fosamil 600 mg q12h 1 h iv infusion, overlaid with ceftaroline MIC distributions for S. aureus collected from the 2013 AWARE surveillance study in Europe and the Asia Pacific region. The ceftaroline MIC90 values for all S. aureus isolates and the MRSA subset were 1 mg/L and 2 mg/L in Europe and the Asia Pacific region, respectively.
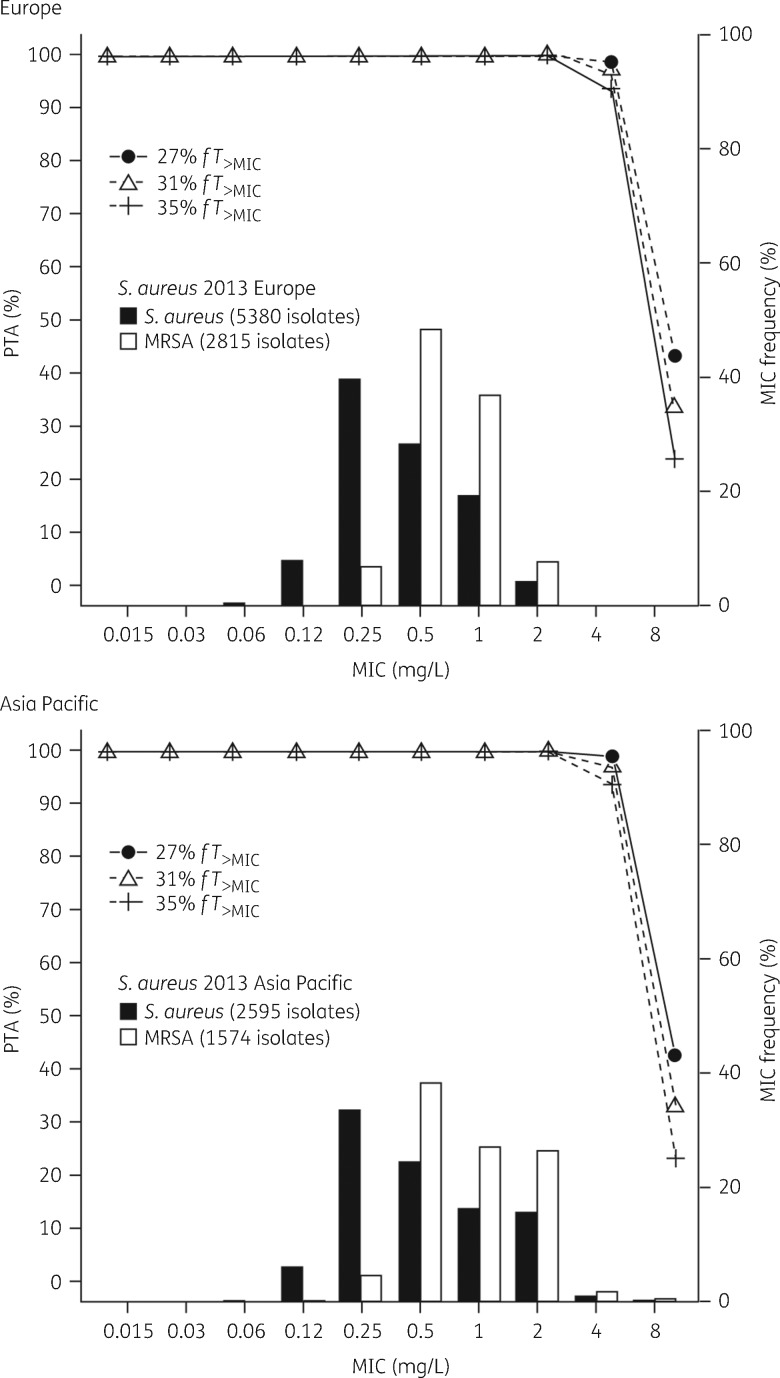
PTA by MIC for patients with normal renal function given ceftaroline fosamil 600 mg q8h by 2 h iv infusion
PTAs by MIC for the S. aureus PK/PD targets in 5000 simulated cSSTI patients with normal renal function (CLCR >80 mL/min) given ceftaroline fosamil 600 mg q8h by 2 h iv infusion are shown in Table 3. Ceftaroline fosamil 600 mg q8h is predicted to achieve >95% PTA for the 1 log10 kill target (31% fT>MIC) and >90% PTA for the 2 log10 kill target against S. aureus isolates with ceftaroline MICs ≤4 mg/L (Table 3). PTA by MIC overlaid with the AWARE 2013 ceftaroline MIC frequency distributions for S. aureus in Europe and the Asia Pacific region shows that ceftaroline fosamil 600 mg q8h provides >90% PTA for the MIC distribution of S. aureus with ceftaroline MICs ≤4 mg/L in both regions (Figure 2). For an MIC of 2 mg/L, >90% PTA was maintained for targets as high as 60% fT>MIC (Table S5). At an MIC of 4 mg/L, PTA was 86% for a target of 40% fT>MIC.
Table 3.
PTA for 5000 simulated cSSTI patients with normal renal function achieving PK/PD targets for S. aureus by MIC following administration of ceftaroline fosamil 600 mg q8h 2 h iv infusion
| Ceftaroline MIC (mg/L) | PTA (%) |
||
|---|---|---|---|
| stasis (27% fT>MIC) | 1 log10 kill (31% fT>MIC) | 2 log10 kill (35% fT>MIC) | |
| 0.015 | 100 | 100 | 100 |
| 0.03 | 100 | 100 | 100 |
| 0.06 | 100 | 100 | 100 |
| 0.125 | 100 | 100 | 100 |
| 0.25 | 100 | 100 | 100 |
| 0.5 | 100 | 100 | 100 |
| 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 |
| 4 | 98.7 | 96.8 | 93.6 |
| 8 | 42.9 | 33.1 | 23.4 |
| 16 | 0.72 | 0.38 | 0.22 |
Figure 2.
PTA for 5000 simulated cSSTI patients with normal renal function achieving PK/PD targets for S. aureus by MIC following administration of ceftaroline fosamil 600 mg q8h 2 h iv infusion, overlaid with ceftaroline MIC distributions for S. aureus collected from the 2013 AWARE surveillance study in Europe and the Asia Pacific region. The ceftaroline MIC90 values for all S. aureus isolates and the MRSA subset were 1 mg/L and 2 mg/L in Europe and the Asia Pacific region, respectively.
PTA by MIC for patients with renal impairment receiving adjusted q12h and q8h doses
Similar PTA analyses were conducted for patients with mild renal impairment receiving ceftaroline fosamil 600 mg q12h or q8h, and in patients with moderate renal impairment, severe renal impairment and ESRD receiving the recommended dosage adjustments.2,3 PTAs by MIC were generally similar or higher in patients with renal impairment compared with subjects with normal renal function (Tables S6 and S7).
Discussion
Ceftaroline PK/PD targets against S. aureus were initially derived based on a single in vivo study against four S. aureus isolates with ceftaroline MICs ranging from 0.12 to 1 mg/L.14 The mean (SD) %fT>MIC values for stasis, 1 log10 kill and 2 log10 kill in that study were 26% (8), 33% (9) and 45% (13). Since then, ceftaroline PK/PD against S. aureus have been evaluated in two further in vitro studies.16,17 The PK/PD targets for S. aureus used in this analysis were derived from these three studies using 24 isolates with a wider range of ceftaroline MICs (0.12–4 mg/L) and genotypes than in the single in vivo study alone. It is expected that there would be some variation in the PK/PD data obtained from in vitro and in vivo approaches; however, the %fT>MIC values across the three models were generally in good concordance. Using data from across three different models provides a robust basis to determine the S. aureus PK/PD targets for use in PTA analysis to support dosage recommendations and breakpoint determination, as each model may compensate for limitations in the others. The ceftaroline S. aureus PK/PD targets for stasis, 1 log10 kill and 2 log10 kill were thus better characterized and defined as 27%, 31% and 35% fT>MIC, respectively.
An early population PK model based on data from 185 healthy subjects and 92 patients with cSSTI was used to support the initial approvals of the ceftaroline fosamil 600 mg q12h dosage regimen.29,30 The population PK model reported here was based on a more substantial clinical dataset comprising 951 subjects, including 463 patients with cSSTI. Similar to the earlier model,30 age, participant status (patients versus healthy volunteers) and NCLCR were identified as significant covariates impacting ceftaroline and ceftaroline fosamil PK. Critical illness caused by severe infection can affect antibiotic PK, resulting in low exposures and a higher risk of not achieving PK/PD targets compared with non-critically ill patients.31 The inclusion of patient PK data from the COVERS study [which included patients with systemic inflammatory response syndrome (SIRS) and bacteraemia]11 ensured that a broad range of comorbid conditions and disease severity covariates were included in the dataset. This provides reassurance that the PTA estimates from this model will be relevant for patients with more severe infections.
Whilst the susceptible breakpoint for the ceftaroline fosamil 600 mg q12h dose is ≤1 mg/L, high PTA (>90%) was predicted for patients with cSSTI treated with the q12h dosage regimen against S. aureus with ceftaroline MICs up to and including 2 mg/L. Ceftaroline fosamil 600 mg q8h is predicted to achieve high PTA (>90%) for S. aureus MICs up to and including 4 mg/L. Whilst the resistant breakpoint for the ceftaroline fosamil q8h dose is MIC >2 mg/L, both the European label and EUCAST indicate that ceftaroline fosamil 600 mg q8h can be used for the treatment of cSSTI caused by rare S. aureus isolates with MICs of 4 mg/L. For both dosage frequencies, similar or higher PTAs are still achieved in cSSTI patients with renal impairment receiving adjusted doses for renal function compared with patients with normal renal function.
The achievement of therapeutic antibiotic concentrations at the infection site is an important consideration for breakpoint setting and dosing recommendations. In line with the PTA results presented here, comparison of ceftaroline PK following administration of ceftaroline 600 mg q12h or q8h in healthy volunteers found that calculated %fT>MIC values in the plasma, muscle and subcutis exceeded the S. aureus PK/PD targets for both dosage regimens up to an MIC of 2 mg/L, while the q8h dosage regimen provided %fT>MIC values ranging from 26.2%–43.0% across the three compartments for an MIC of 4 mg/L.32
The susceptibility of S. aureus to ceftaroline varies geographically.8,9,33,34 In the AWARE 2013 surveillance study data presented here, ceftaroline MIC90s of 1 mg/L and 2 mg/L were reported for S. aureus (including MRSA) in Europe and the Asia Pacific region, respectively. Whilst a low frequency of S. aureus isolates were identified with an MIC of 2 mg/L in Europe, ceftaroline MIC90s of 2 mg/L were reported in Italy, Poland, Romania, Russia and Turkey. In the Asia Pacific region there was a much higher frequency of isolates with a ceftaroline MIC of 2 mg/L: in most countries the MIC90 was 2 mg/L, except in Thailand where the MIC90 was 4 mg/L. Ceftaroline 600 mg q12h was shown to provide high PTA across the MIC distributions of real-life S. aureus isolates with ceftaroline MICs ≤2 mg/L, while the ceftaroline 600 mg q8h dosage regimen provides high PTA for S. aureus isolates with ceftaroline MICs ≤4 mg/L.
The effect of the EUCAST MIC breakpoint change on the interpretation of S. aureus susceptibility to ceftaroline was explored in a recent analysis of MRSA isolates from cSSTI patients collected through the AWARE 2015–16 surveillance programme.35 The percentage of isolates categorized as resistant to ceftaroline across Europe, Latin America, Middle East/Africa and the Asia/South Pacific regions decreased from 3.1%–7.6% when using the q12h breakpoints (resistant >1 mg/L) to 0.0%–1.3% when using the q8h breakpoints (intermediate = 2 mg/L, resistant >2 mg/L). This was most notable in the Latin America and Asia/South Pacific regions, where isolates with a ceftaroline MIC of 2 mg/L are more prevalent and are now classified in the intermediate category. These findings indicate that the frequency of S. aureus resistant to ceftaroline (MIC >2 mg/L) remains low globally.
A limitation of this analysis is the lack of clinical outcomes data available for cSSTI caused by S. aureus with high ceftaroline MICs. The results from COVERS demonstrated the non-inferiority of ceftaroline fosamil 600 mg q8h with respect to vancomycin plus aztreonam in patients with cSSTI and no new safety signals were identified.11 Importantly, clinical outcomes in patient subgroups with more severe infection were comparable between COVERS and the CANVAS studies; clinical cure rates in patients with one or more sign of systemic inflammation in the clinically evaluable population were 88.0% (219/249) in COVERS versus 91.1% (308/338) in CANVAS 1 and 2.36 Consistent with this finding, predicted ceftaroline exposures (AUCss and Cmax, ss) were comparable between COVERS patients with and without signs of severe infection, such as high white blood cell count, high C-reactive protein levels and the presence of fever, SIRS or bacteraemia.37 Together with the findings presented here, these data support that ceftaroline fosamil 600 mg q12h is a robust dosage regimen for most patients with cSSTI, with the 600 mg q8h regimen offering an additional treatment option for patients with cSSTI caused by S. aureus with ceftaroline MICs of 2 or 4 mg/L.
Conclusions
S. aureus with ceftaroline MICs of 2–4 mg/L represent the upper end of the MIC distribution and clinical outcomes data are limited for cSSTI caused by S. aureus isolates with high ceftaroline MICs. Population PK modelling based on an extensive patient dataset and using robustly characterized PK/PD targets indicates that ceftaroline fosamil 600 mg q12h achieves >90% PTA against S. aureus at the EUCAST susceptible breakpoint of 1 mg/L as well as for a ceftaroline MIC of 2 mg/L, and that ceftaroline fosamil 600 mg q8h achieves >90% PTA for ceftaroline MICs ≤4 mg/L. These results provide reassurance that the approved ceftaroline fosamil dosage regimens for cSSTI achieve high PTA against S. aureus at the associated EUCAST MIC breakpoints.
Supplementary Material
Acknowledgements
We would like to thank all the investigators and patients involved in the ceftaroline fosamil clinical trials and Jennifer Hammond, Pfizer, Collegeville, PA, USA for her review and critical input on the manuscript.
These data were presented in part at the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 2015, San Diego, CA, USA (abstracts A-456 and A-455) and at the 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) 2015, Copenhagen, Denmark (poster P1384).
Funding
The population PK modelling and PTA analysis and the global (excluding the US) AWARE surveillance programme were funded by AstraZeneca. Of the studies included in this population PK analysis, NCT01499277, NCT01371838, NCT01458743, NCT01612507 and NCT01664065 were originally sponsored by AstraZeneca and are now sponsored by Pfizer. All other studies included in the population PK analysis were sponsored by Allergan. AstraZeneca’s rights to ceftaroline fosamil were acquired by Pfizer in December 2016.
Medical writing support was provided by Sirisha Bulusu, MBiochem of Prime, Knutsford, Cheshire, UK, funded by Pfizer. Ultimate responsibility for opinions, conclusions and data interpretation lies with the authors.
Transparency declarations
J. I., S. D. and J. L. are former employees of and shareholders in AstraZeneca. G. G. S. is a former employee of and shareholder in AstraZeneca and is currently an employee of and shareholder in Pfizer. D. Z. is an employee of and shareholder in AstraZeneca. D. M. is a former employee of Allergan. J. L. Y. is an employee of and shareholder in Pfizer.
Data sharing
Upon request, and subject to certain criteria, conditions and exceptions see (https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (i) for indications that have been approved in the US and/or EU or (ii) in programmes that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1. Bassetti M, Baguneid M, Bouza E. et al. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin Microbiol Infect 2014; 20 Suppl 4: 3–18. [DOI] [PubMed] [Google Scholar]
- 2. Pfizer. Zinforo 600 mg Powder for Concentrate for Solution for Infusion: Summary of Product Characteristics.2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002252/WC500132586.pdf.
- 3. Allergan. Teflaro™ (Ceftaroline Fosamil) Injection for Intravenous (IV) Use 2016. https://www.allergan.com/assets/pdf/teflaro_pi.
- 4. File TM, Low DE, Eckburg PB. et al. FOCUS 1: a randomized, double-blinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 2011; 66: iii19–32. [DOI] [PubMed] [Google Scholar]
- 5. Low DE, File TM, Eckburg PB. et al. FOCUS 2: a randomized, double-blinded, multicentre, Phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 2011; 66: iii33–44. [DOI] [PubMed] [Google Scholar]
- 6. Corey GR, Wilcox MH, Talbot GH. et al. CANVAS 1: the first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010; 65 Suppl 4: iv41–51. [DOI] [PubMed] [Google Scholar]
- 7. Wilcox MH, Corey GR, Talbot GH. et al. CANVAS 2: the second Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 2010; 65 Suppl 4: iv53–65. [DOI] [PubMed] [Google Scholar]
- 8. Flamm RK, Sader HS, Jones RN.. Ceftaroline activity tested against contemporary Latin American bacterial pathogens (2011). Braz J Infect Dis 2014; 18: 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biedenbach DJ, Alm RA, Lahiri SD. et al. In vitro activity of ceftaroline against Staphylococcus aureus isolated in 2012 from Asia-Pacific countries as part of the AWARE surveillance program. Antimicrob Agents Chemother 2015; 60: 343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones RN, Mendes RE, Sader HS.. Ceftaroline activity against pathogens associated with complicated skin and skin structure infections: results from an international surveillance study. J Antimicrob Chemother 2010; 65 Suppl 4: iv17–31. [DOI] [PubMed] [Google Scholar]
- 11. Dryden M, Zhang Y, Wilson D. et al. A Phase III, randomized, controlled, non-inferiority trial of ceftaroline fosamil 600 mg every 8 h versus vancomycin plus aztreonam in patients with complicated skin and soft tissue infection with systemic inflammatory response or underlying comorbidities. J Antimicrob Chemother 2016; 71: 3575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kahlmeter G. EUCAST proposes to change the definition and usefulness of the susceptibility category ‘Intermediate’. Clin Microbiol Infect 2017; 23: 894–5. [DOI] [PubMed] [Google Scholar]
- 13. EUCAST. Addendum to Breakpoint Table v 7.1, July 2017.2017. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/Addendum_2017-07-14.pdf.
- 14. Andes D, Craig W.. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob Agents Chemother 2006; 50: 1376–83. Erratum in: Antimicrob Agents Chemother 2014; 58: 2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 2003; 17: 479–501. [DOI] [PubMed] [Google Scholar]
- 16. MacGowan AP, Noel AR, Tomaselli S. et al. Pharmacodynamics of ceftaroline against Staphylococcus aureus studied in an in vitro pharmacokinetic model of infection. Antimicrob Agents Chemother 2013; 57: 2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh R, Almutairi M, Alm R. et al. Ceftaroline efficacy against high-MIC clinical Staphylococcus aureus isolates in an in vitro hollow-fibre infection model. J Antimicrob Chemother 2017; 72: 2796–803. [DOI] [PubMed] [Google Scholar]
- 18. Sunzel M, Learoyd M, Li J. et al. An open-label, non-randomised, phase 1, single-dose study to assess the pharmacokinetics of ceftaroline in patients with end-stage renal disease requiring intermittent haemodialysis. Int J Antimicrob Agents 2015; 46: 682–8. [DOI] [PubMed] [Google Scholar]
- 19. Talbot GH, Thye D, Das A. et al. Phase 2 study of ceftaroline versus standard therapy in treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother 2007; 51: 3612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riccobene T, Fang E, Thye D, A single- and multiple-dose study to determine the safety, tolerability, and pharmacokinetics (PK) of ceftaroline (CPT) administered by intramuscular (IM) injection to healthy subjects. In: Abstracts of the 48th ICCAC and 46th IDSA, Washington, DC, USA2008. Abstract A-1888. Washington DC, USA: American Society for Microbiology.
- 21. Yang L, Sunzel M, Xu P. et al. Evaluation of the pharmacokinetics and safety of single and multiple ceftaroline fosamil infusions in healthy Chinese and Western subjects. Int J Clin Pharmacol Ther 2015; 53: 681–91. [DOI] [PubMed] [Google Scholar]
- 22. Riccobene TA, Su SF, Rank D.. Single- and multiple-dose study to determine the safety, tolerability, and pharmacokinetics of ceftaroline fosamil in combination with avibactam in healthy subjects. Antimicrob Agents Chemother 2013; 57: 1496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ClinicalTrials.gov. Pharmacokinetics of a Single Dose of Ceftaroline in Subjects 12 to 17 Years of Age Receiving Antibiotic Therapy (NCT00633126). https://clinicaltrials.gov/ct2/show/NCT00633126.
- 24. Panagiotidis G, Backstrom T, Asker-Hagelberg C. et al. Effect of ceftaroline on normal human intestinal microflora. Antimicrob Agents Chemother 2010; 54: 1811–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riccobene T, Jakate A, Rank D.. A series of pharmacokinetic studies of ceftaroline fosamil in select populations: normal subjects, healthy elderly subjects, and subjects with renal impairment or end-stage renal disease requiring hemodialysis. J Clin Pharmacol 2014; 54: 742–52. [DOI] [PubMed] [Google Scholar]
- 26. Zhong NS, Sun T, Zhuo C. et al. Ceftaroline fosamil versus ceftriaxone for the treatment of Asian patients with community-acquired pneumonia: a randomised, controlled, double-blind, phase 3, non-inferiority with nested superiority trial. Lancet Infect Dis 2015; 15: 161–71. [DOI] [PubMed] [Google Scholar]
- 27. Biedenbach DJ, Iaconis JP, Sahm DF.. Comparative in vitro activities of ceftaroline and ceftriaxone against bacterial pathogens associated with respiratory tract infections: results from the AWARE surveillance study. J Antimicrob Chemother 2016; 71: 3459–64. [DOI] [PubMed] [Google Scholar]
- 28. Karlowsky JA, Biedenbach DJ, Bouchillon SK. et al. In vitro activity of ceftaroline against bacterial pathogens isolated from skin and soft tissue infections in Europe, Russia and Turkey in 2012: results from the Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE) surveillance programme. J Antimicrob Chemother 2016; 71: 162–9. [DOI] [PubMed] [Google Scholar]
- 29. Van Wart SA, Ambrose PG, Rubino CM. et al. Pharmacokinetic-pharmacodynamic target attainment analyses to evaluate in vitro susceptibility test interpretive criteria for ceftaroline against Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother 2014; 58: 885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Wart SA, Forrest A, Khariton T. et al. Population pharmacokinetics of ceftaroline in patients with acute bacterial skin and skin structure infections or community‐acquired bacterial pneumonia. J Clin Pharmacol 2013; 53: 1155–67. [DOI] [PubMed] [Google Scholar]
- 31. Roberts JA, Abdul-Aziz MH, Lipman J. et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 2014; 14: 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matzneller P, Lackner E, Lagler H. et al. Single- and repeated-dose pharmacokinetics of ceftaroline in plasma and soft tissues of healthy volunteers for two different dosing regimens of ceftaroline fosamil. Antimicrob Agents Chemother 2016; 60: 3617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castanheira M, Jones RN, Sader HS.. Activity of ceftaroline and comparator agents tested against contemporary Gram-positive and -negative (2011) isolates collected in Europe, Turkey, and Israel. J Chemother 2014; 26: 202–10. [DOI] [PubMed] [Google Scholar]
- 34. Flamm RK, Sader HS, Farrell DJ. et al. Summary of ceftaroline activity against pathogens in the United States, 2010: report from the Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE) surveillance program. Antimicrob Agents Chemother 2012; 56: 2933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hackel M, Stone G, Sahm D, Impact of EUCAST ceftaroline breakpoint change on MRSA susceptibility of isolates collected from patients with complicated skin and soft tissue infections. In: Abstracts of the 28th European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain2018. Abstract P2503. Basel, Switzerland: European Society of Clinical Microbiology and Infectious Diseases. [DOI] [PubMed]
- 36. Corey G, Wilcox M, Gonzalez J. et al. Ceftaroline fosamil (CPT-F) in patients with acute bacterial skin and skin structure infections (ABSSSI) with systemic inflammatory signs: results across 3 pivotal studies using q8h or q12h. In: Abstracts of the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 2015. Abstract L-839. Washington DC, USA: American Society for Microbiology.
- 37. Zhou D, Dryden M, Gonzalez J. et al. Impact of disease severity on ceftaroline pharmacokinetics in patients with ABSSSI: Phase III COVERS trial. In: Abstracts of the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 2015. Abstract A-966. Washington DC, USA: American Society for Microbiology.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.