Abstract
Objectives
To determine the impact of valaciclovir on HIV disease progression in treatment-naive HIV-positive adults.
Methods
In this fully blind, multicentre, 1:1 randomized placebo-controlled trial, treatment-naive HIV-1-positive adults with CD4 counts 400–900 cells/mm3 and not meeting contemporaneous recommendations for combination ART (cART) were randomized to valaciclovir 500 mg or placebo twice daily, and followed quarterly until having two consecutive CD4 counts ≤350 cells/mm3 or initiating cART for any reason. The primary analysis compared the rate of CD4 count decline by study arm after adjusting for baseline CD4 count and viral load (VL). Secondary analyses compared the rate of CD4 percentage decline, HIV VL, herpes simplex virus (HSV) recurrences and drug-related adverse events. The trial closed after release of the START trial results in August 2015.
Results
We enrolled 198 participants in Canada, Brazil, Argentina and the UK. Median (IQR) age was 35 (30–43) years. Baseline CD4 count was 592 (491–694) cells/mm3 and VL was 4.04 (3.5–4.5) log10 copies/mL. Over 276 person-years of follow-up, CD4 counts declined by 49 cells/mm3/year in the valaciclovir arm versus 58 cells/mm3/year in the placebo arm (P = 0.65). No differences were seen in the rate of change in CD4 percentage (−1.2%/year versus −1.7%/year, P = 0.34). VL was 0.27 log10 copies/mL lower in valaciclovir participants overall (P<0.001). Placebo participants had more HSV recurrences (62 versus 21/100 person-years, P < 0.0001) but similar rates of grade ≥2 drug-related adverse events.
Conclusions
Unlike prior trials using aciclovir, we found that valaciclovir did not slow CD4 count decline in cART-untreated adults, although power was limited due to premature study discontinuation. Valaciclovir modestly lowered HIV VL.
Introduction
Aciclovir and its longer-acting valine ester, valaciclovir, are nucleoside analogues commonly used in the management of herpes simplex virus type 2 (HSV-2). Among people co-infected with HSV-2 and HIV-1, these drugs have been associated with clinically significant decreases in plasma HIV viral load (VL) of 0.33–0.53 log10 copies/mL.1–5
At least two mechanisms underlie these observations. First, much of this effect has been attributed to indirect benefits from suppressing HSV-2 reactivation. The prevalence of HSV-2 co-infection among populations living with HIV is high, ranging from 52%–95%,6–11 and HSV-2 activity is associated with both increases in VL and decreased CD4 counts. Second, valaciclovir has also been shown to have direct anti-HIV activity both in vitro12,13 and in vivo.14
Two placebo-controlled trials showed that aciclovir 400 mg twice daily attenuates HIV disease progression among patients naive to combination ART (cART). In the Partners in Prevention trial, conducted in Kenya and Uganda, this regimen was associated with a moderately decreased progression to the composite endpoint of CD4 < 200 cells/mm3, cART initiation or non-trauma-related death (HR = 0.84, 95% CI = 0.71–0.98).15 Similar findings were found in a trial from Uganda, where aciclovir decreased progression to the composite endpoint of CD4 < 350 cells/mm3 or cART initiation for WHO stage 4 (HR = 0.75, 95% CI = 0.50–0.98).16
However, these trials were conducted in Sub-Saharan Africa, and their applicability to industrialized and middle-income countries has been unclear. Further, valaciclovir might achieve better results because of its improved bioavailability compared with aciclovir (54.5% versus 15%–30%).17
We therefore conducted a randomized, placebo-controlled trial whose primary objective was to compare the rate of CD4 count decline among cART-untreated, HIV-1-seropositive adults randomized to valaciclovir 500 mg twice daily, versus placebo. Secondary objectives were to compare the time to cART initiation or CD4 count ≤350 cells/mm3, annual rate of change in CD4 percentage and VL, drug-related adverse events, frequency of HSV recurrences and overall quality of life between study arms.
Patients and methods
Study design
VALIDATE (VALacyclovir In Delaying Antiretroviral Treatment Entry, CTN-240) was a multicentre, randomized, placebo-controlled, fully blinded clinical trial of twice daily oral valaciclovir 500 mg as an investigational agent versus placebo, with the objective of slowing disease progression and delaying the need for initiating cART among HIV-positive individuals. The trial was conducted at 23 sites in Canada, Brazil, Argentina and the UK. A 13-member Steering Committee provided scientific and logistical oversight. Shortly after the START trial results were released in July 2015, suggesting clear benefit of earlier cART initiation,18 the Data Safety and Monitoring Committee (DSMC) of the Canadian Institutes of Health Research (CIHR) Canadian HIV Trials Network (CTN) recommended that our trial be closed and all participants be offered cART. Sites were instructed on 11 August 2015 to halt recruitment, contact all active participants for a final study visit within 3 months and recommend cART initiation.
Randomization was stratified by site in permuted blocks of variable size (four and six) using a computer-generated list of random numbers, and performed through a secure web site which ensures allocation concealment. All participants and study personnel (investigators, research coordinators and data analysts) were blinded to treatment allocation. The randomization was coordinated by an independent CTN statistician not affiliated with the trial, and the allocation code was released to the study statistician only after the trial was closed and the study database locked.
The trial was registered at both ClinicalTrials.gov (identifier NCT 00860977) and ISRCTN (66756285).
Participants
Eligibility for the trial required individuals to be aged ≥18 years, be HIV-1 seropositive [by enzyme immunoassay (EIA) and Western blot], have a CD4 count of 400–900 cells/mm3 on two consecutive occasions with at least one within 4 weeks of initiating the trial, have neither used chronic anti-HSV therapy for the preceding 6 months nor be anticipated to require it, and not meet local recommendations for initiating cART. At the time of trial design, the definitive CD4 count threshold for initiating cART in asymptomatic adults was 350 cells/mm3 in all participating countries, but the standard of care shifted towards a threshold of 500 cells/mm3 beginning in 2009 in North America,19,20 2012 in Brazil and 2013 in Argentina. Guidelines in the UK did not change until after the trial ended.
At the time of study design, another inclusion criterion was documentation of HSV-2 seropositivity, as determined during screening by the HerpeSelect ELISA (Focus Technologies, Cypress, CA, USA) using the manufacturer’s recommended cut-off index value of 1.10. However, after the release of other studies suggesting direct anti-HIV activity of valaciclovir,12,13 the protocol was amended on 17 December 2012 to remove this requirement and thus permit sensitivity analyses according to baseline HSV-2 serostatus.
Exclusion criteria included current/planned pregnancy, chemotherapy or chronic immunomodulatory medication use, estimated creatinine clearance <30 mL/min, enrolment in any interventional trial related to HIV disease progression and life expectancy <24 months. Finally, individuals with documented duration of HIV infection ≥5 years, CD4 count persistently ≥500 cells/mm3 and persistent VL < 1000 copies/mL in the absence of ART were excluded, in an effort to exclude HIV elite controllers (ECs).21–24 This stringent EC definition was used because a more permissive definition would have excessive overlap with the general HIV-infected population.
Interventions
Participants in the intervention group received oral valaciclovir 500 mg twice daily (purchased from Apotex Inc., Toronto, Canada), the standard dose used for HSV-2 suppression in HIV-infected individuals.10,25 Individuals in the control arm received an odourless placebo tablet identical to valaciclovir in appearance and taste. Use of other anti-HSV medication during the trial was prohibited, except when dispensed by study sites for the treatment of clinical herpes recurrences (described below).
Follow-up and outcome ascertainment
Participants were followed every 3 months until withdrawal, study termination or reaching the primary endpoint, which was a composite of either a CD4 count ≤350 cells/mm3 or initiation of cART for any reason, whichever occurred first. To meet the CD4 count-based definition, a second confirmatory value ≤350 cells/mm3 at least 1 month later was required, and the date of the initial measurement was used as the primary endpoint date.
The original primary outcome measure for the trial was the time from baseline until reaching the primary endpoint defined above. However, because the CD4 count threshold for initiating cART changed over the course of the trial, at different times in different participating countries as described above, the primary outcome measure was amended in December 2012 to the rate of CD4 count decline.
Secondary outcome measures included time to cART initiation or CD4 count ≤350 cells/mm3, the annual rate of change in CD4 percentage, plasma VL in log10 copies/mL, treatment-emergent adverse events, symptomatic HSV recurrences at any anatomical site (oral, genital or anal), aciclovir-resistant HSV recurrences and overall quality of life as measured by biannual Medical Outcomes Study HIV Health Survey (MOS-HIV) questionnaires.26 Adverse events and serious adverse events were graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, and assessed for their relationship to the study drug by site investigators.
To collect data on HSV recurrences, we instructed participants experiencing potential symptoms to continue study drug and present for clinical assessment as soon as possible for microbiological confirmation of the diagnosis according to local laboratory standards (PCR at most sites). Sites were provided with open-label valaciclovir for treatment of such episodes (up to 1 g twice daily for 5 days) at the discretion of site investigators. Although participants in the treatment arm may thus have received a total valaciclovir dose of up to 3 g per day for 5 days, this quantity remains well within the safe therapeutic window of this drug. To maintain blinding, study staff and participants were reminded that HSV recurrences should not be interpreted as meaning the participant was assigned to placebo, because a small proportion of individuals taking chronic suppressive anti-HSV medications can experience herpes recurrences, even in the absence of drug resistance.
We planned to assess adherence with study drug using three methods. First, participants completed a modified version of the ACTG Adherence Questionnaire at each visit, with results scored from 0–100 using previously published methods.27,28 Second, participants were asked to bring all their study drug bottles to every study visit for pill counts. Finally, we collected urine samples from all participants at 6 monthly visits, stored them at −80°C and shipped them to the biochemistry laboratory of the Hospital for Sick Children in Toronto, Canada at the conclusion of the study, for batched qualitative assessment of aciclovir level by mass spectrometry.29 This assay would be expected to give negative results by ∼24–48 h after consuming the last 500 mg dose of valaciclovir.
Sample size considerations
The sample size calculation was performed using an equation designed for studies of longitudinal data with a continuous outcome.30 We assumed an approximate standard deviation in CD4 counts of 150 cells/mm3, correlation of 0.54 among repeated CD4 count observations, and 2.5 years of follow-up per participant (11 visits) based on prior data.21,31,32 Using a two-sided α = 0.0492 (adjusted for multiple analyses by the method of O’Brien and Fleming)33 and 80% power, we anticipated that 115 participants in each study arm or 230 participants overall would permit detection of a minimum detectable difference in CD4 count decline of 14.4 cells/mm3 per year between study groups, which was deemed clinically relevant by the investigators.
Statistical analysis
The primary analysis was a linear mixed model with random effects for intercept and time comparing the annual rate of change in CD4 count between participants randomized to valaciclovir versus placebo, adjusted for baseline CD4 count and VL. Multilevel models grouping participants by clinic were investigated but found to be unnecessary since grouping by participant alone accounted for the majority of between-group variation. A single pre-planned interim efficacy analysis was performed by the DSMC in August 2014, to advise on early discontinuation of the trial only if there was clear evidence of benefit (or harm) using a two-sided statistical significance threshold of 0.0054 and power of 80%.33 The final analysis used an α = 0.0492 level of significance, adjusting for multiple analyses using the O’Brien–Fleming method.33
We also used random effects models to compare the annual rate of change in CD4 percentage and the overall log10 HIV VL between study arms. The time to the primary endpoint was compared between arms using a proportional hazards model. Drug-related adverse events, HSV recurrences and quality-of-life scores at each 6 monthly timepoint were compared between groups using χ2, Fisher’s exact, Cochran–Armitage trend or Wilcoxon rank sum tests as appropriate. All analyses were by ITT.
We assessed effect modification of treatment on the primary outcome by country of enrolment, study drug adherence (≥90% by self-report) and HSV-1 and HSV-2 serological status by adding the corresponding interaction terms into the model separately.
Ethics approval
Ethics approval was obtained from the Research Ethics Boards of the primary study site (University Health Network REB# 09-0587-B) and of each local site prior to commencing any study activities. All participants provided written informed consent.
Results
Between 4 March 2010 and 26 May 2015, we screened 382 individuals; 180 were excluded (Figure 1). The most common reason for study ineligibility was HSV-2 seronegativity (n = 86) in the period before HSV-2 seropositivity was removed from the eligibility criteria. When the DSMC recommended trial closure, 202 individuals had been randomized. Of these, 198 received at least one dose of study drug (97 valaciclovir, 101 placebo) and were included in the analysis, including 81 (40.9%), 72 (36.4%), 15 (7.6%) and 30 (15.2%) in Canada, Brazil, Argentina and the UK, respectively.
Figure 1.
Participant flow diagram.
Overall, 74.2% of participants were HSV-2 seropositive, 80.3% of participants were male and injection drug use was a potential HIV risk factor in 4.0%. Median (IQR) age was 35 (30–43) years, and baseline CD4 count was 592 (491–694) cells/mm3 or 28% (23%–33%), while baseline plasma VL was 4.04 (3.50–4.45) log10 copies/mL. Additional baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics
| Characteristic | Total (N = 198) | Valaciclovir (n = 97) | Placebo (n = 101) |
|---|---|---|---|
| Age (years), median (IQR) | 35 (30–43) | 36 (30–45) | 35 (29–41) |
| Male, n (%) | 159 (80.3) | 77 (79.4) | 82 (81.2) |
| Race, n (%) | |||
| white | 107 (54.0) | 45 (46.4) | 62 (61.4) |
| black | 48 (24.2) | 27 (27.8) | 21 (20.8) |
| Asian | 8 (4.0) | 6 (6.2) | 2 (2.0) |
| other | 35 (17.7) | 19 (19.6) | 16 (15.8) |
| HIV risk factor, n (%) | |||
| MSM | 139 (70.2) | 66 (68.0) | 73 (72.3) |
| heterosexual | 43 (21.7) | 23 (23.7) | 20 (19.8) |
| MSM and IVDU | 3 (1.5) | 1 (1.0) | 2 (2.0) |
| IVDU only | 5 (2.5) | 3 (3.1) | 2 (2.0) |
| other | 8 (4.0) | 4 (4.1) | 4 (4.0) |
| Country, n (%) | |||
| Argentina | 15 (7.6) | 8 (8.3) | 7 (6.9) |
| Brazil | 72 (36.4) | 35 (36.1) | 37 (36.6) |
| Canada | 81 (40.9) | 40 (41.2) | 41 (40.6) |
| UK | 30 (15.2) | 14 (14.4) | 16 (15.8) |
| Baseline CD4, cells/mm3 (IQR) | 592 (491–694) | 580 (476–673) | 609 (522–712) |
| Baseline CD4, % (IQR) | 28 (23–33) | 27 (22–33) | 28 (24–33) |
| Baseline VL, log10 copies/mL (IQR) | 4.04 (3.50–4.45) | 3.98 (3.47–4.49) | 4.04 (3.55–4.42) |
| Clade, n (%) | |||
| B | 65 (32.8) | 34 (35.1) | 31 (30.7) |
| C | 9 (4.6) | 5 (5.2) | 4 (4.0) |
| other | 6 (3.0) | 2 (2.1) | 4 (4.0) |
| missing | 118 (59.6) | 56 (57.7) | 62 (61.4) |
| HSV-2 serostatus, n (%) | |||
| seropositive | 147 (74.2) | 70 (72.2) | 77 (76.2) |
| seronegative | 42 (21.2) | 21 (21.6) | 21 (20.8) |
| missing | 9 (4.6) | 6 (6.2) | 3 (3.0) |
| Clinical history of HSV lesions, n (%) | |||
| oral | 44 (22.2) | 23 (23.7) | 21 (20.8) |
| genital | 23 (11.6) | 13 (13.4) | 10 (9.9) |
| anal | 7 (3.5) | 4 (4.1) | 3 (3.0) |
Participants contributed 276 person-years of follow-up overall. By the time of the final study visit on 10 November 2015, 102 (51.5%) participants had met the primary endpoint, including 91 (46.0%) based on having initiated cART and 11 (5.5%) based on having CD4 counts ≤350 cells/mm3. Thirty-five participants withdrew from the study, while 61 remained in active follow-up (Table 2). Five HIV-related events occurred during follow-up including three in the valaciclovir arm (encephalopathy, seborrhoeic dermatitis and zoster) and two in the placebo arm (lymphadenopathy and seborrhoeic dermatitis). There was a median of 4.5 (2–7) CD4 count and 5 (2–7) VL measurements per participant.
Table 2.
Frequency of primary endpoint and censoring criteria by treatment arm
| Total (N = 198) | Valaciclovir (n = 97) | Placebo (n = 101) | |
|---|---|---|---|
| Met primary endpoint | 102 (51.5) | 47 (48.5) | 55 (54.5) |
| CD4 ≤350 cells/mm3 | 11 (5.56) | 6 (6.19) | 5 (4.95) |
| CD4 ≤350 cells/mm3 and cART initiation | 1 (0.51) | 1 (1.03) | 0 (0) |
| cART initiation | 90 (45.5) | 40 (41.2) | 50 (49.5) |
| Did not meet primary endpoint | 96 (48.5) | 50 (51.5) | 46 (45.5) |
| end of study | 61 (30.8) | 34 (35.1) | 27 (26.7) |
| withdrew consent | 19 (9.60) | 8 (8.25) | 11 (10.9) |
| lost to follow-up | 10 (5.05) | 5 (5.15) | 5 (4.95) |
| moved | 4 (2.02) | 1 (1.03) | 3 (2.97) |
| site withdrew | 2 (1.01) | 2 (2.06) | 0 (0) |
All values are n (%).
Reliable assessments of study drug adherence by pill count were not possible because of a high volume of unreturned study drug bottles. Self-report data suggested that adherence was good and similar between treatment arms; the median (IQR) of participants’ mean ACTG scores were 91 (81–96) and 90 (77–96) for the valaciclovir and placebo arms, respectively (P = 0.70). Qualitative testing of urine suggested that adherence was fair, as aciclovir could be detected in 113/181 (62%) samples from valaciclovir participants and 3/184 (2%) samples from placebo participants.
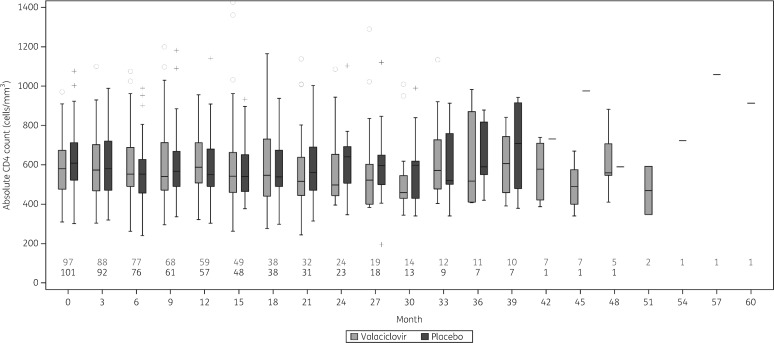
In the primary analysis, we observed no statistically significant difference between groups in the rate of change in CD4 count, which was −49 cells/mm3/year in the valaciclovir arm, and −58 cells/mm3/year in the placebo arm (P = 0.65) after adjusting for baseline CD4 count and viral load. The estimated difference between groups was 9 (95% CI = −29 to +46) cells/mm3. Absolute CD4 counts are shown for each study arm over time in Figure 2.
Figure 2.
CD4 count over time by study arm.
Similarly, there was no difference in the rate of change in CD4 count percentage, which was −1.2%/year in the valaciclovir arm and −1.7%/year in the placebo arm (P = 0.34), giving a difference of 0.5%/year (−0.51% to +1.49%).
The time from baseline until reaching the primary endpoint was not significantly different between study arms (HR = 0.78, 95% CI = 0.52–1.15, P = 0.21). Notably, valaciclovir was associated with an overall decrease in HIV VL of 0.27 (95% CI = 0.12–0.41) log10 copies/mL, although annual rates of change in plasma HIV VL were not different at +0.08 and +0.15 log10 copies/mL/year for valaciclovir and placebo, respectively, giving a difference of −0.07 (95% CI = −0.19–0.04) log10 copies/mL/year.
Thirty HSV recurrences occurred in 19 valaciclovir participants, compared with 82 recurrences in 26 placebo participants; while the number of affected participants was similar between groups (P = 0.30), the rate of HSV recurrences was lower in the valaciclovir arm, at 21 events/100 person-years, than in the placebo arm, at 62 events/100 person-years (P < 0.0001). Notably, however, participants did not attend the study site as recommended for many of these episodes, such that only 9 of the 112 recurrence episodes had samples sent for microbiological testing, and the number of participants with microbiologically confirmed HSV recurrences was very low (one versus two in the valaciclovir and placebo arms respectively, P = 0.99). No participants underwent testing for clinically suspected aciclovir-resistant HSV.
As expected, given the known excellent safety profile of valaciclovir, the number of grade 2 or higher adverse events deemed at least possibly related to study drug was similar between groups, at 6.2% in the valaciclovir arm and 6.9% in the placebo arm (P = 0.83; Table 3). Quality of life was similar between study arms at all timepoints assessed (data not shown).
Table 3.
Adverse events (AEs)
| Total (N = 198) | Valaciclovir (n = 97) | Placebo (n = 101) | P value | |
|---|---|---|---|---|
| Any ≥possibly drug-related AEs ≥grade 2 | 13 (6.57) | 6 (6.19) | 7 (6.93) | 0.83 |
| Any ≥possibly drug-related AEs ≥grade 3 | 4 (2.02) | 3 (3.09) | 1 (0.99) | 0.36 |
| Any ≥probably drug-related AEs ≥grade 2 | 3 (1.52) | 2 (2.06) | 1 (0.99) | 0.62 |
| Number of possibly drug-related AEs ≥grade 2 | ||||
| 0 | 185 (93.4) | 91 (93.8) | 94 (93.1) | 0.70 |
| 1 | 8 (4.04) | 3 (3.09) | 5 (4.95) | |
| 2 | 4 (2.02) | 2 (2.06) | 2 (1.98) | |
| 4 | 1 (0.51) | 1 (1.03) | 0 (0) |
All values shown are n (%).
There was no effect modification of valaciclovir on the rate of change in CD4 count according to country of enrolment, study drug adherence and HSV subtype serostatus (see Table S1 available as Supplementary data at JAC Online).
Discussion
In this placebo-controlled trial, we assessed the effect of valaciclovir 500 mg twice daily on HIV disease progression among cART-untreated adults in four middle- and high-income countries. Our trial was stopped prior to accruing the intended sample size after results from the START trial became available, showing significant benefits to earlier cART initiation among asymptomatic adults with CD4 counts of 350–500 cells/mm3.18 During 276 person-years of follow-up, we observed no statistically significant difference in the rate of change in CD4 count or CD4 percentage between valaciclovir and placebo. The lower-than-expected duration of follow-up may explain why the estimated difference in the rate of CD4 count change of 9 cells/mm3 (95% CI = −29 to +46) did not achieve statistical significance. However, overall plasma VL was 0.27 log10 copies/mL lower in the valaciclovir arm.
The magnitude of this decrease in VL is consistent with prior studies and underscores the potential value of harnessing our understanding of HIV co-infections for therapeutic benefit. In a meta-analysis of seven randomized trials among adults co-infected with HIV and HSV-2, comparable doses of aciclovir and valaciclovir were associated with a 0.33 log10 reduction in plasma VL (95% CI = −0.74 to +0.08).34 Similarly, in a crossover trial among 18 HIV-1-seropositive, HSV-2-seronegative adults, valaciclovir 500 mg twice daily reduced plasma VL by 0.37 log10 copies/mL (95% CI = 0.11–0.62).14 Clinicians may still wish to consider valaciclovir as a low-risk intervention for patients in whom immediate antiretroviral initiation is not feasible.
Our failure to show benefit from valaciclovir in slowing disease progression contrasts with two prior trials of aciclovir 400 mg twice daily in adults co-infected with HIV and HSV-2.15,16 There are several potential explanations for this difference. First, the African trials used considerably lower CD4 counts of 200–250 cells/mm3 as their primary endpoints, thus accruing longer follow-up and increased statistical power to detect an effect of aciclovir over time. Our participant follow-up was further reduced to less than we had anticipated because of evolving trends towards earlier cART initiation during the study and because of the premature trial discontinuation. That our trial generated a similar point estimate for the impact of study drug on time to the primary endpoint as other studies (HR = 0.78, 95% CI = 0.51–1.15, versus HR = 0.84, 95% CI = 0.71–0.98 in Partners in Prevention and HR = 0.75, 95% CI = 0.50–0.98 in Rakai) suggests that inadequate power may have driven the difference in statistical significance of results.15,16
Second, there are differences in the standards of care at the times and geographical locations of our trials. It is also possible that aciclovir and valaciclovir have different effects in different patient populations, viral clades or other cofactors. Our trial included only 24.2% black participants and 81.3% of those with known clade had HIV-1 subtype B infection, whereas the two positive trials were conducted in East Africa, where the main circulating HIV clades are A and D.35
Third, study drug adherence may have differed between the trials. Overall drug coverage was estimated at 90%–97% by pill count in the Partners in Prevention and Rakai trials, and self-reported adherence in our trial was 90%–91%. However, valaciclovir was detectable in only 62% of urine specimens from participants in the active arm of our trial. Nevertheless, the estimated impact of the intervention on plasma VL was similar across studies, with estimated overall decreases of 0.22 (−0.01 to +0.44), 0.16 (0.08–0.24) and 0.25 (0.22–0.29) copies/mL in our trial, the Rakai trial and the Partners in Prevention trial, respectively.
Finally, 21% of our participants were HSV-2 seronegative. If one mechanism by which aciclovir impacts HIV disease progression is through suppression of HSV-2 activity, our participants may have had less opportunity to experience these benefits as a result. The frequency of clinical and subclinical HSV-2 reactivations may also have differed between the trials.
The findings from this trial are broadly consistent with our prior work on HIV/HSV-2 co-infection. In a retrospective cohort study of 218 HIV-positive, antiretroviral-naive adults, we observed no difference in the rate of CD4 count decline or CD4 percentage decline among HSV-2-seropositive versus -seronegative individuals.36 In a systematic review of studies among cART-untreated adults, we further observed no definitive relationship between HSV-2 seropositivity and time to any of cART initiation, CD4 ≤ 350 cells/mm3, CD4 ≤ 200 cells/mm3 or death.37 We have also shown in prior work that among adults with cART-induced virological suppression, HSV-2 seropositivity is not associated with increased inflammation or immune activation,38 nor is valaciclovir associated with decreases in such parameters.39
A key insight we highlighted in those studies was that HSV-2 seropositivity should not be conflated with high HSV-2 activity. Indeed, the rate of HSV recurrences is known to decline over time in seropositive persons.40,41 If reactivation of HSV-2 is harmful to the prognosis of HIV infection, then such effects and the potential benefits of HSV-2 suppression might best be seen in those with more active HSV-2. Eligibility criteria for our trial required participants to have relatively preserved CD4 counts at study entry and may have resulted in a cohort with relatively less active HSV-2 infection. Since the first 3 months after cART initiation are associated with increased HSV reactivations,42 future work should examine whether there are benefits to using anti-HSV medications during this period.
There are several strengths to our study. We conducted the trial in four high- and middle-income country settings in which HIV clinical practice is similar, thus contrasting with the trials from Sub-Saharan Africa. We used a urine biomarker to provide a more realistic estimate of study drug adherence. Our inclusion of HSV-2-seronegative participants supports the notion that valaciclovir has direct anti-HIV activity.
Our study also has limitations. First, only a small number of specimens were sent for microbiological confirmation of HSV, precluding our ability to confirm the expected clinical impact of our intervention. Second, only 3/9 samples from suspected recurrences were confirmed to be HSV-related, suggesting that the reported rates of HSV episodes may be overestimates. Third, urine monitoring suggested only modest study drug adherence. Fourth, we defined HSV-2 seropositivity using the manufacturer’s recommended cut-off index value of >1.1 on the HerpesSelect EIA assay, which may have limited specificity.43,44 Most notably, we experienced challenges with recruitment and terminated the trial early, due to evolving standards of care regarding cART initiation, limiting our ability to observe an effect of the intervention.
The benefits to early cART have been convincingly proven in other studies, including the prevention of AIDS- and non-AIDS-related morbidity and mortality,18,45 preservation of immune function,46 decreased potential for onward HIV transmission47 and peace of mind related to knowing that the infection is being treated. Overall, our findings provide further justification for early cART initiation in treatment-naive people living with HIV, since valaciclovir did not meaningfully attenuate CD4 count decline in this setting.
Supplementary Material
Acknowledgements
D. H. S. T. and S. L. W. conceived the study idea. D. H. S. T., S. L. W. and J. M. R. designed the protocol in consultation with B. G. and P. C.. D. H. S. T., B. G., J. V. M., M. I. F., P. C., S. E. B., A. C., J. F. and S. L. W. recruited and followed participants. W. Z., D. H. S. T. and S. L. W. oversaw the conduct of the trial. L. S. and J. M. R. conducted the statistical analysis. D. H. S. T. wrote the original draft of the manuscript, and all authors critically reviewed and approved the final version.
Members of the VALIDATE Study Group
Additional members of the VALIDATE Study Team are: Jason Brunetta (Maple Leaf Medical Clinic, Toronto), William Cameron (The Ottawa Hospital, Ottawa), Jeff Cohen (Windsor Regional Hospital, Windsor, Canada), Brian Conway (Downtown ID Clinic, Vancouver, Canada), Claude Fortin (Centre Hospitalier de l’Université de Montréal, Montreal, Canada), Chris Fraser (Cool Aid Community Health Centre, Victoria, Canada), Don Kilby (University of Ottawa Health Services, Ottawa, Canada), Marina Klein (McGill University Health Centre, Montreal, Canada), Ken Logue (St. Clair Medical Associates, Toronto, Canada), Neora Pick (Oak Tree Clinic, Vancouver, Canada), Anita Rachlis (Sunnybrook Hospital, Toronto, Canada), Barbara Romanowski (University of Alberta, Edmonton, Canada), Fiona Smaill (Hamilton Health Sciences Centre, Hamilton, Canada), Sylvie Trottier (Centre Hospitalier Universitaire de Quebec, Quebec City, Canada) and Alan Winston (St Mary’s Hospital, London, UK).
Funding
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) (grant number MCT 94245) and by the CIHR Canadian HIV Trials Network. D. H. S. T. is supported by a New Investigator Award from the Canadian Institutes of Health Research and Ontario HIV Treatment Network (grant number NEB 136629). J. M. R. and S. L. W. received salary support from the Ontario HIV Treatment Network. The funding agencies had no role in the design of the trial or writing of this manuscript.
Transparency declarations
D. H. S. T. has received honoraria from Merck, and is a Site Principal Investigator for clinical trials sponsored by Glaxo Smith Kline. D. H. S. T.’s institution has received research support for investigator-initiated research studies from Gilead Sciences and Viiv Healthcare. P. C. reports grants from Merck, Abbvie, and ViiV healthcare and personal fees from Merck and ViiV healthcare. S. E. B. has received grants for educational work from ViiV and Gilead Sciences as well as honoraria for lectures and travel support from ViiV Healthcare, Glaxo Smith Kline and Gilead Sciences. A. C. has received travel bursaries from Gilead Sciences and Janssen, and has advisory roles for Gilead Sciences and Viiv Healthcare. J. F. has received a grant from Gilead Sciences and has advisory roles for Viiv Healthcare. S. L. W. has served on advisory boards, speaking engagements, meetings, symposiums and clinical studies for Viiv Healthcare, Glaxo Smith Kline, Merck and Gilead Sciences. All other authors have none to declare.
Contributor Information
the VALIDATE Study Group:
Jason Brunetta, William Cameron, Jeff Cohen, Brian Conway, Claude Fortin, Chris Fraser, Don Kilby, Marina Klein, Ken Logue, Neora Pick, Anita Rachlis, Barbara Romanowski, Fiona Smaill, Sylvie Trottier, and Alan Winston
References
- 1. Gray RH, Wawer MJ, Sewankambo NK. et al. Relative risks and population attributable fraction of incident HIV associated with symptoms of sexually transmitted diseases and treatable symptomatic sexually transmitted diseases in Rakai District, Uganda. Rakai Project Team. AIDS 1999; 13: 2113–23. [DOI] [PubMed] [Google Scholar]
- 2. Mole L, Ripich S, Margolis D. et al. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis 1997; 176: 766–70. [DOI] [PubMed] [Google Scholar]
- 3. Schacker T, Zeh J, Hu H. et al. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis 2002; 186: 1718–25. [DOI] [PubMed] [Google Scholar]
- 4. Zuckerman RA, Lucchetti A, Whittington WLH. et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis 2007; 196: 1500–8. [DOI] [PubMed] [Google Scholar]
- 5. Nagot N, Ouédraogo A, Foulongne V. et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med 2007; 356: 790–9. [DOI] [PubMed] [Google Scholar]
- 6. Santos FC, de Oliveira SA, Setúbal S. et al. Seroepidemiological study of herpes simplex virus type 2 in patients with the acquired immunodeficiency syndrome in the city of Niterói, Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 2006; 101: 315–9. [DOI] [PubMed] [Google Scholar]
- 7. Chu K, Jiamton S, Pepin J. et al. Association between HSV-2 and HIV-1 viral load in semen, cervico-vaginal secretions and genital ulcers of Thai men and women. Int J STD AIDS 2006; 17: 681–6. [DOI] [PubMed] [Google Scholar]
- 8. Cowan FF, Pascoe SJ, Barlow KL. et al. Association of genital shedding of herpes simplex virus type 2 and HIV-1 among sex workers in rural Zimbabwe. AIDS 2006; 20: 261–7. [DOI] [PubMed] [Google Scholar]
- 9. Ramaswamy M, Sabin C, McDonald C. et al. Herpes simplex virus type 2 (HSV-2) seroprevalence at the time of HIV-1 diagnosis and seroincidence after HIV-1 diagnosis in an ethnically diverse cohort of HIV-1-infected persons. Sex Transm Dis 2006; 33: 96–101. [DOI] [PubMed] [Google Scholar]
- 10. Strick LB, Wald A, Celum C.. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin Infect Dis 2006; 43: 347–56. [DOI] [PubMed] [Google Scholar]
- 11. Romanowski B, Myziuk LN, Walmsley SL. et al. Seroprevalence and risk factors for herpes simplex virus infection in a population of HIV-infected patients in Canada. Sex Transm Dis 2009; 36: 165–9. [DOI] [PubMed] [Google Scholar]
- 12. Lisco A, Vanpouille C, Tchesnokov EP. et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe 2008; 4: 260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McMahon MA, Siliciano JD, Lai J. et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem 2008; 283: 31289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanpouille C, Lisco A, Grivel JC. et al. Valacyclovir decreases plasma HIV-1 RNA in HSV-2 seronegative individuals: a randomized placebo-controlled crossover trial. Clin Infect Dis 2015; 60: 1708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lingappa JR, Baeten JM, Wald A. et al. Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 2010; 375: 824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reynolds SJ, Makumbi F, Newell K. et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis 2012; 12: 441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MacDougall C, Guglielmo BJ.. Pharmacokinetics of valaciclovir. J Antimicrob Chemother 2004; 53: 899–901. [DOI] [PubMed] [Google Scholar]
- 18. The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV Infection. N Engl J Med 2015; 20: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. 1 December 2009. https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL001561.pdf.
- 20. Hammer SM, Eron JJ Jr, Reiss P. et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 2008; 300: 555–70. [DOI] [PubMed] [Google Scholar]
- 21. Rodríguez B, Sethi AK, Cheruvu VK. et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA 2006; 296: 1498–506. [DOI] [PubMed] [Google Scholar]
- 22. Cao Y, Qin L, Zhang L. et al. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med 1995; 332: 201–8. [DOI] [PubMed] [Google Scholar]
- 23. Pantaleo G, Menzo S, Vaccarezza M. et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med 1995; 332: 209–16. [DOI] [PubMed] [Google Scholar]
- 24. Vicenzi E, Poli G.. Regulation of HIV expression by viral genes and cytokines. J Leukoc Biol 1994; 56: 328–34. [DOI] [PubMed] [Google Scholar]
- 25. Workowski K, Berman SM.. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep 2006; 55: 1–94. [PubMed] [Google Scholar]
- 26. Wu AW, Revicki DA, Jacobson D. et al. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Qual Life Res 1997; 6: 481–93. [DOI] [PubMed] [Google Scholar]
- 27. Chesney MA, Ickovics JR, Chambers DB. et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care 2000; 12: 255–66. [DOI] [PubMed] [Google Scholar]
- 28. Reynolds NR, Sun J, Nagaraja HN. et al. Optimizing measurement of self-reported adherence with the ACTG Adherence Questionnaire: a cross-protocol analysis. J Acquir Immune Defic Syndr 2007; 46: 402–9. [DOI] [PubMed] [Google Scholar]
- 29. Kasiari M, Gikas E, Georgakakou S. et al. Selective and rapid liquid chromatography/negative-ion electrospray ionization mass spectrometry method for the quantification of valacyclovir and its metabolite in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 864: 78–86. [DOI] [PubMed] [Google Scholar]
- 30. Diggle PJ, Liang K-Y, Zeger SL.. Analysis of Longitudinal Data. Oxford: Oxford University Press, 1994. [Google Scholar]
- 31. Tan DH, Raboud JM, Kaul R. et al. Can herpes simplex virus type 2 suppression slow HIV disease progression: a study protocol for the VALacyclovir In Delaying Antiretroviral Treatment Entry (VALIDATE) trial. Trials 2010; 11: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mellors JW, Munoz A, Giorgi JV. et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997; 126: 946–54. [DOI] [PubMed] [Google Scholar]
- 33. O’Brien PC, Fleming TR.. A multiple testing procedure for clinical trials. Biometrics 1979; 35: 549–56. [PubMed] [Google Scholar]
- 34. Ludema C, Cole SR, Poole C. et al. Meta-analysis of randomized trials on the association of prophylactic acyclovir and HIV-1 viral load in individuals coinfected with herpes simplex virus-2. AIDS 2011; 25: 1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stebbing J, Moyle G.. The clades of HIV: their origins and clinical significance. AIDS Rev 2003; 5: 205–13. [PubMed] [Google Scholar]
- 36. Tan DH, Raboud JM, Kaul R. et al. Herpes simplex virus type 2 coinfection does not accelerate CD4 count decline in untreated HIV infection. Clin Infect Dis 2013; 57: 448–57. [DOI] [PubMed] [Google Scholar]
- 37. Tan DH, Murphy K, Shah P. et al. Herpes simplex virus type 2 and HIV disease progression: a systematic review of observational studies. BMC Infect Dis 2013; 13: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan DH, Raboud JM, Szadkowski L. et al. Herpes simplex virus type 2 serostatus is not associated with inflammatory or metabolic markers in antiretroviral therapy-treated HIV. AIDS Res Hum Retroviruses 2015; 31: 276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yi TJ, Walmsley S, Szadkowski L. et al. A randomized controlled pilot trial of valacyclovir for attenuating inflammation and immune activation in HIV/herpes simplex virus 2-coinfected adults on suppressive antiretroviral therapy. Clin Infect Dis 2013; 57: 1331–8. [DOI] [PubMed] [Google Scholar]
- 40. Benedetti J, Corey L, Ashley R.. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med 1994; 121: 847–54. [DOI] [PubMed] [Google Scholar]
- 41. Benedetti JK, Zeh J, Corey L.. Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann Intern Med 1999; 131: 14–20. [DOI] [PubMed] [Google Scholar]
- 42. Tobian AA, Grabowski MK, Serwadda D. et al. Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis 2013; 208: 839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ashley-Morrow R, Nollkamper J, Robinson NJ. et al. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect 2004; 10: 530.. [DOI] [PubMed] [Google Scholar]
- 44. Biraro S, Mayaud P, Morrow RA. et al. Performance of commercial herpes simplex virus type-2 antibody tests using serum samples from Sub-Saharan Africa: a systematic review and meta-analysis. Sex Transm Dis 2011; 38: 140–7. [DOI] [PubMed] [Google Scholar]
- 45. Danel C, Moh R, Gabillard D. et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373: 808–22. [DOI] [PubMed] [Google Scholar]
- 46. Le T, Wright EJ, Smith DM. et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368: 218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohen MS, Chen YQ, McCauley M. et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.