Abstract
Testicular torsion, a common urologic emergency, is primarily caused by ischemia/reperfusion (I/R) injury of the testis. Nitric oxide (NO)-derived from nitrite (NO2−) has been reported to have prominent therapeutic effects on I/R injury in the heart, liver, and brain; however, its effects on testicular I/R injury have not been evaluated. This study, therefore, investigated whether NO from NO2 − is beneficial in a rat model of testicular I/R injury which eventually results in impaired spermatogenesis. Male Sprague-Dawley rats were assigned to the following seven groups: group A, sham-operated control group; Group B, I/R with no treatment; Groups C, D, and E, I/R followed by treatment with three different doses of NO2−; Group F, I/R followed by administration of NO2− and NO scavenger (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt [C-PTIO]); and Group G, I/R followed by administration of nitrate (NO3−). NO2−, NO3−, and C-PTIO were intravenously administered. Histological examination of the testes and the western blot analysis of caspase-3 were performed. Levels of antioxidant enzymes and lipid peroxidation were measured. Germ cell apoptosis, oxidative stress, antioxidant enzymatic function, and lipid peroxidation in Group B were significantly higher than those in Group A. Group B exhibited an abnormal testicular morphology and impaired spermatogenesis. In contrast, testicular damages were attenuated in the NO2− treatment groups, which were caused by reduction in superoxide and peroxynitrite levels and an inhibition of caspase-3-dependent apoptosis. The results of this study suggest NO2− to be a promising therapeutic agent with anti-oxidant and anti-apoptotic properties in testicular I/R injury.
Keywords: ischemia/reperfusion injury, nitric oxide, nitrite, rat, testis
INTRODUCTION
Testicular torsion is a common urologic emergency that is primarily induced by torsion of the spermatic cord in infants, children, and adolescents. Its incidence has been estimated at 1 out of 160 to 4000 males per year by 25 years of age.1,2,3 The main etiology of testicular torsion-detorsion is ischemia/reperfusion (I/R) injury of the testis.4,5 Although reperfusion is necessary for the survival of the injured tissue, it paradoxically exacerbates cell and tissue damage.
Nitric oxide (NO) is a freely diffusible, water- and lipid-soluble gaseous molecule and an important free radical with biologically multifunctional effects, such as maintaining vascular tone and homeostasis in many tissues and organs.6,7 Under normoxic conditions, NO is synthesized from L-arginine by three different NO synthase (NOS) isoforms: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS), which are generally expressed in testicular tissues.8,9 Since these isoforms exert catalytic activity via oxygen consumption, they are inhibited under conditions of hypoxia and ischemia. Therefore, alternative sources of NO generation are vital in patients in ischemic states. Emerging evidence has demonstrated that nitrite (NO2−) and nitrate (NO3−) are endogenous materials formed by NO oxidation under an aerobic condition and they represent the major circulating and tissue storage forms of NO.10,11,12 During hypoxia, acidosis, and I/R injury, the NO2− reductase activity of deoxyhemoglobin, deoxymyoglobin, tissue heme proteins, and xanthine oxidoreductase (XOR) results in the reduction of NO2− to NO.10,13,14,15,16,17,18
NO has protective or detrimental effects, depending on the tissue, site, source, and environment.19,20,21 The application of NO2− as a direct NO donor has prominent therapeutic effects on I/R injury in the heart, liver, and brain.10,14,22 In contrast, NO might exert adverse effects by forming peroxynitrite, a strong oxidant produced by the reaction of increased NO and superoxide radicals during the reperfusion period. Because the detrimental effects of peroxynitrite are indiscriminate, several studies have assessed the level of apoptosis and oxidant stress in testicular I/R injury.6,23,24
As the mechanism of cytoprotection provided by NO2− is still unclear, we attempted to examine whether hypoxia-dependent NO production from NO2− attenuates testicular I/R injury.
MATERIALS AND METHODS
Experimental animal model
All experimental procedures and protocols were approved by the Animal Care and Use Committees of Konkuk University (Seoul, Korea) and conformed to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Unilateral testicular ischemia was maintained for 5 h followed by reperfusion for 24 h. Pubertal, 6-week-old, male Sprague-Dawley rats (OrientBio, Seongnam, Korea) were randomly assigned to the following seven groups (10 rats per group): Group A, sham-operated control group with no I/R; Group B, I/R with no treatment; Groups C, D, and E, I/R followed by treatment with three different doses (0.12, 1.2, and 12 nmol g−1 body weight [bwt], 1 min before reperfusion via tail vein) of NO2− administered intravenously; Group F, I/R followed by intravenous administration of NO2− (0.12 nmol g−1 body bwt) and C-PTIO (0.01 μmol g−1 body bwt, 5 min before ischemia); Group G, I/R followed by intravenous administration of NO3− (0.12 nmol g−1 bwt, 1 min before reperfusion via tail vein).
Sodium nitrite and sodium nitrate were obtained from Sigma-Aldrich (St. Louis, MO, USA; catalog number S2252 and S8170, respectively). The NO scavenger C-PTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt) was purchased from Alexis Biochemicals (San Diego, CA, USA; catalog number ALX-430-001). All experiments were performed under zolazepam and tiletamine (Zoletil®, 40 mg kg−1; Virbac, Seoul, Korea) and xylazine (5 mg kg−1; Bayer, Seoul, Korea) anesthesia injected intramuscularly. After a scrotal incision, the left testis was exteriorized. In control Group A, the testis was promptly placed back into the scrotum and suture with 4-0 silk (Ailee, Busan, Korea) was placed through the tunica albuginea. In the remaining six groups, the left testis was rotated 720° in a clockwise direction and maintained in an ischemic state by fixing the testis to the scrotum with a 4-0 silk suture. After 5 h of ischemia, the testis was released in a counter-clockwise direction to initiate reperfusion.25 After surgery, ketoprofen (5 mg kg−1) was injected subcutaneously for reducing pain. All animals experienced 24 h of reperfusion after which orchiectomy was performed using the same anesthetic regime.
Histopathologic evaluation of spermatogenesis
The number of germinal cell layers and Johnsen scores were used to categorize spermatogenesis in the testes by counting 10 consecutive seminiferous tubules (×400 field areas) and calculating the mean number. Each tubular section was given a score ranging from 10 to 1 according to a scoring system based on the degeneration of the germinal epithelium and the presence of germinal cells in the seminiferous tubules.26 Mean seminiferous tubule diameter (MSTD) was determined from 20 tubular diameters. A testicular MSTD below 260 μm was considered a pathologically low value.27
Immunohistochemistry
Serial sections of paraffin-embedded testis were deparaffinized and incubated with an anti-cleaved caspase-3 antibody (rabbit monoclonal, 1:800; Cell Signaling, Beverly, MA, USA; catalog number 9661)for 24 h at 4°C after being blocked with normal goat serum. Then, the slides were incubated with a biotinylated secondary antibody for 1 h followed by avidin-biotin peroxidase complexes (ABC Elite Kit; Vector Labs, Burlingame, CA, USA; catalog number PK-6100) for 30 min. Peroxidase activity was confirmed using 3,3-diaminobenzidine (Vector Labs) followed by a hematoxylin counterstain. Stained germ cells were counted in each group. The number of caspase-3-positive cells was determined by counting 20 consecutive seminiferous tubules (×400 field areas) per slide. Data are expressed as mean of caspase-3-positive cells per seminiferous tubule.
Protein extraction and western blot
Frozen testis tissue was homogenized in a tissue protein extraction reagent (T-PER; Pierce Bioscience, Rockford, IL, USA; catalog number 78510). Homogenates were centrifuged at 10 000 g at 4°C for 10 min, and supernatants were stored at −80°C. Samples were diluted with a reducing sample buffer and then boiled for 10 min. The samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were then transferred onto a PROTRAN nitrocellulose membrane (Whatman GmbH, Dassel, Germany).
After the membranes were blocked by 5% blocking solution (nonfat dry milk/Tris-buffered saline-Tween [TBST]) for 1 h, they were incubated overnight at 4°C in a 2.5% blocking solution containing the primary antibodies against β-actin (mouse monoclonal, 1:1000; Applied Biosystems, CA, USA; catalog number AM4302) and caspase-3 (rabbit polyclonal, 1:500; Abcam, Cambridge, MA, USA; catalog number ab13847). The membranes were then incubated for 1 h at room temperature with goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA; catalog number sc-2004) or goat anti-mouse IgG-HRP (Santa Cruz Biotechnology; catalog number sc-2005) diluted in 2.5% blocking solution.
After washed with TBST, protein was detected using an enhanced chemiluminescence substrate (ELPIS Biotech, Taejon, Korea; catalog number EBP-1071). Protein levels were determined with a LAS-3000 densitometer and Science Laboratory 2001 Image Gauge software (Fuji Photo Film, Tokyo, Japan).
Analysis of peroxynitrite and superoxide formation
Paraffin-embedded, 4-μm thick sections were deparaffinized and then treated with citrate buffer (0.01 mol l−1, pH 6.0) heated in a microwave for antigen retrieval. The sections were incubated in 3% H2O2 in methyl alcohol to block endogenous peroxidase activity and washed with PBS. After incubating with blocking serum for 1 h, the tissue sections were treated overnight at 4°C with anti-3-nitrotyrosine (3-NT) antibody (rabbit polyclonal, 1:1000; Upstate Biotechnology, Lake Placid, NY, USA; catalog number 06-284), a marker of peroxynitrite generation. The secondary antibody DyLight 405-conjugated AffiniPure anti-rabbit (goat polyclonal, 1:800; Jackson ImmunoResearch, West Grove, PA, USA; code number 111-475-003) was applied.
As a principal marker of superoxide, dihydroethidium (DHE) is rapidly oxidized to ethidium bromide (EtBR), which then binds to DNA in the nucleus and emits red fluorescence.28,29 Briefly, frozen, enzymatically intact testes in OCT compound (Leica, Bensheim, Germany) were cut into 7- to 10-μm thick sections and mounted on coating glass slides. The tissues were incubated with 5 mmol l−1 DHE stabilized in dimethyl sulfoxide (Invitrogen, Carlsbad, CA, USA; catalog number D23107) at 1:10 000 dilution in PBS and were stained in a light-protected humidified chamber for 5 min at 37°C. Images were obtained using LSM 710 confocal laser scanning microscopy with ZEN software (Carl Zeiss, Jena, Germany). 3-NT- or DHE–positive cells were manually counted and averaged for 20 seminiferous tubules.
Biochemical parameters
To measure malondialdehyde (MDA), which is an end product of lipid peroxidation, the thiobarbituric acid (TBARS) assay was performed using a commercially available kit (Cell Biolabs, San Diego, CA, USA; catalog number STA-330) following the manufacturer's instructions. A dilution series of MDA standards were prepared ranging from 0 μmol l−1 to 250 μmol l−1, and samples were prepared after treating with PBS containing ×1 butylated hydroxytoluene (BHT). SDS lysis solution was added to both the samples and the MDA standards followed by TBA reagent. All samples were incubated at 95°C for 1 h and then cooled to room temperature. After centrifugation, the supernatant was used for analysis. MDA standards and samples were transferred to a 96-well microplate for spectrophotometric measurement at 532 nm.
Superoxide dismutase (SOD) activity was evaluated using a commercially available kit (Cayman Chemical, Ann Arbor, MI, USA; catalog number 706002) following the manufacturer's instructions. Tissues were homogenized in cold 20 mmol l−1 HEPES buffer (pH 7.2) and centrifuged at 1500 g for 4 min at 4°C for collecting the supernatant. The diluted radical detector was added to both the samples and the SOD standards followed by addition of diluted xanthine oxidase reagent. All samples were incubated for 20 min at room temperature. The SOD standards and samples were analyzed using a plate reader for spectrophotometric measurement at 450 nm.
Catalase (CAT) activities were evaluated using a commercially available kit (Cayman Chemical; catalog number 707002)following the manufacturer's instructions. Tissues were homogenized in a cold buffer and centrifuged at 10 000 g for 15 min at 4°C for collecting the supernatant. The diluted assay buffer and methanol were added to both the samples and the CAT standards followed by addition of diluted hydrogen peroxide reagent. All samples were incubated for 20 min at room temperature. Diluted potassium hydroxide and catalase purpald were added to each well. After incubating for 10 min at room temperature, catalase potassium periodate was added to each well. The standards and samples were analyzed using a plate reader for spectrophotometric measurement at 540 nm.
Statistical analyses
All quantitative data are reported as the mean ± standard deviation (s.d.). The data between the groups were compared using analysis of variance (ANOVA) followed by Tukey's test for CAT level and the number of caspase-3-stained cells, or using nonparametric analysis with Kruskal–Wallis test followed by Dunn's multiple comparison test for other data. A value of P < 0.05 was considered statistically significant.
RESULTS
Histological examination
As expected in ipsilateral testes, the sham Group A had normal testicular architecture and regular seminiferous tubule morphology with an orderly arrangement of germ cells including the presence of primary and secondary spermatocytes, spermatids, and spermatozoa. Group B showed hypospermatogenesis and maturation arrest with a loss of germinal cells and layers and severely impaired seminiferous tubules with many red blood cells due to vessel extravasations in the testes. Unlike Group B rats, Group C rats demonstrated morphology close to normal with well-arranged cell architecture. The testicular structure and the morphology of seminiferous tubules of Group G were similar to those of Group B.
Testicular parameters of spermatogenesis
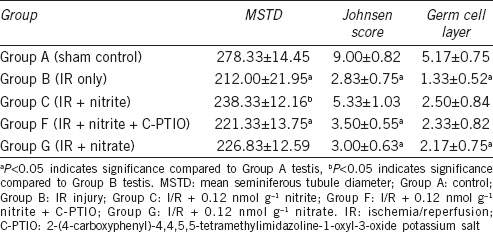
Table 1 summarizes the mean histological changes in the ipsilateral testes. The MSTD and Johnsen scores in Groups B and F were significantly lower (P < 0.05) and the number of germ cell layers in Groups B and G was significantly lower (P < 0.05) than that in Group A. The MSTD was significantly higher in Group C than in Group B (P < 0.05). Furthermore, the Johnsen score in Group E was significantly lower than that in Group A (P < 0.05).
Table 1.
Mean seminiferous tubule diameter value, Johnsen score, and number of germ cell layers in the left testes
Immunohistochemistry and western blot for caspase-3
Figure 1a–1e show the results of immunohistochemical analysis of caspase-3 in the ipsilateral testes. The number of caspase-3-positive cells was significantly higher (P < 0.05) in Groups B (33.33 ± 8.68) and G (27.68 ± 12.13) than that in Group A (2.57 ± 1.26). After I/R, 0.12 nmol g−1 NO2− administration (Group C) showed the most therapeutic effect on the damaged testis, as evidenced by a significant reduction in the number of caspase-3-positive cells (8.51 ± 3.46), as compared to Groups B and G (P < 0.05). No therapeutic effects were observed in the other NO2− and NO3− groups (data not shown). The relative caspase-3/β-actin expression was significantly lower in Groups A (0.48 ± 0.08) and C (0.56 ± 0.08) than that in Group B (1.43 ± 0.11, P < 0.05) (Figure 1f and 1g).
Figure 1.
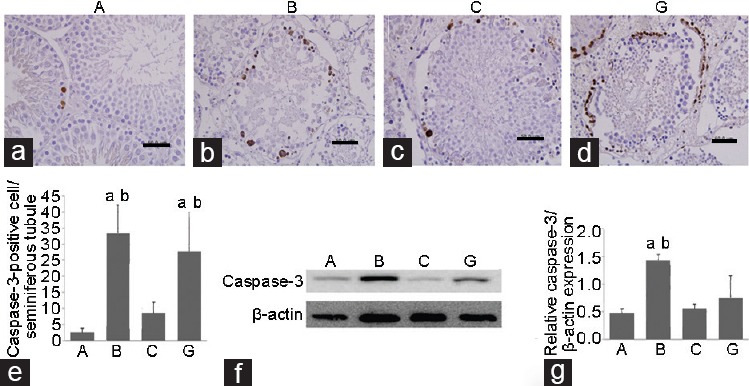
Immunohistochemistry (×400) and western blot analysis of caspase-3 in ipsilateral testis. (a) The sham group has few caspase-3-positive cells. The number of stained cells is significantly higher in groups (b) B and (d) G than that in Group A. (c) The number of stained cells is significantly lower in Group C. Scale bars = 50 μm. (e) The number of caspase-3-positive cells per seminiferous tubule is shown. (f) The western blot image for caspase-3 in left testis is shown. (g) The relative caspase-3/β-actin expression in Groups A and C is significantly lower than that in Group B. aP < 0.05 indicates significance compared to Group A, bP < 0.05 indicates significance compared to Group C. Group A: control; Group B: IR injury; Group C: IR + 0.12 nmol g−1 nitrite; Group G: IR + 0.12 nmol g−1 nitrate. IR: ischemia/reperfusion.
Detection of peroxynitrite and superoxide anion for evaluation of oxidative stress
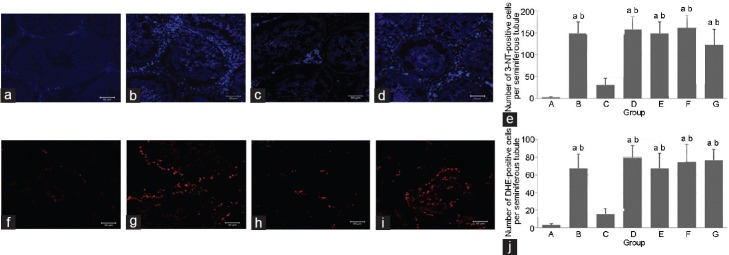
Figure 2 shows the results for 3-NT immunoreactivity and DHE fluorescence in the ipsilateral testes. In the ischemic testes, the number of 3-NT-positive cells in Groups B (148.44 ± 27.09) and G (121.70 ± 36.06) was significantly higher than that in Group A (2.10 ± 1.12, P < 0.05). In contrast, the number in Group C (30.78 ± 15.14) was significantly lower than the corresponding values in Groups B and G (P < 0.05). DHE fluorescence showed the same trend as 3-NT staining. In the ischemic testes, the intensity of DHE fluorescence was significantly higher in Groups B (67.34 ± 15.91) and G (76.71 ± 12.18) than that in Group A (3.04 ± 1.56, P < 0.05). However, Group C (15.74 ± 5.89) had significantly lower DHE fluorescence intensity than Groups B and G (P < 0.05). The extent of oxidative stress in Groups D, E, and F (79.23 ± 13.85, 67.33 ± 16.74, and 74.60 ± 20.07, respectively) was similar to that in Groups B and G.
Figure 2.
Effects of nitrite and nitrate on 3-NT staining and DHE fluorescence in ipsilateral testis. Immunohistochemical staining of 3-NT in the left testes of Groups (a) A, (b) B, (c) C, and (d) G, respectively (scale bars = 50 μm). (e) The number of 3-NT-positive cells per seminiferous tubule in each group is shown. DHE fluorescence in the left testes of groups (f) A, (g) B, (h) C, and (i) G, respectively (scale bars = 50 μm). (j) The number of DHE-positive cells per seminiferous tubule in each group is shown. aP < 0.05 indicates significance compared to Group A, bP < 0.05 indicates significance compared to Group C. Group A: control; Group B: I/R injury; Group C: I/R + 0.12 nmol g−1 nitrite; Group G: I/R + 0.12 nmol g−1 nitrate. 3-NT: 3-nitrotyrosine; DHE: dihydroethidium; IR: ischemia/reperfusion.
Testis MDA levels
The MDA level in the ipsilateral testis was significantly higher in Groups B (2.77 ± 0.84) and F (2.17 ± 0.54) than that in Group A (0.55 ± 0.05) (P < 0.05, Figure 3a). In Group C (0.78 ± 0.29), the MDA levels were significantly lower than those in Groups B and F (P < 0.05).
Figure 3.
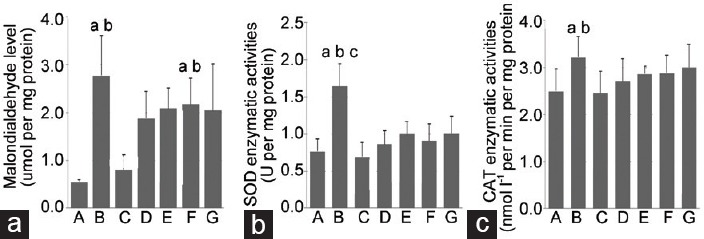
Effects of nitrite and nitrate on MDA, SOD, and CAT levels in ipsilateral testis. (a) MDA values are expressed as micromoles of MDA per mg protein (b) SOD values are expressed as unit of SOD per mg protein. (c) CAT values are expressed as nmol l−1 per min per mg protein. Data are mean ± s.d. aP < 0.05 indicates significance compared to Group A, bP < 0.05 indicates significance compared to Group C, cP < 0.05 indicates significance compared to Group D testis. Group A: control; Group B: I/R injury; Group C: I/R + 0.12 nmol g−1 nitrite; Group D: I/R + 1.2 nmol g−1 nitrite; Group E: I/R + 12 nmol g−1 nitrite; Group F: I/R + 0.12 nmol g−1 nitrite + 0.01 μmol g−1 C-PTIO; Group G: I/R + 0.12 nmol g−1 nitrate. MDA: malondialdehyde; SOD: superoxide dismutase; CAT: catalase; I/R: ischemia/reperfusion; C-PTIO: 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt; s.d.: standard deviation.
Determination of the antioxidant enzymatic activity levels
The SOD level in the ipsilateral testis was significantly higher in Groups A, C, and D (0.77 ± 0.17, 0.69 ± 0.20 and 0.86 ± 0.18, respectively) than that in Group B (1.64 ± 0.30) (P < 0.05, Figure 3b). The CAT levels in Groups A (2.32 ± 0.72) and C (2.48 ± 0.47) were significantly lower than those in Group B (3.31 ± 0.37) (P < 0.05, Figure 3c).
DISCUSSION
The pathway which results in the production of NO from the three NOS isoforms might be compromised under conditions of low oxygen tension, such as acidosis, ischemia, and hypoxia.30 Under these conditions, the following alternative pathways have been found to exhibit NO2− reductase activity to convert NO2− to NO: acidic disproportionation,31 heme-proteins,32 and XOR.33
NO2− is an important source of NO generated through an NOS-independent pathway in the kidney, heart, and liver10,14,15,34 and also has protective effects in murine models of hepatic and myocardial infarction as well as in animal models of cerebral I/R.14,22,35,36 In the present study, we attempted to investigate whether hypoxia-dependent NO production from NO2− confers cytoprotection in testicular I/R injury. To the best of our knowledge, this is the first study to investigate the effects of NO2− and NO3− on testicular I/R injury and determine their therapeutic effects. The doses of 0.12, 1.2, and 12 nmol g−1 and the time of administration for NO2− used in this study were selected based on previous studies regarding liver, heart, and kidney I/R injury.12,14,34
The NO scavenger C-PTIO was used to confirm whether the protection was, indeed, due to NO. C-PTIO inhibited the cytoprotective effect of NO from NO2− in ischemic testis in vivo. These data were in accordance with the results of several studies, which reported that C-PTIO abolished the beneficial effects of NO2−.22,34
In this study, the levels of MDA, SOD, and CAT in the left testes were significantly higher in Group B than the control levels. These results are the same as those of a previous study with ischemic testes.37 However, there was an opposite trend for SOD and CAT values in ischemic brain and intestinal tissue.38,39 These could be due to differences between the organs. Because mammalian testes are highly sensitive to oxidative stress,40 the anti-oxidant enzymes are more likely to increase to compensate for reactive oxygen species (ROS). The fluorescence intensity of 3-NT and DHE-stained cells was also significantly higher in Group B than that in the control group. The formation of peroxynitrite and superoxide radicals also decreased in Group C; as such, our data are in agreement with that of previous research.22
Our study demonstrated that testicular I/R-induced germ cell degeneration and impaired spermatogenesis,41 and an intravenous injection of 0.12 nmol g−1 NO2− exerted therapeutic effects on spermatogenesis in ipsilateral testis. While MSTD was significantly higher in the group treated with NO2− than that in I/R group animals, the differences in the Johnsen scores and number of germ cell layers were not statistically significant. However, these values showed a tendency to increase in the group that received NO2− intravenously. Taken together, these results could support the hypothesis of improved spermatogenesis and increased germ cell layer with intravascular NO2− administration before reperfusion in testis.
Apoptosis is a critical indicator of tissue damage. Testicular I/R can lead to germ cell-specific apoptosis in rats.41,42 In this study, immunohistochemical analyses revealed an increased number of caspase-3-positive cells in the I/R group. Western blot of the germ cell lysate also showed that the relative expression of caspase-3 was significantly higher in the I/R group than that in the control group. The results of our study clearly demonstrated that an intravenous injection of NO2− exerted anti-apoptotic effects, which were consistent with the results of earlier study of liver and heart I/R injury model.14 NO is a multifunctional signaling molecule that inhibits caspase activities to inhibit apoptosis,43 although the underlying mechanism by which NO from NO2− has a beneficial effect on testicular I/R injury is still unclear.
The limitation of the present study is NO derivatives such as S-nitrosothiols, N-nitrosamines, iron-nitrosyl, and nitrated lipid were not evaluated. Since NO-modified proteins, peptides, and lipids have been reported to have cytoprotective effects on I/R injury,44 further analysis is required to discriminate among these NO-related compounds for verifying the exact mechanism. Second, the dose-dependent efficacy of NO2− was demonstrated in other studies,14,22 but not in this study. The organ specificity and sensitivity to NO production should be evaluated. As aforementioned, NO has an effect on the Janus pathway. NO generated in high quantities may form strong oxidant peroxynitrite leading to damage of tissue; however, it can also exert cytoprotection by reducing free radicals. Therefore, the concentrations that are attributable to the protective effect of NO derived from NO2− vary for each organ.
In summary, this study demonstrates that NO from NO2− with anti-oxidant and anti-apoptotic properties has a therapeutic effect under ischemic conditions. NO2− could be an adjunctive therapy for testicular I/R injury.
AUTHOR CONTRIBUTIONS
JWL and JSH were responsible for the study design and the manuscript revision. JWL, DHL, and JGP performed the experiments and analyzed the data. DHL and JGP carried out western blot and performed the statistical analysis. JWL and JSH contributed to all animal experiments including histopathological assessment and solved other technical issues. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by Veterinary Science Research Institute of The Konkuk University, Seoul, Korea.
REFERENCES
- 1.Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age-related delay in the presentation of testicular torsion. J Urol. 1989;142:746–8. doi: 10.1016/s0022-5347(17)38875-4. [DOI] [PubMed] [Google Scholar]
- 2.Ringdahl E, Teague L. Testicular torsion. Am Fam Physician. 2006;74:1739–43. [PubMed] [Google Scholar]
- 3.Wampler SM, Llanes M. Common scrotal and testicular problems. Prim Care. 2010;37:613–26. doi: 10.1016/j.pop.2010.04.009. x. [DOI] [PubMed] [Google Scholar]
- 4.Williamson RC. Death in the scrotum: testicular torsion. N Engl J Med. 1977;296:338. doi: 10.1056/NEJM197702102960611. [DOI] [PubMed] [Google Scholar]
- 5.Lysiak JJ, Nguyen QA, Kirby JL, Turner TT. Ischemia-reperfusion of the murine testis stimulates the expression of proinflammatory cytokines and activation of c-jun N-terminal kinase in a pathway to E-selectin expression. Biol Reprod. 2003;69:202–10. doi: 10.1095/biolreprod.102.013318. [DOI] [PubMed] [Google Scholar]
- 6.Yagmurdur H, Ayyildiz A, Karaguzel E, Akgul T, Ustun H, et al. Propofol reduces nitric oxide-induced apoptosis in testicular ischemia-reperfusion injury by downregulating the expression of inducible nitric oxide synthase. Acta Anaesthesiol Scand. 2008;52:350–7. doi: 10.1111/j.1399-6576.2007.01545.x. [DOI] [PubMed] [Google Scholar]
- 7.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 8.Lissbrant E, Lofmark U, Collin O, Bergh A. Is nitric oxide involved in the regulation of the rat testicular vasculature? Biol Reprod. 1997;56:1221–7. doi: 10.1095/biolreprod56.5.1221. [DOI] [PubMed] [Google Scholar]
- 9.Zini A, Abitbol J, Girardi SK, Schulsinger D, Goldstein M, et al. Germ cell apoptosis and endothelial nitric oxide synthase (eNOS) expression following ischemia-reperfusion injury to testis. Arch Androl. 1998;41:57–65. doi: 10.3109/01485019808988547. [DOI] [PubMed] [Google Scholar]
- 10.Webb A, Bond R, McLean P, Uppal R, Benjamin N, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–8. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefer DJ. Nitrite therapy for protection against ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2006;290:F777–8. doi: 10.1152/ajprenal.00470.2005. [DOI] [PubMed] [Google Scholar]
- 12.Basireddy M, Isbell TS, Teng X, Patel RP, Agarwal A. Effects of sodium nitrite on ischemia-reperfusion injury in the rat kidney. Am J Physiol Renal Physiol. 2006;290:F779–86. doi: 10.1152/ajprenal.00334.2005. [DOI] [PubMed] [Google Scholar]
- 13.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 14.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto M, Tsuchiya K, Kanematsu Y, Izawa Y, Yoshizumi M, et al. Nitrite-derived nitric oxide formation following ischemia-reperfusion injury in kidney. Am J Physiol Renal Physiol. 2005;288:F182–7. doi: 10.1152/ajprenal.00036.2004. [DOI] [PubMed] [Google Scholar]
- 16.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, et al. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci U S A. 2004;101:4308–13. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem. 1981;256:12393–8. [PubMed] [Google Scholar]
- 18.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A. 2008;105:7540–5. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, et al. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32:980–6. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- 20.Buisson A, Margaill I, Callebert J, Plotkine M, Boulu RG. Mechanisms involved in the neuroprotective activity of a nitric oxide synthase inhibitor during focal cerebral ischemia. J Neurochem. 1993;61:690–6. doi: 10.1111/j.1471-4159.1993.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 21.Dimmeler S, Zeiher AM. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide. 1997;1:275–81. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- 22.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, et al. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–50. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 23.Kono T, Saito M, Kinoshita Y, Satoh I, Shinbori C, et al. Real-time monitoring of nitric oxide and blood flow during ischemia-reperfusion in the rat testis. Mol Cell Biochem. 2006;286:139–45. doi: 10.1007/s11010-005-9105-3. [DOI] [PubMed] [Google Scholar]
- 24.Barlas M, Hatiboglu C. The effect of nitric oxide in testicular ischemia-reperfusion injury. Int Urol Nephrol. 2002;34:81–6. doi: 10.1023/a:1021311029572. [DOI] [PubMed] [Google Scholar]
- 25.Dokmeci D, Kanter M, Inan M, Aydogdu N, Basaran UN, et al. Protective effects of ibuprofen on testicular torsion/detorsion-induced ischemia/reperfusion injury in rats. Arch Toxicol. 2007;81:655–63. doi: 10.1007/s00204-007-0189-2. [DOI] [PubMed] [Google Scholar]
- 26.Johnsen SG. Testicular biopsy score count – a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 27.Cosentino MJ, Nishida M, Rabinowitz R, Cockett AT. Histological changes occurring in the contralateral testes of prepubertal rats subjected to various durations of unilateral spermatic cord torsion. J Urol. 1985;133:906–11. doi: 10.1016/s0022-5347(17)49278-0. [DOI] [PubMed] [Google Scholar]
- 28.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 29.Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, et al. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–7. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 30.Mikula I, Durocher S, Martasek P, Mutus B, Slama-Schwok A. Isoform-specific differences in the nitrite reductase activity of nitric oxide synthases under hypoxia. Biochem J. 2009;418:673–82. doi: 10.1042/BJ20080987. [DOI] [PubMed] [Google Scholar]
- 31.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–9. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 32.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–47. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, et al. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 2000;275:7757–63. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 34.Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, et al. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007;18:570–80. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 35.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, et al. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–93. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 36.Jung KH, Chu K, Lee ST, Sunwoo JS, Park DK, et al. Effects of long term nitrite therapy on functional recovery in experimental ischemia model. Biochem Biophys Res Commun. 2010;403:66–72. doi: 10.1016/j.bbrc.2010.10.116. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu S, Saito M, Kinoshita Y, Shomori K, Satoh I, et al. Ischemic preconditioning and post-conditioning to decrease testicular torsion-detorsion injury. J Urol. 2009;182:1637–43. doi: 10.1016/j.juro.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Xing B, Chen H, Zhang M, Zhao D, Jiang R, et al. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39:2362–9. doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]
- 39.Tunc T, Uysal B, Atabek C, Kesik V, Caliskan B, et al. Erdosteine and ebselen as useful agents in intestinal ischemia/reperfusion injury. J Surg Res. 2009;155:210–6. doi: 10.1016/j.jss.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Parlaktas BS, Atilgan D, Ozyurt H, Gencten Y, Akbas A, et al. The biochemical effects of ischemia-reperfusion injury in the ipsilateral and contralateral testes of rats and the protective role of melatonin. Asian J Androl. 2014;16:314–8. doi: 10.4103/1008-682X.122202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sukhotnik I, Miselevich I, Lurie M, Nativ O, Coran AG, et al. The time relationship between ipsilateral testicular ischemia and germ cell apoptosis in the contralateral testis in rat. Pediatr Surg Int. 2005;21:512–6. doi: 10.1007/s00383-005-1477-7. [DOI] [PubMed] [Google Scholar]
- 42.Turner TT, Tung KS, Tomomasa H, Wilson LW. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod. 1997;57:1267–74. doi: 10.1095/biolreprod57.6.1267. [DOI] [PubMed] [Google Scholar]
- 43.Guan LY, Fu PY, Li PD, Li ZN, Liu HY, et al. Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg. 2014;6:122–8. doi: 10.4240/wjgs.v6.i7.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, et al. Fas-induced caspase denitrosylation. Science. 1999;284:651–4. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]