Abstract
Circulating tumor cells (CTC) have become an important biomarker in patients with advanced prostate cancer. CTC count has been demonstrated to be a prognostic factor for overall survival in patients with metastatic castration-resistant prostate cancer (mCRPC). In localized prostate cancer, a clear correlation between CTC counts and clinicopathological risk parameters and outcome has not been observed. Currently, the focus of research is shifting from CTC enumeration towards molecular characterization of CTC leading to the discovery of markers predicting treatment response. The role of androgen receptor splice variants expressed by CTC as markers of resistance to abiraterone and enzalutamide has been assessed by various studies. The identification of CTC markers predicting treatment response represents a key step to guide the selection of treatment (e.g., abiraterone/enzalutamide vs taxanes), particularly in patients with mCRPC. As an alternative to CTC, the analysis of circulating tumor DNA has been shown to enable a noninvasive disease characterization having high potential to promote precision oncology.
Keywords: AR-V7, biomarker, cell-free DNA, cfDNA, circulating tumor cells, ctDNA, liquid biopsy, prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the most prevalent malignancy and the third leading cause of cancer death in men in the United States.1 Morbidity and mortality by PCa are mainly caused by PCa metastases.2 One important step in the sequence leading to the development of distant metastases is loss of adhesion and entry into the systemic vasculature with migration of tumor cells away from the primary tumor.2 This step results in entry of tumor cells into the blood stream. Circulating tumor cells (CTC) are potentially detectable in the peripheral blood before the occurrence of clinically relevant metastases, and these cells are capable of forming new metastases.3,4,5,6,7 The evidence of CTC in the peripheral blood may, therefore, be a hint for the progress of tumor although clear data from clinical studies are missing in this context.
In the past, most of the studies focused on quantification of CTC (CTC enumeration), which has been shown to correlate with disease outcome but does not exploit the full potential of circulating cells as biomarkers.8,9 Molecular analysis of the CTC may provide a real-time overview of the tumor characteristics and its mutations.10 This information might help predicting the tumor's response to different treatment options, particularly in cases of metastatic castration-resistant prostate cancer (mCRPC). Therefore, qualitative molecular analysis of CTC collected through liquid biopsies might be an important step for the implementation of personalized treatment strategies in the more and more complex therapeutic landscape of PCa.
Molecular analyses of CTC are useful because several studies have shown that the genomic information of CTC is comparable to the primary tumor tissue and/or metastases.11 It is assumed that CTC are able to reflect all tumor characteristics including the intratumoral and intertumoral heterogeneity.12,13,14,15,16 However, it is still not known whether CTC represent characteristics of all metastases or only the most invasive/aggressive clones. Because the half-life of CTC has been estimated to be in the range of hours, it is suggested that CTC provides a real-time representation of the tumor's characteristics, which offers the opportunity to take tumor snapshots at various time-points during the therapy.17,18,19
The diagnostic and therapeutic potential of CTC as promising biomarkers with prognostic and predictive value for potential clinical outcome and therapy response is a highly relevant topic for solid malignancies.20,21 Concerning urologic oncology, there is a rising number of studies investigating the potential role of CTC, particularly in local and metastatic PCa.10,22,23,24 The aim of this review is to give an overview on the current role of CTC in localized and metastatic PCa.
METHODS OF ISOLATION AND ENRICHMENT OF CTC
CTC are rare in the peripheral blood, estimated to account for one in a billion nucleated cells.25 Therefore, one key step for a “liquid biopsy” aiming to detect CTC in the peripheral blood is the enrichment and isolation of these cells. There are different techniques for this purpose.
The CellSearch® system (Janssen Diagnostics, Raritan, NJ, USA) is the only Food and Drug Administration (FDA) approved CTC detection platform. After an immunomagnetic enrichment step, based on epithelial cell adhesion molecule (EpCAM), the second step is staining of the isolated cells with specific fluorescent antibody conjugates against cluster of differentiation 45 (CD45) and cytokeratin (CK) 8, 18, 19. In the next step, the sample is scanned on an analyser. In this system, CTC are defined as nucleated cells lacking the leukocyte marker CD45 and expressing cytokeratins. The size of a CTC has to be at least 4 μm × 4 μm and it has to show specific morphological features, judged by trained operators.26,27 There is evidence that smaller and CK-negative CTC exist, which are even more aggressive. They seem to appear after epithelial-mesenchymal transition (EMT) or neuroendocrine differentiation.28,29,30 These cells do not get isolated by the CellSearch® system.
In addition to the CellSearch® system, a variety of other approaches for CTC enrichment and detection exist. Polymerase chain reaction (PCR)-based approaches with or without previous enrichment steps have been assessed in various studies. CTC presence varies depending on the used mRNA marker to detect CTC and the method of enrichment.31 The Adnatest platform (Adnatest®, Qiagen GmbH, Hilden, Germany) combines immunomagnetic enrichment of CTC with subsequent RNA isolation and reverse transcription PCR for PCa associated transcripts.23,32 Recently, various groups have used mRNA analysis of whole blood-derived ribonucleic acid (RNA) to detect CTC-associated transcripts without prior enrichment of CTC.33,34
The EpicScience platform (Epic Science, San Diego, CA 92121, USA) uses high-throughput imaging, in which nucleated cells are plated on glass slides. It is based on the separation between nucleated cells (e.g., white blood cells, and CTC) and red blood cells. After lysis of red blood cells, it uses cytokeratin, leukocyte marker CD45, and nucleus staining with 4’6-diamidino-2-phenylindole (DAPI). Following this step, each slide is scanned by a fiber-optic array scanning technology (FAST), which records the precise coordinates of each cell and analyzes each cell for different parameters. This algorithm proposes potential CTC which have to be confirmed by a trained reader. It is therefore independent of EpCAM expression.35
Moreover, there are several label-free methods, based on cell size and morphology.36 Separation of CTC from the peripheral blood is based on three-dimensional microfilters and bilayers. The success rate depends on several parameters, such as pore size, blood flow rate (high flow rates may lead to squeezing of CTC through pores, the slow flow rate can cause accumulation of leukocytes and clotting of the blood), and rigidity of the membrane.36,37 In microfluidic devices, CTC seem to be detected in a higher proportion of patients and in higher counts.17,38 Isolation of CTC by these devices offers the opportunity for detailed molecular analysis of tumor diseases. As the majority of these label-free techniques lead to the isolation of viable cells, these platforms have a significant potential for assays requiring viable cells (such as in vitro culture and xenografts).
The gold standard until today is the CellSearch® system. In the future, the focus will switch from quantification (like CTC enumeration) to molecular analyses. Because the CellSearch® system is mainly designed for quantification and not characterization, development and further improvements of other approaches, which enable extensive molecular analyses of CTC, are needed.
SIGNIFICANCE OF CTC IN LOCALIZED PCa
CTC have been most intensively investigated in metastatic PCa. In localized disease, the role of CTC has been addressed only by few studies, showing conflicting data on the added value of CTC analysis to the currently established risk stratifications, depending on the technique used for enrichment and isolation of CTC.
A significant amount of patients with localized PCa develop tumor recurrence despite curative intended surgical therapy.39 Current risk stratification is mainly based on clinical parameters and evaluation of pathological tumor specifications after prostatectomy. Most of the published studies investigated the correlation between CTC in localized PCa with other clinical and pathological risk stratification parameters (e.g., prostate-specific antigen [PSA] concentration, pathological tumor stage [pT-stage], lymph node stage [pN-stage], Gleason score) aiming to evaluate the value of CTC as an additional parameter.
In studies using the CellSearch® system to enrich and isolate CTC, no correlation was found between CTC count and other clinical-pathological parameters:
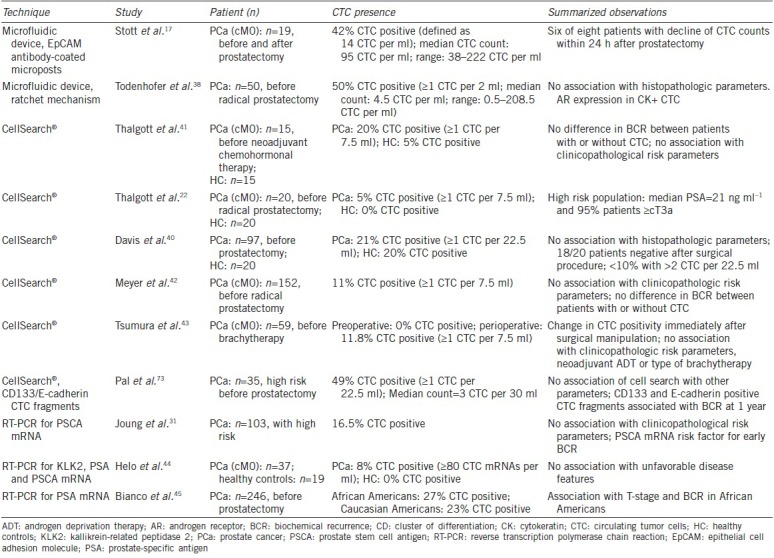
Thalgott et al.22 analyzed CTC in a group of twenty patients with localized PCa before radical prostatectomy. They observed only one patient (5.0%) with one CTC per 7.5 ml blood. Consistent with the study from Davis et al.40 (see below), there was no difference in the CTC-positive rate between patients with localized PCa and the healthy control group. In a second study from the same authors,41 20% of a cohort of 15 patients with localized PCa undergoing neoadjuvant docetaxel chemotherapy were CTC positive at baseline compared to 5% in the healthy control group. There was no difference in biochemical recurrence, independent from the patient status (CTC positive or not), before therapy. Moreover, they did not see any correlation between CTC and clinicopathological risk parameters.
Davis et al.40 not only detected CTC in 21% of patients but also in a comparable proportion of the control group comprising patients without PCa (20%). In addition, the authors used more than the usually processed blood (22.5 ml instead of 7.5 ml) probably increasing the rate of CTC-positive patients compared to studies using only 7.5 ml. The authors did not observe any correlation with established pathological or clinical risk parameters.
Meyer et al.42 analyzed preoperative CTC in a cohort of 152 patients with localized PCa. Eleven percent of this cohort had CTC preoperatively. Again, they could not observe a significant correlation of CTC presence with T-stage, Gleason score, or PSA level. Furthermore, there was no correlation between CTC positivity and biochemical recurrence (median follow-up: 48 months).
In a cohort of 59 patients with localized PCa, Tsumura et al.43 evaluated the CTC count before and immediately after brachytherapy. They could show a change in CTC positivity after surgical manipulation (insertion of needles) in 11.8% of the patients. There was no correlation of CTC status with other variables such as PSA at diagnosis, previous use of neoadjuvant androgen deprivation therapy (ADT), type of brachytherapy, Gleason score, and biopsy positivity core rate.
Studies using a microfluidic device for CTC detection identified CTC in a higher proportion of patients and found considerably higher CTC counts than studies using CellSearch® system (Stott et al.17; 42% of patients with ≥14 CTC per ml, median count = 95 CTC per ml; Todenhofer et al.39; 50% of patients with ≥1 CTC per ml, median count = 4.5 CTC per ml). Stott et al.17 could show a decline of CTC in six of eight patients within 24 h after prostatectomy. Todenhöfer et al.38 could not find any correlation between the presence of CTC with age, serum PSA level, pT stage, pN stage, Gleason score, or risk category. Furthermore, the number of CTC in patients with CTC positivity was not associated with pT-stage, N-stage, or Gleason score. There was no surveillance for clearance of putative CTC from the blood after prostatectomy in this study.
In PCR-based approaches, associations with clinicopathological risk parameters depend on the cohort and the genes used for CTC detection. Joung et al.31 could not find any association between prostate stem cell antigen (PSCA) mRNA levels with other clinicopathological risk parameters. Helo et al.44 used PCR for kallikrein-related peptidase 2 (KLK2), PSA, and PSCA mRNA. They were not able to demonstrate an association between presence of these transcripts with unfavorable disease features. Bianco et al.45 could show an association of PSA mRNA and T-stage/biochemical recurrence (BCR) in a cohort of African Americans.
In localized PCa, the clinical value of CTC analyses remains to be determined. Compared with the prognostic value of CTC in metastatic PCa, where several studies showed that CTC detected by the CellSearch® system is associated with an unfavorable outcome, no clear association with outcome has been reported in localized disease. In studies analyzing prostate-cell associated transcripts as a surrogate parameter for CTC like in Joung et al.31 or Bianco et al.,45 a prognostic value for biochemical recurrence could be shown. The question whether this should result in more frequent follow-up examinations after surgery in CTC-positive patients remains to be elucidated in a clinical study. There is currently no clear rationale for a different treatment of CTC-positive patients with localized PCa compared to CTC-negative patients (e.g., receiving adjuvant chemotherapy).
Due to the low median number of CTC encounted using the CellSearch® system, a use of its CTC count as a continuous parameter is unlikely to add additional information in the localized setting. Microfluidic-based devices for CTC detection and enrichment lead to the detection of a higher number of CTC. Therefore, these techniques could provide a broader dynamic range, which prospectively may lead to a longitudinal assessment of CTC count as an additional continuous risk parameter regarding disease status and therapy response.
In addition to the quantitative analyses of the CTC count, qualitative analyses of molecular features of CTC may provide additional information about the tumor. In view of the fact that most of the localized PCa develop multifocally, CTC may represent the quintessence of molecular heterogeneity. Furthermore, CTC are discussed as originated cells from the most aggressive subpopulation inside of the prostate as they are cells which already left the prostate and infiltrated the blood circulating system.38 However, the potential additional information that CTC may provide compared to analysis of the different tumor foci is unclear. Moreover, there is not sufficient evidence yet for a clear correlation of the molecular characteristics of CTC and multiple tumor foci.11,46
Table 1 summarizes results from studies assessing CTC in patients with localized PCa.
Table 1.
The summary of results from studies assessing circulating tumor cells (CTC) in patients with localized prostate cancer
ROLE OF CTC IN METASTATIC PCa
In an advanced disease such as mCRPC, CTC have been demonstrated as a valuable biomarker. Table 2 summarizes studies on CTC in metastatic PCa. In the past, most of the studies focused on the prognostic value of CTC enumeration and the role of CTC count as an early treatment response biomarker for patients with metastatic PCa. de Bono et al.47 published a landmark study in 2008 contributing to the FDA approval of the CTC enumeration by the CellSearch® system for clinical use in patients with mCRPC. In this study, it was demonstrated that a favorable CTC count (<5 cells per 7.5 ml blood) predicts a significantly improved progression-free survival (PFS) and overall survival (OS) compared to an unfavorable CTC count (≥5 cells per 7.5 ml blood) in abiraterone-treated patients. CTC were defined as nucleated (DAPIpos), CKpos, and CD45neg cells with a diameter ≥4 μm. Moreover, the authors could show that conversions of the CTC count from favorable to unfavorable and vice versa were associated with an improvement or deterioration of the prognosis. This change could already be shown 2–5 weeks after the treatment's start, which means that the CTC count as early response marker outperforms a 30%–50% decline in PSA (significant for the prognosis after 6–8 weeks).
Table 2.
Role of circulating tumor cell (CTC) in metastatic prostate cancer
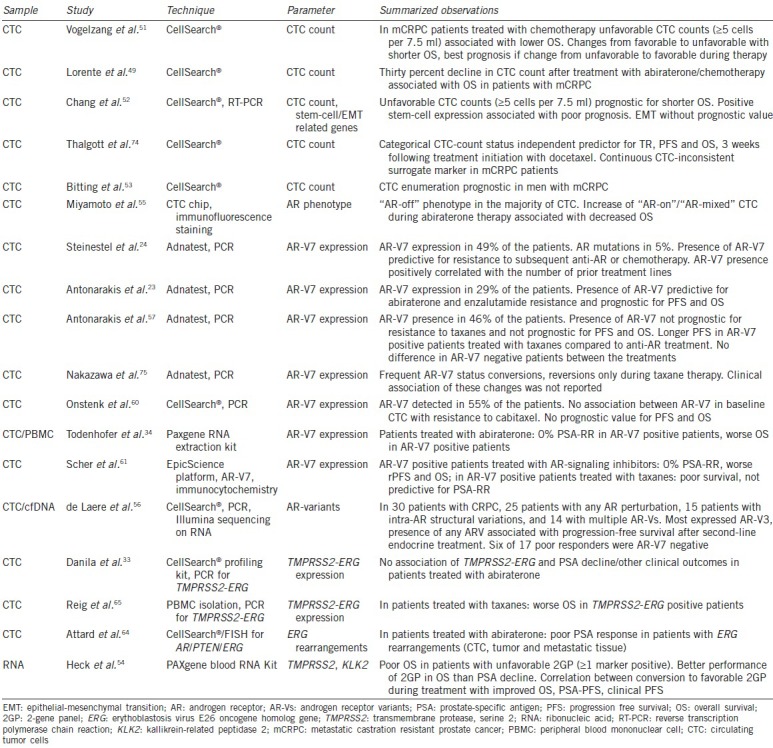
The prognostic value of CTC count and the value as early therapy response biomarkers have been confirmed in other studies. Danila et al.9 showed that baseline CTC count was associated with OS. Olmos et al.48 demonstrated that CTC count changes predict a change in prognosis in patients treated with any chemotherapy. Scher et al.8 observed that changes in CTC count were strongly associated with OS at various time points during therapy with docetaxel monotherapy (or combination therapy). Furthermore, they demonstrated again that CTC count can outperform PSA as an early treatment response marker. A recently published study by Lorente et al.49 showed that a 30% CTC decline after treatment from an initial unfavorable CTC count (≥5 cells per 7.5 ml blood) is independently associated with OS after abiraterone/chemotherapy in patients with CRPC. These results were confirmed in several other studies.22,26,50,51,52,53
Heck et al.54 recently developed a prognostic model derived from PCa-enhanced transcripts in whole blood of CRPC patients and explored its applicability as a surrogate of treatment response. They showed that an unfavorable 2-gene panel (2GP) (meaning: ≥1 marker positive) correlates with poor OS. A conversion to favorable 2GP during treatment correlated with improved OS, PSA-PFS, and clinical PFS.
The characterization of CTC significantly broadens the options for their use in patients with PC as a sole enumeration provides only limited information. By limiting CTC analysis on their quantification, molecular features of these cells with potential predictive value for the selection of the most promising treatment for individual patients are ignored. This led to the shift of the research focus from pure CTC quantification toward qualitative analyses. In the dense therapeutic landscape of the mCRPC, molecular analyses of CTC may be a key step for the implementation of personalized treatment strategies.
In the context of CTC characterization in mCRPC, the most intensively evaluated target is the androgen receptor (AR):
Miyamoto et al.55 studied the transition of AR phenotypes in CTC under different therapies and in different disease stages. They showed that the AR phenotype of CTC was highly heterogeneous with a majority being of the “AR off” phenotype (PSAneg, PSMApos). In hormone-naοve patients, “AR-on” cells (immunofluorescence: PSApos, PSMAneg) are dominant. Most of the “AR-on” CTC changed into “AR-off” after androgen deprivation therapy. After the onset of CRPC, an increasing quantity of “AR-mixed” (PSApos, PSMApos) and “AR-on” cells could be observed. The increase of these cells during abiraterone therapy was associated with a decreased OS.
de Laere et al.56 demonstrated that in most of CRPC patients, AR pertubations can be detected.56 In 50% of the patients, intra-AR structural variations were present, including AR variants (AR-V) in most of the patients. AR-V positive patients express multiple AR-V, and in most of the cases, AR-V3 could be found. The presence of any AR-V was associated with PFS after the second-line endocrine treatment.
AR splice variant 7 (AR-V7) is coding for a truncated and constitutively active AR and shows a higher transactivating activity than a full-length AR. Various studies have evaluated the potential predictive value of CTC by investigating the association between the presence of AR-V7 and treatment outcome. The presence of AR-V7 in CTC was highly predictive for resistance to anti-AR treatments.23,24 While Antonarakis et al.23 could prove a predictive value of AR-V7 concerning an abiraterone/enzalutamide resistance, the same group could demonstrate that an AR-V7 expression is not predictive for resistance to taxanes.57 In the latter cohort, the PFS of AR-V7pos patients was comparable to AR-V7neg. AR-V7pos patients treated with taxanes showed a longer PFS compared to patients treated with abiraterone or enzalutamide. Because the enzalutamide/abiraterone group was taken from the first study, this kind of comparison between two different cohorts has to be interpreted carefully. In a recent update from the Baltimore group the correlation of the presence of AR-V7 positivity with response to abiraterone or enzalutamide could be confirmed. However, in this larger cohort, a considerable proportion of AR-V7 positive patients (13.9%) showed a PSA response.58 In these studies, the Adnatest system was used for CTC- and AR-V7-detection. Recently, the analytical validation of AR-V7 analysis in a Clinical Laboratory Improvement Amendments (CLIA) setting has been published showing the stable performance of the assay.59
Onstenk et al.60 measured the AR-V7 expression in patients after they have been treated with at least docetaxel and starting with cabazitaxel. Their hypothesis was that treatment with AR-independent mechanisms (such as cabazitaxel) remains effective, due to the lack of any dependency to the missing ligand-binding domain in AR-V7. AR-V7 presence was detected in 55% of the patients and was more frequent in patients who had received abiraterone before (100% vs 35%). In terms of CTC- response rate (RR) or PSA-RR no association could be found between AR-V7 presence in baseline CTC and response to cabazitaxel. OS was not impacted by the presence of AR-V7.
Todenhofer et al.34 used whole blood mRNA to detect AR-V7 mRNA in patients treated with abiraterone. Detection of AR-V7 transcripts was associated with inferior outcomes. AR-V7pos patients had a PSA RR of 0%. These results confirm the potential usefulness of AR-V7 as a prognostic and predictive biomarker for mCRPC using an alternative approach.
A predictive ability of AR-V7 as biomarker in CTC has been also observed by Scher et al.61 They showed that CTC nuclear expression of the AR-V7 protein in men with mCRPC as a treatment-specific biomarker is associated with superior survival on taxane therapy over ARS-directed therapy using the EpicScience platform.61 In summary, recent studies indicate that AR-V7 has an impact on the response to enzalutamide and abiraterone but does not impact the effect of chemotherapy and can be detected in CTC.
Gene fusion between transmembrane protease, serine 2 (TMPRSS2), and erythoblastosis virus E26 oncogene homolog gene (ERG) seems to represent a prostate-specific alteration in patients with advanced PCa.62 This androgen-driven expression of the ERG oncogene after fusion with TMPRSS2 occurs in 30%–70% of therapy-naοve prostate cancers.63 The results concerning the prognostic role of this fusion remain inconsistent: in 41 patients treated with abiraterone after docetaxel failure, Danila et al.33 analyzed a TMPRSS2-ERG status. They could not show that TMPRSS2-ERG status was able to predict PSA response or other clinical outcome parameters. Attard et al.64 could show an association of ERG rearrangements with the magnitude of PSA response. However, this analysis correlates ERG status not only from CTC, but also from primary tumor tissue and metastatic lesions.
Reig et al.65 analyzed TMPRSS2-ERG expression in a peripheral blood mononuclear cell (PBMC) of 72 patients treated with taxanes. They observed a significantly worse PSA-PFS in patients positive for TMPRSS2-ERG. Because this study did not include an enrichment of CTC, it is not comparable to the previous studies, which means that until now it is not yet possible to draw a conclusion of the predictive value of ERG rearrangements in CTC.
As with ERG, the value of tensin homolog (PTEN) loss in CTC as a potential marker of therapy resistance remains unclear. Loss of tumor-suppressor phosphatase and PTEN is frequently associated with ERG rearrangements and frequently occurs in CRPC progression.63 To date, there are only limited data on the clinical value of PTEN analyses in CTC. A correlation between PTEN status and survival was found by Punnoose et al.11
In contrast to localized PCa, several studies have already demonstrated an added clinical value of CTC analysis in metastatic PCa. It could be shown that a favorable CTC count is a prognostic marker for improved PFS and OS in metastatic PCa, whereas no clear association between outcome and CTC count could be found in localized disease.
At present, the research focus is shifting away from quantitative analysis toward molecular characterization to evaluate if molecular information of CTC can be used as a predictive marker for different therapy strategies in the complex therapeutic landscape of mCRPC.
FUTURE PERSPECTIVES
The recent progress in the development of CTC isolation and characterization techniques makes it likely that the information obtained by analyzing CTC will increase in the future. Beside the enumeration and molecular analysis of CTC on cellular level, one promising approach for the future might be in vitro cultivation of CTC. In vitro grown cells, as molecular copy of the tumor and metastases in vivo might be a key step for the diagnostic and therapeutic future of PCa, although it is known that in vitro conditions differ from the microenvironment in the blood. Nevertheless, drug sensitivity of tumor-specific tissue might be tested ex vivo as a step toward individualized treatment.
Gao et al.66 described the successful long-term culture of CTC from patients with advanced PCa; however, future improved methods for isolation and enrichment of CTC are required to guarantee viable, for cultivation feasible, CTC. Xenograft models using CTC for further in vivo drug testing may then provide a valuable tool for investigating the individual responsiveness of a tumor.
As the sequencing of single cells (e.g., CTC) is challenging, circulating DNA fragments from tumor cells (so-called circulating tumor DNA [ctDNA]) has been discussed as an alternative approach to obtain molecular information on tumor-associated DNA alterations in metastatic patients.67 ctDNA is composed of small fragments of the nucleic acid of both nuclear and mitochondrial DNA and is not associated with cells or cell fragments.68 This DNA contains coding and noncoding DNA-sequences and can be used to analyze microsatellite instability, mutations, methylation, polymorphisms and loss of heterozygosity and DNA integrity.68,69 In recent studies, analysis of ctDNA has been used to assess AR alterations in patients with CRPC. Azad et al.70 studied patients progressing after abiraterone, enzalutamide, or other agents. They could show that in those switching to enzalutamide, a higher copy number or mutation in AR exon 8 meant lower PSA-RR.
The results of Romanel et al.71 are in accordance to this, showing that the presence of specific mutations of the AR and an increased number of AR copies were associated with abiraterone resistance in terms of PSA response.
Wyatt et al.72 used cfDNA to detect copy number variations and mutations in AR and other PCa associated genes. They could show, that detection of PCa associated genes such as MYC proto-oncogene (MYC) (MYC gain), retinoblastoma protein 1 (RB1) (RB1 loss), tyrosine-protein kinase Met (MET) (MET gain) was associated with poor PFS in 65 patients receiving enzalutamide. Analyses and monitoring of cfDNA, therefore, might be an attractive alternative to CTC, although, to date, most of the available studies address CTC and not cfDNA.
CONCLUSIONS
Although the number of studies including CTC analysis has been constantly increased during the last decade, the optimal use of this biomarker in patients with PCa is still unclear. Although correlations of CTC numbers with outcome have been reported, CTC numbers and their changes are still not recommended to serve as parameters for clinical decision making in patients with PCa. The recent developments in the molecular analysis of CTC have significantly broadened the options for the use of CTC as biomarkers. The recent discovery of AR-V7 as a predictor of outcome response for treatment with abiraterone/enzalutamide led to a significantly increased interest in the discovery of predictive biomarkers. Beside CTC, circulating tumor DNA enables the noninvasive characterization of CTC and first studies indicate a potential role as markers used for precision oncology.
Although the CellSearch® system has been approved by the FDA, the use of analysis of CTC is limited in patients with PCa and has not been recommended yet in current guidelines for daily clinical practice. To promote the use of CTC analysis in standard practice, a clear influence of CTC test results on clinical decision making should be demonstrated. The identification of AR-V7 as a potential marker of resistance for treatment with abiraterone and enzalutamide has significantly promoted the interest in CTC as a tool for promoting personalized treatment in PCa patients. However, further data on the clinical relevance of AR-V7 positive CTC and the optimal technique for determination is required before being implemented in daily clinical practice.
AUTHOR CONTRIBUTIONS
MM was mainly responsible for data acquisition, drafting the manuscript and setup of tables. TT contributed to the planning of the manuscript, data acquisition, and writing of the manuscript. MH, SR, and JB contributed to data acquisition and reviewing the manuscript. AS was responsible for the planning of the review, selection of subtopics, and final review of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
Dr. T Todenhöfer has received research funds from Astellas. Prof. A Stenzl has served as consultant for Astellas.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Arya M, Bott SR, Shergill IS, Ahmed HU, Williamson M, et al. The metastatic cascade in prostate cancer. Surg Oncol. 2006;15:117–28. doi: 10.1016/j.suronc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Muller V, Stahmann N, Riethdorf S, Rau T, Zabel T, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005;11:3678–85. doi: 10.1158/1078-0432.CCR-04-2469. [DOI] [PubMed] [Google Scholar]
- 4.Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–44. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano M, Herrera S, Christiny P, Shaw C, Creighton CJ, et al. Circulating and disseminated tumor cells from breast cancer patient-derived xenograft-bearing mice as a novel model to study metastasis. Breast Cancer Res. 2015;17:3. doi: 10.1186/s13058-014-0508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho FL, Simons BW, Antonarakis ES, Rasheed Z, Douglas N, et al. Tumorigenic potential of circulating prostate tumor cells. Oncotarget. 2013;4:413–21. doi: 10.18632/oncotarget.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–9. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–8. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 10.Onstenk W, de Klaver W, de Wit R, Lolkema M, Foekens J, et al. The use of circulating tumor cells in guiding treatment decisions for patients with metastatic castration-resistant prostate cancer. Cancer Treat Rev. 2016;46:42–50. doi: 10.1016/j.ctrv.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Punnoose EA, Ferraldeschi R, Szafer-Glusman E, Tucker EK, Mohan S, et al. PTEN loss in circulating tumour cells correlates with PTEN loss in fresh tumour tissue from castration-resistant prostate cancer patients. Br J Cancer. 2015;113:1225–33. doi: 10.1038/bjc.2015.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helzer KT, Barnes HE, Day L, Harvey J, Billings PR, et al. Circulating tumor cells are transcriptionally similar to the primary tumor in a murine prostate model. Cancer Res. 2009;69:7860–6. doi: 10.1158/0008-5472.CAN-09-0801. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt H, DeAngelis G, Eltze E, Gockel I, Semjonow A, et al. Asynchronous growth of prostate cancer is reflected by circulating tumor cells delivered from distinct, even small foci, harboring loss of heterozygosity of the PTEN gene. Cancer Res. 2006;66:8959–65. doi: 10.1158/0008-5472.CAN-06-1722. [DOI] [PubMed] [Google Scholar]
- 14.Dago AE, Stepansky A, Carlsson A, Luttgen M, Kendall J, et al. Rapid phenotypic and genomic change in response to therapeutic pressure in prostate cancer inferred by high content analysis of single circulating tumor cells. PLoS One. 2014;9:e101777. doi: 10.1371/journal.pone.0101777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paris PL, Kobayashi Y, Zhao Q, Zeng W, Sridharan S, et al. Functional phenotyping and genotyping of circulating tumor cells from patients with castration resistant prostate cancer. Cancer Lett. 2009;277:164–73. doi: 10.1016/j.canlet.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Lohr JG, Adalsteinsson VA, Cibulskis K, Choudhury AD, Rosenberg M, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014;32:479–84. doi: 10.1038/nbt.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2:25ra3. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623–31. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 19.Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–62. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 20.Banys-Paluchowski M, Krawczyk N, Fehm T. Potential role of circulating tumor cell detection and monitoring in breast cancer: a review of current evidence. Front Oncol. 2016;6:255. doi: 10.3389/fonc.2016.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Economopoulou P, Georgoulias V, Kotsakis A. Classifying circulating tumor cells to monitor cancer progression. Expert Rev Mol Diag. 2017;17:153–65. doi: 10.1080/14737159.2017.1275572. [DOI] [PubMed] [Google Scholar]
- 22.Thalgott M, Rack B, Maurer T, Souvatzoglou M, Eiber M, et al. Detection of circulating tumor cells in different stages of prostate cancer. J Cancer Res Clin Oncol. 2013;139:755–63. doi: 10.1007/s00432-013-1377-5. [DOI] [PubMed] [Google Scholar]
- 23.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinestel J, Luedeke M, Arndt A, Schnoeller TJ, Lennerz JK, et al. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget. 2015 doi: 10.18632/oncotarget.3925. doi: 10.18632/oncotarget.3925 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alix-Panabieres C, Vendrell JP, Pelle O, Rebillard X, Riethdorf S, et al. Detection and characterization of putative metastatic precursor cells in cancer patients. Clin Chem. 2007;53:537–9. doi: 10.1373/clinchem.2006.079509. [DOI] [PubMed] [Google Scholar]
- 26.Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the cellsearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attard G, de Bono JS. Utilizing circulating tumor cells: challenges and pitfalls. Curr Opin Genet Dev. 2011;21:50–8. doi: 10.1016/j.gde.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Crespo M, van Dalum G, Ferraldeschi R, Zafeiriou Z, Sideris S, et al. Androgen receptor expression in circulating tumour cells from castration-resistant prostate cancer patients treated with novel endocrine agents. Br J Cancer. 2015;112:1166–74. doi: 10.1038/bjc.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltran H, Jendrisak A, Landers M, Mosquera JM, Kossai M, et al. The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clin Cancer Res. 2016;22:1510–9. doi: 10.1158/1078-0432.CCR-15-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joung JY, Cho KS, Kim JE, Seo HK, Chung J, et al. Prostate stem cell antigen mRNA in peripheral blood as a potential predictor of biochemical recurrence in high-risk prostate cancer. J Surg Oncol. 2010;101:145–8. doi: 10.1002/jso.21445. [DOI] [PubMed] [Google Scholar]
- 32.Todenhofer T, Hennenlotter J, Feyerabend S, Aufderklamm S, Mischinger J, et al. Preliminary experience on the use of the Adnatest ® system for detection of circulating tumor cells in prostate cancer patients. Anticancer Res. 2012;32:3507–13. [PubMed] [Google Scholar]
- 33.Danila DC, Anand A, Sung CC, Heller G, Leversha MA, et al. TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur Urol. 2011;60:897–904. doi: 10.1016/j.eururo.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todenhofer T, Azad A, Stewart C, Gao J, Eigl BJ, et al. AR-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. J Urol. 2017;197:135–42. doi: 10.1016/j.juro.2016.06.094. [DOI] [PubMed] [Google Scholar]
- 35.Greene SB, Dago AE, Leitz LJ, Wang Y, Lee J, et al. Chromosomal instability estimation based on next generation sequencing and single cell genome wide copy number variation analysis. PLoS One. 2016;11:e0165089. doi: 10.1371/journal.pone.0165089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin X, Park S, Duffy SP, Matthews K, Ang RR, et al. Size and deformability based separation of circulating tumor cells from castrate resistant prostate cancer patients using resettable cell traps. Lab Chip. 2015;15:2278–86. doi: 10.1039/c5lc00226e. [DOI] [PubMed] [Google Scholar]
- 37.De Giorgi V, Pinzani P, Salvianti F, Panelos J, Paglierani M, et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol. 2010;130:2440–7. doi: 10.1038/jid.2010.141. [DOI] [PubMed] [Google Scholar]
- 38.Todenhofer T, Park ES, Duffy S, Deng X, Jin C, et al. Microfluidic enrichment of circulating tumor cells in patients with clinically localized prostate cancer. Urol Oncol. 2016;34:483. doi: 10.1016/j.urolonc.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 40.Davis JW, Nakanishi H, Kumar VS, Bhadkamkar VA, McCormack R et al. Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: initial results in early prostate cancer. J Urol. 2008;179:2187–91. doi: 10.1016/j.juro.2008.01.102. [DOI] [PubMed] [Google Scholar]
- 41.Thalgott M, Rack B, Horn T, Heck MM, Eiber M, et al. Detection of circulating tumor cells in locally advanced high-risk prostate cancer during neoadjuvant chemotherapy and radical prostatectomy. Anticancer Res. 2015;35:5679–85. [PubMed] [Google Scholar]
- 42.Meyer CP, Pantel K, Tennstedt P, Stroelin P, Schlomm T, et al. Limited prognostic value of preoperative circulating tumor cells for early biochemical recurrence in patients with localized prostate cancer. Urol Oncol. 2016;34:235. doi: 10.1016/j.urolonc.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Tsumura H, Satoh T, Ishiyama H, Tabata KI, Takenaka K, et al. Perioperative search for circulating tumor cells in patients undergoing prostate brachytherapy for clinically nonmetastatic prostate cancer. Int J Mol Sci. 2017;18:128. doi: 10.3390/ijms18010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helo P, Cronin AM, Danila DC, Wenske S, Gonzalez-Espinoza R, et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: concordance with CellSearch assay and association with bone metastases and with survival. Clin Chem. 2009;55:765–73. doi: 10.1373/clinchem.2008.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bianco FJ, Jr, Powell IJ, Cher ML, Wood DP., Jr Presence of circulating prostate cancer cells in African American males adversely affects survival. Urol Oncol. 2002;7:147–52. doi: 10.1016/s1078-1439(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 46.Jiang R, Lu YT, Ho H, Li B, Chen JF, et al. A comparison of isolated circulating tumor cells and tissue biopsies using whole-genome sequencing in prostate cancer. Oncotarget. 2015;6:44781–93. doi: 10.18632/oncotarget.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 48.Olmos D, Arkenau HT, Ang JE, Ledaki I, Attard G, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol. 2009;20:27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]
- 49.Lorente D, Olmos D, Mateo J, Bianchini D, Seed G, et al. Decline in circulating tumor cell count and treatment outcome in advanced prostate cancer. Eur Urol. 2016;70:985–92. doi: 10.1016/j.eururo.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okegawa T, Itaya N, Hara H, Tambo M, Nutahara K. Circulating tumor cells as a biomarker predictive of sensitivity to docetaxel chemotherapy in patients with castration-resistant prostate cancer. Anticancer Res. 2014;34:6705–10. [PubMed] [Google Scholar]
- 51.Vogelzang NJ, Fizazi K, Burke JM, De Wit R, Bellmunt J, et al. Circulating tumor cells in a phase 3 study of docetaxel and prednisone with or without lenalidomide in metastatic castration-resistant prostate cancer. Eur Urol. 2017;71:168–71. doi: 10.1016/j.eururo.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 52.Chang K, Kong YY, Dai B, Ye DW, Qu YY, et al. Combination of circulating tumor cell enumeration and tumor marker detection in predicting prognosis and treatment effect in metastatic castration-resistant prostate cancer. Oncotarget. 2015;6:41825–36. doi: 10.18632/oncotarget.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bitting RL, Healy P, Halabi S, George DJ, Goodin M, et al. Clinical phenotypes associated with circulating tumor cell enumeration in metastatic castration-resistant prostate cancer. Urol Oncol. 2015;33:e1–9. doi: 10.1016/j.urolonc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Heck MM, Thalgott M, Schmid SC, Oh WK, Gong Y, et al. A 2-gene panel derived from prostate cancer-enhanced transcripts in whole blood is prognostic for survival and predicts treatment benefit in metastatic castration-resistant prostate cancer. Prostate. 2016;76:1160–8. doi: 10.1002/pros.23202. [DOI] [PubMed] [Google Scholar]
- 55.Miyamoto DT, Lee RJ, Stott SL, Ting DT, Wittner BS, et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2012;2:995–1003. doi: 10.1158/2159-8290.CD-12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Laere B, van Dam PJ, Whitington T, Mayrhofer M, Diaz EH, et al. Comprehensive profiling of the androgen receptor in liquid biopsies from castration-resistant prostate cancer reveals novel Intra-AR structural variation and splice variant expression patterns. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.01.011. doi: 10.1016/j.eururo. 2017.01.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–91. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. J Clin Oncol. 2017;35:2149–56. doi: 10.1200/JCO.2016.70.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lokhandwala PM, Riel SL, Haley L, Lu C, Chen Y, et al. Analytical validation of androgen receptor splice variant 7 detection in a clinical laboratory improvement amendments (CLIA) laboratory setting. J Mol Diagn. 2017;19:115–25. doi: 10.1016/j.jmoldx.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJ, et al. Efficacy of Cabazitaxel in Castration-resistant Prostate Cancer Is Independent of the Presence of AR-V7 in Circulating Tumor Cells. Eur Urol. 2015;68:939–45. doi: 10.1016/j.eururo.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2:1441–9. doi: 10.1001/jamaoncol.2016.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scheble VJ, Braun M, Beroukhim R, Mermel CH, Ruiz C, et al. ERG rearrangement is specific to prostate cancer and does not occur in any other common tumor. Mod Pathol. 2010;23:1061–7. doi: 10.1038/modpathol.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barbieri CE, Bangma CH, Bjartell A, Catto JW, Culig Z, et al. The mutational landscape of prostate cancer. Eur Urol. 2013;64:567–76. doi: 10.1016/j.eururo.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–8. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 65.Reig O, Marin-Aguilera M, Carrera G, Jimenez N, Pare L, et al. TMPRSS2-ERG in blood and docetaxel resistance in metastatic castration-resistant prostate cancer. Eur Urol. 2016;70:709–13. doi: 10.1016/j.eururo.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 66.Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–87. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta. 2001;313:139–42. doi: 10.1016/s0009-8981(01)00665-9. [DOI] [PubMed] [Google Scholar]
- 69.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–37. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 70.Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clinical Canc Res. 2015;21:2315–24. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- 71.Romanel A, Gasi Tandefelt D, Conteduca V, Jayaram A, Casiraghi N, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re10. doi: 10.1126/scitranslmed.aac9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyatt AW, Azad AA, Volik SV, Annala M, Beja K, et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. 2016;2:1598–606. doi: 10.1001/jamaoncol.2016.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pal SK, He M, Wilson T, Liu X, Zhang K, et al. Detection and phenotyping of circulating tumor cells in high-risk localized prostate cancer. Clin Genitourin Cancer. 2015;13:130–6. doi: 10.1016/j.clgc.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thalgott M, Rack B, Eiber M, Souvatzoglou M, Heck MM, et al. Categorical versus continuous circulating tumor cell enumeration as early surrogate marker for therapy response and prognosis during docetaxel therapy in metastatic prostate cancer patients. BMC Cancer. 2015;15:458. doi: 10.1186/s12885-015-1478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakazawa M, Lu C, Chen Y, Paller CJ, Carducci MA, et al. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol. 2015;26:1859–65. doi: 10.1093/annonc/mdv282. [DOI] [PMC free article] [PubMed] [Google Scholar]


