Abstract
This study comprises a systematic review and meta.analysis of microsurgical vasoepididymostomy outcomes in epididymal obstructive azoospermia. A comprehensive literature search was performed using Medline, Embase, and the Cochrane library that included all studies related to microsurgical vasoepididymostomy. Keywords included “vasoepididymostomy” “epididymovasostomy” “epididymal obstruction” and “epididymis obstruction” Event rate and risk ratio (RR) were estimated. Patency rate and pregnancy rate were investigated. The analysis comprised 1422 articles, including 42 observational studies with 2298 enrolled patients performed from November 1978 to January 2017. The overall mean patency rate was 64.1% (95% confidence interval [CI]: 58.5%.69.3%; I2=83.0%), and the overall mean pregnancy rate was 31.1% (95% CI: 26.9%.35.7%; I2=73.0%). We performed a meta-analysis comparing the patency rate of bilateral microsurgical vasoepididymostomy and unilateral microsurgical vasoepididymostomy and found an RR of 1.38% (95% CI: 1.21%–1.57%; P < 0.00001). A comparison of the site of microsurgical vasoepididymostomy showed that caudal or corpus area was favorable for patency rate (RR = 1.17%; 95% CI: 1.01%–1.35%; P = 0.04). Patients with motile sperm in epididymal fluid exhibited an RR of 1.53% (95% CI: 1.11%–2.13%; P = 0.01) with respect to patency rate. Microsurgical vasoepididymostomy is an effective treatment for epididymal obstructive azoospermia that can improve male fertility. We find that performing microsurgical vasoepididymostomy bilaterally, anastomosing a larger caudal area, and containing motile sperm in epididymis fluid can potentially achieve a superior patency rate.
Keywords: azoospermia, meta-analysis, vasoepididymostomy
INTRODUCTION
Obstructive azoospermia is associated with obstruction of the vas deferens, epididymis, or ejaculatory duct system. Epididymal obstruction is the second most common cause of obstructive azoospermia behind epididymal infection, which is considered to be the most frequent cause of the acquired forms.1
Surgery, specifically microsurgical vasoepididymostomy (MVE), is the treatment of choice for epididymal obstructive azoospermia (EOA) patients. Because of rapid developments in assisted reproductive technology (ART), especially in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in combination with sperm extraction from testis or epididymis, patients with azoospermia who were previously untreatable can now achieve fertility. However, it is important to comprehensively evaluate the cause of male infertility prior to IVF-ICSI. Without careful evaluation, IVF-ICSI may result in increased medical costs for patients and involve additional risks for the female partner, such as ovarian hyperstimulation syndrome (OHSS), and for the fetus, including multiple gestations, prematurity, and genetic abnormalities.2,3
MVE is technically the most challenging procedure of all urological microsurgeries, and in many fertility centers it is also highly dependent on ART. Therefore, a comprehensive evaluation is required to assess the effectiveness of MVE.
The objective of this study is to systematically review the evidence supporting MVE in treating EOA and to provide a meta-analysis of its effectiveness. To the best of our knowledge, this is the first systematic review and meta-analysis of MVE in patients with EOA.
MATERIALS AND METHODS
Literature search
We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses recommendations) and MOOSE Guidelines for Meta-analyses and Systematic Reviews of Observational studies.
We performed a comprehensive literature search using Medline, Embase, and the Cochrane library that included all studies related to MVE from November 1978 to January 2017. The search term was (“vasoepididymostomy” [All Fields]) OR (“epididymovasostomy” [All Fields]) OR (“epididymal obstruction” [All Fields]) OR (“epididymis obstruction” [All Fields]). Event rate and risk ratio (RR) were estimated using a random-effects model. Heterogeneity was investigated using the Q statistic and I2 values.
Inclusion and exclusion criteria and outcome measures
We included articles that evaluated the effects of MVE on patency rate or pregnancy rate, as well as those that compared different surgical techniques and different intraoperative methods during MVE. We excluded articles not written in English, animal studies, review articles, letters, and one article including a majority of vasovasostomy cases.
Included studies were independently selected by two investigators (DK Kim and YE Yoon). Any disagreements were discussed by the two investigators and resolved by consensus with HH Lee. We extracted the following data from the retrieved studies: author's name, publication year, country, sample size, follow-up period, diagnostic criteria for patency, surgical technique, patency rate, and pregnancy rate. We also extracted intraoperative findings, which included bilateral vs unilateral anastomosis, anastomosis location in the epididymis, and presence or absence of motile sperm in the epididymis.
Quality assessment
Inclusion of studies in the meta-analysis comparing patency rate and pregnancy rate after MVE was determined using the Quality Assessment Tool for Before-After (Pre-Post) Studies with no Control Group (available at https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular risk-reduction/tools/before-after), which considers 11 “yes/no” items and gives 1 point for each affirmative answer. The total scores of each study were converted into a quality rank between 0 and 1 by dividing each score by the score of the highest scoring study in the group. The quality of each noncontrol study, based on study characteristics of “no control group” obstructive azoospermia patients who underwent MVE, was also assessed using the same tool.
Statistical analyses
The meta-analysis was performed using RevMan 5.3 (Cochrane Community, London, UK) and Comprehensive Meta-Analysis 3.0 (CMA; Biostat, Englewood, NJ, USA). Event rates or RR and 95% confidence interval (CI) for dichotomous variables were investigated. The Mantel–Haenszel random-effects model was used due to heterogeneity of included studies. All P values are two-sided, and P < 0.05 was considered statistically significant.
The Q statistic was used to test between-study homogeneity. Homogeneity was rejected in cases where the Q statistic P value was <0.10.
Forest plots were used to show the effects of MVE on patency rates and pregnancy rates. Forest plots contain a pooled estimate of the effect (event rate or RR) as a dashed vertical line with a diamond at the bottom representing the 95% CI, and individual studies are represented as squares with their CIs such that the surface of the square is proportional to the weight of the study.
RESULTS
Eligible studies
Figure 1 shows the PRISMA flow chart depicting the identification of studies according to their inclusion in the meta-analysis. We initially identified a total of 1422 articles; after deleting duplications, we analyzed abstracts of 895 articles. Subsequently, we excluded 143 abstracts, 106 review articles, 51 letters or editorials, 144 animal studies, and 4 nonEnglish studies. After review of 447 abstracts that matched our inclusion and exclusion criteria, we then assessed the full text and each of the remaining 62 articles for eligibility. Of these, 20 articles were excluded: 13 studies in which the main text was not English; 6 that focused mainly on vasovasostomy, and 1 that reported neither patency rate nor pregnancy rate. Ultimately, we included 42 observational studies (9 prospective cohort studies and 33 retrospective cohort studies) with 2298 patients in qualitative as well as quantitative synthesis in the meta-analysis (Figure 1 and Supplementary Table 1 (7.4MB, tif) ). The publication interval was from 1978 to 2017, and cohort sizes ranged from 6 to 249 patients. The study participants were from various countries, including the United States (14 studies), China (11 studies), Japan (4 studies), India (2 studies), Sweden (2 studies), Korea (2 studies), Canada (1 study), Italy (1 study), Belgium (1 study), Saudi Arabia (1 study), Romania (1 study), Slovenia (1 study), and Egypt (1 study).
Figure 1.
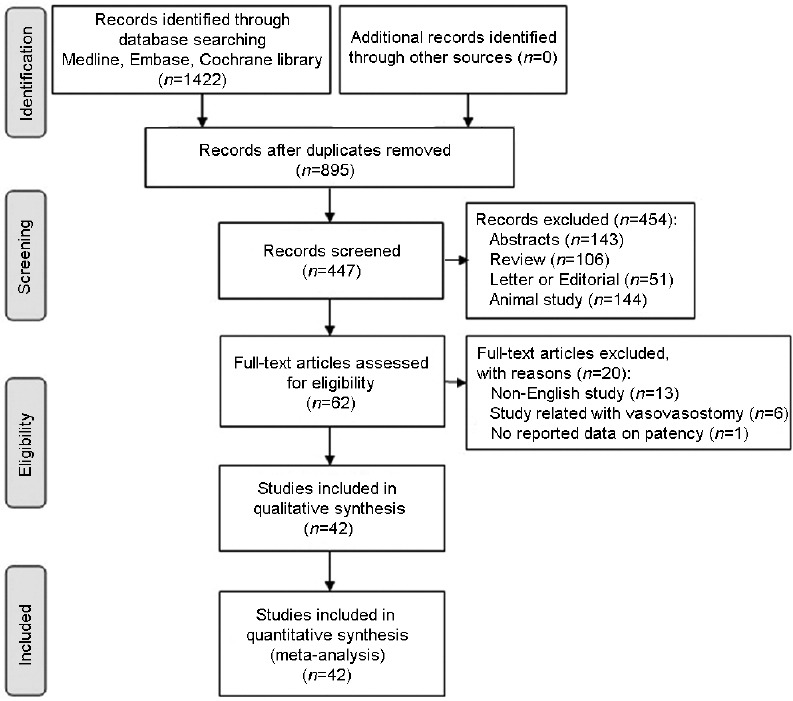
Flow diagram of articles included in the systematic review and meta-analysis.
Characteristics of the studies included in the systematic review
Quality assessment and publication bias
Supplementary Table 1 (7.4MB, tif) presents the quality of each article, as measured by The Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group final results. The median quality score was 7 (interquartile range 6–8) out of a total possible score of 11.
We investigated the possibility of publication bias. As shown in Figure 2, funnel plots showed no publication bias with respect to overall patency rate (P = 0.26, Egger's test) or overall pregnancy rate (P = 0.2, Egger's test).
Figure 2.
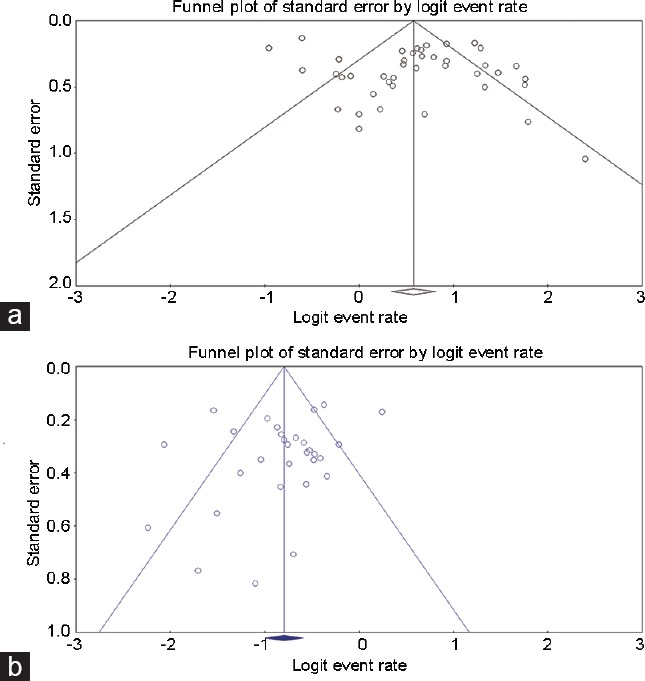
Funnel plots for patency group and pregnancy group. (a) Patency group; (b) pregnancy group.
Overall patency rate after MVE
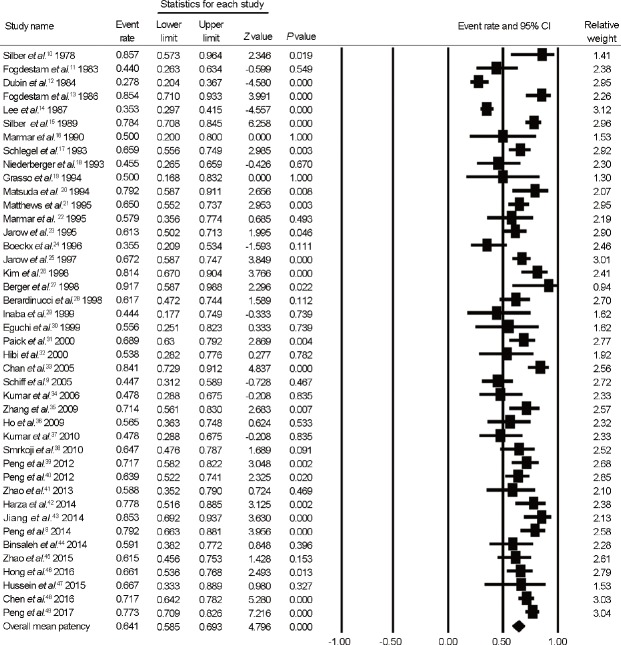
We included all 42 observational studies in analyses of patency rate after MVE. As shown in Figure 3, which presents forest plots of patency rate after MVE, the overall mean patency rate, calculated as the mean weighted by sample size, was 64.1% (95% [CI]: 58.5%–69.3%; I2 = 83.0%).
Figure 3.
Forest plots of overall patency rate after MVE. MVE: microsurgical vasoepididymostomy; CI: confidence interval.
Mean time to reach patency was from 2.8 months to 9.6 months (Supplementary Table 1 (7.4MB, tif) ). However, the definition of patency varied among studies. Fifteen studies did not specifically define patency; 10 defined it as the presence of spermatozoa; and 4 defined it as the presence of motile spermatozoa. Other studies defined patency in terms of the following threshold spermatozoa concentrations: >104 spermatozoa per ml (5 studies); >105 spermatozoa per ml (3 studies); >106 spermatozoa per ml (1 study); >107 spermatozoa per ml (3 studies); and >108 spermatozoa per ml (1 study).
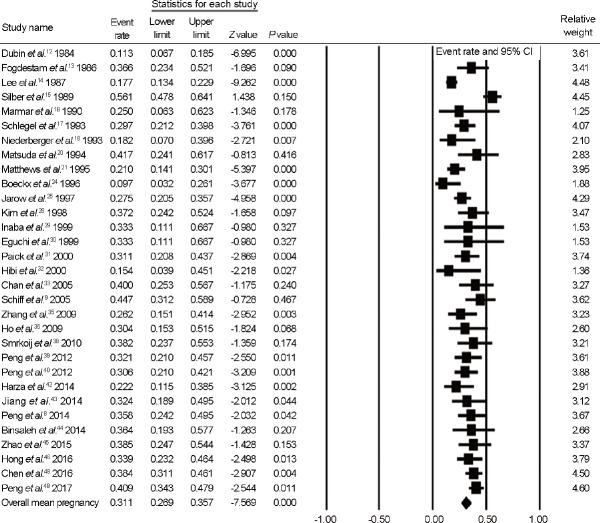
Pregnancy rate after MVE
A total of 31 observational studies (7 prospective cohort studies and 24 retrospective cohort studies) were included in analysis of pregnancy rate after MVE. As shown in Figure 4, which presents forest plots of pregnancy rate after MVE, the overall mean pregnancy rate, calculated as the mean weighted by sample size, was 31.1% (95% CI, 26.9%–35.7%; I2 = 73.0%). Mean time to reach pregnancy was from 6.9 months to 9.9 months (Supplementary Table 1 (7.4MB, tif) ). However, not all studies clearly indicated whether the definition of pregnancy was biochemical pregnancy or clinical pregnancy detected by ultrasonography.
Figure 4.
Forest plots of overall pregnancy rate after MVE. MVE: microsurgical vasoepididymostomy; CI: confidence interval.
Outcomes related to MVE surgical technique
Details of surgical techniques were clearly described in all studies included in the analysis. From 1978 to 2005, majority of EOA surgeries utilized the end-to-end or end-to-side technique. After 2005, the majority of studies employed the intussusception technique.
The patency rate for end-to-end/end-to-side and intussusception techniques were 61.1% (95% CI, 52.4%–69.2%; I2 = 86.3%) and 69.1% (95% CI, 64.1%–73.8%; I2 = 54.5%), respectively (Supplementary Figure 1 (854.8KB, tif) ). The pregnancy rates for end-to-end/end-to-side and intussusception techniques were 26.9% (95% CI, 20.1%–35.1%; I2 = 83.3%) and 35.9% (95% CI, 32.8%–39.2%; I2 = 54.5%), respectively (Supplementary Figure 2 (890KB, tif) ). Thus, introduction of the newer intussusception surgical technique improved both patency rate and pregnancy rate after MVE.
Forest plots of patency rates compared by surgical techniques. (a) patency rates by end-to-end/end-to-side technique; (b) patency rates by intussusception technique.
Forest plots of pregnancy rates compared by surgical techniques. (a) pregnancy rates by end-to-end/end-to-side technique; (b) pregnancy rates by intussusception technique.
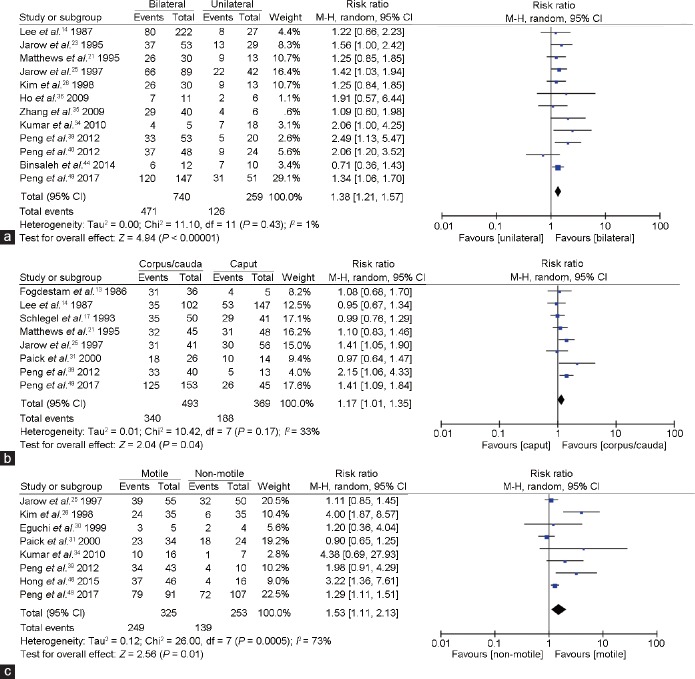
Figure 5 presents forest plots of intraoperative findings after MVE. Twelve articles analyzed the patency rate after MVE, according to use of bilateral or unilateral anastomosis techniques. Compared with the unilateral group, the patency rate of patients in the bilateral MVE group exhibited higher RR of 1.38% (95% CI, 1.21%–1.57%; P < 0.00001).
Figure 5.
Forest plots of patency rate (a) comparing unilateral and bilateral MVE, (b) comparing anastomosis location after MVE, and (c) comparing motility of epididymal fluid after MVE. MVE: microsurgical vasoepididymostomy; CI: confidence interval.
Eight studies analyzed the patency rate after MVE according to the location of the operated anastomosis. Compared with the caput anastomosis group, the patency rate of patients in cauda or corpus MVE group exhibited a higher RR of 1.17% (95% CI, 1.01%–1.35%; P = 0.04).
Finally, eight studies analyzed the patency rate after MVE according to the intraoperative presence or absence of motile sperm in epididymis fluid. Compared with patients in the non-motile group, patients in the motile-sperm MVE group exhibited higher patency, with RR of 1.53% (95% CI, 1.11%–2.13%; P = 0.01).
DISCUSSION
Our research analysis indicates that MVE is capable of achieving an overall patency rate of 64.1% and an overall pregnancy rate of 31.1% in EOA patients. To the best of our knowledge, this is the first systematic review of MVE in EOA. We note that an earlier study investigated the effect of IVF-ICSI with surgically retrieved epididymal sperm in obstructive azoospermia on fertility, reporting a pregnancy rate of 34%.4
Before the era of IVF, the primary treatment for infertile men with obstructive azoospermia was vasovasostomy or MVE for reconstructable cases. Using IVF, matured oocytes can be aspirated and fertilized in vitro. This effort resulted in the first successful delivery of an IVF child in the United Kingdom in 1978.5 As the ART field progresses in male infertility, couples who had been previously considered irreversibly infertile, such as those eligible for artificial insemination of donor sperm, may now have the opportunity to parent a genetically related child.6 ARTs, such as intra-uterine insemination (IUI), in vitro fertilization and embryo transfer (IVF-ET), and ICSI, can overcome barriers to fertilization. However, surgical treatment of obstructive azoospermia patients remains an important issue because it retains the natural sperm selection during fertilization.
Surgical techniques of MVE have evolved and advanced over the last 40 years. However, the technique remains among the most technically challenging microsurgical procedures in urology. From 1978 to 2004, MVE was primarily performed using end-to-end or end-to-side techniques and yielded patency and pregnancy rates of 61.1% and 26.9%, respectively. Since that time MVE has been predominantly performed using transverse intussusception vasoepididymostomy (TIVE) or longitudinal intussusception vasoepididymostomy (LIVE) techniques. This change in anastomotic techniques has improved both the overall patency rate (69.1%) and pregnancy rate (35.9%).
The goal of this systematic review was to gather published data on MVE to provide improved counsel for EOA patients. We found that the average incidence of postoperative patency across all publications included in our analysis was 64.1% while the average pregnancy rate was 31.1%. However, these results are difficult to interpret because the definition of patency varies among studies. The heterogeneity in definitions and the use of different surgical techniques was a limitation to drawing more definitive conclusions.
Given these interpretative challenges, we investigated improvements in patency rate after MVE by focusing on intraoperative findings. First, in cases of bilateral anastomosis, MVE resulted in significantly higher patency rates compared with unilateral anastomosis cases. Second, anastomoses located to a more caudal area were associated with significantly higher sperm patency rates after MVE. Third, cases in which motile sperm was present in anastomosis areas exhibited significantly higher patency rates. By carefully applying these findings, it should be possible to achieve improved patency and pregnancy rates.
However, even if reconstruction surgery for EOA is carefully performed, it is difficult to compare pregnancy rates following MVE with those for IVF-ICSI for a number of reasons, including female-related fertility issues and the couple's age. Nevertheless, pregnancy through natural intercourse engages physiologic mechanisms that result in natural selection of the best sperm. IVF-ICSI is a complicated technique involving ovarian hyperstimulation, oocyte retrieval, and embryo implantation.7 Pregnancy after MVE avoids the high cost and associated complications of repeated IVF-ICSI, such as OHSS and multiple gestations.8 Even patients who do not achieve natural pregnancy after successful MVE will have additional options, such as IUI and IVF-ICSI using freshly ejaculated sperm instead of sperm retrieved surgically from the testis or epididymis. However, in cases of congenital bilateral agenesis of the vas deferens or intratesticular obstruction, IVF-ICSI with testicular sperm extraction remains the only treatment option.
This study has value as the first systematic review of MVE in EOA. MVE is the most challenging microsurgical technique in urology; thus, the resulting patency rate is dependent on the surgeon's microsurgical skill.9 Continuing efforts on the part of urologists to improve the sperm patency rate and pregnancy rate through MVE are important in managing EOA patients and helping them to achieve natural pregnancy.
We recognize several limitations to the current study. First, only observational studies were included. However, because MVE could create “normozoospermia from azoospermia,” it is impossible and unnecessary to perform a randomized controlled trial to evaluate the effectiveness of MVE. Second, the definition of patency varied among included studies, from the mere presence of spermatozoa to a threshold of more than 108 spermatozoa per ml. Third, the surgical method employed changed over time, with initial studies performing end-to-end MVE and a majority of recent studies performing the LIVE technique. Fourth, there have been significant advances in ART over the preceding years, which make it misleading to compare pregnancy rates from the 1980s with those of the present day. Finally, a majority of included studies did not consider female factors in the pregnancy rate, and none of the included studies reported live birth rates.
CONCLUSIONS
Even in the IVF-ICSI era, MVE remains an effective treatment for EOA that achieves improved male fertility from an azoospermia status, thereby allowing natural selection of healthy sperm. In addition, performing MVE bilaterally, anastomosing a more caudal area, and containing motile sperm in epididymis fluid potentially achieves a superior patency rate.
AUTHOR CONTRIBUTIONS
DKK designed the study. YEY coordinated the study and performed data acquisition. HHL, DKK, YEY, HSM, and SYP participated in collecting and interpreting the data, drafted and critically revised the paper. SHS and DSK reviewed the paper. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea (NRF-2017R1C1B5018097).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, et al. European Association of urology guidelines on male infertility: the 2012 update. Eur Urol. 2012;62:324–32. doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 2.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725–30. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 3.Sutcliffe AG, Ludwig M. Outcome of assisted reproduction. Lancet. 2007;370:351–9. doi: 10.1016/S0140-6736(07)60456-5. [DOI] [PubMed] [Google Scholar]
- 4.Woldringh GH, Kremer JA, Wetzels AM, Meuleman EJ, Ramos L, et al. Obstructive azoospermia in men who wish to father children; initial clinical results of intracytoplasmatic sperm injection (ICSI) with surgically retrieved epididymal semen. Ned Tijdschr Geneeskd. 2003;147:2587–91. Article in Dutch. [PubMed] [Google Scholar]
- 5.Fauser BC, Edwards RG. The early days of IVF. Hum Reprod Update. 2005;11:437–8. doi: 10.1093/humupd/dmi026. [DOI] [PubMed] [Google Scholar]
- 6.Schlegel PN, Girardi SK. Clinical review 87: in vitro fertilization for male factor infertility. J Clin Endocrinol Metab. 1997;82:709–16. doi: 10.1210/jcem.82.3.3785. [DOI] [PubMed] [Google Scholar]
- 7.Boyle KE, Vlahos N, Jarow JP. Assisted reproductive technology in the new millennium: part II. Urology. 2004;63:217–24. doi: 10.1016/j.urology.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Peng J, Yuan Y, Zhang Z, Cui W, Song W, et al. Microsurgical vasoepididymostomy is an effective treatment for azoospermic patients with epididymal obstruction and prior failure to achieve pregnancy by sperm retrieval with intracytoplasmic sperm injection. Hum Reprod. 2014;29:1–7. doi: 10.1093/humrep/det385. [DOI] [PubMed] [Google Scholar]
- 9.Schiff J, Chan P, Li PS, Finkelberg S, Goldstein M. Outcome and late failures compared in 4 techniques of microsurgical vasoepididymostomy in 153 consecutive men. J Urol. 2005;174:651–5. doi: 10.1097/01.ju.0000165573.53109.92. [DOI] [PubMed] [Google Scholar]
- 10.Silber SJ. Microscopic vasoepididymostomy: specific microanastomosis to the epididymal tubule. Fertil Steril. 1978;30:565–71. [PubMed] [Google Scholar]
- 11.Fogdestam I, Fall M. Microsurgical end-to-end and end-to-side epididymovasostomy to correct occlusive azoospermia. Scand J Plast Reconstr Surg. 1983;17:137–40. doi: 10.3109/02844318309013109. [DOI] [PubMed] [Google Scholar]
- 12.Dubin L, Amelar RD. Magnified surgery for epididymovasostomy. Urology. 1984;23:525–8. doi: 10.1016/s0090-4295(84)80015-1. [DOI] [PubMed] [Google Scholar]
- 13.Fogdestam I, Fall M, Nilsson S. Microsurgical epididymovasostomy in the treatment of occlusive azoospermia. Fertil Steril. 1986;46:925–9. doi: 10.1016/s0015-0282(16)49836-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee HY. A 20-year experience with epididymovasostomy for pathologic epididymal obstruction. Fertil Steril. 1987;47:487–91. doi: 10.1016/s0015-0282(16)59060-8. [DOI] [PubMed] [Google Scholar]
- 15.Silber SJ. Results of microsurgical vasoepididymostomy: role of epididymis in sperm maturation. Hum Reprod. 1989;4:298–303. doi: 10.1093/oxfordjournals.humrep.a136892. [DOI] [PubMed] [Google Scholar]
- 16.Marmar JL, DeBenedictis TJ, Praiss DE. A modified vasoepididymostomy performed with the sling and blanket technique. J Urol. 1990;143:320–2. doi: 10.1016/s0022-5347(17)39946-9. [DOI] [PubMed] [Google Scholar]
- 17.Schlegel PN, Goldstein M. Microsurgical vasoepididymostomy: refinements and results. J Urol. 1993;150:1165–8. doi: 10.1016/s0022-5347(17)35715-4. [DOI] [PubMed] [Google Scholar]
- 18.Niederberger C, Ross LS. Microsurgical epididymovasostomy: predictors of success. J Urol. 1993;149(5 Pt 2):1364–7. doi: 10.1016/s0022-5347(17)36393-0. [DOI] [PubMed] [Google Scholar]
- 19.Grasso M, Lania C, Castelli M, Rigatti P. New technical expedient for epididymovasostomy. Br J Urol. 1994;73:207–8. doi: 10.1111/j.1464-410x.1994.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda T, Horii Y, Muguruma K, Komatz Y, Yoshida O. Microsurgical epididymovasostomy for obstructive azoospermia: factors affecting postoperative fertility. Eur Urol. 1994;26:322–6. doi: 10.1159/000475408. [DOI] [PubMed] [Google Scholar]
- 21.Matthews GJ, Schlegel PN, Goldstein M. Patency following microsurgical vasoepididymostomy and vasovasostomy: temporal considerations. J Urol. 1995;154:2070–3. [PubMed] [Google Scholar]
- 22.Marmar JL. Management of the epididymal tubule during an end-to-side vasoepididymostomy. J Urol. 1995;154:93–6. [PubMed] [Google Scholar]
- 23.Jarow JP, Sigman M, Buch JP, Oates RD. Delayed appearance of sperm after end-to-side vasoepididymostomy. J Urol. 1995;153:1156–8. [PubMed] [Google Scholar]
- 24.Boeckx W, Van Helden S. Microsurgical vasoepididymostomy in the treatment of occlusive azoospermia. Br J Urol. 1996;77:577–9. doi: 10.1046/j.1464-410x.1996.93417.x. [DOI] [PubMed] [Google Scholar]
- 25.Jarow JP, Oates RD, Buch JP, Shaban SF, Sigman M. Effect of level of anastomosis and quality of intraepididymal sperm on the outcome of end-to-side epididymovasostomy. Urology. 1997;49:590–5. doi: 10.1016/s0090-4295(97)80001-5. [DOI] [PubMed] [Google Scholar]
- 26.Kim ED, Winkel E, Orejuela F, Lipshultz LI. Pathological epididymal obstruction unrelated to vasectomy: results with microsurgical reconstruction. J Urol. 1998;160(6 Pt 1):2078–80. doi: 10.1097/00005392-199812010-00037. [DOI] [PubMed] [Google Scholar]
- 27.Berger RE. Triangulation end-to-side vasoepididymostomy. J Urol. 1998;159:1951–3. doi: 10.1016/S0022-5347(01)63205-1. [DOI] [PubMed] [Google Scholar]
- 28.Berardinucci D, Zini A, Jarvi K. Outcome of microsurgical reconstruction in men with suspected epididymal obstruction. J Urol. 1998;159:831–4. [PubMed] [Google Scholar]
- 29.Inaba Y, Fujisawa M, Okada H, Arakawa S, Kamidono S. Clinical outcome of microsurgery for obstructive azoospermia. Int J Urol. 1999;6:139–44. doi: 10.1046/j.1442-2042.1999.06332.x. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi J, Nomata K, Hirose T, Nishimura N, Igawa T, et al. Clinical experiences of microsurgical side-to-end epididymovasostomy for epididymal obstruction. Int J Urol. 1999;6:271–4. doi: 10.1046/j.1442-2042.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 31.Paick JS, Hong SK, Yun JM, Kim SW. Microsurgical single tubular epididymovasostomy: assessment in the era of intracytoplasmic sperm injection. Fertil Steril. 2000;74:920–4. doi: 10.1016/s0015-0282(00)01534-x. [DOI] [PubMed] [Google Scholar]
- 32.Hibi H, Yamada Y, Honda N, Fukatsu H, Katsuno S, et al. Microsurgical vasoepididymostomy with sperm cryopreservation for future assisted reproduction. Int J Urol. 2000;7:435–9. doi: 10.1046/j.1442-2042.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 33.Chan PT, Brandell RA, Goldstein M. Prospective analysis of outcomes after microsurgical intussusception vasoepididymostomy. BJU Int. 2005;96:598–601. doi: 10.1111/j.1464-410X.2005.05691.x. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Gautam G, Gupta NP. Early patency rates after the two-suture invagination technique of vaso-epididymal anastomosis for idiopathic obstruction. BJU Int. 2006;97:575–7. doi: 10.1111/j.1464-410X.2006.05952.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang GX, Bai WJ, Xu KX, Wang XF, Zhu JC. Clinical observation of loupe-assisted intussusception vasoepididymostomy in the treatment of obstructive azoospermia (analysis of 49 case reports) Asian J Androl. 2009;11:193–9. doi: 10.1038/aja.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho KL, Wong MH, Tam PC. Microsurgical vasoepididymostomy for obstructive azoospermia. Hong Kong Med J. 2009;15:452–7. [PubMed] [Google Scholar]
- 37.Kumar R, Mukherjee S, Gupta NP. Intussusception vasoepididymostomy with longitudinal suture placement for idiopathic obstructive azoospermia. J Urol. 2010;183:1489–92. doi: 10.1016/j.juro.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 38.Smrkolj T, Virant-Klun I, Sinkovec J, Oblak C, Zorn B. Epididymovasostomy as the first-line treatment of obstructive azoospermia in young couples with normal spermatogenesis. Reprod Biomed Online. 2010;20:594–601. doi: 10.1016/j.rbmo.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Peng J, Yuan Y, Zhang Z, Gao B, Song W, et al. Patency rates of microsurgical vasoepididymostomy for patients with idiopathic obstructive azoospermia: a prospective analysis of factors associated with patency – single-center experience. Urology. 2012;79:119–22. doi: 10.1016/j.urology.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 40.Peng J, Yuan Y, Cui W, Zhang Z, Gao B, et al. Causes of suspected epididymal obstruction in Chinese men. Urology. 2012;80:1258–61. doi: 10.1016/j.urology.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, Deng CH, Sun XZ, Chen Y, Wang WW, et al. A modified single-armed technique for microsurgical vasoepididymostomy. Asian J Androl. 2013;15:79–82. doi: 10.1038/aja.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harza M, Voinea S, Ismail G, Gagiu C, Baston C, et al. Predictive factors for natural pregnancy after microsurgical reconstruction in patients with primary epididymal obstructive azoospermia. Int J Endocrinol. 2014;2014:873527. doi: 10.1155/2014/873527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang HT, Yuan Q, Liu Y, Liu ZQ, Zhou ZY, et al. Multiple advanced surgical techniques to treat acquired seminal duct obstruction. Asian J Androl. 2014;16:912–6. doi: 10.4103/1008-682X.139256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Binsaleh S. Two-suture single-armed longitudinal intussusception vasoepididymostomy for obstructive azoospermia: report of patients characteristics and outcome. Int Urol Nephrol. 2014;46:2271–7. doi: 10.1007/s11255-014-0835-6. [DOI] [PubMed] [Google Scholar]
- 45.Zhao L, Tu XA, Zhuang JT, Chen Y, Wang WW, et al. Retrospective analysis of early outcomes after a single-armed suture technique for microsurgical intussusception vasoepididymostomy. Andrology. 2015;3:1150–3. doi: 10.1111/andr.12111. [DOI] [PubMed] [Google Scholar]
- 46.Hong K, Zhao LM, Xu SX, Tang WH, Mao JM, et al. Multiple factors affecting surgical outcomes and patency rates in use of single-armed two-suture microsurgical vasoepididymostomy: a single surgeon's experience with 81 patients. Asian J Androl. 2016;18:129–33. doi: 10.4103/1008-682X.159718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussein A. A new one-layer epididymovasostomy technique. BJU Int. 2015;115:653–8. doi: 10.1111/bju.12839. [DOI] [PubMed] [Google Scholar]
- 48.Chen XF, Chen B, Liu W, Huang YP, Wang HX, et al. Microsurgical vasoepididymostomy for patients with infectious obstructive azoospermia: cause, outcome, and associated factors. Asian J Androl. 2016;18:759–62. doi: 10.4103/1008-682X.175095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng J, Zhang Z, Yuan Y, Cui W, Song W. Pregnancy and live birth rates after microsurgical vasoepididymostomy for azoospermic patients with epididymal obstruction. Hum Reprod. 2017;32:284–9. doi: 10.1093/humrep/dew331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the studies included in the systematic review
Forest plots of patency rates compared by surgical techniques. (a) patency rates by end-to-end/end-to-side technique; (b) patency rates by intussusception technique.
Forest plots of pregnancy rates compared by surgical techniques. (a) pregnancy rates by end-to-end/end-to-side technique; (b) pregnancy rates by intussusception technique.