Abstract
Background
Metaplastic breast cancer (MBC) is a rare type of breast cancer, characterized histologically by the presence of two or more malignant cell types (epithelial and mesenchymal). This retrospective study aimed to review the imaging and histological features of MBC, with a review of the literature.
Material/Methods
Nineteen patients with MBC (age range, 28–75 years; mean, 55 years) underwent review of their clinical records, histopathology, immunohistochemistry, and imaging findings, which included mammography, sonography, and magnetic resonance imaging (MRI) with T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and diffusion restriction determined by the apparent diffusion coefficient (ADC) and a time-intensity curve (TIC) for signal intensity.
Results
The mammographic features of MBC were oval shaped (54.5%), with indistinct margin (45.5%), and high tumor density (72.7%), and on sonography, they were oval shaped (57.1%), with hypo-echogenic areas (85.8%). On MRI, MBC showed moderate hyper-intensity with a high signal intensity in the center of the tumor on T2WI (100%), an indistinct margin (75.0%), and rim enhancement (58.3%). Using a TIC, the early phase showed rapid enhancement, and the delay phase showed a signal plateau (91.7%). DWI showed diffusion restriction in all cases determined by the ADC. Immunohistochemistry showed negative expression of estrogen receptor (ER) (91.0%), progesterone receptor (PR) (81%), and HER2 (erbB-2) (80.0%).
Conclusions
Imaging features of MBC on mammography and ultrasound were benign. The use of T2WI MRI showed characteristic features of signal intensity using TIC curve and ADC analysis, which may support biopsy and histological analysis for definitive diagnosis.
MeSH Keywords: Breast; Constriction, Pathologic; Diffusion Magnetic Resonance Imaging; Mammography; Metaplasia
Background
In 1974, Huvos first described metaplastic breast cancer (MBC), which is a rare type of breast cancer [1]. MBC is characterized histologically by the presence of two or more malignant cell types, epithelial and mesenchymal [2–5]. Compared with invasive ductal carcinoma (IDC), MBC is a rare subtype of breast cancer, accounting for less than 5% of all cases of breast cancer [2,3,6]. Recently published studies have reported the incidence of MBC as less than 1% of all cases of breast cancer [7,8]. According to the 2012 World Health Organization (WHO) system for classifying breast tumors, MBC can be further sub-divided histologically into MBC of no specific type (including low-grade adenosquamous, fibromatosis-like, squamous cell and spindle cell carcinoma), mesenchymal metaplastic carcinoma (with chondroid and osseous metaplasia), and mixed epithelial-myoepithelial cell carcinoma. Oberman reported that the inclusion of mesenchymal components in the tumor should be regarded as metaplastic [9].
Most cases of MBC are triple-negative breast cancers (TNBCs), which are negative for the expression of estrogen receptor (ER), progesterone receptor (PR), and negative for the receptor tyrosine-protein kinase erbB-2, also known as human epidermal growth factor receptor 2 (HER2), using immunohistochemistry. TNBCs are resistant to many currently available systemic therapies, including chemotherapy, hormonal therapy, or targeted molecular therapy, which explains the poor prognosis for women with MBC and makes clinical management a challenge. Therefore, it is particularly important to diagnose MBC accurately at an early stage [10,11].
Therefore, this retrospective study aimed to review the histological features of MBC and the imaging features, including the use of mammography, sonography, and magnetic resonance imaging (MRI) to identify the optimal approach to early diagnosis, and to review the literature on the diagnosis of MBC.
Material and Methods
Patient clinical characteristics
This study was approved by the Ethics Committee of the Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute (No.2013010). Data were collected from the computerized pathology database of our hospital over a period of three years.
Twenty-two patients were initially diagnosed with metaplastic breast carcinoma (MBC) by biopsy, and two patients were then diagnosed with invasive ductal carcinoma (IDC) by histopathology following surgery, and one patient was excluded from the study as imaging data was unavailable. Nineteen patients with MBC were included in the study, who were in the age range of 28–75 years, with a mean age of 55 years. All 19 patients presented with palpable breast lumps, of between one day and one year in duration, with 12 masses identified in the left breast and seven in the right breast. On palpation, the size of the breast masses ranged from between 1 cm to 6 cm (mean, 3 cm). One patient had palpable axillary lymph node metastases. However, all the patients were without nipple discharge. One patient had a previous mastectomy due to medullary carcinoma of the contralateral breast 13 years previously, and one patient had undergone a modified radical mastectomy for phyllodes tumor (cystosarcoma phyllodes) of the contralateral breast, three years earlier.
Breast imaging and image acquisition
Mammograms were performed with standard craniocaudal and mediolateral oblique views acquired using the GE Senographe DS Mammography System (GE Healthcare, Chicago, Ill, USA). The ultrasound images were obtained using a 12 MHZ linear array transducer and Philips Elite scanner (Philips Healthcare Solutions, Bothell, WA, USA). Magnetic resonance imaging (MRI) was carried out using a GE 1.5T Signa Excite MRI scanner and dedicated bilateral breast surface coil (GE Healthcare, Chicago, Ill, USA) with the patient placed in the prone position. After the conventional three-plane positioning MRI scanning, T2-weighted imaging (T2WI) with sagittal fat-suppressed fast spin echo (FSE) images were obtained with repetition time (TR)/echo time (TE) of 4650/85 ms, slice thickness of 4 mm, layer spacing of 1.0 mm, field of view of 20×20, image matrix of 320×224, and the number of excitations (NEX) being 4.
The axial diffusion-weighted imaging (DWI) with single-shot echo-planar imaging was performed on pre-contrast scans with beta values of 800 s/mm, spin echo (SE) echo-planar imaging (EPI) was used with a TR/TE of 5000/63.2 ms. Dynamic contrast-enhanced MRI (DCE-MRI) was performed after injection of 0.1 mmol/kg of diethylenetriamine pentaacetate (DTPA) at the rate of 2.0 ml/second by pressure injection. One scan was performed before injection of the DTPA and eight further scans were performed after injection of DTPA, with a TR of 6.1 ms, a TE of 2.9 ms, a field of view (FOV) of 36×36 cm, slice number of 52, an image matrix of 350×350, NEX of 0.8. A time-signal intensity curve (TIC) of the lesions were recorded.
Imaging analysis and processing
Imaging data measurement and imaging processing were performed using a GE Healthcare Advantage Workstation (AW) 4.3 using Functool software. Imaging data were analyzed according to the American College of Radiologists (ACR) Breast Imaging and Reporting Data System-Magnetic Resonance Imaging (BI-RADS-MRI) atlas for reporting breast MRI imaging. On pre-contrast imaging, lesions were compared with normal glandular tissue. The signal intensity was divided into hypo-intensity, iso-intensity, moderately hyper-intense and significantly hyper-intense. On enhanced imaging, the tumor shape, tumor margin, imaging enhancement characteristics, incidence of early-phase enhancement and TIC (steady, plateau, and washout) were observed. The apparent diffusion coefficient (ADC) values were automatically calculated by placing a region of interest (ROI) well within the confines of the lesion. ROIs were drawn manually on non-interpolated DWI images in all lesions and additional normal tissue regions. The solid components showing maximum enhancement were chosen, while necrotic areas were avoided. The scanner software provided the mean value within the ROI, which equals the ADC value in millimeter square per second (mm2/s). All the imaging data were reviewed with consensus by two radiologists.
Histopathology analysis
Histopathology diagnosis was confirmed following surgery, as the histology of postoperative specimens were diagnostic. Axillary lymph node metastases were evaluated in the dissected lymph node specimens. Routine diagnostic immunohistochemical methods were used to evaluate the expression of estrogen receptor (ER), progesterone receptor (PR), and receptor tyrosine-protein kinase erbB-2, also known as human epidermal growth factor receptor 2 (HER2). All the histopathology data were reviewed with consensus by two pathologists. When the two pathologists held different views, a third pathologist was consulted, who had at least 20 years of experience in breast pathology.
Results
Mammographic findings in cases of metaplastic breast cancer (MBC)
Mammography was performed in 13 patients. The amount of fibroglandular tissue of the entire breast accounted for <25%, 25–50%, 51–75%, and >75% in four, five, three, and one patient, respectively. Eleven patients had tumor masses identified by mammography, and two patients had false-negative findings for metaplastic breast cancer (MBC), including chondroid differentiation and squamous cell carcinoma.
The mammographic characteristics of MBCs included an oval and irregular shape, identified in six and five patients, respectively; an indistinct tumor margin, in four and five patients respectively; and a well-circumscribed tumor in two patients. High and equal density were each identified in eight and three patients, respectively. The range of the maximum diameter of lesions was between 2.34 cm and 6.89 cm (mean, 3.43 cm). Significantly enlarged axillary lymph nodes were found in three patients. Micro-calcifications were found in three patients including fine pleomorphic, coarse heterogeneous, and fine-linear branching calcification, respectively (Figure 1). Mammographic characteristics and pathological subtypes are shown in Table 1.
Figure 1.
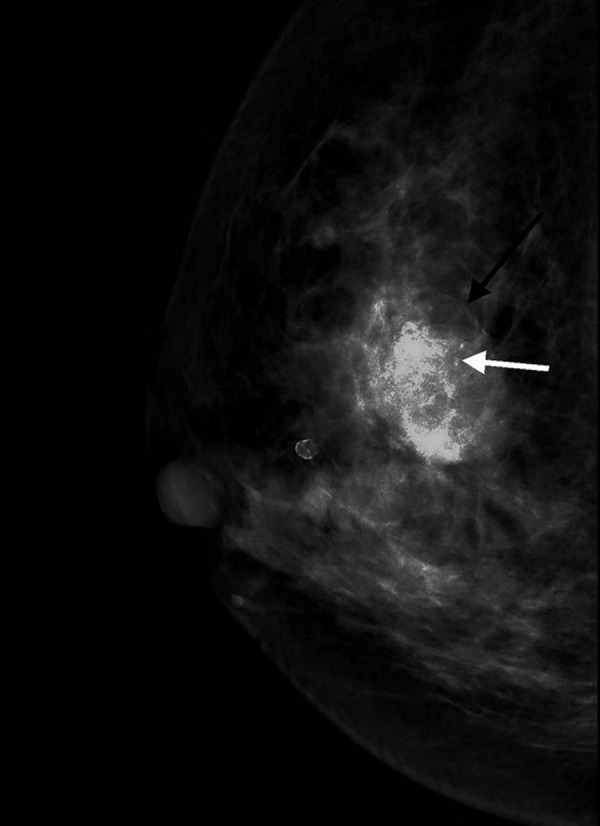
Craniocaudal mammogram of the right breast shows a obscure oval high density mass in the upper quadrant(balck arrow). coarse heterogeneous are seen in the lesion(white arrow).
Table 1.
Mammography features and pathological subtypes of 13 patients with mataplastic breast carcinoma.
| Mammography feature | Pathological subtype of carcinoma | Total N (%) | |||
|---|---|---|---|---|---|
| Squamous | Chondroid differentiation | Spindle | Mixed | ||
| Total, n | 6 | 2 | 2 | 3 | 13 |
| Mass | 5 | 1 | 2 | 3 | 11 (84.6) |
| Shape | |||||
| Round | 0 | 0 | 0 | 0 | 0 (0.0) |
| Oval | 2 | 1 | 1 | 2 | 6 (54.5) |
| Irregular | 3 | 0 | 1 | 1 | 5 (45.5) |
| Margin | |||||
| Circumscribed | 1 | 0 | 1 | 0 | 2 (18.2) |
| Obscured | 2 | 0 | 0 | 2 | 4 (36.4) |
| Indistinct | 2 | 1 | 1 | 1 | 5 (45.5) |
| Spiculated | 0 | 0 | 0 | 0 | 0 (0.0) |
| Density | |||||
| High | 4 | 0 | 2 | 2 | 8 (72.7) |
| Equal | 1 | 1 | 0 | 1 | 3 (27.3) |
| Low | 0 | 0 | 0 | 0 | |
| Microcalcification | |||||
| Yes | 2 | 0 | 1 | 0 | 3 (27.3) |
| No | 3 | 1 | 1 | 3 | 8 (72.7) |
| Non-mass | 1 | 1 | 0 | 0 | 2 (15.4) |
Ultrasound findings in cases of MBC
Ultrasound imaging was performed in 14 patients. The mean size of the tumors was 2.71 cm (range, 1.52–3.99 cm). The ultrasound features of MBC included an irregular-shaped mass in six patients, an oval shape in eight patients, and a circumscribed and indistinct margin in seven patients. Ultrasound showed a hypoechogenic area in 12 tumors, while two lesions showed complex echogenicity including solid and cystic areas. Color Doppler ultrasound showed blood flow in 13 patients. Six patients were found to have enlarged lymph nodes on ultrasound. Ultrasound characteristics and pathological subtypes are shown in Table 2.
Table 2.
Sonographic features and pathological subtypes of 14 patients with mataplastic breast carcinoma.
| Sonographic features | Pathological subtype of carcinoma | Total N (%) | ||||
|---|---|---|---|---|---|---|
| Squamous | Chondroid differentiation | Spindle | Low-adenosquamous | Mixed | ||
| Total,n | 8 | 2 | 1 | 1 | 2 | 14 |
| Shape | ||||||
| Round | 0 | 0 | 0 | 0 | 0 | 0 (0.0) |
| Oval | 4 | 1 | 1 | 0 | 2 | 8 (57.1) |
| Irregular | 4 | 1 | 0 | 1 | 0 | 6 (42.8) |
| Margin | ||||||
| Circumscribed | 3 | 1 | 1 | 0 | 2 | 7 (50.0) |
| Indistinct | 5 | 1 | 0 | 1 | 0 | 7 (50.0) |
| Spiculated | 0 | 0 | 0 | 0 | 0 | |
| Echogenicity | ||||||
| Hypo | 7 | 2 | 0 | 1 | 2 | 12 (85.8) |
| Mixed | 1 | 0 | 1 | 0 | 0 | 2 (14.2) |
| Lymph Node | ||||||
| Yes | 4 | 1 | 1 | 0 | 0 | 6 (42.9) |
| No | 4 | 1 | 0 | 1 | 2 | 8 (57.1) |
Magnetic resonance imaging (MRI) findings in cases of MBC
Magnetic resonance imaging (MRI) data were available for 11 patients with 12 lesions (one patient with two lesions) (Figure 2). The mean size of the 12 lesions was 2.9 centimeters (range, between 1.5 cm and 4.2 cm). On T1-weighted imaging (T1WI), four MBC tumors showed iso-intensity and scatted hypo-intensity in the center, five lesions showed hypo-intensity in the center, and iso-intensity in the periphery, and three lesions showed iso-intensity. On T2-weighted imaging (T2WI), the most common imaging features included moderate hyperintensity with high signal intensity in the center, in 12 lesions. Six lesions showed an irregular shape, and six lesions showed an oval shape. Nine lesions showed an irregular margin, one lesion showed circumscribed margins, and two lesions showed spiculated margins. On post-contrast imaging, five lesions exhibited heterogeneous enhancement, and seven lesions had rim enhancement. The area of peripheral iso-intensity on the T1WI sequence showed enhancement, with an area of hypo-intensity in the center, without significant enhancement. The area of moderate hyperintensity on T2WI sequences, showed enhancement, while the hyperintensity on T2WI sequences was without enhancement. With dynamic contrast-enhanced (DCE) curve analysis, all the lesions showed rapid enhancement in the early phase, eleven lesions showed a Type II curve, with a Type III curve in one patient. On diffusion-weighted imaging (DWI), all lesions that showed enhancement partly showed diffusion restriction, and the range of apparent diffusion coefficient (ADC) values were between 0.000528–0.00162 mm2/s (0.000945±0.0489mm2/s), with non-enhancement partly without diffusion restriction. Involved axillary lymph nodes were found in five patients. Ten patients showed thickened blood vessels and one lesion involved the pectoralis major. The MRI characteristics and pathological subtypes are shown in Table 3.
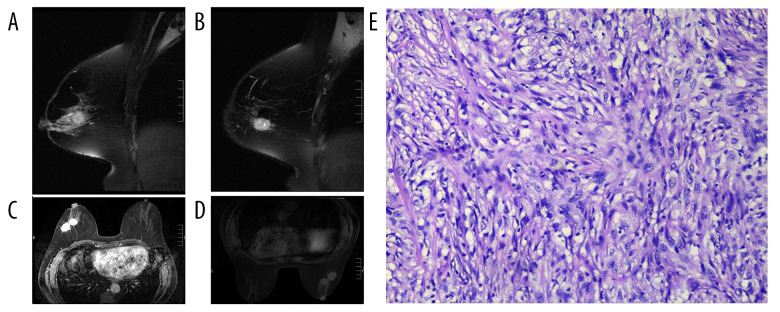
Figure 2.
(A–B) T2WI showed two masses with moderately high intensity and obviously high intensity, and spiculated can be observed. (C) Masses showed heterogeneous enhancement. (D) DWI showed isointensity. (E) HE staining (×40), spindle cell be observed under microscope.
Table 3.
MRI features and tumor subtypes of 11 patients of mataplastic breast carcinoma with 12 lesions.
| MRI features | Pathological subtype of carcinoma | Total N (%) | ||||
|---|---|---|---|---|---|---|
| Squamous | Chondroid differentiation | Spindle | Low-adenosquamous | Mixed | ||
| Total | 6 | 2 | 2 | 0 | 2 | 12 |
| Mass | 6 | 2 | 2 | 0 | 2 | 12 (100.0) |
| Shape | ||||||
| Round | 0 | 0 | 0 | 0 | 0 | 0 (0.00) |
| Oval | 0 | 2 | 2 | 0 | 2 | 6 (50.0) |
| Irregular | 6 | 0 | 0 | 0 | 0 | 6 (50.0) |
| Margin | ||||||
| Circumscribed | 0 | 1 | 0 | 0 | 0 | 1 (8.3) |
| indistinct | 6 | 1 | 0 | 0 | 2 | 9 (75) |
| spiculated | 0 | 0 | 2 | 0 | 0 | 2 (16.7) |
| Enhancement | ||||||
| Heterogeneous | 2 | 0 | 2 | 0 | 1 | 5 (41.7) |
| Rim | 4 | 2 | 0 | 0 | 1 | 7 (58.3) |
| TIC curve | ||||||
| Type I | 0 | 0 | 0 | 0 | 0 | 0 (0.00) |
| Type II | 5 | 2 | 2 | 0 | 2 | 11 (91.7) |
| Type III | 1 | 0 | 0 | 0 | 0 | 1 (8.3) |
Histopathology and immunohistochemistry findings in cases of MBC
All final pathological diagnoses were obtained by postoperative histopathology. The subtype of the epithelial component of MBC was squamous cell carcinoma in ten patients, mesenchymal differentiation with chondroid and mixed metaplasia were identified in three patients (one patient with sweat gland with squamous cell differentiation, squamous cell with spindle cell differentiation in two patients) and two patients had spindle carcinoma, and one patient had a diagnosis of low-grade adenosquamous carcinoma. Axillary lymph node metastases were found in four cases.
Routine diagnostic immunohistochemical methods were used to determine the expression of estrogen receptor (ER), progesterone receptor (PR), and receptor tyrosine-protein kinase erbB-2, also known as human epidermal growth factor receptor 2 (HER2). The patients in the study had immunohistochemical features that included negative ER and PR expression levels in 17 tumor samples. HER2 (erbB-2) showed negative expression in tumors from 15 patients with MBC, HER2 (2+) in three patients, and HER2 (3+) in one patient. Cytokeratin (CK) and CK5/6 were positively expressed in tumor tissues from 18 patients. Ki67 was positively expressed in tumor tissues from 13 patients (45±21%). Two spindle cell carcinomas and two squamous cell carcinomas with spindle cell differentiation showed positive expression for vimentin and S-100. Ten patients with squamous carcinoma, one patient with spindle cell carcinoma, one patient with chondroid differentiation and two mixed cell carcinomas with squamous differentiation expressed p63.
Clinical findings in cases of MBC
Among the 19 patients in this study, three patients were treated with neoadjuvant therapy before surgery. One patient underwent postoperative chemotherapy, radiotherapy, and endocrine therapy. One patient underwent chemotherapy and radiotherapy, and one patient received radiotherapy. Eleven patients received postoperative chemotherapy alone. Five patients refused postoperative adjuvant treatment.
One patient with spindle cell carcinoma refused to receive adjuvant therapy after surgery, and local recurrence occurred after four months, followed by surgical treatment and adjuvant chemoradiotherapy. Three months later, a further relapse occurred, and chemoradiotherapy was performed again after surgery. One month later, the patient developed local recurrence and chest wall metastases and was treated with surgery, radiotherapy, chemotherapy, and biologic therapy. However, four months later, the patient’s thoracic wall mass increased, the patient underwent genetic testing after everolimus and platinum drug treatment, which did not control the tumor. The patient refused targeted therapy. The imaging findings, pathological subtypes, and treatment of thirteen patients are summarized in Table 4. A summary of the literature review of the findings from published studies on MBC is summarized in Table 5. The pathological subtypes of MBC, from a review of the literature, are summarized in Table 6.
Table 4.
Thirteen patients with imaging grade, pathological methods of clinical treatment table.
| Patients’ number | Age | BI-RADS-X-ray | BI-RADS-US | BI-RADS-MRI | Pathological subtype of carcinoma | Lymph node metastasis | Surgery | Chemo-therapy | Radio-therapy | Endocrine therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | a | a | 5 | Squamous | No (0/7) | Yes | Yes | No | No |
| 2 | 54 | 4C | 4B | a | Spindle | No (0/10) | Yes | Yes | No | No |
| 3 | 52 | 4C | a | 5 | Spindle | No (0/7) | Yes | Yes | Yes | No |
| 4 | 41 | 4C | a | 3 | Chondroid differentiation | No (0/11) | Yes | Yes | No | No |
| 5 | 54 | 4C | 4C | 5 | Squamous | No (0/16) | Yes | Yes | No | No |
| 6 | 62 | a | 4C | a | Squamous | Yes (1/14) | Yes | Yes | No | No |
| 7 | 58 | 4C | 4C | 5 | Squamous | Yes (3/35) | Yes | Yes | No | No |
| 8 | 63 | 4B | 4B | a | Squamous | Yes (2/18) | Yes | Yes | Yes | Yes |
| 9 | 54 | 4C | 4C | 5 | Chondroid differentiation | Yes (1/23) | Yes | Yes | Yes | No |
| 10 | 49 | 4C | 4C | a | Squamous | No (0/6) | Yes | Yes | No | No |
| 11 | 64 | 4B | a | a | Mixed (sweat + squamous) | No (0/6) | Yes | Yes | No | No |
| 12 | 44 | 4B | 4B | 5 | Mixed (squamous + spindle) | No (0/7) | Yes | No | No | No |
| 13 | 48 | a | a | 5 | Squamous | No (0/9) | Yes | Yes | No | No |
| 14 | 75 | 5 | 5 | 5 | Mixed (squamous + spindle) | No (0/13) | Yes | No | No | No |
| 15 | 67 | a | a | a | Chondroid differentiation | No (0/4) | Yes | No | No | No |
| 16 | 60 | 0 | 0 | a | Squamous | No (0/16) | Yes | Yes | No | No |
| 17 | 61 | 5 | 5 | 5 | Squamous | No (0/16) | Yes | Yes | No | No |
| 18 | 46 | a | a | 5 | Squamous | No (0/27) | Yes | Yes | No | No |
| 19 | 57 | a | a | a | Low-grade adenosquamous | No (0/6) | Yes | No | No | No |
a – not examination.
Table 5.
Majority published a summary of the imaging findings of metaplastic breast carcinoma.
| Data | First author | Cases number | X-ray | US | MRI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oval | Circumscribed/ obscured | High-density | Microcalcification | Irregular | Circumscribed | Mixed echogenicity | Irregular | Indistinct | T2WI high intensity | Rim enhancement | |||
| 1997 | Stephanie K. Patterson | 9 | 44% (4/9) | 56% (5/9) | 89% (8/9) | 0% (0/9) | No | No | |||||
| 2000 | Jeong Mi Park | 16 | 69% (11/16) | 19% (3/16) | 94% (15/16) | 25% (4/16) | 9% (1/11) | 9% (1/11) | 55% (6/11) | No | |||
| 2002 | Isil Günhan-Bilgen | 8 | 100% (8/8) | 63% (5/8) | 100% (8/8) | 0% (0/8) | 75% (6/8) | 25% (2/8) | 13% (1/8) | No | |||
| 2005 | Martín Velasco | 12 | 25% (3/12) | No | No | 25% (3/12) | No | No | 75% (6/8) | 100% (12/12) | 100% (12/12) | 100% (12/12) | 73% (8/11) |
| 2011 | Bo Bae Choi | 33 | 37% (10/27) | 59% (16/27) | 74% (20/27) | 23% (7/30) | 60% (20/30) | 27% (9/33) | 82% (27/33) | 52% | 57% | 57% | 19 (4/21) |
| 2015 | Tiantian Bian | 13 | 31% (4/13) | 31% (4/13) | 69% (9/13) | 8% (1/13) | 69% (9/13) | 46% (6/13) | 77% (10/13) | No | |||
| 2018 | Henrique Donato | 11 | 56% (5/9) | 33% (3/9) | 56% (5/9) | 44% (4/9) | 36% (4/11) | 55% (6/11) | 82% (9/11) | a | a | 100% (2/2) | a |
No – the study was not conducted; a – not mention in article.
Table 6.
Majorty Published papers pathological type summary.
| Date (year) | First author | Case number | Squamous | Mixed matrix subtypes | Spindle | Fibromatosis-like | Chondroid and osseous alteration | Mixed epithelial | Other | Undefined |
|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | Stephanie K. Patterson | 9 | 0 | 0 | 6 | 0 | 0 | 3 | 0 | 0 |
| 2000 | Jeong Mi Park | 16 | 3 | 1 | 0 | 0 | 2 | 9 | 1 | 0 |
| 2002 | Isil Günhan-Bilgen | 8 | 3 | 2 | 0 | 0 | 0 | 1 | 2 | 0 |
| 2011 | Bo Bae Choi | 33 | 18 | 10 | 0 | 0 | 0 | 0 | 4 | 1 |
| 2015 | Tiantian Bian | 15 | 8 | 2 | 3 | 1 | 0 | 0 | 1 | 0 |
| 2018 | Henrique Donato | 11 | 3 | 3 | 2 | 0 | 0 | 0 | 3 | 0 |
Discussion
Metaplastic breast cancer (MBC) is a rare type of breast cancer, characterized histologically by the presence of two or more malignant cell types (epithelial and mesenchymal). A retrospective study of 19 patients with MBC included the evaluation of the tumor histopathology and imaging findings. Imaging included mammography, sonography, and magnetic resonance imaging (MRI) with T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and diffusion restriction determined by the apparent diffusion coefficient (ADC) and a time-intensity curve (TIC) for signal intensity. The findings showed that the imaging features of MBC on mammography and ultrasound tended to show benign features. The use of T2WI MRI showed characteristic features of signal intensity using TIC curve and ADC analysis.
Toumi et al. reviewed fifteen cases of MBC and reported that larger tumor size was one of the imaging characteristics [12]. Pezzi et al. compared the biological behavior of 892 patients with MBC with 255,164 patients with invasive ductal carcinoma (IDC) and reported that 5.5% of patients with MBC had a tumor size of less than 10 mm, compared with 26.9% of patients IDC [13]. Donato et al. reported that the median tumor size in cases of IDC was 27 mm [14]. In the present study, the mean diameter of the tumor was 3.43 cm on mammography, which is similar to the findings in previous studies.
Choi et al. analyzed the mammographic X-ray features of MBC for 30 patients and reported that MBC showed an oval mass on imaging with a circumscribed margin [15]. In the present study, six patients with MBC (54.5%) had tumors with an oval shape on imaging, and four patients with MBC (36.4%) had tumors with ill-defined margins on imaging, which is similar to a previously published study [15]. Donato et al. reported that in MBC, an oval dense mass with a non-circumscribed margin was most common on imaging [14]. Petterson et al. reported the imaging findings on mammography in nine patients and showed that cases of MBC that had an irregular or spiculated margin had an IDC component of metaplastic carcinoma [4]. Also, on imaging, cases of MBC with a squamous carcinoma component showed an irregularly-shaped mass with a spiculated margin, while spindle cell carcinoma and matrix-producing carcinoma showed an oval-shaped mass with a circumscribed margin [9]. Langland et al. investigated the imaging findings in 71 cases of MBC and showed that an irregular and spiculated margin was associated with a rapidly growing tumor and with less of a desmoplastic reaction [16].
Yang et al. compared the mammographic X-ray features of 43 patients with MBC with 43 patients with IDC and showed that 25% of patients with MBC had microcalcification when compared with 51% patients with IDC [17]. In the present study, three (23.1%) patients had imaging findings associated with malignant calcification. False-negative cases on imaging included squamous carcinoma and chondroid metaplasia. On retrospective review, these two cases showed masses of equal density on imaging, and the case of chondroid metaplasia showed scattered round calcifications. Decreased sensitivity of mammography was found, with round calcification being mistaken for typical benign calcification.
Park et al. [5] and Donato et al. [14] studied the ultrasound characteristics of 11 patients with MBC and reported the most common finding were an oval shape with a circumscribed margin and posterior enhancement. Choi et al. studied the ultrasound characteristics of 33 patients with MBC and reported the most common finding was an indistinct tumor margin [15]. In the present study, the most common feature on ultrasound was an oval mass. Recently published studies have shown that more circumscribed tumor masses might represent higher histologic tumor grades, while lesions that appear benign on imaging might be aggressive malignancy associated with poorer prognosis and survival rates [17–19].
Velaslo et al. reviewed the MRI findings in 12 patients with MBC, and reported that the characteristics on T2WI included homogeneous high-intensity signals that were similar to simple cysts (in 16% of patients), mixed high-intensity signals (in 75% of patients) and equal intensity signals (in 8% of patients) [20]. In the present study, on T1WI, four tumors (33.3%) showed iso-intensity and scattered hypo-intensity in the center, five tumors (41.6%) showed hypo-intensity in the center and iso-intensity in the periphery, and three tumors (25.0%) showed iso-intensity. The most common imaging feature in cases of MBC was a mixed high signal intensity on T2WI (in 100% of patients). On dynamic contrast-enhanced MRI (DCE-MRI), the most common internal enhancement pattern was heterogeneous enhancement (71%). On imaging, tumor rim-enhancement was approximately 19% in MBC, as reported by Choi et al. [15]. In the present study, five tumors (41.7%) showed heterogeneous enhancement and seven tumors (58.3%) showed rim enhancement. Rim enhancement on imaging might be explained by central necrosis or a hemorrhagic component, and non-enhancing solid portions in the peripheral areas of the tumor might be explained by the presence of metaplastic tissue.
In the present study, nine patients with MBC (75.0%) had thickened blood vessels in the tumor mass on subtraction imaging, which was associated with rapid growth of the tumor, central necrosis, and high malignant grade. Choi et al. reported the most common patterns on kinetic curve analysis were Type I and Type II [15]. In the present study, MRI included T2WI, DWI, and diffusion restriction determined by the apparent diffusion coefficient (ADC) and a time-intensity curve (TIC) for signal intensity. Velasco et al. reported that Type II and Type III patterns were common findings [20]. In the present study, the Type II pattern was the most common finding in cases of MBC (91.7%).
However, MBC showed benign tumor features including oval shape and a circumscribed margin on mammography and ultrasound imaging, indicating that using MRI techniques could distinguish between MBC and benign tumors or other types of breast cancer. Also, using MRI techniques, heterogenous imaging signals or rim enhancement and diffusion restriction were significant for a diagnosis of MBC. Phyllodes tumor (cystosarcoma phyllodes) and mucinous adenocarcinoma showed circumscribed oval or round masses, with phyllodes tumor showing homogeneous imaging signal enhancement, which could distinguish it from MBC. Mucinous adenocarcinoma showed a high signal intensity on T2WI, with no enhancement, or mild enhancement in the center, possibly due to the presence of mucus, which has a similar degree of signal intensity as MBC, which may be distinguished on DCE-MRI. A summary of the literature review of the findings from published studies on MBC is summarized in Table 5.
Choi et al. summarized the pathological features in 33 patients with MBC, and reported that the most common epithelial component of MBC was squamous carcinoma (40%), followed by matrix-producing MBC (30%), and in three patients (9%) MBC included spindle-cell components accompanied by squamous cell, osseous, and neuroendocrine cells [15]. In the present study, the most common metaplastic components were squamous metaplasia (46.1%), followed by chondroid differentiation (15.3%), mixed metaplastic carcinoma (15.3%), myoepithelioma, adenosquamous carcinoma, and spindle cell carcinoma each identified in one patient (7.6%), which was consistent with previously reported studies.
Choi et al. reported that most cases of MBC did not express estrogen receptor (ER) (91%), progesterone receptor (PR) (81%) and HER2 (erbB-2) (>80%). In the cases in this study, 17 patients with MBC (89.4%) did not express ER and PR, and 15 patients with MBC (78.9%) had tumors that showed negative expression of HER2 (erbB-2). ER, PR and HER2 (erbB-2) were positively expressed in association with an IDC component [15]. Tse et al. reported that p63 was expressed in all squamous and spindle cell carcinomas, and was associated with chondroid mesenchymal differentiation in a study that included 34 patients with MBC [20]. In the present study, ten patients with squamous carcinoma (100%), one patient with spindle cell carcinoma (50.0%), one patient with chondroid differentiation (33.3%), and two patients with mixed carcinoma with squamous components (66.7%) expressed P63. Song et al. reported that Ki67 overexpression was one of the characteristics of MBC in a study that analyzed the clinicopathological features of 55 patients with MBC and 767 age-matched patients with IDC [21]. Song et al. noted that Ki67 expression was more than 14% in MBC and IDC, in 87% and 63% of cases of MBC and IDC, respectively [21]. In the present study, a mean value of up to 45% of patients who had MBC, t expressed a Ki67-positive rate of more than 14%. The pathological subtypes of MBC from a review of the literature are summarized in Table 6.
Axillary lymph node metastasis occurs less frequently in cases on MBC when compared with IDC. Leddy et al. reported the incidence of axillary lymph node metastasis was between 8% and 40% [2]. In the present study, the axillary lymph nodes were involved in four patients with MBC (21.1%), which is consistent with previously published findings. Song et al. [21], and Luini et al. [18] reported that metaplastic carcinoma tends to metastasize by hematogenous invasion and spread instead of lymphatic invasion and spread, resulting in bone and lung metastasis. In the present study, 13 (92.8%) patients showed blood flow on color Doppler imaging, which may be related to hematogenous metastasis. Also, in this study, tumor rim enhancement was significantly increased in MBC. Kobayashi et al. conducted a study on the histopathological features of tumor rim enhancement on MRI and reported that the blood vessel density of the rim-enhanced mass were significantly increased when compared with the remaining tumor mass, while the lymphatic vessel densities were significantly reduced when compared with the normal breast tissue, which might contribute to lymph node metastasis [22].
Jha et al. showed that MBC was more insensitive to chemotherapy, radiotherapy, and hormone therapy, and so extensive resection is important as the primary treatment of MBC [23]. Toumi et al. [12] reviewed the literature and showed that the incidence of disease-free survival was between 42–84% and the overall survival was between 64–83% after five years of follow-up [12]. Langlands et al. conducted a follow-up study on 71 cases of MBC with the median follow-up of 62 months (range, 3–151 months), and a local recurrence rate of 7%, with 21% of the patients developing metastatic disease [16].
On review of the previously published literature, combined with the present study, the reduced prognosis for patients with MBC is associated with a larger tumor size, diverse histopathology, triple negative breast cancer (TNBC) with negative immunohistochemistry for ER, PR, and HER2, lack of effective targeted treatment, and a Ki67-positive rate of >14%, high cell proliferation rate, aggressive tumor behavior, and young age [2,8,18,21,24].
Conclusions
Metaplastic breast carcinoma (MBC) has benign morphological features on X-ray mammographic and ultrasound imaging. Magnetic resonance imaging (MRI) can provide more diagnostic information, which is the most effective method of imaging examination for the diagnosis of MBC. Imaging, combined with immunohistochemistry can provide guidance for the diagnosis, clinical treatment, and prognosis for patients with MBC.
Footnotes
Source of support: National Key Research and Development Program of China (No. 2017YFC1309100)
Conflict of interest
None.
References
- 1.Sherwell-Cabello S, Maffuz-Aziz A, Hernandez-Hernandez B, et al. Metaplastic carcinoma of the breast and the impact of the p63 and cytokeratin 5/6: Experience of 40 patients. Gynecol Obstet Mex. 2016;84:127–35. [PubMed] [Google Scholar]
- 2.Leddy R, Irshad A, Rumboldt T, et al. Review of metaplastic carcinoma of the breast: Imaging findings and pathologic features. J Clin Imaging Sci. 2012;2:21. doi: 10.4103/2156-7514.95435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunhan-Bilgen I, Memis A, Ustun EE, et al. Metaplastic carcinoma of the breast: Clinical, mammographic, and sonographic findings with histopathologic correlation. Am J Roentgenol. 2002;178:1421–25. doi: 10.2214/ajr.178.6.1781421. [DOI] [PubMed] [Google Scholar]
- 4.Patterson SK, Tworek JA, Roubidoux MA, et al. Metaplastic carcinoma of the breast: Mammographic appearance with pathologic correlation. Am J Roentgenol. 1997;169:709–12. doi: 10.2214/ajr.169.3.9275883. [DOI] [PubMed] [Google Scholar]
- 5.Park JM, Han BK, Moon WK, et al. Metaplastic carcinoma of the breast: Mammographic and sonographic findings. J Clin Ultrasound. 2000;28:179–86. doi: 10.1002/(sici)1097-0096(200005)28:4<179::aid-jcu5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Santamaria G, Velasco M, Bargallo X, et al. Radiologic and pathologic findings in breast tumors with high signal intensity on T2-weighted MR images. Radiographics. 2010;30:533–48. doi: 10.1148/rg.302095044. [DOI] [PubMed] [Google Scholar]
- 7.Lai HW, Tseng LM, Chang TW, et al. The prognostic significance of metaplastic carcinoma of the breast (MCB) – a case-controlled comparison study with infiltrating ductal carcinoma. Breast. 2013;22:968–73. doi: 10.1016/j.breast.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Esbah O, Turkoz FP, Turker I, et al. Metaplastic breast carcinoma: Case series and review of the literature. Asian Pac J Cancer Prev. 2012;13:4645–49. doi: 10.7314/apjcp.2012.13.9.4645. [DOI] [PubMed] [Google Scholar]
- 9.Oberman HA. Metaplastic carcinoma of the breast. A clinicopathologic study of 29 patients. Am J Surg Pathol. 1987;11:918–29. doi: 10.1097/00000478-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Bian T, Lin Q, Wu Z, et al. Metaplastic carcinoma of the breast: Imaging and pathological features. Oncol Lett. 2016;12:3975–80. doi: 10.3892/ol.2016.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzanninis IG, Kotteas EA, Ntanasis-Stathopoulos I, et al. Management and outcomes in metaplastic breast cancer. Clin Breast Cancer. 2016;16:437–43. doi: 10.1016/j.clbc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Toumi Z, Bullen C, Tang AC, et al. Metaplastic breast carcinoma: A case report and systematic review of the literature. Pathol Int. 2011;61:582–88. doi: 10.1111/j.1440-1827.2011.02698.x. [DOI] [PubMed] [Google Scholar]
- 13.Pezzi CM, Patel-Parekh L, Cole K, et al. Characteristics and treatment of metaplastic breast cancer: Analysis of 892 cases from the National Cancer Data Base. Ann Surg Oncol. 2007;14:166–73. doi: 10.1245/s10434-006-9124-7. [DOI] [PubMed] [Google Scholar]
- 14.Donato H, Candelária I, Oliveira P, et al. Imaging findings of metaplastic carcinoma of the breast with pathologic correlation. J Belg Soc Radiol. 2018;102(1):46. doi: 10.5334/jbsr.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi BB, Shu KS. Metaplastic carcinoma of the breast: Multimodality imaging and histopathologic assessment. Acta Radiol. 2012;53:5–11. doi: 10.1258/ar.2011.110341. [DOI] [PubMed] [Google Scholar]
- 16.Langlands F, Cornford E, Rakha E, et al. Imaging overview of metaplastic carcinomas of the breast: A large study of 71 cases. Br J Radiol. 2016 doi: 10.1259/bjr.20140644. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang WT, Hennessy B, Broglio K, et al. Imaging differences in metaplastic and invasive ductal carcinomas of the breast. Am J Roentgenol. 2007;189:1288–93. doi: 10.2214/AJR.07.2056. [DOI] [PubMed] [Google Scholar]
- 18.Luini A, Aguilar M, Gatti G, et al. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: The experience of the European Institute of Oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349–53. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- 19.Kaas R, Kroger R, Hendriks JH, et al. The significance of circumscribed malignant mammographic masses in the surveillance of BRCA 1/2 gene mutation carriers. Eur J Radiol. 2004;14:1647–53. doi: 10.1007/s00330-004-2307-3. [DOI] [PubMed] [Google Scholar]
- 20.Velasco M, Santamaria G, Ganau S, et al. MRI of metaplastic carcinoma of the breast. Am J Roentgenol. 2005;184:1274–78. doi: 10.2214/ajr.184.4.01841274. [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Liu X, Zhang G, et al. Unique clinicopathological features of metaplastic breast carcinoma compared with invasive ductal carcinoma and poor prognostic indicators. World J Surg Oncol. 2013;11:129. doi: 10.1186/1477-7819-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi M, Kawashima H, Matsui O, et al. Two different types of ring-like enhancement on dynamic MR imaging in breast cancer: Correlation with the histopathologic findings. J Magn Reson Imaging. 2008;28:1435–43. doi: 10.1002/jmri.21622. [DOI] [PubMed] [Google Scholar]
- 23.Jha A, Agrawal V, Tanveer N, et al. Metaplastic breast carcinoma presenting as benign breast lump. J Cancer Res Ther. 2017;13(3):593–96. doi: 10.4103/0973-1482.183221. [DOI] [PubMed] [Google Scholar]
- 24.Oon ML, Thike AA, Tan SY, Tan PH. Cancer stem cell and epithelial-mesenchymal transition markers predict worse outcome in metaplastic carcinoma of the breast. Breast Cancer Res Treat. 2015;150(1):31–41. doi: 10.1007/s10549-015-3299-1. [DOI] [PubMed] [Google Scholar]