Abstract
Background
Normothermic machine perfusion (NMP) preservation is superior to cold preservation during reduced-size liver transplantation (RSLT) in pigs. However, the mechanism of this protective effect has not been explained. We aimed to compare the effects of NMP preservation with that of cold preservation (CS) in protecting against ischemia-reperfusion injury (IRI) during RSLT in pigs.
Material/Methods
Twenty-four healthy Bama miniature pigs were randomized into 2 groups: 1) the NMP group in which donor livers harvested without warm ischemia time and cardiac activity were connected to the NMP system to reduce liver size under normothermic conditions, and 2) the CS group in which donor livers harvested without warm ischemia time and cardiac activity were perfused using the University of Wisconsin (UW) solution and then preserved in the 0–4°C UW solution to reduce liver size under cold conditions. Livers were then transplanted without veno-venous bypass. Amounts of bile secretion for the NMP groups were recorded hourly. The serological indices were measured. Expressions of cytochrome C, caspase 3, and NF-κB p65 in liver tissue were observed.
Results
The levels of bile secretions were gradually diminished from 16.50±2.66 mL/h before splitting to 6.35±1.24 mL/h after splitting. With the exception of TNF-α on postoperative day 2, overall, levels of TNF-α, IL-1, IL-6, and MDA were significantly lower in the NMP group versus CS group for all 5 days postoperatively. Finally, cytochrome C, caspase 3, and NF-κB p65 expressions were all significantly suppressed in the NMP group as compared with the CS group.
Conclusions
MP preservation is superior to cold preservation in protecting against liver IRI during RSLT in pigs.
MeSH Keywords: Animal Experimentation; Organ Preservation; Reperfusion Injury; Surgical Procedures, Operative
Background
Ischemia-reperfusion injury (IRI) is a key factor contributing to liver dysfunction and failure after transplantation [1]. The initial component of IRI represents a pathophysiological process resulting from hypoxia, an effect which is further worsened with the resumption of blood flow to the damaged tissue [2].
Liver IRI is a complex process involving multiple cell types and a variety of molecular pathways and mediators. During the period between hepatic ischemia and reperfusion, Kupffer cells, CD4+ lymphocytes, neutrophils, and hepatocytes are the major cell types involved in the inflammatory response. In addition, various cytokines, chemokines, and complement proteins act as liaisons between cells to participate in the inflammatory response [3].
Although liver transplants remain the only therapeutic approach for end-stage liver disease, the paucity of donors severely limits this procedure. As a result, mortality for adult patients on waiting lists is over 10%, while this rate increases to greater than 20% in children [4,5]. The increased mortality rate in children is attributable to a combination of even fewer donors being available for children in whom biliary atresia was the main indication for liver transplantation [6] and differences in size between a small recipient and a large liver graft, which makes abdominal closure problematic and can result in respiratory impairment [7]. One initial approach to resolve this dilemma has involved heterotopic partial liver transplants [8,9]. In 1984, the first orthotopic partial liver transplant was performed, as reported by Bismuth and Houssin [10]. With use of a hemiliver, it was possible to tailor the fit of this implant within the recipient’s abdominal space [6]. Following the success indicated in this report, reduced-size liver transplantation (RSLT) has emerged as a relatively routine procedure, with some centers reporting reductions in mortality rates of patients on waiting lists [11,12]. While reductions in graft size can be accomplished by ex vivo partial liver resection, this procedure has some limitations. Notably, the extended time required for performing this procedure significantly increases the cold ischemia time of the donor liver. As a result, aggravated ischemia-reperfusion injuries to the donor liver can occur, which increases the rates of posttransplantation vascular and biliary complications and graft dysfunction.
Endo et al. [13] have shown that in the early stages of liver cell damage, aerobic metabolism of liver cells can be enhanced and damage of hepatocytes reduced by restoring the supply of oxygen and energy. Such a procedure, which can be achieved through a simulated normothermic mechanical perfusion (NMP) procedure, enables maintenance of a more physiological state of the liver. Accordingly, by reducing the damage caused by ischemia, this approach provides an effective environment for maintaining the overall viability of the donor liver [14]. In a previous study, the effectiveness and stability of the NMP device developed by our group and the superiority of the NMP preservation during RSLT in pigs was verified. Some results of the present study, which are not shown in this manuscript were presented in previously published papers [15]. However, the mechanism of this protective effect is not explained in previous papers. In this study, we assessed the potential gains that can result from combining NMP with RSLT as a means to alleviate liver injury induced by ischemia-reperfusion in Bama miniature pigs after performing correlation tests on previous experimental specimens, hoping to provide a foundation for the clinical application of the NMP-based RSLT.
Material and Methods
All animal work was conducted according to relevant national and international guidelines and was in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health [16]. The protocol was approved by the Committee on the Ethics of Animal Experiments of Tianjin First Central Hospital.
Twenty-four healthy Bama miniature pigs at 13–16 months of age and weighing 25–35 kg were used in this experiment. The pigs were provided by the Experimental Animal Center of the Academy of Military Medical Sciences (Beijing, China). The pigs were randomly divided into 2 groups. In the NMP group, donor livers were harvested from 6 pairs of pigs in the absence of warm ischemia and cardiac activity. An intravenous (IV) infusion of 30 mL 10% potassium chloride was administered after clamping the aorta at the cardiac base. Donor livers were then connected to the NMP system to perform liver size reductions under normothermic conditions. After washing the donor livers with 4°C University of Wisconsin (UW) solution, liver transplantation without veno-venous bypass was performed. In the CS (control) group, donor livers were harvested from 6 pairs of pigs in the absence of warm ischemia and cardiac activity after being perfused with the UW solution. Liver donors were then preserved in 0–4°C UW solution and liver size reduction was performed during the preservation period. Subsequently, liver transplantation was performed without veno-venous bypass. Procedures involving surgeries of donor liver acquisition and donor recipient, postoperative management, machine perfusion, and size reduction in livers were performed as previously described.
Surgical procedure for liver donor acquisition
Anesthetized pigs were maintained in a supine position while their carotid arteries and internal carotid veins were dissected and catheterized in preparation for liver removal. Following a longitudinal midline abdominal incision, the perihepatic ligaments were detached. The hepatoduodenal ligament was dissected, the hepatic artery separated from its origin at the abdominal aorta, the portal vein was severed at the site of confluence between the splenic and superior mesenteric veins, and the inferior vena cava detached at the level of the renal vein. Approximately 1000 mL of blood was extracted from the carotid artery after a rapid fluid infusion via the carotid vein. Potassium chloride (1 g; 10 mL of 10% potassium chloride) was injected intravenously. Perfusion cannulas were first inserted into the hepatic artery and portal vein (which arrests cardiac function), while the hepatic artery, portal vein, and suprahepatic vena cava, and the infrahepatic vena cava were immediately severed under normothermic conditions. The liver was then rapidly harvested followed by coupling with the NMP system for perfusion preservation.
Machine perfusion
The NMP system included: 1) a peristaltic pump (PD5206; Heidolph, Schwabach, Germany), 2) a membrane oxygenator (70058174; Maquet, Rastatt, Germany), and 3) a cannula system. The cannula system included: 1) a German-made extracorporeal membrane oxygenator (ECMO) with an altered cannula system (the portal vein and hepatic artery cannulas were replaced by medical ECMO cannulas with different calibers), 2) a softshell reservoir, 3) a gate clamp, 4) a pressure sensor (DPT-248; SCW Medicath, Shenzhen, China), 5) a multilead physiological recorder (MP150;Biopac, USA), and 6) a heat exchange water tank (Hico-Variotherm 550; Hirtz and Co, Cologne, Germany). A dual-perfusion procedure was used, with the cannula system being divided into 2 pathways following transport through the membrane oxygenator. The narrower pathway was used for perfusion of the hepatic artery, while the wider pathway was used for perfusion of the portal vein. In order to control perfusion pressures, as accomplished by adjustments of the inner caliber of the pathway, a gate clamp was inserted in the portal vein perfusion pathway.
The perfusion fluid included: 1) 800 mL of blood drawn from the donor prior to liver harvest, 2) 800 mL of normal saline, 3) 600 mg of reduced glutathione sodium injection, 4) 1.5 g of cefuroxime sodium injection, and 4) 10 μg of alprostadil injection. A heat exchange water tank was used to maintain the blood temperature at 39°C. Flow rates of the portal vein were maintained at 320–580 mL/min at a pressure of approximately 8–10 mmHg, while flow rates of the hepatic artery were maintained at 129–239 mL/min at a perfusion pressure of 85–100 mmHg. A continuous perfusion of heparin (12 500 U of heparin +50 mL of normal saline at 1000 U/h), insulin (100 U of insulin +47.5 mL of normal saline at 6–10U/h), 5% glucose (at 10 mL/h), and compound amino acid (composition of the 250 mL of 5% compound amino acid; 1.8 g of alanine; 1.23 g of arginine; 0.38 g of aspartate; 0.05 g of cystine; 0.63 g of glutamic acid; 0.88 g of glycine; 0.75 g of histidine; 0.63 g of isoleucine; 0.85 g of leucine; 1.38 g of lysine acetate; 0.63 g of methionine; 0.88 g of phenylalanine; 0.73 g of proline; 0.48 g of serine; 0.63 g of threonine; 0.21 g of tryptophan; 0.05 g of tyrosine; 0.8 g of valine at 10 mL/h) were maintained with use of a microinjection pump. Doses of glucose and insulin were adjusted according to glucose levels within the perfusion fluid. Blood gas analysis and electrolyte levels were monitored hourly to avoid acid–base imbalances and electrolyte disturbances. Partial pressures of oxygen and carbon dioxide were maintained at 80–100 and 30–50 mmHg, respectively.
Procedure for liver size reduction
Following removal, the liver was inserted within a perfusion container with the diaphragmatic surface exposed. Liver reduction was achieved through a separation along the median liver fissure, with removal of the hepatic capsule along the resection line. Hepatic ducts and left lateral portal vein were then ligated. The left hepatic vein was clamped at its origin and the left hepatic vein was transected at the left side of the clamp followed by suturing with 5–0 Prolene sutures. Prolene sutures and the bipolar coagulation were also used to prevent any other bleeding from the transected surface during the NMP, while only bipolar coagulation was used to cauterize the vessels on the transected surface during the reduction of the cold-preserved liver because blood oozing from the transected surface could not be observed.
Surgical procedures of recipients
Following an initial NMP perfusion, the graft was perfused with 1000 mL of normothermic lactate Ringer’s solution and then implanted into a recipient pig with a total hepatomy. Methylprednisolone (10 mg/kg IV) was administered during the anhepatic phase, which was no longer than 20 min. Neither veno-venous bypass nor vasoactive substances were applied during the anhepatic phase, as recommended in Fondevila’s [19] porcine transplant protocol.
Postoperative management
For the initial 48 h postoperatively, animals were permitted free access to water but no food. An IV infusion with tacrolimus (0.04 mg/kg) and methylprednisolone sodium succinate (administered in decreasing doses from 200 mg, 100 mg to 50 mg on postoperative days 1–3, respectively) were used to inhibit rejection. To avoid infection, cefuroxime sodium (0.75 g every 12 h) was injected intravenously over the initial 3 days following transplant. Fluids were infused as needed based upon daily urinary output and hematocrit value. Typically, these fluid administrations consisted of 1000–1500 mL/day for the first 2 days after transplant. By day 3, treatments with fluids were terminated and the animals were freely eating and drinking.
Observation indices
Changes of bile secretion for the NMP groups
Every hour, we recorded amounts of bile secretion for the NMP groups prior to liver procurement, as well as before, during, and after splitting.
Serological examination
Venous blood of recipient pigs from the 2 groups were sampled at postoperative days 1–5. Blood samples were centrifuged at 3000 rpm for 10 min, and supernatants were harvested for determining serological levels of tumor necrosis factor-a (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6) and malondialdehyde (MDA) were detected using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (Pig TNF-α ELISA kit Ab100756, IL-1 Beta Pig ELISA kit Ab10075, IL-6 Pig ELISA kit Ab100755, Abcam, Cambridge, UK, and Malondialdehyde assay kit (A003-1 Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Levels obtained are expressed as either pg/mL or mmol/mL.
Western blot analysis
Liver tissues were lysed with a protein extract reagent according to the manufacturer’s directions (Bioteke Corporation, Beijing, China). The protein concentration was measured using a Pierce BCA protein assay kit (Solarbio, Beijing, China). Tissues lysates containing equal amounts of total proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in TBST for 2 h at 37ºC and incubated at 4ºC with primary antibodies overnight. The primary antibodies included cytochrome C rabbit mAb (CST, Danvers MA, USA), caspase 3 (Santa, Dallas, Texas, USA), and Phospho-NF-κB p65 (Ser536) rabbit mAb (CST, Danvers MA, USA). After 5 washes, membranes were incubated with the appropriate HRP-conjugated secondary antibody for 2 h at room temperature and developed using the ECL System (Millipore, USA). Band densities were quantified using Image J software.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) and were analyzed using SPSS16.0 statistical software (IBM, Armonk, NY). Differences between the 2 groups were analyzed with use of a 2-tailed t test using an a-level of 0.05.
Results
Changes of bile secretion for the NMP groups
A profile of the hourly bile secretions is presented in Figure 1. Levels of bile secretions were gradually diminished with splitting (before splitting: 16.50±2.66 mL/h, during splitting: 10.37±1.58 mL/h, and after splitting: 6.35±1.24 mL/h).
Figure 1.
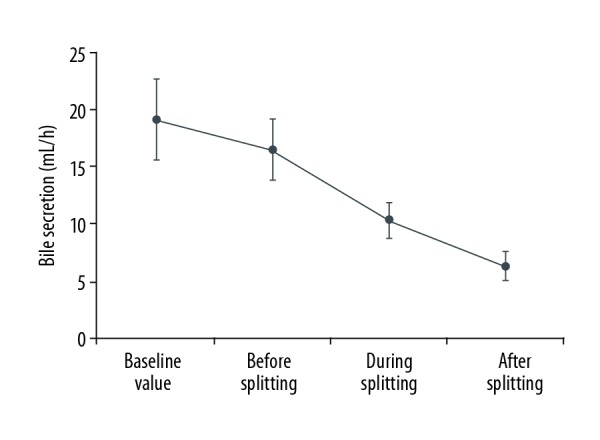
Changes of bile secretion.
Serum TNF-α, IL-1, IL-6 and MDA levels
Levels of TNF-α in the CS group were significantly increased as compared with that of the NMP group on days 1 (0.020±0.001 pg/ml versus 0.019±0.001 pg/ml), 3 (0.019±0.000 pg/ml versus 0.018±0.001 pg/ml), 4(0.021±0.001pg/ml versus 0.019±0.000 pg/ml), and 5 (0.020±0.001 pg/ml versus 0.018±0.000 pg/ml) postoperatively (P<0.05, Figure 2). Levels of IL-1 (day 1: 37.87±16.47 pg/ml versus 14.08±1.69 pg/ml, day 2: 11.88±3.19 pg/ml versus 6.59±1.17 pg/ml, day 3: 7.45±1.42 pg/ml versus 0.58±0.35 pg/ml, day 4: 15.26±1.83 pg/ml versus 2.68±1.83 pg/ml, day 5: 16.17±7.10 pg/ml versus 5.57±1.88 pg/ml) (P<0.05, Figure 3), IL-6 (day 1: 451.29±93.02 pg/ml versus 158.18±76.62 pg/ml, day 2: 338.35±52.86 pg/ml versus 143.77±120.92 pg/ml, day 3: 282.60±20.47 pg/ml versus 21.19±5.35 pg/ml, day 4: 223.94±36.42 pg/ml versus 107.07±43.69 pg/ml, day 5: 247.53±36.39 pg/ml versus 61.19±33.72 pg/ml) (P<0.05, Figure 4) and MDA (day 1: 14.73±3.02 mmol/ml versus 6.47±1.21 mmol/ml, day 2: 13.03±1.80 mmol/ml versus 7.12±0.59 mmol/ml, day 3: 10.53±2.05 mmol/ml versus 8.33±0.57 mmol/ml, day 4: 8.85±1.22 mmol/ml versus 5.20±0.49 mmol/ml, day 5: 12.35±0.84 mmol/ml versus 9.00 ± 0.63 mmol/ml) (P<0.05, Figure 5) in the CS group were significantly greater than those of the NMP group on each of the 5 days sampled.
Figure 2.
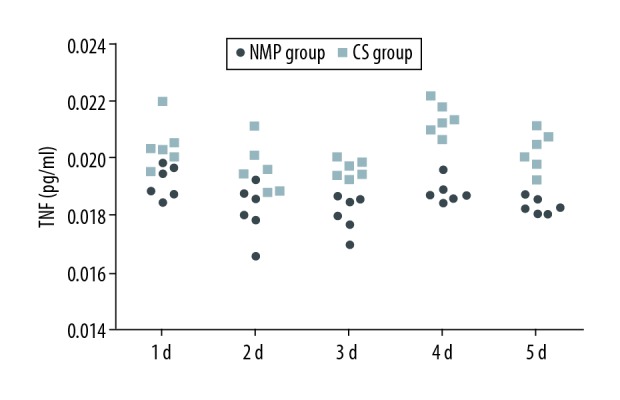
TNF-α levels in sera from the blood samples as assessed using ELISA.
Figure 3.
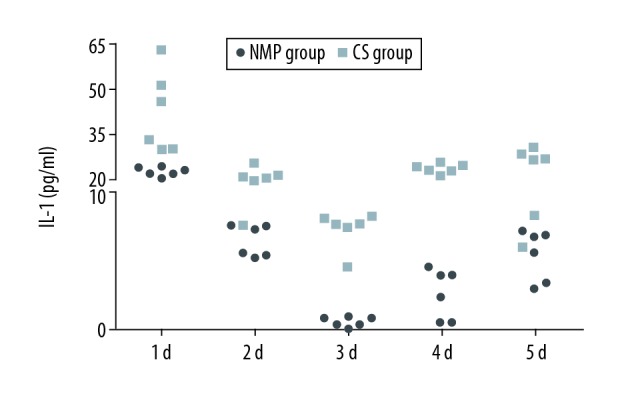
IL-1 levels in sera from blood samples as assessed using ELISA.
Figure 4.

IL-6 levels in sera from blood samples as assessed using ELISA.
Figure 5.

MDA levels in sera from blood samples as assessed using ELISA.
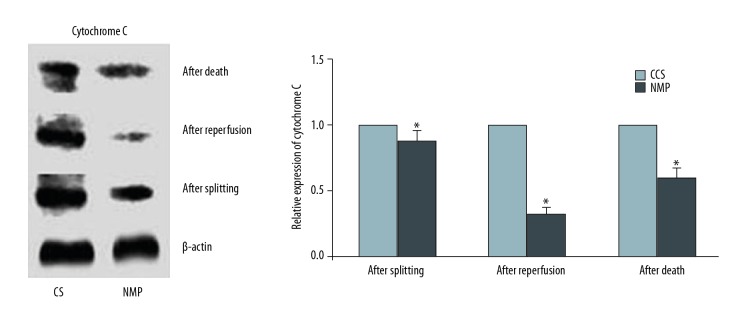
Expression of cytochrome C in liver tissue
Levels of cytochrome C from liver tissue of the CS and NMP groups were determined after splitting, after reperfusion, and after death. Relative to that of the CS group, levels of cytochrome C were significantly decreased in the NMP group as determined after splitting, after reperfusion, and after death (P<0.05, Figure 6).
Figure 6.
Western blot analysis of relative Cytochrome C expression in liver tissue of the CS and NMP groups as determined after splitting, after reperfusion and after death (* P<0.05). All experiments were repeated three times.
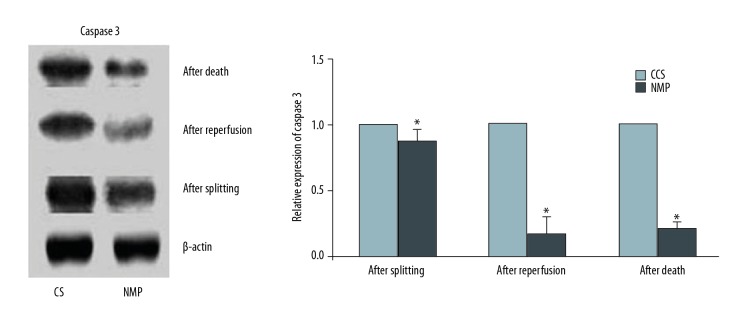
Expression of caspase 3 in liver tissue
Levels of caspase 3 in liver tissue of the CS and NMP groups were also determined after splitting, after reperfusion, and after death. Similar to that obtained for cytochrome C, levels of caspase 3 as determined after splitting, after reperfusion, and after death were found to be significantly decreased in the NMP group versus the CS group (P<0.05, Figure 7).
Figure 7.
Western blot analysis of relative caspase 3 expression in liver tissue of the CS and NMP groups as determined after splitting, after reperfusion and after death (* P<0.05). All experiments were repeated three times.
Expression of NF-κB p65 in liver tissue
Finally, levels of NF-κB p65 in liver tissues of the CS group and NMP group were determined after splitting, after reperfusion, and after death. Levels of NF-κB p65 as determined after splitting, after reperfusion, and after death were found to be significantly decreased in the NMP group versus the CS group (P<0.05, Figure 8).
Figure 8.
Western blot analysis of relative NF-κB p65 expression in liver tissue of the CS and NMP groups as determined after splitting, after reperfusion and after death (* P<0.05) All experiments were repeated three times.
Discussion
In 1963, Starzl [17] first proposed liver transplantation as a means for treatment of end-stage liver disease, and this remains the only therapeutic approach for this condition. While advances in surgical techniques and procedures have increased the effectiveness of liver transplantation, the limited number of liver donors continues to be a pervasive problem. This deficit is particularly insidious for children and small adult recipients, as large livers from the adult donor pool cannot be effectively used in these groups. One approach to resolve this issue has been the development of RSLT to maximize use of donor organs [18]. While the problem of the limited donor pool remains, RSLT does provide a means for livers from adults to be available for smaller recipients [19]. RSLT has proved to be as effective as full-size livers transplantation, and has significantly reduced waiting times and mortality rates of pediatric recipients [11,12]. A number of advantages are associated with in situ resection as compared with that of ex vivo resection: (1) reductions in cold ischemia durations; (2) an enhanced recognition of the bile duct and vascular tissues, more precise liver transected surface management, and reductions in surface bleeding and bile leakage; (3) reductions in liver damage during rewarming [20]; (4) as blood supplies and their return at each hepatic segment after liver size reduction can be better visualized, there is a more effective allocation of hepatic blood vessels; and (5) complete hemostasis of the transected surface during the liver size reduction, thereby reducing transected surface bleeding after recipient reperfusion [21]. NMP is not a new technique for use in organ preservation. In fact, the first successful liver transplant using diluted oxygenated blood to preserve the liver was performed in 1967 [22]. Since that time, UW has been used as a means to significantly improve liver preservation. However, this method is burdened with a number of limitations, such as limited preservation time, irreversible injury from warm ischemia, and the existence of time-dependent damage during preservation. As a result, NMP has once again become the focus of organ transplant techniques, especially given the improvements in cardiopulmonary bypass technology. Here, we report our results using a self-established NMP system to assimilate the resecting process under in vivo conditions while fully exerting the advantages of an in situ resection. We demonstrate that following liver size reduction, a near complete hemostasis of the cut surface was present, and significantly less intraoperative bleeding was observed after graft transplantation in the NMP group versus the cold preservation group.
As bile secretions and drainage can provide important indices regarding liver function and biliary tracts [23], they can serve as a means to assess the effect of NMP preservation. For example, reductions in liver function can initially involve decreases, followed by complete cessation, in bile secretion [24]. In the present study we found that a continuous flow in bile secretions was present at the onset of extracorporeal perfusion. Thereafter, bile secretions decreased and stabilized with splitting due to reductions in the efficacy of the liver cells to secrete bile. Such a profile of bile secretions indicates that the donor liver in the splitting process is both functional and viable.
NMP provides a means to restore the energy supply of the liver by continuously supplying oxygen and nutrients, and reversing the damage caused by disruption of ATP supply during warm ischemia. In this way, it can prevent further damage to hepatocytes during preservation [25]. As hepatic enzyme detection can serve as a basis for testing liver viability, we compared ALT and AST levels between the CS group and NMP group in our previous experiment. For both ALT and AST, we found significantly lower levels in the NMP group versus the CS group on days 3–5 postoperatively.
It has been reported that IRI increases the incidence of vascular and biliary complications and graft dysfunction after transplantation [26]. Thus, minimizing the adverse effects of IRI can significantly increase the success rate in patients receiving liver transplants. Although the precise mechanisms of liver IRI have not been fully elucidated, inflammatory responses have been shown to play a critical role in its pathogenesis [27]. Kupffer cells, which are liver macrophages, can be activated during ischemia and reperfusion, and may also play a significant role in the IRI process. After activation, free radicals such as ROS and inflammatory cytokines, including tumor necrosis factor-a (TNF-α), interleukin 1 (IL-1) and interleukin-6 (IL-6), can be produced [28]. These activated oxygen substances and inflammatory cytokines exert a relatively widespread influence on the occurrence and development of IRI. In specific, they initiate a cascade of events, a primary one being the activation of neutrophils, CD4+T lymphocytes, and sinusoidal endothelial cells that gather in the circulation and result in the expression of cell surface adhesion molecules. Such processes stimulate the production of reactive oxygen species in liver cells and promote the adhesion of platelets to hepatic sinusoidal endothelial cells, which further aggravates the injury [29]. TNF-α and IL-6, as important inflammatory factors affecting the IRI process, are mainly responsible for the induction of neutrophil sequestration in the liver and directly mediate tissue injury [19]. In many models of liver injury, TNF-α levels are elevated and are correlated with the degree of injury, while inhibition of TNF-α activity attenuates liver injury, protects hepatic morphology, and decreases mortality associated with liver transplants [30]. Results from our present experiments demonstrate that, with the exception of TNF-α levels on day 2 postoperatively, overall levels of TNF-α, IL-1, and IL-6 were significantly elevated in the CS group as compared with the NMP group. These results not only provide evidence for the beneficial effects of NMP, but also show some of the potential mechanisms involved in reducing IRI during RSLT.
Oxidative stress is considered an important causative factor for IRI in a variety of organs, such as the heart, liver, and kidneys [31–33]. Accordingly, attempts have been directed at reducing reactive oxygen species as a means to minimize liver IRI [34]. In this study, we examined the production of MDA, the lipid peroxidation product of oxidative stress, and found that MDA levels were significantly decreased in the NMP group. Such findings indicate that NMP can reduce the occurrence of oxidative stress associated with liver IRI.
Among pro-apoptotic signaling molecules, cytochrome C represents one that is released through mitochondrial permeability transition in response to pro-apoptotic stimuli. As a result, cytochrome C in turn activates caspase 9 and eventually caspase 3 en route to the production of apoptosis [35,36]. It has been reported that caspase 3 inhibition improves survival and reduces early graft injury resulting from IRI of the liver [37]. In this study we showed that, compared with that observed in the CS group, there was an inhibition in the expressions of cytochrome C and caspase 3 in the NMP group, an effect associated with a reduction of apoptosis in liver cells.
Findings from recent studies have revealed that hepatic IRI is characterized by a series of inflammatory reactions [3], with cytokines and chemokines functioning as “liaisons” among the cells involved in these inflammatory responses. NF-κB is an important transcription factor that regulates the synthesis and secretion of various cytokines and chemokines in immunologic stimuli [38,39]. NF-κB activation requires the nuclear translocation of the Rel/p65 subunit of NF-κB, and nuclear NF-κB p65 levels indirectly reflect NF-κB activation [40]. In this study, we assessed NF-κB p65 levels and found that expression levels of NF-κB p65 were inhibited in the NMP group. Therefore, the capacity for NMP perfusion to inhibit the expression of NF-κB in liver tissues would have the effect of reducing the synthesis and secretion of cytokines and chemokines, thus reducing another source responsible for liver IRI following transplantation.
Conclusions
The results of the present study not only provide clear evidence demonstrating the beneficial effects of NMP in protecting against liver IRI, but also identify a number of important molecular factors and signaling pathways associated with this protective effect of NMP in RSLT. The information provided in our study increase understanding of the protection mechanism of NMP, which has great potential to successfully reduce graft size accomplished by ex vivo partial liver resection and improve post-transplant outcomes by reducing IRI. Our study has some limitations. First, the sample size was small. Second, animal experiments currently only focus on the short-term effects of the model, and long-term postoperative complications such as biliary stricture formation cannot be evaluated after only 5 days of observation. Third, optimum and maximum perfusion duration need to be explored, and both clinical and animal experiments have shown that biliary stricture formation is related to prolonged preservation time. Therefore, studies with larger sample sizes are needed to evaluate the long-term benefits of NMP in RSLT.
Footnotes
Source of support: This study was supported by the Tianjin Science and Technology Plan Project (No: 12ZCZDSY02600, 14RCGFSY00147), Science and Technology Fund of Tianjin Municipal Health Bureau (2012KZ114), the International S&T Cooperation Program of China (No. 2015DFG31850), and the Tianjin Clinical Research Center for Organ Transplantation (No. 15ZXLCSY00070)
Conflict of interests
None.
References
- 1.Rao J, Qin J, Qian X, et al. Lipopolysaccharide preconditioning protects hepatocytes from ischemia/reperfusion injury (IRI) through inhibiting ATF4-CHOP pathway in mice. PLoS One. 2013;8:e65568. doi: 10.1371/journal.pone.0065568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selzner N, Rudiger H, Graf R, et al. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–36. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Amara M, Yang SY, Tapuria N, et al. Liver ischemia/reperfusion injury: Processes in inflammatory networks – a review. Liver Transpl. 2010;16:1016–32. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- 4.Foster R, Zimmerman M, Trotter JF. Expanding donor options: Marginal, living, and split donors. Clin liver Dis. 2007;11:417–29. doi: 10.1016/j.cld.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Alkofer B, Samstein B, Guarrera JV, et al. Extended-donor criteria liver allografts. Semin Liver Dis. 2006;26:221–33. doi: 10.1055/s-2006-947292. [DOI] [PubMed] [Google Scholar]
- 6.Garcea G, Nabi H, Maddern GJ. Russell Strong and the history of reduced-size liver transplantation. World J Surg. 2009;33:1575–80. doi: 10.1007/s00268-009-0139-7. [DOI] [PubMed] [Google Scholar]
- 7.Moreno E, García I, Loinaz C, et al. Reduced-size liver transplantation in children and adults. Transplant Proc. 1991;23:1953. [PubMed] [Google Scholar]
- 8.Gans H. Development of modern liver surgery. Lancet. 2002;360:805. doi: 10.1016/S0140-6736(02)09920-8. [DOI] [PubMed] [Google Scholar]
- 9.Fortner JG, Yeh SD, Kim DK, et al. The case for and technique of heterotopic liver grafting. Transplant Proc. 1979;11:269–75. [PubMed] [Google Scholar]
- 10.Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367–70. [PubMed] [Google Scholar]
- 11.Langnas AN, Marujo WC, Inagaki M, et al. The results of reduced-size liver transplantation, including split livers, in patients with end-stage liver disease. Transplantation. 1992;53:387–91. doi: 10.1097/00007890-199202010-00023. [DOI] [PubMed] [Google Scholar]
- 12.de Ville de Goyet J, Hausleithner V, Reding R, et al. Impact of innovative techniques on the waiting list and results in pediatric liver transplantation. Transplantation. 1993;56:1130–36. doi: 10.1097/00007890-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Endoh T, Ohkohchi N, Katoh H, et al. Graft conditioning of liver in non-heart-beating donors by an artificial heart and lung machine in situ. Transplant Proc. 1996;28:110–15. [PubMed] [Google Scholar]
- 14.St Peter SD, Imber CJ, Lopez I, et al. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg. 2002;89:609–16. doi: 10.1046/j.1365-2168.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZB, Gao W, Liu L, et al. Development and assessment of normothermic machine perfusion preservation for extracorporeal splitting of pig liver. Ann Transplant. 2017;22:507–17. doi: 10.12659/aot.904483. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan K, Burt de Perera T, Carere C, et al. Guidelines for the treatment of animals in behavioural research and teaching. Animal Behaviour. 2013;85:287. [Google Scholar]
- 17.Starzl TE, Marchioro TL, Vonkaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Marwan IK, Fawzy AT, Egawa H, et al. Innovative techniques for and results of portal vein reconstruction in living-related liver transplantation. Surgery. 1999;125:265–70. [PubMed] [Google Scholar]
- 19.Pappas SC, Rouch DA, Stevens LH. New techniques for liver transplantation: Reduced-size, split-liver, living-related and auxiliary liver transplantation. Scand J Gastroenterol Suppl. 1995;208:97–100. doi: 10.3109/00365529509107769. [DOI] [PubMed] [Google Scholar]
- 20.Nesher E, Island E, Tryphonopoulos P, et al. Split liver transplantation. Transplant Proc. 2011;43:1736–41. doi: 10.1016/j.transproceed.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Emre S, Umman V. Split liver transplantation: An overview. Transplant Proc. 2011;43:884–87. doi: 10.1016/j.transproceed.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Starzl TE, Groth CG, Brettschneider L, et al. Extended survival in 3 cases of orthotopic homotransplantation of the human liver. Surgery. 1968;63:549–63. [PMC free article] [PubMed] [Google Scholar]
- 23.Schoening WN, Feige I, Schubert T, et al. Iloprost donor treatment reduces ischemia-reperfusion injury in an isolated extracorporeal pig liver perfusion model. Exp Clin Transplant. 2015;13:51–61. [PubMed] [Google Scholar]
- 24.Sutton ME, op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. 2014;9:e110642. doi: 10.1371/journal.pone.0110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogel T, Brockmann JG, Quaglia A, et al. 24-hour normothermic machine perfusion of discarded human liver grafts. Liver Transpl. 2017;23:207–20. doi: 10.1002/lt.24672. [DOI] [PubMed] [Google Scholar]
- 26.Rao J, Qian X, Wang P, et al. All-trans retinoic acid preconditioning protects against liver ischemia/reperfusion injury by inhibiting the nuclear factor kappa B signaling pathway. J Surg Res. 2013;180:e99–106. doi: 10.1016/j.jss.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: New insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–69. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llacuna L, Mari M, Lluis JM, et al. Reactive oxygen species mediate liver injury through parenchymal nuclear factor-kappaB inactivation in prolonged ischemia/reperfusion. Am J Pathol. 2009;174:1776–85. doi: 10.2353/ajpath.2009.080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanschen M, Zahler S, Krombach F, et al. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710–18. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- 30.Nickkholgh A, Li Z, Yi X, et al. Effects of a preconditioning oral nutritional supplement on pig livers after warm ischemia. HPB Surg. 2012;2012 doi: 10.1155/2012/783479. 783479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao G, Shen X, Nan H, et al. Remifentanil protects liver against ischemia/reperfusion injury through activation of anti-apoptotic pathways. J Surg Res. 2013;183:827–34. doi: 10.1016/j.jss.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 32.Braunersreuther V, Jaquet V. Reactive oxygen species in myocardial reperfusion injury: From physiopathology to therapeutic approaches. Curr Pharm Biotechnol. 2012;13:97–114. doi: 10.2174/138920112798868782. [DOI] [PubMed] [Google Scholar]
- 33.Basile DP, Leonard EC, Beal AG, et al. Persistent oxidative stress following renal ischemia-reperfusion injury increases ANG II hemodynamic and fibrotic activity. Am J Physiol Renal Physiol. 2012;302:F1494–502. doi: 10.1152/ajprenal.00691.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando) 2012;26:103–14. doi: 10.1016/j.trre.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caroppi P, Sinibaldi F, Fiorucci L, et al. Apoptosis and human diseases: mitochondrion damage and lethal role of released cytochrome C as proapoptotic protein. Curr Med Chem. 2009;16:4058–65. doi: 10.2174/092986709789378206. [DOI] [PubMed] [Google Scholar]
- 36.Hüttemann M, Pecina P, Rainbolt M, et al. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion. 2011;11:369–81. doi: 10.1016/j.mito.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller TH, Kienle K, Beham A, et al. Caspase 3 inhibition improves survival and reduces early graft injury after ischemia and reperfusion in rat liver transplantation. Transplantation. 2004;78:1267–73. doi: 10.1097/01.tp.0000141095.06273.10. [DOI] [PubMed] [Google Scholar]
- 38.Chandel NS, Trzyna WC, McClintock DS, et al. Role of oxidants in NF-κB activation and TNF-α gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–21. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 39.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 40.Molestina RE, Payne TM, Coppens I, et al. Activation of NF-kappaB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IkappaB to the parasitophorous vacuole membrane. J Cell Sci. 2003;116:4359–71. doi: 10.1242/jcs.00683. [DOI] [PubMed] [Google Scholar]