Abstract
Background
Hypertension is a leading global disease, and myocardial fibrosis is an important adverse effect of hypertension, seriously threatening human health. The IL-6/STAT3 pathway and endothelin-1 (ET-1) were previously suggested to play a part in myocardial fibrosis.
Material/Methods
To investigate the role of Atorvastatin (Ato) in spontaneous hypertension, systolic blood pressure (SBP) and left ventricular mass index (LVMI) were measured, and Masson trichrome staining was performed. Furthermore, the relative protein levels of the IL-6/STAT3/ET-1 pathway were tested.
Results
Ato prevented myocardial fibrosis in spontaneous hypertension rats, especially at the dosage of 50 mg/kg/d. The IL-6/STAT3 pathway was observed to be suppressed by Ato, and ET-1 level in myocardial tissues was also downregulated by Ato. The phosphorylation status of STAT3 was tested after Ato treatment, showing that Ato mainly stimulated the tyr-705 phosphorylation of STAT3.
Conclusions
Results of this study may help promote myocardial fibrosis therapy and provide insights into the IL-6/STAT3/ET-1-mediated mechanism in Ato-induced myocardial fibrosis inhibition.
MeSH Keywords: Endomyocardial Fibrosis; Endothelin-1; Hypertension; Receptors, Interleukin-6; STAT3 Transcription Factor
Background
Hypertension is medically defined as a blood pressure higher than 130 over 80 mmHg and is a leading global cause of disease [1]. Myocardial fibrosis (MF), a serious adverse effect of hypertension on the heart, is mainly caused by pressure overload and associated hormonal actions. MF is an essential feature of hypertension-induced ventricular remodeling and reveals hypertensive target organ injury. Therefore, MF research and treatment are very important to global health.
Atorvastatin (Ato) is a statin that is a widely used cholesterol-lowering drug. Similar to other statins, Ato functions via inhibiting the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. However, the beneficial effects of Ato on cardiovascular disease are not limited to lowering plasma cholesterol [2]; various effects, including plaque thrombogenicity reduction, and anti-inflammatory effects, as well as myocardial remodeling suppression, are also involved [3]. Zhang et al. [4] demonstrated that Ato ameliorates radiation-induced cardiac fibrosis in Sprague-Dawley rats. Similarly, Chang et al. [5] showed that medium-dose Ato treatment for 12 weeks resulted in a significant reduction in myocardial fibrosis in patients. However, the specific mechanisms are not fully understood.
Interleukin-6 (IL-6) is a well-known interleukin that acts as an important mediator in fever and the acute phase response (APR), with multiple functions. It is secreted by T cells and macrophages to stimulate immune response. When IL-6 binds to IL-6R on target cells, it boosts subsequent activation of intracellular signaling such as STAT3 phosphorylation by JAK [6]. It is well-established that the IL-6/STAT3 pathway is involved in various physiological processes, mainly influencing immune and epithelial cells [7]. Recently, multiple studies showed that IL-6 plays an important role in myocardial fibrosis [8,9], suggesting that IL-6/STAT3 participates in hypertension-induced myocardial fibrosis. Endothelin-1 (ET-1) is a potent vasoconstrictor produced by vascular endothelial cells, being 1 of 3 isoforms of endothelin, and is predominantly responsible for cardiovascular effects. Previous studies showed ET-1 plays a role in myocardial fibrosi s[10] and could be a potential therapeutic target in fibroblast activation [11].
The present study aimed to evaluate the efficacy of Ato in myocardial fibrosis suppression in spontaneous hypertension in rats. In addition, we investigated whether the IL-6/STAT3/ET-1 pathway was involved in the mechanism. This study may help promote myocardial fibrosis treatment and expand the use of Ato. Our study is an in-depth exploration of the pathogenesis of myocardial fibrosis.
Material and Methods
Animals and treatments
We purchased 16-week-old male spontaneously hypertensive rats (SHRs) and Wistar-Kyoto rats (WKYs) from the Model Animal Research Center of Nanjing University, China. Animals were allowed to adapt for 1 week, then the SHRs were randomly divided into 5 groups and fed for 6 weeks. Ato was produced by Pfizer (Dalian, China) and administered to the rats in their drinking water. The SHR group (n=8) and WKY group (n=8) received 1 mL distilled water/day by gavage. The Ato-treated SHR group (n=8) received a low dosage of 10 mg/kg/day [12] i.g. The Ato-treated SHR group (n=8) received a medium dosage of 25 mg/kg/day i.g. The Ato-treated SHR group (n=8) received a high dosage of 50 mg/kg/day i.g. The 10 mg/kg/day dose used in rats was equivalent to the dosage used in humans. All animal experiments were approved by the Institutional Animal Care and Use Committee of Zhejiang Chinese Medical University (Hangzhou China).
Systolic blood pressure (SBP) and left ventricular mass index (LVMI) measurements
The SBP was measured by a tail-cuff method with a noninvasive blood pressure measurement system (Chengdu TME Technology Company, China). The rats remained conscious and were placed in a holding device at 37°C for at least 5 min before the measurement. SBP measurement was repeated 3 times in a manner blind to the randomization sequence on each time point, and the mean values were used. After 6 week’s treatment, all rats were sacrificed by anesthesia and the hearts were removed. The left ventricular mass index (LVMI) was obtained by determining the ratio of body weight (BW) and left ventricular mass (LVM) in each rat.
Masson trichrome
Hearts were harvested and kept in liquid nitrogen for later use. Frozen tissues were cut into 5-μm-thick sections and fixed in 4% polyoxymethylene and then embedded in paraffin. Paraffin-section slides were stained with Masson’s trichrome. Photomicrographs were analyzed in a blinded manner. Collagen volume fraction (CVF) was observed with the image analysis system (Motic MED 6.0, Xiamen, China). CVF=collagen area/total area (total area is exclusive of perivascular collagen area and luminal area).
Determination of IL-6, ET-1 in plasma
Plasma IL-6 and ET-1 levels were determined via commercial rat ELISA kit (Thermo Fisher). The results were then calculated by Bioelisa reader Elx800 with the provided standard curve.
Western blot analysis
In brief, total protein was extracted with RIPA buffer containing 1% protease inhibitor PMSF, and a BCA Protein Assay Kit (Beyotime, Shanghai, China) was used to measure the protein concentration. We used 10% SDS-PAGE gel to separate the protein strips, which were then transferred to PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked the with 5% BSA and then incubated with primary antibodies ET-1 (1: 1000, Abcam), IL-6 (1: 1000, Abcam), STAT3 (1: 5000, Abcam), p-STAT3 (1: 2000, Abcam), and GAPDH (1: 5000, Abcam, ab8245) at 4°C overnight. After washing, corresponding HRP-conjugated secondary antibodies (1: 5000, Abcam) were used, and then chemiluminescence (Beyotime) was detected. Western blot analysis was done in 3 animals per group.
Statistical analysis
GraphPad Prism software was used to analyze data. The measurement data are presented as mean ±SEM. ANOVA was used to compare variation between groups. Results with P<0.05 were considered to have statistically significant differences.
Results
Ato improved hypertension in SHR
At age 16 weeks, systolic blood pressure in WKY rats as a control was stably in the normal range, while SHR was significantly higher than in the control (P<0.001). After treating with Ato for 2 weeks, SBP in the group administered 25 mg/kg/d (P<0.01) and 50 mg/kg/d (P<0.001) was significantly improved (Figure 1).
Figure 1.
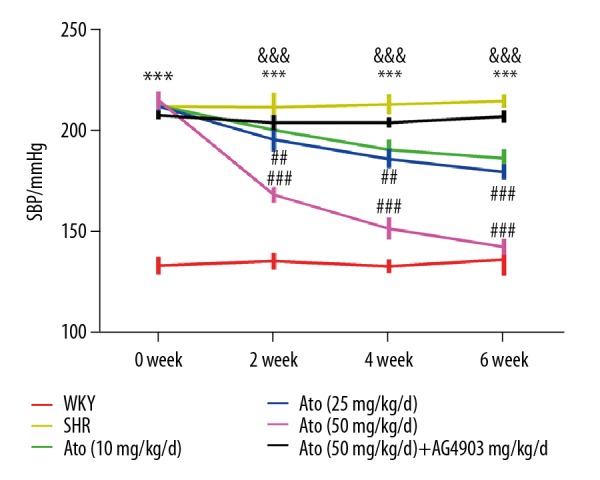
Atorvastatin improved hypertension in SHR rats. Systolic blood pressure was monitored for 6 weeks. *** P<0.001 SHR vs. WKY. ## P<0.01, ### P<0.001 Ato (10, 25, 50 mg/kg/d) vs. SHR. &&& P<0.001 Ato (50 mg/kg/d) + AG490 vs. Ato (50 mg/kg/d). WKY – control group; SHR – SHR model group; Ato – atorvastatin with 10, 25, 50 mg/kg per day. Ato+Ag490 – AG490 as a STAT3 inhibitor co-treated with Atorvastatin in SHR.
Ato attenuated myocardial fibrosis in SHR
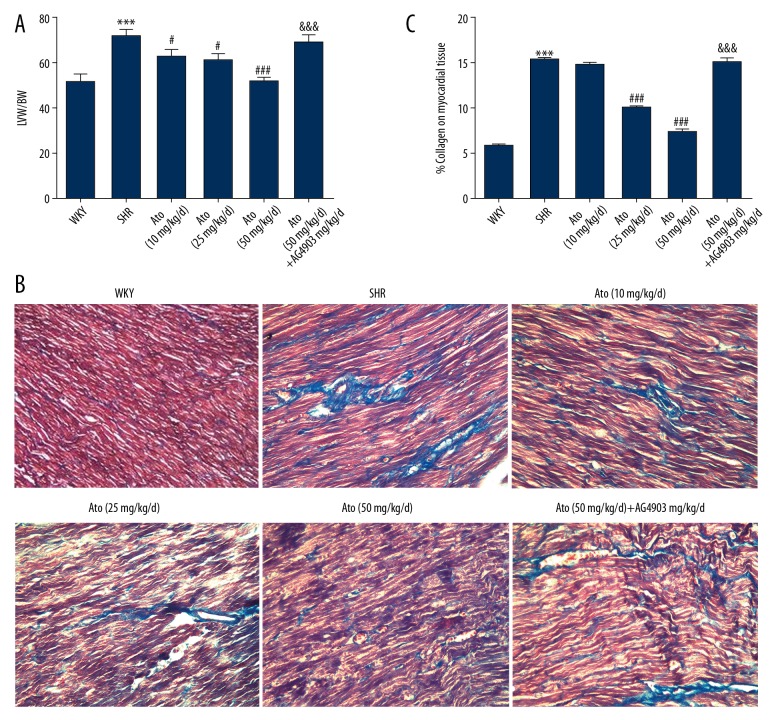
To evaluate whether the changes of SBP after treating with Ato were affected via myocardial fibrosis, the ratio of LVW/BW and morphological index was assessed.
LVW/BW was significantly higher in hypertension rats (71.33±2.33 vs. 51.67±3.67, P<0.001). A dramatic decrease in LVW/BW ratio appeared after administration of Ato at 25 or 50 mg/kg/d (Figure 2A). Masson’s trichrome staining illustrated that the increased interstitial fibrosis in the SHR was attenuated by Ato treatment (Figure 2B), and the ratio of collagen area to heart area was significantly decreased by Ato treatment (Figure 2C).
Figure 2.
Left ventricular mass index and morphological index changed in SHR. (A) Left ventricular mass index. (B) Masson’s trichrome stain was used to detect interstitial fibrosis. (C) The area ratio of collagen and myocardium. *** P<0.001 SHR vs. WKY. # P<0.05, ### P<0.001 Ato (10, 25, 50 mg/kg/d) vs. SHR. &&& P<0.001 Ato (50 mg/kg/d) + AG490 vs. Ato (50 mg/kg/d). LVW – left ventricular weight; BW – body weight. WKY – control group; SHR – SHR model group; Ato – atorvastatin with 10, 25, 50 mg/kg per day. Ato+Ag490 – AG490 as a STAT3 inhibitor co-treated with Atorvastatin in SHR.
Myocardial fibrosis in SHR was prevented via IL6/STATE3/ET-1 pathway
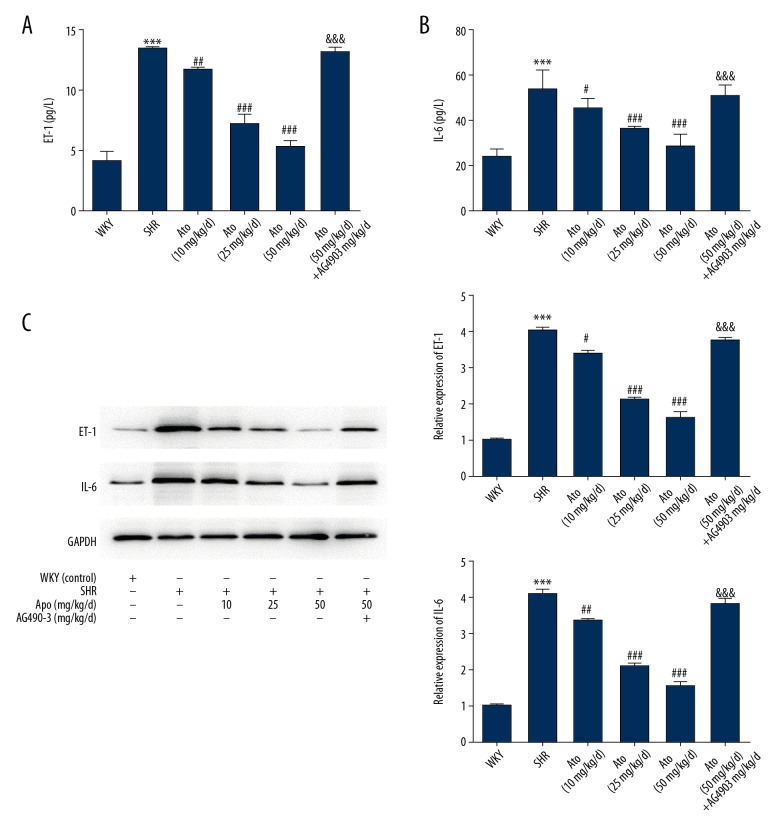
ELISA was used to evaluate the expression of IL-6 and ET-1 in rat plasma. The expression of IL-6 and ET-1 was significantly reduced after treatment with Ato (P<0.001), especially at doses of 25 and 50 mg/kg/d (P<0.001, Figure 3A, 3B).
Figure 3.
Atorvastatin regulated myocardial fibrosis via IL-6/ET-1. The secretion of ET-1 (A) and IL-6 (B) in plasma of the different groups. (C) IL-6 and ET-1 in myocardial tissue were detected via Western blot. *** P<0.001 vs. WKY. # P<0.05, ## P<0.01, ### P<0.001 vs. SHR. WKY – control group; SHR – SHR model group; Ato – Atorvastatin with 10, 25, 50 mg/kg per day; Ato+Ag490 – AG490 as a STAT3 inhibitor co-treated with Atorvastatin in SHR.
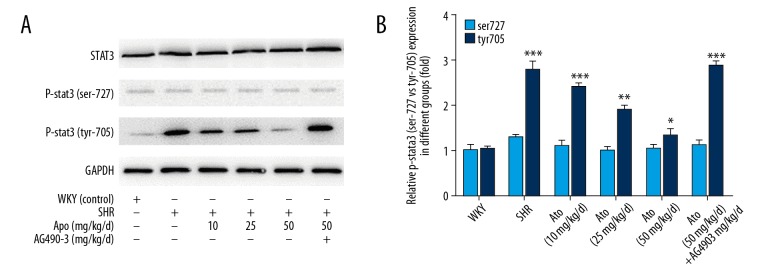
To evaluate whether the IL-6 and ET-1 levels were associated with myocardial fibrosis, IL-6 and ET-1 were detected in myocardial tissues via Western blot, showing the same trend as ELISA analysis in plasma with Ato (Figure 3C). Further investigation of the underlying mechanism showed that phosphorylation proteins of STAT 3 in 2 states, ser-727 and tyr-705, were downregulated after treatment with Ato, and the phosphorylation of STAT3 mainly occurred in the tyr-705 state (Figure 4A, 4B). The STAT 3 inhibitor AG490 showed that effects of Ato in IL-6 and ET-1 in plasma and tissues were reversed (Figure 3). After co-treating with AG490, the SBP, LVW/BW, and morphological changes with Ato were all reversed (Figures 1, 2).
Figure 4.
Atorvastatin regulated myocardial fibrosis via phosphorylation of STAT3. (A) The expression of STAT3 and p-STAT3 in plasma of the different groups by Western blot. (B) Quantitative analysis of STAT3 and p-STAT3 protein levels. * P<0.05, ** P<0.01, *** P<0.001 tyr705 vs. ser727. WKY – control group; SHR – SHR model group; Ato – atorvastatin with 10, 25, 50 mg/kg per day; Ato+Ag490 – AG490 as a STAT3 inhibitor co-treated with Atorvastatin in SHR.
Discussion
The main findings of our study are: 1) Ato significantly reduced SBP and myocardial fibrosis in a dose dependent manner in SHR rats, and the STAT3 inhibitor AG490 canceled the Ato-induced SBP decrease in SHR rats. 2) Ato reduced the levels of IL-6 and ET-1 in plasma of SHR rats, which was also abolished by AG490. 3) Ato inhibited activation of the IL-6/STAT3/ET-1 pathway in myocardial tissue of SHR rats, and the inhibition was also abolished by AG490. 4) Western blot results showed that STAT-3 activation in SHR rats predominantly fell in tyr-705 phosphorylation.
Cardiac fibrosis is an important pathological process in hypertensive heart remodeling and other heart diseases. Current treatments for hypertension and the related cardiac fibrosis are still far from satisfactory. Therefore, it is necessary to search for novel therapy options. Ato is a widely used lipid-lowering drug, and recent reports suggested it might be a potential alternative treatment for cardiac fibrosis.
In the present study, different dosages of Ato were given to SHR rats. As shown in Figures 1 and 2, the drug significantly relieved high blood and cardiac fibrosis in rats, which shows that Ato might effectively inhibit myocardial fibrosis in spontaneous hypertension. So, its clinical use for hypertension patients is expectable. As early as 2001 [13], Takemoto et.al raised that statins could be used as antioxidant therapy to prevent cardiac myocyte hypertrophy. A latest report [14] also showed that Ato helped improve hypertensive cardiac remodeling by Regulating MMPs/TIMPs in SHR Rats. Similarly, a study [4] of radiation-induced cardiac fibrosis revealed an obvious therapeutic effect of Ato, which also partly supports our findings. However, the plasma concentrations of Ato in rats were not measured during this study, so the equivalent doses in humans was not achieved.
After achieving clear results, we further explored the possible mechanism. Blood tests showed that IL-6 and ET-1 was greatly elevated in SHR rats and Ato treatment significantly reduced them. Therefore, we speculated that the IL-6 relative pathway might play a role in Ato-induced myocardial fibrosis inhibition. The STAT3 pathway is well known to be a direct downstream effector of IL-6 [15, 16] and the pathway can induce a variety of vital biological activities, including inflammation and cancer. Importantly, STAT3 also plays an important role in cardiac fibrosis development [17,18]. Thus, we assessed the activity of the STAT3 pathway to determine whether it is involved. As shown in Figure 3, IL-6 and ET-1 were highly expressed in myocardial tissues in SHR rats and were markedly decreased after Ato treatment, with no significant change in total STAT3 levels. AG490 abolished the Ato-induced drop in IL-6 and ET-1 levels. Next, the phosphorylation of STAT3 was measured by Western blot analysis (Figure 4). Since there are 2 forms of phosphorylation in STAT3, 2 corresponding antibodies were used. Results showed that the phosphorylation status of STAT3 was predominated by the tyr-705 sites, and p-STAT3 (tyr-705) showed the same change trend as with IL-6. Taken together, our findings reveal that the IL-6/STAT3 pathway contributes to the inhibition effect of Ato in cardiac fibrosis.
Conclusions
In conclusion, the present study shows that Ato prevented myocardial fibrosis in spontaneous hypertension rats, and IL-6/STAT3/ET-1 signaling may play a part in this; however, more research is needed to draw a clearer and more specific conclusion as to whether other signaling pathways are involved. Our findings suggest that Ato is a potential treatment for cardiac fibrosis and expanding its range of application wide is promising.
Footnotes
Source of support: Departmental sources
References
- 1.Lacruz ME, Kluttig A, Hartwig S, et al. Prevalence and incidence of hypertension in the general adult population: Results of the CARLA – cohort study. Medicine (Baltimore) 2015;94(22):e952. doi: 10.1097/MD.0000000000000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen TR. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S) Atherosclerosis Supplements. 2004;5:81–87. doi: 10.1016/j.atherosclerosissup.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan CJ, Gotto AM, Jr, Basson CT. The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol. 2000;35:1–10. doi: 10.1016/s0735-1097(99)00525-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhang KY, He XY, Zhou Y, et al. Atorvastatin ameliorates radiation-induced cardiac fibrosis in rats. Radiat Res. 2015;184:611–20. doi: 10.1667/RR14075.1. [DOI] [PubMed] [Google Scholar]
- 5.Chang YY, Wu YW, Lee JK, et al. Effects of 12 weeks of atorvastatin therapy on myocardial fibrosis and circulating fibrosis biomarkers in statin-naïve patients with hypertension with atherosclerosis. J Investig Med. 2016;64:1194–99. doi: 10.1136/jim-2016-000092. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat Rev Cancer. 2014;14:736–46. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Sun Y. The IL-6/JAK/STAT3 pathway: Potential therapeutic strategies in treating colorectal cancer (Review) Int J Oncol. 2014;44:1032–40. doi: 10.3892/ijo.2014.2259. [DOI] [PubMed] [Google Scholar]
- 8.Wang JH, Lan Z, Xin P, et al. Hypoxia-stimulated cardiac fibroblast production of IL-6 promotes myocardial fibrosis via the TGF-{beta}1 signaling pathway. Lab Invest. 2016;96:839–52. doi: 10.1038/labinvest.2016.65. [DOI] [PubMed] [Google Scholar]
- 9.Meléndez GC, Mclarty JL, Levick SP, et al. Interleukin-6 mediates myocardial fibrosis, concentric hypertrophy and diastolic dysfunction in rats. Hypertension. 2010;56:225–31. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang LL, Husain M, Stewart DJ. The role of endothelin-1 in myocardial inflammation and fibrosis. In: Feuerstein GZ, Libby P, Mann DL, editors. Inflammation and cardiac diseases. Progress in Inflammation Research. Birkhäuser; Basel: 2003. [Google Scholar]
- 11.Leask A. Potential therapeutic targets for cardiac fibrosis TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–80. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Hao J, Du H, et al. Amlodipine and atorvastatin improved hypertensive cardiac remodeling through regulation of MMPs/TIMPs in SHR rats. Cell Physiol Biochem. 2016;39:47–60. doi: 10.1159/000445604. [DOI] [PubMed] [Google Scholar]
- 13.Takemoto M, Node K, Nakagami H, et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108:1429–37. doi: 10.1172/JCI13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J, Hao J, Du H, et al. Amlodipine and atorvastatin improved hypertensive cardiac remodeling through regulation of MMPs/TIMPs in SHR rats. Cell Physiol Biochem. 2016;39:47–60. doi: 10.1159/000445604. [DOI] [PubMed] [Google Scholar]
- 15.Tadokoro T, Wang Y, Barak LS, et al. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci USA. 2014;111:E3641–49. doi: 10.1073/pnas.1409781111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby JJ, Kalinowski A, Liu M, et al. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA. 2003;100:12929–34. doi: 10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obana M, Maeda M, Takeda K, et al. Therapeutic activation of signal transducer and activator of transcription 3 by Interleukin-11 ameliorates cardiac fibrosis after myocardial infarction. Circulation. 2010;121:684–91. doi: 10.1161/CIRCULATIONAHA.109.893677. [DOI] [PubMed] [Google Scholar]