Abstract
Background
Worldwide, the increasing use of antibiotics has resulted in antimicrobial resistance, leading to studies to find alternative antimicrobial treatments. Tea polyphenols have antibacterial properties. Bacteriocins produced by probiotic lactobacilli can inhibit Gram-positive bacteria. This study used a rabbit model of infection, following femoral fracture with internal fixation, to evaluate the efficacy of the combined use of tea polyphenols and Lactobacillus plantarum ST8SH bacteriocin.
Material/Methods
Twenty-four New Zealand White rabbits underwent femoral fracture, internal fixation, and insertion of a mini-titanium implant, and were inoculated intravenously with suspensions of Staphylococcal bacteria. Four treatment groups included group A, injected with tea polyphenols and bacteriocins (N=6); group B, injected with cefradine and bacteriocins (N=6); group C, injected with tea polyphenols and cefradine (N=6); and group D (controls), injected with saline (N=6). Blood samples were collected at 1, 6, 12, 24, and 48 hours after the injection of bacteriocins. Biofilms that formed on the mini-titanium implant were studied by fluorescence microscopy. Serum levels of level of interleukin (IL)-8, IL-6, and tumor necrosis factor-α (TNF-α) were measured using an enzyme-linked immunosorbent assay (ELISA).
Results
The combination of tea polyphenols and bacteriocins (group A) had a significant inhibitory effect on Staphylococcus aureus (P<0.05) and significant differences in serum levels of IL-8, TNF-α, and IL-6 levels in serum (P<0.05) when compared with groups, B, C, and D.
Conclusions
In a rabbit model of femoral fracture with internal fixation, the combined use of tea polyphenols and Lactobacillus plantarum ST8SH bacteriocin effectively controlled Staphylococcus aureus infection.
MeSH Keywords: Anti-Infective Agents, Biofilms, Polyphenols
Background
Tea, especially green tea, contains polyphenols, which are now known to have many pharmacological properties [1,2]. Studies have shown that polyphenols that are derived from tea have antioxidant properties, and can have a protective role in preventing tumor formation and liver damage, and also have anti-aging effects, and have a role in immune regulation, as well as having a broad-spectrum of antibacterial effects [2].
Bacteriocins. produced by probiotic lactobacilli, are synthesized and secreted during bacterial metabolism [3]. Bacteriocins have been shown to have broad antimicrobial activity against both Gram-positive and Gram-negative bacteria [4]. Worldwide, the increasing use of antibiotics has resulted in antimicrobial resistance, leading to studies to find alternative antimicrobial treatments [5]. However, bacteria rarely develop resistance to bacteriocins, and due to their low toxicity, bacteriocins are now widely used in the food industry to preserve food from bacterial contamination. Therefore, the use of bacteriocins may be a promising way to control infections clinically.
Worldwide, as there are more people who drive, there has been an increasing number of fractures caused by road traffic accidents. Rigid internal fixation is one of the most important ways to treat fractures [6]. However, following rigid internal fixation, infection occasionally occurs, resulting in non-union of the fracture [7]. Therefore, finding effective antimicrobial drugs to control these types of orthopedic infections remains an important problem. There has been a previously reported study on the antibacterial effects of green tea polyphenols on methicillin-resistant Staphylococcus aureus infection [8]. However, there have been no previous studies on the antibacterial effects of a combination of tea polyphenols and bacteriocins.
Therefore, because bacteriocins can inhibit Gram-positive bacteria, the aims of this study were to use a rabbit model of infection, following femoral fracture with internal fixation, to evaluate the efficacy of the combined use of tea polyphenols and Lactobacillus plantarum ST8SH bacteriocin, including measurement of serum levels of interleukin (IL)-8, IL-6, and tumor necrosis factor-α (TNF-α).
Material and Methods
Bacterial culture conditions
Lactobacillus plantarum ST8SH, a bacteriocin-producing strain isolated from Bulgarian salami (Shpek), was grown overnight in Difco™ Lactobacilli De Man, Rogosa, and Sharpe (MRS) broth (Fisher Scientific, Waltham, MA, USA) at 37°C with gentle agitation and maintained on MRS agar plates. The Staphylococcus aureus strain used in this study (subsp. aureus Rosenbach ATCC® 29213™) was grown in BD Difco™ brain heart infusion (BHI) broth (Fisher Scientific, Waltham, MA, USA) at 37°C for 24 h, and maintained on blood agar plates (BioMérieux, Marcy-l’Étoile, France). Bacteria were stored at −80°C in the presence of 20% glycerol.
Isolation of bacteriocin from Lactobacillus plantarum ST8SH
Lactobacillus plantarum ST8SH was grown in MRS broth at 37°C for 24 h. The supernatant was centrifuged at 8000×g for 10 min to remove the cells, after which the supernatants were adjusted to pH 6.5, treated with catalase (5 mg/ml), and filtered using a filter of 0.22 μm pore size (MilliporeSigma, Burlington, MA, USA).
The supernatant was first purified by freeze-drying under reduced pressure and the bacteriocin fraction was dissolved in 0.1% trifluoroacetic acid (TFA). This active fraction was used for final purification by reverse-phase high-performance liquid chromatography (HPLC) on a Waters Nucleosil C18 column (250×4.6 mm) (Waters, Milford, MA, USA). Fractions were collected, vacuum dried, dissolved in 1 mL of deionized water and stored at −20°C
The molecular size of the bacteriocin was determined by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (tricine-SDS-PAGE). A low molecular weight marker, with sizes ranging from 10.0–120.0 kDa was used.
Rabbit model groups and model validation
This study was approved by and followed the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University and Zhejiang Medical Academy (approval number 20180324-12).
Twenty-four New Zealand White rabbits underwent femoral fracture, internal fixation, and insertion of a mini-titanium implant, and were inoculated intravenously with suspensions of Staphylococcal bacteria. Four treatment groups included group A, injected with tea polyphenols and bacteriocins (N=6); group B, injected with cefradine and bacteriocins (N=6); group C, injected with tea polyphenols and cefradine (N=6); group D (controls) were injected with saline (N=6). Blood samples were collected at 1, 6, 12, 24, and 48 hours after the injection of bacteriocins.
Rabbits were anesthetized via inhalation of isoflurane (2%). The surgical procedure was modified from previous studies conducted in our laboratory. A skin incision was made at the site of the right femur. The bone fracture was made using a drill with a diameter of 1 mm. After fixing the guide with a temporary screw to the distal end of the femur, four holes were drilled. A compression element was used to achieve anatomical reduction and compression.
After drilling a hole with 0.5 mm in diameter into the femur, a mini-titanium plate (0.5 mm in diameter) was implanted over the holes to stabilize the fracture. An inoculum of S. aureus (3×105 CFU/ml) in 2 ml of normal saline was pipetted into the femoral space containing the cut end of the implant. The surgical site was closed with Dexon 5-0 sutures. Buprenorphine (0.1 mg/kg) was administered subcutaneously every six hours as analgesia for the duration of the experiment.
Evaluation of biofilm formation on the mini-titanium plate
Samples from the mini-titanium plate were collected 48 h after the injection of the bacteriocins. The mini-titanium plate was placed in 5 ml of PBS and sonicated to remove unattached cells. The specimen was then fixed with 2.5% glutaraldehyde at 4°C for 1 h. After fixation, the tissue was stained with a 0.01% acridine orange solution (Sigma, USA) and washed three times with PBS [9]. The biofilm on the mini-titanium plate was observed with a Nikon 80i microscope at an excitation wavelength of 488 nm for double-strand DNA (green fluorescence). All images were captured and saved.
Detection of cytokines in rabbit serum
Blood samples were collected at 1 h, 6 h, 12 h, 24 h, and 48 h after rigid internal fixation surgery. Blood samples were first centrifuged at 6,000×g for 5 minutes and stored at −70°C. To detect the level of serum interleukin (IL)-8, IL-6 and tumor necrosis factor-α (TNF-α), a quantitative sandwich enzyme-linked immunoassay (ELISA) kit (Invitrogen Biosource, Carlsbad, CA, USA) was used. The levels of each cytokine were expressed as pg/mg of protein.
Statistical analysis
All experiments were performed in triplicate. SPSS 14.0 software for Windows was used for data analysis. The results were compared statistically using a Student’s t-test. P-values <0.05 were considered to be statistically significant.
Results
Biofilm formation
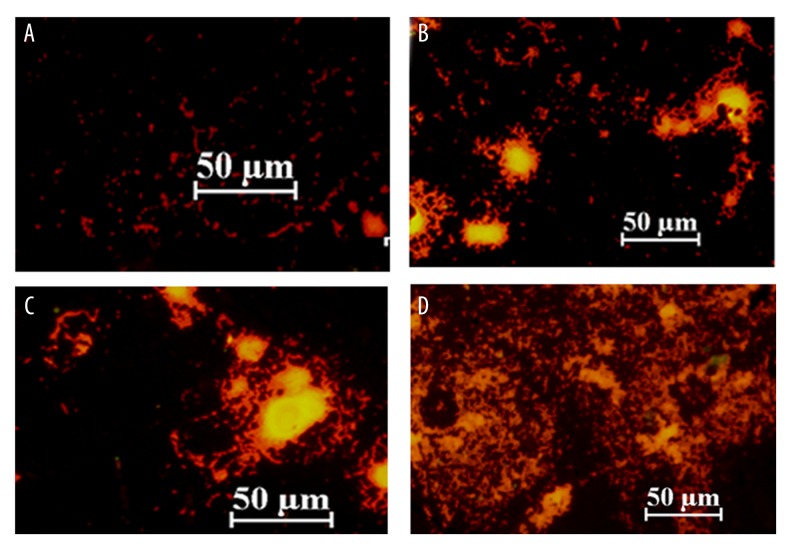
Forty-eight hours after infection in group A, B and C, the integrated optical density (IOD) of Staphylococcus aureus biofilms were significantly different when compared with the control group (group D) (p<0.05) (Figure 1). The number of bacteria in group A was significantly decreased when compared with groups B and C (p<0.0) (Figure 1). Fluorescence images of the biofilms are shown in Figure 2.
Figure 1.
(A–D) Fluorescence microscopy images of the biofilms of Staphylococcus aureus that formed on the mini-titanium implants in rabbits in the four study groups at 48 hours following surgery. The four study groups were group A, injected with tea polyphenols and bacteriocins (N=6); group B, injected with cefradine and bacteriocins (N=6); group C, injected with tea polyphenols and cefradine (N=6); group D (controls) were injected with saline (N=6).
Figure 2.
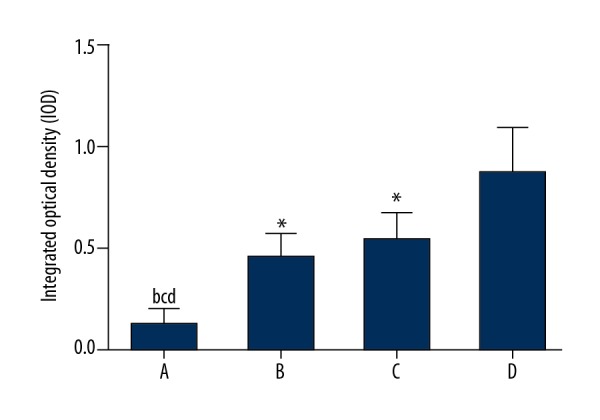
The integrated optical density (IOD) of the biofilms of Staphylococcus aureus that formed on the mini-titanium implants in rabbits in the four study groups at 48 hours following surgery. (A) The four study groups were group A, injected with tea polyphenols and bacteriocins (N=6); group B, injected with cefradine and bacteriocins (N=6); group C, injected with tea polyphenols and cefradine (N=6); group D (controls) were injected with saline (N=6). (B) Group A compared with group B (P<0.05). (C) Group A compared with group C (P<0.05). (D) Group A compared with Group D (P<0.05). * Compared with Group D (P<0.05).
Serum levels of pro-inflammatory cytokines in the four study groups
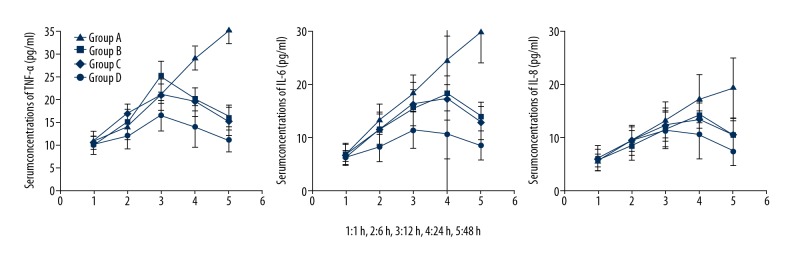
Serum levels of tumor necrosis factor-α (TNF-α) in the serum of rabbits in group A were significantly decreased when compared with groups B, C, and D at 48 h, and TNF-α levels reached a minimum at 48 h following the operation (p<0.05) (Table 1, Figure 3). Interleukin (IL)-6 levels in the serum of rabbits in group A were significantly decreased when compared with those in groups B, C, and D at 48h, and IL-6 levels reached a minimum at 8 h following the operation (p<0.05) (Table 2, Figure 3). The level of IL-8 in the serum of rabbits in group A was increased and IL-8 levels reached a maximum at 8 h following the operation, after 48h, IL-8 levels decreased to the same level before the operation and IL-8 levels were significantly decreased when compared with group B, C, and D (p<0.05) (Table 3, Figure 3).
Table 1.
Serum concentrations of TNF-α (pg/ml) in rabbits of four groups at 1 h, 6 h, 12 h, 24 h and 48 h following operation (Group A: Tea polyphenols and bacteriocins; Group B: cefradine and bacteriocins Group C: cefradine and bacteriocins Group D: control mean ±SD; b, Group A compared with Group B, P<0.05; C, Group A compared with Group C, P<0.05; d, Group A compared with Group D, P<0.05; * compared with Group D, P<0.05).
| 1 h | 6 h | 12 h | 24 h | 48 h | |
|---|---|---|---|---|---|
| Group A | 10.07±2.12 | 12.23±2.87 | 16.43±3.43 | 14.34±3.78bcd | 10.24±2.76bcd |
| Group B | 9.98±2.76 | 16.34±3.34 | 21.22±4.45 | 19.32±4.43 | 15.54±3.98* |
| Group C | 10.87±1.98 | 17.45±4.23 | 25.26±4.98 | 20.01±5.32 | 16.34±4.32* |
| Group D | 11.67±2.03 | 14.23±2.56 | 21.21±3.34 | 29.43±4.54 | 36.43±5.67 |
Figure 3.
Serum concentrations of interleukin (IL)-8, IL-6, and tumor necrosis factor-α (TNF-α) in rabbits in the four study groups at 48 hours following surgery. The four study groups were group A, injected with tea polyphenols and bacteriocins (N=6); group B, injected with cefradine and bacteriocins (N=6); group C, injected with tea polyphenols and cefradine (N=6); group D (controls) were injected with saline (N=6).
Table 2.
Serum concentrations of IL-6 (pg/ml) in rabbits of four groups at 1 h, 6 h, 12 h, 24 h and 48 h following operation (Group A: Tea polyphenols and bacteriocins; Group B: cefradine and bacteriocins Group C: cefradine and bacteriocins Group D: control mean ±SD, b, Group A compared with Group B, P<0.05; C, Group A compared with Group C, P<0.05; d, Group A compared with Group D, P<0.05;*, compared with Group D, P<0.05).
| 1 h | 6 h | 12 h | 24 h | 48 h | |
|---|---|---|---|---|---|
| Group A | 6.17±1.32 | 8.14±2.65 | 11.34±3.47 | 10.45±3.54bcd | 8.42±2.79bcd |
| Group B | 6.48±2.46 | 11.59±3.39 | 16.29±5.56 | 17.39±4.98 | 12.64±3.78* |
| Group C | 6.87±1.45 | 11.47±4.35 | 15.45±4.67 | 18.22±6.39 | 13.83±4.67* |
| Group D | 6.68±2.14 | 13.32±4.46 | 18.27±4.39 | 24.45±5.57 | 29.45±6.68 |
Table 3.
Serum concentrations of IL-8 (pg/ml) in rabbits of four groups at 1 h, 6 h, 12 h, 24 h and 48 h following operation (Group A: Tea polyphenols and bacteriocins; Group B: cefradine and bacteriocins Group C: cefradine and bacteriocins Group D: control mean ±SD, b, Group A compared with Group B, P<0.05; C, Group A compared with Group C, P<0.05; d, Group A compared with Group D, P<0.05; *, compared with Group D, P<0.05).
| 1 h | 6 h | 12 h | 24 h | 48 h | |
|---|---|---|---|---|---|
| Group A | 6.17±2.32 | 9.43±2.65 | 11.43±3.32 | 10.45±3.43 | 7.43±2.49bcd |
| Group B | 5.78±2.56 | 9.64±3.49 | 12.38±3.49 | 13.32±3.49 | 10.50±3.67* |
| Group C | 5.77±1.93 | 8.47±3.54 | 11.59±4.54 | 14.26±4.09 | 10.31±4.12* |
| Group D | 5.68±2.12 | 9.43±2.76 | 13.28±3.76 | 17.23±4.68 | 19.23±4.47 |
Discussion
Worldwide, with the increasing use of antibacterial agents, the incidence of nosocomial infections caused by Gram-positive bacteria that are resistant to antimicrobials are increasing [10]. Of the Gram-positive bacteria, Staphylococcus aureus has become one of the important nosocomial pathogens [11]. Therefore, finding an effective way to control Staphylococcus aureus infections is a priority.
Tea polyphenols are broad-spectrum antimicrobials with limited toxicity that have been recognized and used in several countries in the world [12]. Several studies have shown that tea polyphenols were active against a wide range of bacteria, including E. coli, Staphylococcus aureus, Staphylococcus epidermidis, Lactobacilli spp., and Vibrio cholerae, including an effect on intestinal pathogenic bacteria [13]. Tea polyphenols can also effectively prevent antibiotic-resistant Staphylococcus infections and are active against a range of skin fungi [14,15]. Probiotic Lactobacilli can adjust the balance of the intestinal microflora, enhance the body’s immunity and resistance, and promote the growth of normal gut flora [16]. Bacteriocins are antibacterial proteins or peptides with biological activity, which are secreted in the process of metabolism by probiotic Lactobacilli, and can inhibit many Gram-positive bacteria [17]. The findings of the present study have shown that a combination of tea polyphenols and bacteriocins might be a promising treatment for bacterial infections, particularly for infection with S. aureus.
Tumor necrosis factor-α (TNF-α) is a cytokine with a broad range of biological activities and is involved in immunity, inflammation, among other pathological and physiological processes [18]. Increased expression of TNF-α has been shown to be associated with B-cell hyperplasia and to modify the ability of B-cells to produce antibodies [19]. TNF-α can also induce the generation of reactive oxygen species and the synthesis of prostaglandins, which further aggravates the pathology of immune disease [20]. Interleukin-8 (IL-8) promotes the accumulation of inflammatory cells in the tissues, including neutrophils and T-cells, which then release their active products [21]. IL-6 plays a complicated role in infectious diseases, and its protective effect may be due to promoting the function of cytotoxic T-cells, natural killer (NK) cells and macrophages, promoting the generation of specific antibodies, and enhancing defense against pathogenic microorganisms [22]. In this study, the combination of tea polyphenols and bacteriocins significantly changed the levels of the three inflammatory cytokines, TNF-α, IL-8, and IL-6, which is a finding supported by previous studies.
This study had several limitations. The rabbit internal fracture fixation and infection model is quite different from clinical infections, as clinical infections are caused by a variety of infectious organisms. The results of this study, in an animal model, may not reflect the clinical situation of specific infections that occur after the fixation of internal fractures. Further studies are required to extend these in vivo findings in animal models and in clinical studies.
Conclusions
In a rabbit model of femoral fracture with internal fixation, the combined use of tea polyphenols and Lactobacillus plantarum ST8SH bacteriocin effectively controlled Staphylococcus aureus infection, when assessed by the reduction in the formation of biofilms on mini-titanium implants and serum levels of inflammatory cytokines. The findings of this study indicated that the combination of bacteriocins and tea polyphenols may be a promising way to control postoperative fracture infections, but further studies are required to support the findings of this preliminary study.
Footnotes
Source of support: Departmental sources
References
- 1.Afzal M, Safer AM, Menon M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology. 2015;23(4):151–61. doi: 10.1007/s10787-015-0236-1. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui MW, Sharangi AB, Singh JP, et al. Antimicrobial properties of teas and their extracts in vitro. Crit Rev Food Sci Nutr. 2015;56(9):1428–39. doi: 10.1080/10408398.2013.769932. [DOI] [PubMed] [Google Scholar]
- 3.Heng NCK, Wescombe PA, Burton JP, et al. The diversity of bacteriocins in gram-positive bacteria. In: Riley MA, Chavan MA, editors. Bacteriocins. Springer; Berlin, Heidelberg: 2007. pp. 45–92. [Google Scholar]
- 4.Line JE, Svetoch EA, Eruslanov BV, et al. Isolation and purification of enterocin E-760 with broad antimicrobial activity against Gram-positive and Gram-negative bacteria. Antimicrob Agents Chemother. 2008;52(3):1094–100. doi: 10.1128/AAC.01569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palaniappan K, Holley RA. Use of natural antimicrobials to increase antibiotic susceptibility of drug-resistant bacteria. Int J Food Microbiol. 2010;140(2–3):164–68. doi: 10.1016/j.ijfoodmicro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 6.McMillan TE, Johnstone AJ. Primary screw perforation or subsequent screw cut-out following proximal humerus fracture fixation using locking plates: A review of causative factors and proposed solutions. Int Orthop. 2018;42(8):1935–42. doi: 10.1007/s00264-017-3652-6. [DOI] [PubMed] [Google Scholar]
- 7.Schenkel JS, Jacobsen C, Rostetter C, et al. Inferior alveolar nerve function after open reduction and internal fixation of mandibular fractures. J Craniomaxillofac Surg. 2016;44(6):743–48. doi: 10.1016/j.jcms.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Cho Y-S, Schiller NL, Oh K-H. Antibacterial effects of green tea polyphenols on clinical isolates of methicillin-resistant Staphylococcus aureus. Curr Microbiol. 2008;57(6):542–46. doi: 10.1007/s00284-008-9239-0. [DOI] [PubMed] [Google Scholar]
- 9.Vickerman MM, Mansfield JM, Min Z, et al. Codon-optimized fluorescent mTFP and mCherry for microscopic visualization and genetic counterselection of Streptococci and Enterococci. J Microbiol Methods. 2015;116:15–22. doi: 10.1016/j.mimet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice LB. Antimicrobial resistance in Gram-positive bacteria. Am J Infect Control. 2006;34(5):S11–19. doi: 10.1016/j.ajic.2006.05.220. [DOI] [PubMed] [Google Scholar]
- 11.Edmond MB, Wallace SE, McClish DK, et al. Nosocomial bloodstream infections in United States hospitals: A three-year analysis. Clin Infect Dis. 1999;29(2):239–44. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 12.Mandel SA, Amit T, Weinreb O, Youdim MBH. Understanding the broad-spectrum neuroprotective action profile of green tea polyphenols in aging and neurodegenerative diseases. J Alzheimers Dis. 2011;25(2):187–208. doi: 10.3233/JAD-2011-101803. [DOI] [PubMed] [Google Scholar]
- 13.Sakanaka S, Kim M, Taniguchi M, Yamamoto T. Antibacterial substances in Japanese green tea extract against Streptococcus mutans, a cariogenic bacterium. Agric Biol Chem. 1989;53(9):2307–11. [Google Scholar]
- 14.Radji M, Agustama RA, Elya B, Tjampakasari CR. Antimicrobial activity of green tea extract against isolates of methicillin-resistant Staphylococcus aureus and multidrug-resistant Pseudomonas aeruginosa. Asian Pac J Trop Biomed. 2013;3(8):663–67. doi: 10.1016/S2221-1691(13)60133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan X, Niu G, Liu H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chemical Engineering and Processing: Process Intensification. 2003;42(2):129–33. [Google Scholar]
- 16.Isolauri E, Sütas Y, Kankaanpää P, et al. Probiotics: Effects on immunity. Am J Clin Nutr. 2001;73(2):444s–50s. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 17.Patricia AS, Manuel ML, Mu D, Kuipers OP. Bacteriocins of lactic acid bacteria: Extending the family. Appl Microbiol Biotechnol. 2016;100(7):2939–51. doi: 10.1007/s00253-016-7343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabry A, El-husseini A, Mahmoud K, et al. Proinflammatory cytokines (TNF-α and IL-6) in Egyptian patients with SLE: its correlation with disease activity. Cytokine. 2006;35(3–4):148–53. doi: 10.1016/j.cyto.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Rider P, Carmi Y, Cohen I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol. 2016;2016 doi: 10.1155/2016/9259646. 9259646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Li Y, Chen X, et al. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7(2):113–17. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 22.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005(5):273–79. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]