Abstract
Introduction:
Type 2 diabetes mellitus and obesity may increase risks for cognitive decline as individuals age. It is unknown whether this results in different prevalences of cognitive impairment for women and men.
Methods:
The Action for Health in Diabetes, a randomized controlled clinical trial of a 10-year intensive lifestyle intervention, adjudicated cases of cross-sectional cognitive impairment (mild cognitive impairment or dementia) 10–13 years after enrollment in 3802 individuals (61% women).
Results:
The cross-sectional prevalences of cognitive impairment were 8.3% (women) and 14.8% (men): adjusted odds ratio 0.55, 95% confidence interval [0.43, 0.71], P < .001. Demographic, clinical, and lifestyle risk factors varied between women and men but did not account for this difference, which was limited to individuals without apolipoprotein E (APOE)-ε4 alleles (interaction P =.034).
Conclusions:
Among overweight and obese adults with type 2 diabetes mellitus, traditional risk factors did not account for the lower prevalence of cognitive impairment observed in women compared with men.
Keywords: Sex differences, Type 2 diabetes mellitus, Obesity, Cognitive impairment, Risk factors
1. Background
In both women and men, type 2 diabetes (T2DM) and midlife overweight and obesity increase long-term risks for cognitive decline and cognitive impairment. Studies disagree, however, whether these effects vary by sex and, if so, whether this may be attributable to differences in risk factor distributions or strengths of risk factor relationships [1–8].
The Action for Health in Diabetes (Look AHEAD) enrolled overweight and obese individuals with T2DM, aged 45–76 years, in a randomized controlled clinical trial of a 10-year multidomain behavioral intervention. When their cognitive impairment status (mild cognitive impairment [MCI] or dementia) was ascertained after intervention, its prevalence was 30% lower in women than in men (P = .006) [9]. Here, we explore factors that might account for this striking finding: whether it could be attributed to differences between women and men in demography, lifestyle, medical care, and genotype and whether its magnitude varied across risk factors. We also examined whether markers related to intervention adherence (weight, waist girth, and physical activity) and markers of diabetes treatment and control over time (fasting glucose, glycated hemoglobin (HbA1c), and medications) might have differentially affected risks for cognitive impairment. Identification of sex-specific differences may aid in developing better strategies to prevent cognitive decline and dementia.
2. Methods
The design, Consolidated Standard of Reporting Trials diagram, and primary results of Look AHEAD have been published [10,11]. It was a single-masked randomized controlled trial that recruited 5145 individuals during 2001–2004 with body mass index (BMI) > 25 kg/m2 (>27 kg/m2 if on insulin), HbA1c < 11%, systolic/diastolic blood pressure <160/100 mm Hg, and triglycerides <600 mg/dL. During screening, each participant completed a 2-week run-in, during which they successfully recorded information about diet and physical activity. Each met a behavioral psychologist or interventionist to confirm whether they understood intervention requirements and exclude those with issues likely to impair adherence. Participants provided written informed consent. Local institutional review boards approved protocols.
2.1. Interventions
Participants were randomly assigned with equal probability to Intensive Lifestyle Intervention (ILI) or Diabetes Support and Education (DSE). The ILI included diet modification and physical activity designed to induce weight loss to average ≥ 7% at 1 year and maintain this over time [12]. ILI participants were assigned a daily calorie goal (1200–1800 based on initial weight), with <30% of total calories from fat (<10% from saturated fat) and ≥15% from protein. The physical activity goal was ≥175 min/week through activities similar in intensity to brisk walking.
DSE participants were invited (but not required) to attend three annual group sessions focused on diet, physical activity, and social support [13]. They received no specific diet, activity, or weight goals or information on behavioral strategies.
Interventions ended in September 2012. The mean (range) lengths of intervention for ILI and DSE participants reported in this article were both 9.8 (8.4 to 11.1) years.
2.2. Risk factors for cognitive decline
Certified clinic staff, masked to intervention assignment, collected data [10]. Digital scales recoded annual measures of weight. BMI and waist girths were obtained with standard protocols. The Paffenbarger Physical Activity Questionnaire was used to estimate weekly minutes of moderate-to-vigorous physical activity [14]. Participants’ current prescription medication use was recorded. At enrollment, women were asked whether they had used hormone therapy previously; however, age at use and its duration were not queried. The Beck Depression Inventory-II assessed depression symptoms [15]. Blood specimens were collected after a ≥ 12-hour fast and analyzed centrally for HbA1c and glucose. A maximal graded exercise test was administered during screening. For participants who provided consent (80% of women; 86% of men, P < .001), TaqMan genotyping for apolipoprotein E (APOE)-ε4 (the rs7412 allele) was performed.
2.3. Cognitive function
Cross-sectional standardized assessments of cognitive function in the full cohort occurred between August 2013 and December 2014 [16]. The Rey Auditory Verbal Learning Test evaluated verbal learning. The Digit Symbol Coding test evaluated speed of processing and working memory. The modified Stroop Color and Word Test and the Trail Making Test-Part B evaluated executive function. The Modified Mini–Mental State Examination evaluated global cognitive functioning. Test results were standardized, using z-scores and ordered so that higher scores reflected better performance [16]. The primary cognitive measure for Look AHEAD was an average of these z-scores (composite cognitive function).
2.4. Cognitive impairment adjudication
A masked expert panel adjudicated cognitive status to identify cognitive impairment and dementia [9]. Participants whose modified Mini–Mental State Examination scores fell below age- and education-specific cutpoints underwent review, which was supplemented by telephone administration of the Functional Assessment Questionnaire to a friend or family member to query functional status and instrumental activities of daily living [17]. Additional cases were identified through adjudicating Functional Assessment Questionnaire scores from participants who had died before cognitive testing.
Two adjudicators independently reviewed all cognitive tests and Functional Assessment Questionnaire scores, and all relevant data (physical function, medications, depression, and hospitalizations) to make their primary classification (no impairment, MCI, and probable dementia), using a successful protocol from other multicenter trials [18]. When MCI was identified, they made a secondary classification of sub-type: amnestic single domain, amnestic multidomain, nonamnestic single domain, or nonamnestic multiple domain. Adjudicators used classification of “cannot classify” if they could not make a confident classification.
2.5. Statistical analysis
Differences between women and men in risk factors for cognitive impairment (markers of demography, lifestyle, clinical care, and disease) at enrollment were evaluated with chi-squared and t-tests. Mean differences in changes in weight, waist girth, and physical activity, averaged across 4-year spans during follow-up were compared between women and men with t-tests. We assessed whether women or men had greater cross-sectional odds of MCI and dementia using logistic regression. We examined whether sex-related differences varied across risk factors using tests for interactions in models. To explore whether sex differences in the odds of MCI and dementia could be accounted for by differences in risk factor profiles, we used stepwise selection (backward and forward) to develop a risk factor prediction model and then examined whether sex-related differences in the odds of MCI and dementia remained after adjustment for estimates from the model. We also assessed sex-related differences in cognitive test scores using analyses of covariance (adjusting for age, education, race/ethnicity, and intervention assignment) overall and for normal and cognitively impaired subgroups. Inverse probability weighting was used to assess the sensitivity of findings to differential retention [19].
3. Results
As described in Table 1, 3802 Look AHEAD participants who underwent cognitive assessments (N = 3771; 99%) and/or whose proxies were interviewed for adjudication of cognitive impairment were included in analyses. At enrollment, compared with men (39% of the cohort), women were significantly younger, less fit, and had higher BMI (all P < .001). Women were also significantly more likely than men to be from a minority racial/ethnic group, to have less formal education, to have shorter durations of diabetes, and to report more symptoms of depressed mood. Compared with men, women were significantly less likely to have a history of cardiovascular disease or smoking and reported less alcohol intake.
Table 1.
Characteristics at the time of Look AHEAD enrollment of participants who had cognitive assessments: mean (standard deviation) or N (percent)
| Women | Men | ||
|---|---|---|---|
| Baseline characteristic | N = 2323 | N = 1479 | P value |
| Age at enrollment | <.001 | ||
| 45–54 | 684 (29.4) | 303 (20.5) | |
| 55–64 | 1327 (57.1) | 839 (56.7) | |
| 65–76 | 312 (13.4) | 337 (22.8) | |
| Race/ethnicity | <.001 | ||
| African-American | 476 (20.5) | 148 (10.0) | |
| American Indian | 169 (7.3) | 43 (2.9) | |
| Hispanic | 381 (16.4) | 130 (8.8) | |
| Non-Hispanic white | 1222 (52.6) | 1112 (75.2) | |
| Other, multiple | 75 (3.2) | 46 (3.1) | |
| Education, miss = 94 | <.001 | ||
| High school or less | 1501 (66.6) | 646 (44.4) | |
| College graduate | 417 (18.5) | 425 (29.2) | |
| Post college | 335 (14.9) | 384 (26.4) | |
| Body mass index, kg/m2 | <.001 | ||
| 25–29 | 333 (14.3) | 258 (17.4) | |
| 30–39 | 1403 (60.4) | 971 (65.6) | |
| ≥40 | 587 (25.3) | 250 (16.9) | |
| HbAlc, percent | .070 | ||
| <7.0 | 1074 (46.2) | 731 (49.4) | |
| 7.0–8.9 | 1031 (44.4) | 634 (42.9) | |
| ≥9.0 | 218 (9.4) | 114 (7.7) | |
| Diabetes duration, years, miss = 27 | .017 | ||
| <5 | 1116 (48.4) | 654 (44.5) | |
| ≥5 | 1188 (51.6) | 817 (55.5) | |
| Hypertension | 1902 (81.9) | 1223 (82.7) | .520 |
| History of cardiovascular disease | 174 (7.5) | 267 (18.0) | <.001 |
| Smoking, miss = 8 | <.001 | ||
| Never | 1399 (60.3) | 577 (39.1) | |
| Former | 829 (35.7) | 834 (56.5) | |
| Current | 93 (4.0) | 64 (4.3) | |
| Alcohol intake | <.001 | ||
| None | 1840 (79.2) | 756 (51.1) | |
| <1/day | 442 (19.0) | 551 (37.2) | |
| ≥1/day | 41 (1.8) | 172 (11.6) | |
| Fitness from graded exercise testing | <.001 | ||
| <7.5 METS/day | 1621 (69.8) | 599 (40.5) | |
| ≥7.5 METS/day | 702 (30.2) | 880 (59.5) | |
| APOE genotype, miss = 671 | .793 | ||
| No ε4 allele | 1425 (76.5) | 982 (77.4) | |
| 1 ε4 allele | 404 (21.7) | 263 (20.7) | |
| 2 ε4 alleles | 33 (1.8) | 24 (1.9) | |
| Beck depression inventory, miss =11 | <.001 | ||
| 0–10 | 1922 (83.1) | 1349 (91.3) | |
| ≥11 | 392 (16.9) | 128 (8.7) | |
| Intervention assignment | .356 | ||
| Diabetes Support and Education | 1155 (50.2) | 719 (48.6) | |
| Intensive Lifestyle Intervention | 1158 (49.8) | 760 (51.4) |
Abbreviation: HbA1c, glycated hemoglobin.
Table 2 summarizes changes in weight, waist girth, and physical activity from enrollment throughout follow-up before cognitive status ascertainment. At enrollment, women tended to weigh less, have smaller waist girths, and reported less physical activity than men (all P < .05). Within the ILI group, changes in weight and physical activity were initially smaller among women; however, these differences attenuated during follow-up.
Table 2.
Markers related to intervention adherence by intervention assignment and sex
| Markers of weight loss and intervention adherence | ||||
|---|---|---|---|---|
| Change from baseline, Mean (SD) | ||||
| Baseline, Mean (SD) | Year 1–4 mean | Year 5–8 mean | Year 9–12 mean | |
| Diabetes Support and Education | ||||
| Weight, kg | ||||
| Women | 95.45 (17.45) | −1.10 (6.61) | −2.26 (9.47) | −4.76 (10.50) |
| Men | 108.89 (17.97) | −0.74 (5.29) | −1.11 (8.24) | −3.08 (10.36) |
| P value | .001 | .210 | .007 | .001 |
| Waist girth, cm | ||||
| Women | 111.0 (13.7) | −1.03 (6.58) | −0.49 (8.09) | −0.76 (9.21) |
| Men | 117.8 (13.0) | −0.59 (6.93) | 0.94 (8.64) | 0.82 (9.86) |
| P value | .001 | .163 | .001 | .001 |
| Physical activity, kcal* | ||||
| Women | 675.1 (890.2) | 68.2 (888.9) | −48.8 (1003.6) | −205.6 (1115.1) |
| Men | 1166.2 (1290.3) | 180.1 (1366.8) | −121.0 (1549.8) | −274.1 (1584.7) |
| P value | .001 | .147 | .410 | .462 |
| Intensive Lifestyle Intervention | ||||
| Weight, kg | ||||
| Women | 94.43 (17.74) | −5.58 (6.69) | −4.54 (8.55) | −6.29 (9.97) |
| Men | 108.40 (18.98) | −7.50 (7.51) | −4.89 (8.21) | −5.95 (9.52) |
| P value | .001 | .001 | .377 | .466 |
| Waist girth, cm | ||||
| Women | 109.9 (13.2) | −4.55 (7.42) | −2.00 (8.28) | −1.78 (9.51) |
| Men | 117.8 (13.8) | −6.20 (7.67) | −1.84 (8.19) | −1.25 (8.89) |
| P value | .001 | .001 | .668 | .229 |
| Physical activity, kcal* | ||||
| Women | 715.7 (914.3) | 544.7 (1163.1) | 125.8 (1788.9) | −156.9 (1118.5) |
| Men | 1033.7 (1147.4) | 774.8 (1433.2) | 235.0 (1826.1) | −30.9 (1589.1) |
| P value | .001 | .010 | .383 | .180 |
Kilocalories per week from moderate or intensive physical activity.
Cognitive status was assessed an average [range] of 11.4[9.5, 13.5] years after enrollment. Central adjudication classified 159 (6.9%) women as meeting criteria for MCI and 32 (1.4%) women as meeting criteria for dementia. Among men, 179 (12.3%) met criteria for MCI and 36 (2.5%) met criteria for dementia. The odds ratio (OR) [95% confidence interval] for MCI or dementia in women compared with men, after adjusting for age, race/ethnicity, education, and intervention assignment, was 0.55 [0.43, 0.71] (P < .001). With similar covariate adjustment, the OR for dementia was 0.79 [0.47, 1.33] (P = .37). Among the 159 women classified as MCI, subtypes were assigned to 111 (70%). Of these 111 cases, 22 (20%) were subtyped as amnestic single domain, 39 (35%) as amnestic multiple domain, 44 (40%) as nonamnestic single domain, and 6 (6%) as nonamnestic multiple domain. Among the 179 men classified as MCI, subtypes were assigned to 135 (75%). Of these 135 cases, 37 (27%) were subtyped as amnestic single domain, 63 (47%) were subtyped as amnestic multiple domain, 31 (23%) were subtyped as nonamnestic single domain, and 4 (1%) were subtyped as nonamnestic multiple domain.
Sex-specific differences in intervention response did not explain the higher odds of cognitive impairment among men. With adjustment for age, education, and race/ethnicity, the ORs for cognitive impairment among women compared with men was 0.50 [0.35, 0.70] among DSE participants and0.61 [0.43, 0.86] among ILI participants (interaction P = .450). Additional adjustment for baseline markers related to intervention adherence or change from baseline (Table 2) did not attenuate these differences. We explored whether sex-related differences in diabetes treatment and control over time could account for differences in the prevalence of cognitive impairment, focusing on HbA1c, fasting glucose, and use of insulin and biguanide therapy. In models including measures of intervention adherence (Table 2) and diabetes treatment and control (Supplementary Table 1) as covariates, sex-related differences in cognitive impairment remained highly significant (all P < .001), and the ORs varied little among models (range 0.48 to 0.56).
Women significantly (P < .05) outperformed men in all cognitive tests except the Stroop test of executive function (Table 3). The largest mean difference was for measures of verbal learning and memory. Among cognitively normal participants, women outperformed men in verbal learning and memory, processing speed, and global cognitive function. Among those classified as cognitively impaired, only sex-related differences in verbal learning and memory were evident.
Table 3.
Cognitive function test scores (transformed into z-scores), with covariate adjustment for age, education, race/ethnicity, and intervention assignment
| Women | Men | ||
| Cognitive measure | N = 2323 | N = 1479 | P value |
| Overall | |||
| Composite cognitive function | 0.12 (0.02) | −0.18 (0.02) | <.001 |
| Rey Auditory Verbal Learning | |||
| Test | |||
| Immediate | 0.23 (0.02) | −0.36 (0.02) | <.001 |
| Delayed | 0.21 (0.02) | −0.33 (0.02) | <.001 |
| Trail Making Test, seconds | |||
| Part A | 0.03 (0.02) | −0.04 (0.02) | .032 |
| Part B | 0.03 (0.02) | −0.04 (0.02) | .031 |
| Modified Stroop Color and | 0.01 (0.02) | −0.03 (0.03) | .155 |
| Word Test | |||
| Digit Symbol Coding | 0.09 (0.02) | −0.15 (0.02) | <.001 |
| Modified Mini-Mental State | 0.08 (0.02) | −0.12 (0.02) | <.001 |
| Examination | |||
| Women | Men | ||
| N = 2152 | N = 1281 | ||
| Cognitively normal | |||
| Composite cognitive function | 0.22 (0.02) | −0.02 (0.02) | <.001 |
| Rey Auditory Verbal Learning | |||
| Test | |||
| Immediate | 0.30 (0.02) | −0.24 (0.02) | <.001 |
| Delayed | 0.28 (0.02) | −0.22 (0.03) | <.001 |
| Trail Making Test, seconds | |||
| Part A | 0.08 (0.02) | 0.04 (0.03) | .174 |
| Part B | 0.10 (0.02) | 0.08 (0.02) | .385 |
| Modified Stroop Color and | 0.06 (0.02) | 0.04 (0.03) | .615 |
| Word Test | |||
| Digit Symbol Coding | 0.15 (0.02) | −0.08 (0.02) | <.001 |
| Modified Mini-Mental State | 0.22 (0.02) | 0.08 (0.02) | <.001 |
| Examination | |||
| Women | Men | ||
| N= 159 | N= 179 | ||
| Cognitively impaired | |||
| Composite cognitive function | −1.32 (0.06) | −1.43 (0.05) | .164 |
| Rey Auditory Verbal Learning | |||
| Test | |||
| Immediate | −0.73 (0.07) | −1.23 (0.06) | <.001 |
| Delayed | −0.79 (0.07) | −1.21 (0.07) | <.001 |
| Trail Making Test, seconds | |||
| Part A | −0.79 (0.07) | −0.67 (0.07) | .239 |
| Part B | −1.07 (0.07) | −0.96 (0.07) | .275 |
| Modified Stroop Color and | −0.75 (0.08) | −0.76 (0.08) | .924 |
| Word Test | |||
| Digit Symbol Coding | −0.62 (0.07) | −0.71 (0.07) | .338 |
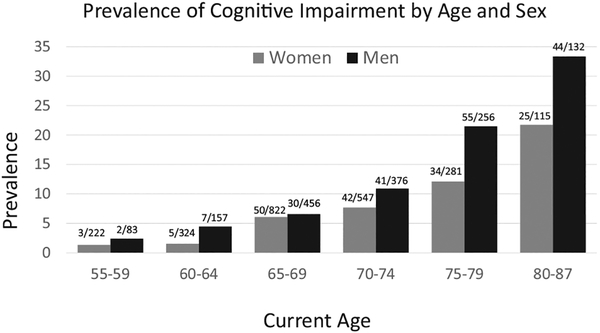
Fig. 1 portrays the prevalence of cognitive impairment (either MCI or dementia) for women and men grouped by age at assessment. The lower prevalence among women compared was evident for all age categories, and the interaction between age and sex was not significant (P = .669).
Fig. 1.
Prevalence of cognitive impairment (mild cognitive impairment or dementia) by age and sex (interaction P = .669).
We also examined whether risk factor relationships with cognitive impairment, for each factor in Table 1, varied by sex using tests of interaction in logistic regression models with covariate adjustment for age, education, race/ethnicity, and intervention assignment. There was modest evidence for an interaction (nominal P <.05) only for APOE-ε4 (interaction P = .034). The OR for cognitive impairment in women compared with men who were not APOE-ε4 carriers was 0.49 [0.36, 0.68]. There was little difference in odds of cognitive impairment for women and men among APOE-ε4 allele carriers: OR = 0.92 [0.35, 1.50]. A 3-way interaction for sex, APOE-ε4 allele, and intervention assignment was not significant (P = .453).
Next, we used stepwise logistic regression to develop, from among the risk factors in Table 1, a multivariable risk factor model (selection based on nominal P < .05). We did not include APOE-ε4 initially, due to the large amount (12%) of missing data. Both forward and backward algorithms yielded the same set of predictors: age (OR = 1.13/year [1.11, 1.15]); history of cardiovascular disease (OR = 1.79 [1.33, 2.43]); education (relative to post college education) high school or less (OR = 1.84, [1.29, 2.62] and college graduate (OR = 1.42, [0.94, 2.16]); and symptoms of depressed mood, that is, Beck Depression Inventory-II ≥11 (OR = 1.44,[1.03, 2.02]). An interaction between intervention assignment and baseline BMI was forced into the model based on prior publication [9] and reached nominal significance (P = .059).
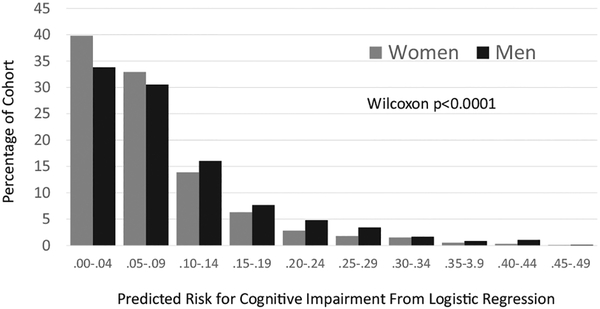
Overall, women had lower scores from this risk factor model: P < .0001 (Fig. 2). However, after controlling for these scores, sex-related differences remained statistically significant: OR = 0.60 [0.47, 0.76], P < .001. When changes in weight and physical activity at each time period in Table 2 were also included in the model, sex-related differences remained: OR = 0.54 [0.36, 0.83], P = .004. Finally, among individuals with genotyping, including APOE-ε4 as a covariate did not diminish the sex-related differences: OR = 0.60 [0.39, 0.92], P = .020.
Fig. 2.
Probability estimates from a multivariable logistic regression model for cognitive impairment (mild cognitive impairment or dementia) that includes age, history of cardiovascular disease, education, and depression symptoms as predictors.
Because our findings are based on a subset of the original Look AHEAD enrollees, there is a potential for bias due to differential retention by sex. This subset contains 54.7% of the 3063 women and 45.3% of the 2082 men originally enrolled in Look AHEAD (P < .001). Those lost included 239 women (7.8%) and 290 (13.9%) men who died before cognitive assessment. To gauge the potential influence of this lost follow-up, we applied inverse probability weighing, using logistic regression to model the probability of lost follow-up, utilizing predictors from Table 1, baseline diabetes medication use, and clinical site. This had little effect on estimates. For example, the OR for sex differences in the prevalence of cognitive impairment with inverse probability weighting was 0.56 compared with the unweighted value reported above 0.55.
4. Discussion
In this large cohort of adults with T2DM, there was a markedly lower prevalence of cognitive impairment (MCI or dementia) among women compared with men and correspondingly better performance in tests of cognitive function, especially memory. These sex-related differences did not appear to attenuate with age. The only risk factor we examined that moderated these sex-related differences was APOE genotype: they were attenuated among APOE-e4 carriers. Sex-related differences could not be explained by differences in risk factor profiles, intervention assignment or adherence, or how T2DM was treated and controlled over time.
At least two other studies have found evidence that obesity accelerates cognitive decline more in men than women. The Three City Study in France followed a large cohort aged ≥65 years for 4 years. Obesity was related to an increased incidence of MCI among men, but not among women [4]. Rodriguez-Fernandez, et al. [20], in the large US National Health and Aging Trends Study cohort aged ≥65 years, found that higher BMI was protective of cognitive decline over 3 years among women but not among men. However, others have not found sex differences in the association between obesity and cognitive decline [3,21].
It is not clear whether T2DM accelerates cognitive decline more in men than women. Kim et al. [8], in a large Korean cohort, found that T2DM was an independent risk factor for dementia only in women. Sherzai et al. [22], in the large US Nationwide Inpatient Sample, found no sex-related difference in the association between T2DM and non-Alzheimer’s dementia (AD), but a stronger association of T2DM with increased risk for AD among women than men. Wang et al. [23], in a large Taiwanese study of medical claims data, found that the association between T2DM and increased risk for AD was similar between sexes. A large meta-analysis found no sex-related differences between diabetes and nonvascular dementias, but a 19% increased risk for vascular-related dementia among women compared with men [7]. In the Three City Study, T2DM was independently related to the prevalence of MCI in men, but not in women [4]. Others have found no sex-related differences in the association of T2DM with MCI [6].
The prevalence of many risk factors for cognitive impairment differed between women and men in Look AHEAD. Some differences, such as lower age, shorter duration of diabetes, less cardiovascular disease, and fewer smokers would be expected to benefit women. Others, such as lower educational attainment, greater membership in racial/ethnic groups at increased risk, greater obesity, and more symptoms of depression might be expected to put women at greater risk. However, we found no evidence that any of these individual differences in risk factor distributions accounted for the lower prevalence of cognitive impairment among women. When accumulated in a multivariable prediction model, overall differences in risk factor profiles similarly did not account for the sex-related differences in prevalence.
We found no evidence that the sex-related differences were associated with intervention assignment or adherence, or with how T2DM was treated or controlled over time. The overall long-term impact of the ILI on cognitive function and cognitive impairment has been documented elsewhere [9,16,24]. Relative to the control condition, the intervention appeared to benefit cognitive function among individuals who weighed less and who had less vascular disease, but to not benefit, and perhaps harm, cognitive function among the heaviest individuals with vascular disease. There is no evidence that these relative intervention effects on cognitive impairment varied by sex.
It has been shown that the increased risk for dementia associated with the APOE-ε4 allele is elevated more among women than men [2,25,26]. APOE-ε4 alleles are associated with an increase in β-amyloidosis that is expressed later in life and which is separable from adverse effects related to brain structure and vascularization [27]. In Look AHEAD, presence of APOE-e4 alleles appeared to eliminate any sex-related advantages for women.
No risk factor other than APOE-ε4 carrier status appeared to affect the magnitude of the sex-related difference. In other cohorts, some such interactions have been described. For example, in a population-based cohort (28% diabetes), midlife hypertension was associated with an increased risk of dementia for women but not for men [28]. In the Rotterdam cohort, smoking was more associated with risk of AD for men than women [29]. Overall, the similarity in sex-related differences across many risk factors in Look AHEAD signals that strategies targeting risk factor reduction in such a cohort might impact outcomes for both sexes comparably.
There are many potential mechanisms that may differentially affect risks between women and men that Look AHEAD did not assess directly, such as those related to neuroanatomy, brain connectivity, brain metabolism, sex chromosomes, and early life course exposures [25,26,30]. As one example, we speculate on the potential role of sex hormones.
Endogenous estrogens are important for maintaining vascular function, repair of vascular damage, and promoting neurogenesis [31] and may preserve cognitive functioning in older women [32]. The deleterious impact of insulin resistance on brain function may begin to occur relatively early in the disease process, during insulin resistance before diabetes [33–35] and thus may have been ongoing for some time, both before and after menopause among Look AHEAD women. It is possible that before menopause, endogenous estrogens provided a buffer against brain aging that translated to a lower rate of cognitive impairment later in life compared with men. As noted earlier, there is some evidence that obesity may be protective against cognitive decline and cognitive impairment in older women but not in men [4,20]. This may be linked to increased production of endogenous estrogens in adipose tissue [36]. Even as estrogen levels decline with age, females may compensate for T2DM-related neurodegeneration by maintaining estrogen receptor signaling in the brain that may protect against the accumulation of neuropathology [37].
Prior use of postmenopausal hormone therapy was queried at baseline, and current use of prescription medications was assessed annually. Based on these data, 62% of women had at least some exposure to hormone therapy. With covariate adjustment for current age, intervention assignment, education, and race/ethnicity, hormone therapy exposure was associated with a lower odds of cognitive impairment: OR = 0.55 [0.39, 0.78]. To date, all well-powered clinical trials across a variety of regimens have found no evidence that random assignment to hormone therapy provides cognitive benefits [38–42], and among older women, particularly those with diabetes, hormone therapy may increase risks for atrophy and cognitive impairment through mechanisms related to energy metabolism [43,44]. However, the lower prevalence of cognitive impairment among women with a history of using hormone therapy in the Look AHEAD cohort is intriguing and warrants further scrutiny, as there are prior findings in some, but not all, studies that women who use these therapies to treat menopausal symptoms have better cognitive functioning[45]. This finding resonates with a potentially protective effect of endogenous estrogens discussed previously.
Testosterone may also have a role in fostering sex-related differences in cognitive impairment. T2DM lowers testosterone levels in older men, most markedly among those who are overweight or obese [46]. Lower testosterone levels are associated with losses in neuroprotection and with increased plasma levels of amyloid β in men [47]. It is possible that an accelerated loss of testosterone in the cohort males left them more vulnerable to cognitive impairment than women.
We note several strengths, including a large, diverse, and richly phenotyped cohort, standardized cognitive assessments, and central adjudication of cognitive impairment. There are also potential limitations of this cross-sectional study. As volunteers for whom ILI was safe, participants may not represent general populations. Eligibility criteria related to weight, fitness, and risk factor control may have differentially screened participants by gender. No baseline cognitive assessments were made; however, screening enhanced the likelihood that enrollees were cognitively intact. While analyses suggest that differential retention of women versus men did not markedly bias findings, we cannot rule out that unmeasured imbalances or biases related to mortality may have existed. The accuracy for classifying cognitive impairment may have been affected by the lack longitudinal cognitive measures and neuroimaging. We have no information on the timing and duration of pretrial hormone therapy.
We found a lower prevalence of MCI and dementia in women compared with men and that differences were evident across age, and only evident among APOE-ε4 non-carriers. Although there were differences in the frequencies of common risk factors for cognitive impairment between sexes, these did not account for differences in the prevalence of cognitive impairment. This suggests that how diabetes accelerates the development of cognitive impairment, in the context of obesity, is related, at least in part, to male sex, independent of conventional risk factor relationships. The future research necessary to identify mechanisms that underlie this finding may lead to new targets for prevention and treatment, including the development of tailored interventions to reduce risks for cognitive impairment.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: A rich literature identifies type 2 diabetes mellitus and midlife obesity as potent risk factors that increase individual’s risks for cognitive impairment later in life. Less is known, however, whether this leads to differences in the prevalence of cognitive impairment between women and men with these conditions.
Interpretation: Our findings that, in a large cohort defined by these conditions, the prevalence of cognitive impairment was markedly lower in women than men across an over 20-year age range later is novel. Furthermore, we could not account for this sex-related difference despite extensive risk factor adjustment.
Future directions: This suggests that how diabetes accelerates the development of cognitive impairment, in the context of obesity, is related, at least in part, to male sex. The future research needed to identify mechanisms that underlie this finding may lead to new targets for prevention and treatment, including the development of tailored interventions to reduce risks for cognitive impairment.
Acknowledgments
Funding and support: The Action for Health in Diabetes is supported through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: the National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; the Centers for Disease Control and Prevention; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); Frederic C. Bartter General Clinical Research Center (M01RR01346); and the Wake Forest Alzheimer’s Disease Core Center (P30AG049638–01A1). C.H. received funding from K01-AG043547.
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST®of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
The Look AHEAD study group is listed in Supplementary Appendix 1.
Footnotes
Conflicts of interest: The authors have no conflicts to report. ClinicalTrials.gov Identifier: NCT00017953.
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jalz.2018.05.015.
References
- [1].Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RD. Obesity, diabetes, and cognitive deficit: the Framingham Heart Study. Neurobiol Aging 2005;26:11–6. [DOI] [PubMed] [Google Scholar]
- [2].Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord 2006; 20:93–100. [DOI] [PubMed] [Google Scholar]
- [3].Azad NA, Bugami MA, Loy-English I. Gender differences in dementia risk factors. Gend Med 2007;4:120–9. [DOI] [PubMed] [Google Scholar]
- [4].Artero S, Ancelin M-L, Portet F, Dupuy A, Berr C, Dartigues JF, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender-specific. J Neurol Neurosurg Psychiatry 2008;79:979–84. [DOI] [PubMed] [Google Scholar]
- [5].West NA, Haan MN. Body adiposity in late life and risk of dementia or cognitive impairment in a longitudinal community-based study. J Geront A Biol Med Sci 2009;64:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Winkler A, Dlugaj M, Weimar C, J€ockel KH, Erbel R, Dragano N, et al. Association of diabetes mellitus and mild cognitive impairment in middle-aged men and women. J Alz Dis 2014;42:1269–77. [DOI] [PubMed] [Google Scholar]
- [7].Chatterjee S, Peters SA, Woodward M, Mejia-Arango S, Batty GD, Beckett N, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016;39:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim YH, Kim NH, Jung MH, Kim HJ. Sex differences in metabolic risk indicator of dementia in an elderly Korean population: a community-based cross-sectional study. Geriatr Gerontol Int 2017; 17:2136–42. [DOI] [PubMed] [Google Scholar]
- [9].Espeland MA, Luchsinger JA, Baker LD, Neiberg R, Kahn SE, Arnold SE, et al. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology 2017;88:2026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].The Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–28. [DOI] [PubMed] [Google Scholar]
- [11].The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Eng J Med 2013; 369:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].The Look AHEAD Research Group. The Look AHEAD Study: a description of the lifestyle intervention and the evidence supporting it. Obesity 2006;14:737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].The Look AHEAD Research Group. The development and description of the diabetes support and education (comparison group) intervention for the Action for Health in Diabetes (Look AHEAD) Trial. Clin Trials 2011;8:320–9.21730080 [Google Scholar]
- [14].Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 1986; 314:605–13. [DOI] [PubMed] [Google Scholar]
- [15].Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100. [Google Scholar]
- [16].Rapp SR, Luchsinger JA, Baker LD, Blackburn GL, Hazuda HP, Demos-McDermott KE, et al. Effect of a long-term intensive lifestyle intervention on cognitive function: Action for Health in Diabetes Study. J Am Geriatr Soc 2017;65:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Geront Med Sci 1982;37:323–9. [DOI] [PubMed] [Google Scholar]
- [18].Sink K, Espeland MA, Rushing J, Castro CM, Church TS, Cohen R, et al. The LIFE Cognition Study: design and baseline characteristics. Clin Interv Aging 2014;9:1425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Weuve J, Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology 2012; 23:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rodriguez-Fernandez J, Danies E, Martinez-Ortega J, Chen WC. Cognitive decline, body mass index, waist circumference in community-dwelling elderly participants: results from a nationally representative sample. J Geriatr Psychiatry 2017;30:67–76. [DOI] [PubMed] [Google Scholar]
- [21].Rafnsson SB, Deary I, Smith FB, Whiteman MC, Fowkes FGR. Cardiovascular diseases and decline in cognitive function in an elderly community population: the Edinburgh Artery Study. Psychosomat Med 2007;69:425–34. [DOI] [PubMed] [Google Scholar]
- [22].Sherzai D, Sherzai A, Lui K, Pan D, Chiou D, Bazargan M, et al. The association between diabetes and dementia among elderly individuals: a nationwide inpatient sample analysis. J Geriatr Psych 2016; 29:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang KC, Woung LC, Tsai MT, Liu CC, Su YH, Li CY. Risk of Alzheimer’s disease in relation to diabetes: a population-based cohort study. Neuroepidemiol 2012;38:237–44. [DOI] [PubMed] [Google Scholar]
- [24].Espeland MA, Rapp SR, Bray GA, Houston DK, Johnson KC, Kitabchi AE, et al. Long-term impact of behavioral weight loss intervention on cognitive function: the Action for Health in Diabetes Movement and Memory Study. J Geront A Biol Sci Med Sci 2014; 69:1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol 2014;6:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Snyder HM, Asthana S, Bain L, Brinton R, Craft S, Dubal DB, et al. Sex biology contributions to vulnerability to Alzheimer’s disease: a think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimer’s Dement 2016;12:1186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jack CR, Wiste HJ, Weigand SD, Therneau TM, Knopman DS, Lowe V, et al. Age, sex, and apoe-e4 effects on memory, brain structure, and b-amyloid across the adult lifespan. JAMA Neurol 2015; 72:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Mungas DM, DeCarli C, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology 2017;89:1886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ott A, Slooter AJ, Hofman A, van Harskamp F, Witteman JC, van Broeckhoven C, et al. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study. Lancet 1998;351:1840–3. [DOI] [PubMed] [Google Scholar]
- [30].Zhao L, Mao Z, Woody SK, Brinton RC. Sex difference in metabolic aging of the brain: insights into female susceptibility to Alzheimer’s disease. Neurobiol Aging 2016;42:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li R, Singh M. Sex differences in cognitive impairment and Alzheimer’s disease. Front Neuroendocrinology 2014;35:385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lebrun CE, van der Schouw YT, de Jong FH, Pols HA, Grobbee DE, Lamberts SW. Endogenous oestrogens are related to cognition in healthy elderly women. Clin Endocrinol 2005;63:50–5. [DOI] [PubMed] [Google Scholar]
- [33].Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 2011;68:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tiehuis AM, van der Graaf Y, Mali WP, Vincken K, Muller M, Geerlings MI. Metabolic syndrome, prediabetes, and brain abnormalities on MRI in patients with manifest arterial disease: the SMART-MR study. Diabetes Care 2014;37:2515–21. [DOI] [PubMed] [Google Scholar]
- [35].Frosch OH, Yau PL, Osorio RS, Rusinek H, Storey P, Convit A. Insulin resistance among obese middle-aged is associated with decreased cerebrovascular reactivity. Neurology 2017;89:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liedtke S, Schmidt ME, Vrieling A, Lukanova A, Becker S, Kaaks R, et al. Postmenopausal sex hormones in relation to body fat distribution. Obesity (Silver Spring) 2012;20:1088–95. [DOI] [PubMed] [Google Scholar]
- [37].Candeias E, Duarte AL, Sebastiao I, Fernandes MA, Pl acido AI, Carvalho C, et al. Middle-aged diabetic females and males present distinct susceptibility to Alzheimer disease-like pathology. Mol Neurobiol 2017;54:6471–89. [DOI] [PubMed] [Google Scholar]
- [38].Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;20:2663–72. [DOI] [PubMed] [Google Scholar]
- [39].Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2959–68. [DOI] [PubMed] [Google Scholar]
- [40].Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, LeBlanc ES, et al. Long term effect on cognitive function of postmenopausal hormone therapy prescribed to women aged 50–55 years. JAMA Intern Med 2013;173:1429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. Plos Med 2015;12:e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology 2016; 87:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Espeland MA, Brinton RD, Hugenschmidt C, Manson JE, Craft S, Yaffe K, et al. Impact of type 2 diabetes and postmenopausal hormone therapy on incidence of cognitive impairment in older women. Diabetes Care 2015;38:2316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Espeland MA, Brinton RD, Manson JE, Yaffe K, Hugenschmidt C, Vaughan L, et al. Postmenopausal hormone therapy, type 2 diabetes mellitus, and brain volumes. Neurology 2015;85:1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Miller VM, Garovic VD, Kantarci K, Barnes JN, Jayachandran M, Mielke MM, et al. Sex-specific risk of cardiovascular disease and cognitive decline: pregnancy and menopause. Biol Sex Diff 2013;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008;93:1843–940. [DOI] [PubMed] [Google Scholar]
- [47].Asih PR, Tegg ML, Sohrabi H, Carruthers M, Gandy SE, Saad F, et al. Multiple mechanisms linking type 2 diabetes and Alzheimer’s disease: testosterone as a modifier. J Alz Dis 2017;59:445–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.