Abstract
Research has identified early-appearing differences in gross and fine motor abilities in infants at heightened risk (HR) for autism spectrum disorder (ASD) because they are the younger siblings of children with ASD, and it suggests that such differences may be especially apparent among those HR infants themselves eventually diagnosed with ASD. The present study examined overall and item-level performance on the Gross (GM) and Fine Motor (FM) subscales of the Mullen Scales of Early Learning (MSEL) administered at 6 months to a large, geographically diverse sample of HR infants with varying developmental outcomes (ASD, Elevated ADOS without ASD, Low ADOS without ASD) and to infants with low ASD risk (Low Risk; LR). We also explored whether motor abilities assessed at 6 months predicted ASD symptom severity at 36 months. FM (but not GM) performance distinguished all three HR groups from LR infants with the weakest performance observed in the HR-Elevated ADOS children, who exhibited multiple differences from both LR and other HR infants in both gross and fine motor skills. Finally, 6-month FM (but not GM) scores significant predicted 36-month ADOS severity scores in the HR group; but no evidence was found of specific early-appearing motor signs associated with a later ASD diagnosis. Vulnerabilities in infants’ fine and gross motor skills may have significant consequences for later development not only in the motor domain but in other domains.
Keywords: ASD, infant siblings, gross motor, fine motor, early identification
General Scientific Summary:
Infants with an older sibling with autism spectrum disorder (ASD) are at heightened risk (HR) for receiving an ASD diagnosis, and research has focused on identification of early markers of risk for a future ASD diagnosis. One area of focus has been motor development, but existing research has involved small sample sizes and reported varying results. Using data from a large sample of HR infants, this study found that as a group, 6 month-old HR infants do not perform as well as infants with low ASD risk on an assessment of fine motor skill, and that poorer performance is not specific to the subgroup of HR infants later diagnosed with ASD.
Although motor difficulties are not included among the diagnostic criteria for autism spectrum disorder (ASD), evidence indicating their presence in individuals across the full range of the autism spectrum has been accumulating (Fournier et al., 2010). This evidence has led to the suggestion that motor impairment may be a component of the ASD phenotype (Hilton et al., 2011). It has also increased interest in characterizing the nature of these differences and determining whether there is a ‘motor signature’ specific to ASD (e.g., Ament et al., 2015).
This interest in motor difficulties has carried over into research on infant siblings of children with ASD, for whom the risk of ASD recurrence is elevated (18.9%) relative to the general population (Messinger et al., 2015). The prospective, longitudinal study of infants at heightened risk (HR) has enabled researchers to evaluate potential early markers of risk for later ASD diagnosis. While many studies have focused on behaviors conceptually related to atypicalities characteristic of ASD (e.g., eye gaze, joint attention), the focus has begun to shift to possible sensory and motor precursors of later impairments (see Jones et al., 2014, for a review). During the first year of life, infants rapidly acquire new perceptual and motor skills. The rapidity with which these changes occur, coupled with the sheer number of skills that emerge, make the motor system an excellent candidate for prodromal signs of ASD prior to 12 months of age. The present study is designed to address this issue by leveraging data on motor abilities and their development from large samples of HR infants and infants with low ASD risk followed longitudinally by member sites of the Baby Siblings Research Consortium (BSRC; e.g., Messinger et al., 2013).
As research interest in the relevance of motor skills to early identification of ASD has grown, emerging studies have been characterized by considerable methodological variability. Some researchers have compared performance of HR infants to that of a group of infants at low risk (LR) for ASD (i.e., no immediate family history of ASD). These studies have typically reported significantly weaker performance in HR compared to LR infants on measures of both fine motor (Kaur, Srinivasan, & Bhat, 2015; Libertus et al., 2014, Experiment 2) and gross motor skills (Bhat, Galloway, & Landa, 2012). However, these studies have not provided information on HR infants’ diagnostic outcomes; and thus the question of whether observed group differences were representative of HR infants’ performance in general or driven by a subgroup later diagnosed with ASD is not addressed.
Other researchers have identified HR infants who ultimately received an ASD diagnosis (from assessments completed at 24 months or later; HR-ASD) and compared their data to those from LR and unaffected infants. These studies, focusing primarily on gross motor skills (Heathcock et al., 2015; Nickel et al., 2013), have reported multiple differences in postural development between HR-ASD infants and both LR and unaffected HR infants during the first year, suggesting that HR-ASD infants in particular may have difficulty with postural control. Interestingly, there was also some indication that unaffected HR infants manifest more subtle delays relative to LR peers, particularly in the development of sitting (Nickel et al., 2013). While these findings suggest that HR-ASD infants may exhibit more pronounced difficulties in early motor development, they do not address the issue of whether these early-appearing differences are specific to ASD.
Finally, several studies have compared the motor performance of subgroups of HR infants, a comparison critical for evaluating the specificity of differences associated with HR-ASD infants. Estes et al. (2015) compared 6-month MSEL GM and FM scores for HR infants later classified as having the highest ASD symptom levels (HR-high ASD) to those for LR, infants, HR infants with moderate ASD symptom levels, and HR infants without ASD symptoms. They found that relative to LR infants GM but not FM scores were lower for HR-high ASD but not other HR subgroups (see also Boin Choi, Leech, Tager-Flusberg, & Nelson, 2018). Landa and Garrett-Meyer (2006) found that both 6 month-old HR infants with a later ASD diagnosis (HR-ASD) and HR infants without an ASD diagnosis but with delayed language development (HR-LD) performed worse than typically-developing HR infants (HR-TD) on the Fine Motor (FM) but not Gross Motor (GM) subscales of the Mullen Scales of Early Learning (MSEL; Mullen, 1995). Leonard et al. (2014) compared GM and FM scores of LR infants to those in three HR sub-groups: HR-TD, HR-ASD, and a group that performed atypically on the ADOS or MSEL but did not receive a consensus ASD diagnosis (HR-AT). At 7 months, the HR group as a whole obtained significantly lower scores than LR infants (though scores were within the average range) on both the GM and FM subscales, but there were no differences between any of the HR subgroups on either subscale (see also Libertus et al., 2014).
Taken together, these findings suggest that there may be subtle disruptions in early motor development among HR relative to LR infants (West, in press), but leave open the question of whether differences are more pronounced among HR infants later diagnosed with ASD. While previous work has identified the existence of these disruptions, little is known about the specific behaviors contributing to early motor delays in HR infants. Because subscale scores reflect aggregated item performance, they provide no information about patterns of performance on individual skills. Examining item-level performance would provide theoretically and clinically important information about whether there are specific motor skills (or components of skills) that may be particularly vulnerable in HR infants. The promise of an item-level approach has been illustrated by previous work identifying potentially specific sources of gross and fine motor delay in HR infants, such as grasping (Libertus et al., 2014) and head lag (Flanagan et al., 2012). Differences in motor development of thjs sort are likely to be missed when research is restricted to the examination of conventional composite scores.
The Present Study
The research reviewed above has identified early-appearing differences in gross and fine motor abilities in HR infants and provided preliminary indications that such differences may be especially apparent among HR infants eventually diagnosed with ASD. However, it has also been limited by inconsistencies in patterns of results across studies, small sample sizes, variability in the availability of diagnostic outcome data for HR infants, differences in methodology, and reliance on composite subscale scores yielding little information about the performance of specific motor skills. Finally, relatively few studies have included a comparison group of HR infants without ASD but representing a continuum of risk to examine the specificity of differences observed among subgroups of HR infants.
The present study is designed to address these issues. First, it employs data from large, well-characterized samples of HR and LR infants followed longitudinally as part of the BSRC. Because the MSEL is a core measure administered at 6 months of age at participating sites, this will be the first study to examine gross and fine motor abilities at 6 months in a large and geographically diverse group of infants. Second, in addition to examining overall performance on the GM and FM subscales, we analyze item-level data from both subscales to examine difficulties with specific skills and determine whether particular items distinguish HR from LR infants and subgroups of HR infants from one another. Third, to further evaluate the question of whether motor delays are specific to HR-ASD infants, we have included a group of HR infants whose ADOS scores at 36 months were elevated (i.e., at or above the cut-off for ASD) but who did not receive a clinical best estimate (CBE) diagnosis of ASD. This procedure allowed us specifically to examine gross and fine motor abilities across a continuum of risk for ASD.
Method
Participants
Data for the present study were obtained from a potential sample of 648 infants gathered at 8 BSRC member sites (Boston University; Kennedy Krieger Institute; New York State Institute for Basic Research; University of Calgary; University of California, Davis; University of California, Los Angeles; University of Pittsburgh; Yale University; Boston Children’s Hospital) and at the 4 member sites of the Infant Brain Imaging Study (Children’s Hospital of Philadelphia; University of North Carolina; University of Washington; Washington University) whose recruitment procedures and common assessment measures allowed for data pooling. Infants at participating sites were enrolled at a mean age of 4.6 months (SD= 2.7; enrollment age was missing for 109 infants). At approximately 6 months of age, infants completed a study visit that included the MSEL; and, at 36 months, they received complete clinical evaluations that included the MSEL and the ADOS.
HR infants were recruited by identifying affected older siblings (probands) and their families through clinics and agencies serving individuals with ASD, community events, or other research studies. All sites verified the older sibling’s ASD diagnosis using the ADOS and/or parent report measures such as the Autism Diagnostic Interview–Revised (Lord et al., 1994) or the Social Communication Questionnaire (Rutter, Bailey, & Lord, 1994). Low Risk (LR) infants with no known family history of ASD were recruited by mailings, fliers, media announcements, and word-of-mouth. Infants were excluded from the broader sample if a neurologic or genetic condition (e.g., fragile X syndrome; tuberous sclerosis) was present in the infant or proband or if item-level scoring from the Gross or Fine Motor subscales from the MSEL (or both) at the 6-month assessment or 36-month outcome evaluation data were unavailable. The final sample consisted of 437 HR and 188 LR infants.
Measures
MSEL.
The MSEL is a standardized developmental assessment for children from birth to 68 months with excellent internal consistency and test-retest reliability. It assesses abilities in five domains: Visual Reception, Receptive Language, Expressive Language, Fine Motor, and Gross Motor. The four cognitive scales (visual reception, fine motor, receptive language, and expressive language) are combined to yield an Early Learning Composite (ELC) that indexes overall cognitive functioning.
The MSEL employs a baseline and ceiling approach, and not all items are administered to all infants. For 6 month-old infants, Gross Motor (GM) administration begins with Item 5 and Fine Motor (FM) administration with Item 4. All previous items are not administered and infants are credited with passing scores. However, failure on any of the first three administered items (FM items 4, 5, or 6; GM items 5, 6, or 7) requires administration of the prior items beginning with Item 1. Administration ends when the infant fails three items in succession. Brief descriptions of GM and FM items 1 to 13 with scoring information are presented in Table 1.
Table 1.
Brief Descriptions of Selected Mullen Scales of Early Learning Gross Motor and Fine Motor Items.
| Gross Motor Items | ||
|---|---|---|
| Item | Description | Scoring |
| 1 | Enjoys being held/realigns | 0 or 1 |
| 2 | Rotates head | 0 or 1 |
| 3 | Moves arms and legs vigorously | 0 or 1 |
| 4 | Holds head steady while held upright | 0 or 1 |
| 5 | Supports self on forearms while positioned on belly | 0 or 1 |
| 6 | Sits with support and with head steady | 0 or 1 |
| 7 | Rolls over (belly to back) | 0 or 1 |
| 8 | Holds on to examiner’s fingers and pulls self from lying on back to sitting position | 0 or 1 |
| 9 | Shifts weight to one side while reaching for toy | 0 or 1 |
| 10 | Stands with hands held and bounces | 0 or 1 |
| 11 | Sits alone with arms not used for support | 0 or 1 |
| 12 | Pulls self to stand | 0 or 1 |
| 13 | Gets from sitting to hands and knees | 0 or 1 |
| Fine Motor Items | ||
| Item | Description | Scoring |
| 1 | Arms held close to body with hands fisted | 0 or 1 |
| 2 | Reflexively grasps a ring | 0 or 1 |
| 3 | Brings fist to mouth | 0 or 1 |
| 4 | Brings hands together at midline | 0 or 1 |
| 5 | Hands held open with fingers extended | 0 or 1 |
| 6 | Nonreflexive grasping of a small peg using ulnar palmar grasp | 0 or 1 |
| 7 | Reach for and grasp a block using radial-palmar grasp | 0 or 1 |
| 8 | As child plays with blocks, s/he transfers, bangs, and/or drops a block (at least 2 behaviors must be observed) | 0 or 1 |
| 9 | Reach for and grasp a block using radial-digital grasp | 0 or 1 |
| 10 | Pick up small object using partial or refined pincer grasp | 0–2 |
| 11 | Bangs blocks together in a horizontal movement at midline | 0 or 1 |
| 12 | Takes blocks out or puts them in a container | 0–3 |
| 13 | Uses two hands together to manipulate an object | 0 or 1 |
Standardized T scores from the GM and FM subscales were used in our primary analyses at 6 months of age. The ELC was calculated as part of the 36-month outcome assessment procedures at each site (see below). Because the GM subscale is not included when calculating the ELC, it was not administered at 2 of the 10 sites. Thus, our sample size was smaller for GM analyses (see Table 2 below).
Table 2.
Characteristics of the sample (N = 625)
| LR | HR-LA | HR-EA | HR-ASD | |
|---|---|---|---|---|
| N | 188 | 317 | 51 | 69 |
| Age at enrollment in months, mean (SD) | 4.8 (2.3) | 4.4 (2.8) | 5.2 (2.1) | 4.7 (3.3) |
| Age at 6-month visit in months, mean (SD) | 6.0 (0.4) | 6.1 (0.5) | 6.1 (0.4) | 6.1 (0.4) |
| Age at 36-month visit in months, mean (SD) | 36.4 (1.2) | 36.6 (1.4) | 36.7 (1.4) | 36.5 (1.2) |
| Gender, n (%) | ||||
| Male | 107 (57%) | 174 (55%) | 33 (65%) | 49 (71%) |
| Race, n (%) | ||||
| Caucasian | 153 (81%) | 267 (84%) | 44 (86%) | 50 (73%) |
| Non-Caucasian | 33 (18%) | 43 (14%) | 6 (12%) | 15 (21%) |
| Missing | 2 (1%) | 7 (2%) | 1 (2%) | 4 (6%) |
| Hispanic, n (%) | ||||
| No | 166 (88%) | 273 (86%) | 42 (82%) | 53 (76%) |
| Yes | 11 (6%) | 26 (8%) | 32 (4%) | 8 (12%) |
| Missing | 2 (1%) | 18 (6%) | 7 (14%) | 8 (12%) |
| ADOS mean scores (SD) | ||||
| SA | 1.7 (.8) | 1.8 (1.0) | 5.3 (1.9) | 6.6 (1.7) |
| RRB | 2.6 (2.2) | 2.9 (2.3) | 6.2 (1.9) | 7.3 (2.4) |
| Severity | 1.3 (.5) | 1.4 (.7) | 5.2 (1.5) | 7.0 (1.8) |
| MSEL ELC mean (SD) | 108.9 (18.9) | 106.3 (19.6) | 100.2 (20.9) | 84.4 (20.1) |
Note. LR = Low-Risk; HR-LA = High-Risk, Low ADOS; HR-EA = High-Risk, Elevated ADOS;HR-ASD = High-Risk, ASD Diagnosis; ADOS SA= ADOS Social Affect Severity Score; ADOS, RRB= ADOS Restrictive and Repetitive Behavior Severity Score; MSEL ELC= Mullen Scales of Early Learning Early Learning Composite.
Autism Diagnostic Observation Scale (ADOS).
The ADOS is a semi-structured, standardized assessment of social interaction, communication, play skills, and repetitive behaviors diagnostic of ASD. At 36 months, either Module 1 or Module 2 was used depending on the child’s spoken language abilities. To permit comparisons of symptom severity across modules, calibrated severity scores ranging from 1 to 10 (with 10 being most severe) were calculated across the social interaction, communication, and repetitive behavior domains (Gotham, Pickles, & Lord, 2009). Severity scores of 4 or greater are within the clinical range and reflect moderate to high severity of autism characteristics.
Outcome assessment.
Diagnostic outcome classification at 36 months was based on a combination of CBE and ADOS scores. CBE was assigned at each site by an experienced clinician and typically required that the child meet diagnostic criteria according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; American Psychiatric Association, 2000). Using this information, infants were assigned to one of 4 outcome groups using the following algorithm. Infants in the HR-ASD group had an older sibling with ASD and scored at or above the ASD threshold on the ADOS (calibrated severity score ≥ 4; Gotham, Pickles, & Lord, 2009) and met CBE criteria for ASD. Infants in the HR-Elevated ADOS (HR-EA) group had an older sibling with ASD and exhibited abnormal scores on the ADOS (with severity scores ≥ 4) but did not meet CBE criteria for ASD. Infants in the HR-Low ADOS (HR-LA) group had an older sibling with ASD and scored in the typical range on the ADOS (severity scores ≤ 3) and did not receive an ASD diagnosis. The LR-Low ADOS (hereafter LR) group had no family history of ASD, scores in the typical range on the ADOS, and no ASD diagnosis.1 Based on these criteria, the sample for the present study consisted of 69 HR-ASD, 51 HR-EA, 317 HR-LA, and 188 LR infants. Demographic data for the four outcome groups and characteristics of the sample at 36 months are presented in Table 2. Ages at enrollment, 6-month visit, and outcome assessment were similar across all groups (enrollment: F(3, 512) = 1.55, p = 0.20; 6-month visit: F(3, 621) = 2.33, p = 0.073; outcome: F(3, 621) = 0.058, p = 0.982).
Analyses
Our primary aim was to examine GM and FM skills at 6 months in relation to outcome classification at 36 months in a large sample of HR and LR infants. We addressed this aim in three sets of analyses. The first was designed to characterize overall GM and FM performance by comparing total scores and percentages of infants with scores suggesting clinically significant delays across the four groups. The second examined item-level performance on the two subscales by examining failure rates across groups both in terms of item difficulty (performance curve analysis) and for sets of preselected items (item analysis). Finally, we determined whether GM and/or FM skills at 6 months were predictive of ASD symptom severity at 36 months.
Results
Group Level Comparisons
Descriptive statistics for GM and FM standardized T-scores for the four groups of infants are presented in Table 3. Although GM T-scores for all three HR subgroups were slightly lower than those of LR infants, the differences were not significant F(3, 474) = 2.34, p = .072.
Table 3.
Mean Gross Motor and Fine Motor T Scores (and Standard Deviations) by Group at 6 Months.
| LR | HR-LA | HR-EA | HR-ASD | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Gross Motor T score | 49.0 | 8.8 | 47.0 | 9.1 | 45.4 | 9.8 | 46.6 | 9.8 |
| N | 121 | 248 | 48 | 61 | ||||
| Fine Motor T score | 50.1 | 7.9 | 48.5 | 9.4 | 44.4 | 9.8 | 45.9 | 9.1 |
| N | 188 | 317 | 51 | 69 | ||||
Note: The Gross Motor subscale was not administered at 2 of the 10 sites, so the N for this measure is smaller than that for Fine Motor.
With regard to the FM subscale, a univariate ANOVA confirmed that the four group means were not equal, F(3,621) = 7.4, p < .001 (see Table 3). Pairwise comparisons using Holm-Bonferroni correction indicated that the LR group scored significantly higher than the HR-EA (p .001, d = .605, 95% CI: 0.290, 0.918) and HR-ASD groups (p = .004, d = .481, 95% CI: 0.201, 0.759). The HR-LA scores were significantly higher than those in the HR-EA group (p = .010, d 0.424, 95% CI: 0.127, 0.721). The HR-EA and HR-ASD groups did not significantly differ from one another (p = .376, d = .155; 95% CI: −0.208, 0.517). The HR-LA group did not differ from the LR (p = .120, d = 0.183, 95% CI: 0.002, 0.364) or HR-ASD groups (p = .080, d = 0.291, 95% CI: 0.029, 0.552).2
The data in Table 3 also indicate greater variability in the scores of all three groups of HR infants, especially for FM. To explore the nature of this variability, we examined the distributions of scores within each group to identify all infants who received scores indicative of a clinically significant motor delay (i.e., T-score ≤ 35, or 1.5 SDs below the scale mean). This procedure was conducted separately for the GM and FM subscales. The percentages of infants in each group who met this criterion are presented in Table 4.
Table 4.
Percentages of Infants by 36-month Outcome Scoring 1.5 SDs Below the Mean at 6 months
| Gross Motor | Fine Motor | |
|---|---|---|
| LR | 7.4% | 2.1% |
| HR-LA | 13.3% | 8.2% |
| HR-EA | 20.8% | 21.6% |
| HR-ASD | 14.8% | 15.9% |
The percentages of HR infants who received GM scores 1.5 SDs or more below the mean were 2–3 times higher than the percentage of infants in the LR group. However, Fisher’s exact tests with Holm-Bonferroni corrections only revealed one significant difference: the percentage of HR-EA infants who fell in this range on the GM subscale was higher than that for LR infants (p = .027). For FM scores, significant differences were found between the LR and HR-LA, HR-EA, and HR-ASD groups, as well as between the HR-LA and HR-EA groups (all ps < .01), with higher percentages for all HR compared to LR infants.
GM and FM Item Performance Curves
Probabilities of failure across items.
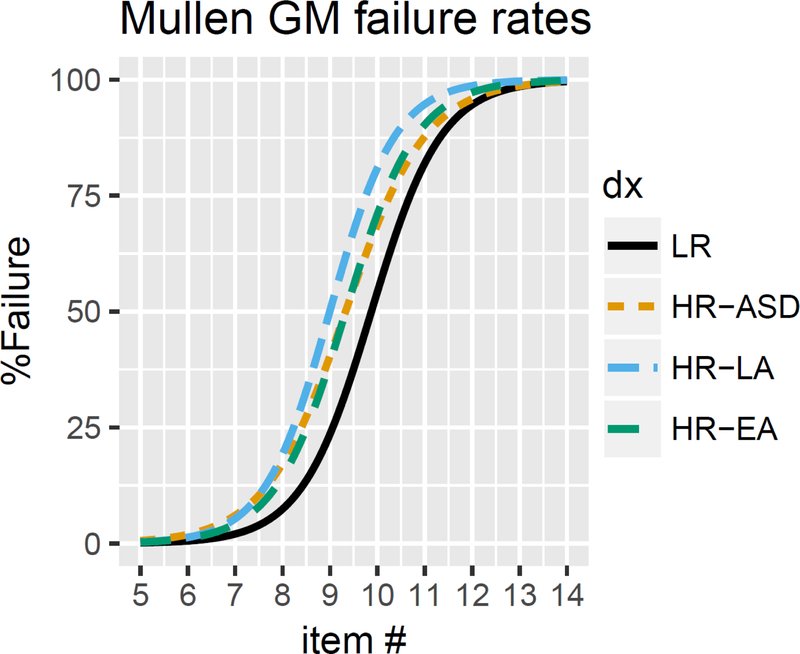
Our next set of analyses examined performance curves on GM and FM items in the four groups of infants. We first examined failure probabilities for each group across the sets of items on each subscale for which at least one child in any group passed the item (i.e., up to and including item 14 for both GM and FM). A generalized linear mixed model with a logit link function was then used to model failure probabilities separately for each subscale. Fixed effects were outcome group, MSEL item number, and participant age. Participant intercept and item slope were included as random effects. Figure 1 presents failure rates on the GM subscale for the four groups. Likelihood ratio tests indicated significant effects of Group (p < .01) and Item (p < .001), but no Group x Item interaction (p = .24). Post-hoc comparisons using Holm-Bonferroni corrections presented in Table 5 suggest overall failure rate intercept differences between the LR and all three HR groups (vs. HR-LA, p = .047; vs. HR-EA, p = .038; vs. HR-ASD, p = .029), such that failure rates for earlier GM items were increased for all of the HR outcome groups. None of the HR groups differed from one another. There were no differences in failure rate slopes between groups, suggesting no differential scaling of failure probabilities with item difficulty between groups.
Figure 1.
Failure rates on the Gross Motor subscale for LR, HR-LA, HR-EA, and HR-ASD infants at 6 months of age.
Table 5.
Post-hoc Comparisons of Failure Rate Intercepts and Slopes Between Outcome Groups for the MSEL Gross Motor subscale
| Failure Rate Intercept Differences |
Failure Rate Slope Differences |
|||
|---|---|---|---|---|
| Value | χ2 | Value | χ2 | |
| LR vs. HR-LA | 0.26 | 6.4* | −0.09 | 0.5 |
| LR vs. HR-EA | 0.32 | 7.1* | 0.01 | 0.1 |
| LR vs. HR-ASD | 0.26 | 8.0* | 0.17 | 2.5 |
| HR-LA vs. HR-EA | 0.57 | 0.64 | 0.10 | 0.7 |
| HR-LA vs. HR-ASD | 0.50 | <0.001 | −0.26 | 3.4 |
| HR-EA vs. HR-ASD | 0.57 | 0.84 | −0.16 | 0.4 |
Note.
p< .05.
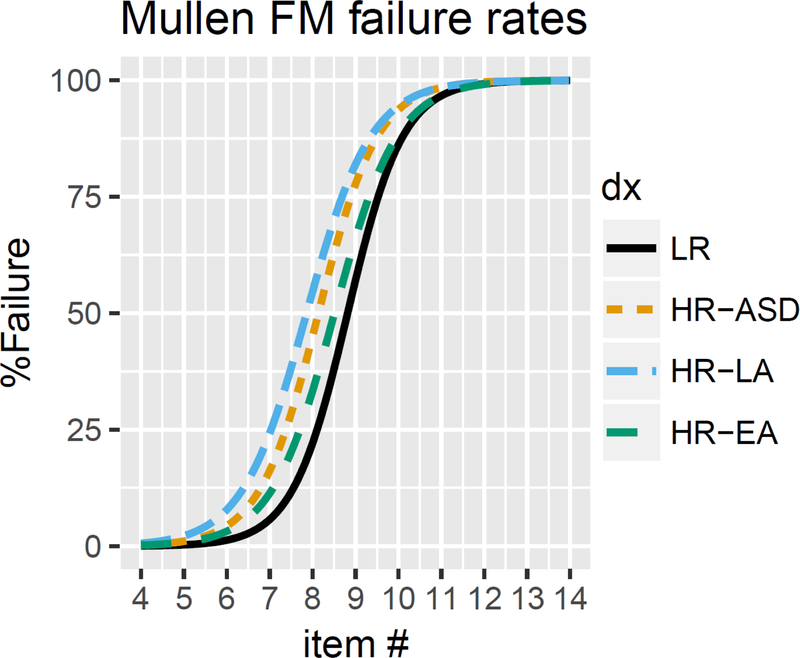
Data on failure rates for the FM subscale are presented in Figure 2. Likelihood ratio tests indicated significant effects of Group (p < .001) and Item (p < .001) and no Group x Item interaction (p = .10). Post-hoc comparisons presented in Table 6 revealed overall failure rate intercept differences between LR and all HR groups (vs. HR-ASD, p < .001; HR-EA, p = .001; HR-LA, p <. 001), indicating that failure rates for earlier FM items were increased for all of the HR groups relative to LR infants. There was also a significant intercept difference between HR-LA and HR-EA infants (p= .002). As with GM performance curves, there were no failure slope differences between groups.
Figure 2.
Failure rates on the Fine Motor subscale for LR, HR-LA, HR-EA, and HR-ASD infants at 6 months of age.
Table 6.
Post-hoc Comparisons of Failure Rate Intercepts and Slopes Between Outcome Groups for the MSEL Fine Motor subscale
| Failure Rate Intercept Differences |
Failure Rate Slope Differences |
|||
|---|---|---|---|---|
| Value | χ2 | Value | χ2 | |
| LR vs. HR-LA | 0.17 | 29.1*** | 0.22 | 3.1 |
| LR vs. HR-EA | 0.34 | 12.7** | 0.18 | 5.3 |
| LR vs. HR-ASD | 0.25 | 17.3*** | 0.10 | 0.7 |
| HR-LA vs. HR-EA | 0.71 | 11.4** | −0.04 | 0.1 |
| HR-LA vs. HR-ASD | 0.39 | 1.8 | 0.12 | 0.7 |
| HR-EA vs. HR-ASD | 0.61 | 3.5 | 0.08 | 0.6 |
Note.
p < .05.
p < .01
p < .001.
Failure rates among infants in each group.
For this analysis, we identified subsets of GM and FM items that assess skills known to be rapidly developing at 6 months of age and for which there is evidence of delays in HR infants both with and without ASD. The selected GM items (5–9 inclusive) focus on control of the head and torso and the emergence of sitting (e.g., Flanagan et al., 2012; Nickel et al., 2013). The selected FM items evaluate refinements in reaching and grasping skills (items 5–9 inclusive; Libertus et al., 2014, Bhat, Galloway, & Landa, 2012). We then calculated failure rates for each item (i.e., the percentages of infants in each group who failed a given item) and conducted a series of pairwise comparisons on the data. Results are presented in Table 7.
Table 7.
Item Level Failure Rates by Group and Results of Pairwise Comparisons
| Failure Rates | Comparison p-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LR | HR-LA | HR-EA | HR-ASD | LR vs. HR-LA | LR vs.HR-EA | LR vs.HR-ASD | HR-LA vs. HR-EA | HR-LA vs. HR-ASD | HR-EA vs. HR-ASD | ||
| Gross motor | |||||||||||
| 5: Supports on forearms | 1.0% | 3.2% | 0.0% | 4.9% | .28 | 1.0 | .11 | .36 | .46 | .25 | |
| 6: Sits supported, head steady | 0.8% | 0.4% | 2.1% | 5% | .55 | .48 | .11 | .30 | .02* | .63 | |
| 7: Rolls over | 12.4% | 13.3% | 14.6% | 11.5% | .87 | .80 | 1.0 | .81 | .83 | .77 | |
| 8: Holds on to fingers/pulls self to sit | 14.9% | 25% | 27.1% | 32.8% | .03* | .08 | .007** | .86 | .26 | .54 | |
| 9: Shifts weight, reaches | 27.3% | 36.7% | 50% | 34.4% | .07 | .007** | .39 | .11 | .77 | .12 | |
| Fine Motor | |||||||||||
| 5: Grasp reflex integrated | 0.0% | 0.9% | 3.9% | 1.4% | .30 | .04* | .27 | .14 | .55 | .57 | |
| 6: Grasps peg (ulnar palmar) | 4.3% | 12.3% | 19.6% | 11.4% | .002** | .001** | .04* | .18 | 1.0 | .30 | |
| 7: Reaches for and grasps blocks | 3.2% | 9.5% | 15.7% | 21.4% | .007** | .003** | <.001** | .21 | .007** | .48 | |
| 8: Transfers, bangs, drops | 42.6% | 49.2% | 64.7% | 54.3% | .17 | .007** | .12 | .05* | .59 | .26 | |
| 9: Refined grasp/thumb opposition | 45.7% | 49.8% | 64.7% | 65.7% | .41 | .02* | .007** | .05* | .02* | 1.0 | |
Note. Pairwise comparisons are Holm-Bonferroni corrected.
As can be seen in Table 7, significant differences in failure rates for all pairwise comparisons were apparent on only three GM items. HR-LA infants had significantly lower failure rates than HR-ASD infants on GM item 6 (sits supported, head steady). LR children were less likely to fail GM item 8 (holds on to fingers/pulls self to sit) than HR-LA and HR-ASD infants and less likely to fail GM item 9 (shifts weight, reaches) than HR-EA infants.
A similar pattern of failure rates was apparent across the four groups on the selected FM items. Relative to LR infants, all groups of HR infants were significantly more likely to fail items 6 (grasps peg, ulnar palmar) and 7 (reaches for and grasps blocks); HR-EA infants were more likely to fail items 5 (grasp reflex integrated), 8 (transfers, bangs, drops), and 9 (refined grasp/thumb opposition); and HR-ASD infants were more likely to fail item 9. Relative to HR-LA infants, HR-EA and HR-ASD infants had significantly higher failure rates on items 8 and 9 and 7 and 9, respectively. There were no significant differences in failure rates between the HR-EA and HR-ASD groups.
Relations between 6-month motor skills and 36-month ASD symptom severity in HR children
In our final analysis, we used negative binomial regression to examine bivariate relations between MSEL GM and FM subscale standardized scores at 6 months of age and ASD symptom severity at 36 months of age as indexed by ADOS severity scores. Standardized scores were utilized due to slight variations in infant age at the 6-month assessment. In order to focus on understanding relations within the autism spectrum, this analysis was restricted to HR children. Negative binomial regression was selected in order to compensate for the strong right skew of outcome variables (biased towards 0, indicating few symptoms of ASD in the sample overall), and was found to fit the data better than other models including Poisson and zero-inflated negative binomial (after minimal score zeroing) regression models. Results of this analysis are shown in Table 8. Although GM scores at 6 months did not predict ASD symptom severity at 36 months, FM scores at 6 months did.
Table 8.
Results of Negative Binomial Regressions Predicting 36-month ADOS Outcomes from 6-month MSEL T-scores
| Gross Motor | Fine Motor | |||||
|---|---|---|---|---|---|---|
| B | z | p | B | z | p | |
| SA Severity | −0.0014 | −0.34 | .74 | −0.012 | −3.25 | .001** |
| RRB Severity | −0.0002 | −0.004 | .60 | −0.014 | −3.59 | <.001** |
| Total Severity | −0.0010 | −0.22 | .83 | −0.015 | −3.65 | <.001** |
Note.
p < .01. SA = Social Affect. RRB = Restricted and Repetitive Behaviors. Z = z statistic on the parameter estimate. For each one point increase in T-score, there was a corresponding change in the log of the ADOS outcome score of B. For example, an increase of Fine Motor t-score from 30 to 40 (ΔT = +10) points would result in a predicted total severity change of exp(ΔT x B) = exp(10 x −.015) = .860, or a 14% reduction.
Discussion
The present study examined overall and item-level performance on the GM and FM subscales of the MSEL administered at 6 months to a large, geographically diverse sample of infants at varying degrees of risk for ASD. We also explored the extent to which motor abilities assessed at 6 months predicted ASD symptom severity at 36 months within the HR group. There were five main findings. First, on average, total scores on the GM subscale did not differ statistically between groups, but HR-EA and HR-ASD infants performed significantly worse than LR peers on the FM subscale. Second, while average scores for all groups on both subscales were within the average range, relative to LR infants, failure rates for earlier FM items were increased for all HR outcome groups, and all HR outcome groups contained significantly higher percentages of infants whose FM scores fell within the range for clinical concern. For GM scores, relative to LR infants, failure rates for earlier items were increased for all HR outcome groups, but only the HR-EA group contained a significantly higher percentage of infants with scores falling within the range for clinical concern. Third, when we examined performance on an individual basis and at the level of individual items, clear patterns of difference emerged between LR and HR infants and among the three HR outcome groups. Interestingly, the weakest profile of performance was observed in the HR-EA children, who exhibited multiple differences from both LR and HR-LA infants in both gross and fine motor skills. Fourth, there were no significant differences between the HR-EA and HR-ASD groups on any variable, and descriptively they often appeared similar to one another. That is, gross and fine motor skills, as assessed on the MSEL at 6 months, did not distinguish infants who were later judged to have ASD from infants who performed similarly on the ADOS but received no CBE diagnosis. We found no evidence of early-appearing motor signs specific to a later ASD diagnosis. Indeed, the presence of early motor differences in both the HR-ASD and HR-EA groups suggests that motor behaviors may be affected across a broad spectrum of disorders (including those with and without ASD-related developmental concerns). Finally, 6-month FM score was a significant predictor of 36-month ADOS severity scores. Each of these findings will be discussed in turn.
Early motor skill differences are associated with ASD risk
Consistent with prior research (Leonard et al., 2014), we observed weaker gross and fine motor performance relative to LR infants in all 3 HR groups, and even among HR-LA infants for whom there were no developmental concerns at 36 months. The most extensive profiles of difference were evident among HR-EA and HR-ASD infants, who performed more poorly relative to LR infants on a broader set of gross and fine motor measures and relative to HR-LA infants on some fine motor variables.
With regard to gross motor ability, analysis of failure rates on individual GM items revealed intercept differences between all 3 HR subgroups and the LR group, indicating that failure rates were higher for HR than LR infants on earlier-administered GM items. Items typically administered at 6 months assess the development of postural control, with a particular focus on head and trunk control critical for the development of independent sitting (see Adolph Berger, 2015). The relatively weaker performance of HR infants on these items is consistent with the observation that a many HR infants are delayed in the onset of independent sitting (Iverson & Wozniak, 2007) and the development of sitting control (Nickel et al., 2013).
We also found that HR infants were significantly more likely than LR infants to fail GM item 8 or 9 (pull to sit; reaching for a toy while prone). Increased failure rates on GM item 8 (e.g., head lag during pull to sit) among HR infants have also been reported by Flanagan et al. (2012). What distinguishes these two items is that while most earlier-administered items focus on static postural control (e.g., supports on forearms while prone), GM 8 and 9 evaluate the ability to coordinate movements of multiple body segments (head, neck, arms, trunk) to counteract gravity and maintain balance while moving — skills fundamental for later self-generated mobility (e.g., crawling, walking).
With regard to fine motor ability, relative to LR infants, failure rates for earlier FM items were increased for all HR outcome groups and all HR outcome groups contained significantly higher percentages of infants whose FM scores fell within the range for clinical concern. HR infants were more likely than LR infants to fail two items assessing grasp quality: grasping peg with ulnar palmar grasp (FM 6); and reaching with grasping (FM 7). In addition, HR-EA and HR-ASD infants had significantly higher failure rates on these items than their HR-LA peers and also exhibited weaker performance on several other FM items assessing grasping (e.g. FM 9; refined grasp with thumb opposition) (see also Libertus et al., 2014).
Grasping and reaching are fundamental fine motor achievements of the first year, and while both exhibit continued development over more extended timeframes (Sacrey et al., 2012), early-emerging disruptions in these skills have implications for another significant development: the ability to explore objects in progressively more sophisticated ways (e.g., Rochat, 1989). The development of reaching provides infants with opportunities to engage spontaneously with objects, and the ability to grasp those objects (and to adjust the shape of the grasp to the features of the object) provides a means for maintaining control while engaging in exploratory behavior. Difficulties with reaching may therefore constrain infant-driven interactions with objects; and weaknesses in grasping ability may limit infants’ ability to explore objects effectively.
Early motor differences are not specific to ASD
We found no significant differences between the HR-EA and HR-ASD groups on any of the variables examined here. The fact that the pattern of motor differences observed among HR-ASD infants was not specific to this group is consistent not only with studies of HR infants that have included a contrast group with non-ASD-related developmental concerns (Landa & Garrett-Meyer, 2006; Leonard et al., 2014) but also with studies that have compared early gross and fine motor abilities in infants later diagnosed with ASD (Ozonoff et al., 2008) and young toddlers with ASD (Provost et al., 2007) to comparison groups of children with developmental delay.
In the present study, although HR-EA infants’ performance did not differ statistically from that of the HR-ASD group, as a group they exhibited a broader array of differences from the LR and HR-LA groups, and relatively more differences in gross motor skills than the HR-ASD group. It is unclear why this was the case, but inspection of the data suggests that the HR-EA group is quite heterogeneous, consisting of children with a variety of different developmental concerns (e.g., language delay; social concerns). The extent to which the motor profiles observed here are characteristic of the group rather than reflecting the aggregation of disparate subtypes is a question for future research.
Results suggesting that delays or alterations in early motor development are not specific to ASD might lead to the conclusion that motor difficulties observed in infancy are not clinically useful or important for understanding symptomology unique to ASD (see Hocking & Caeyenberghs, 2017). In our view, such a conclusion would be premature. While motor delays alone may not specifically predict ASD, aspects of motor behavior in combination with measures of social responsiveness (Baranek, 1999) or measures of reactivity and difficulty making transitions (Brian et al., 2008) appear to improve prediction of ASD diagnoses. In addition, Sacrey et al. (2015) reported that the number of reported parental concerns about motor development distinguished HR infants later diagnosed with ASD from other HR and LR infants as early as 6 months of age. Thus, while motor skills may not be specific indicators of ASD, they clearly contribute valuable information to the complex problem of early identification of ASD.
Are fine motor skills particularly impacted in HR infants?
The fact that we observed significant motor skill differences in fine motor ability is consistent with other research using a variety of methods and examining a broad range of fine motor behaviors appearing in the first two years (e.g., midline play, grasping, stacking blocks, clapping with controlled movement; Heathcock et al., 2015; Kaur et al., 2015; LeBarton & Iverson, 2013; Libertus et al., 2014). However, our results should not necessarily be taken to imply that fine motor skills are more heavily impacted in HR infants than gross motor skills. Our data were derived from a single measure, the MSEL. Scoring of FM items on the MSEL focuses on relatively fine-grained aspects of the target behavior (e.g., evaluation of the position of the thumb relative to the palm and fingers). By contrast, scoring of GM items typically involves a global assessment of presence/absence of a target behavior (i.e., does the infant roll over or not). More fine-grained scoring criteria (e.g., leg position) might capture subtle yet important differences in GM behavior. Indeed, studies that have utilized more detailed measures of gross motor ability (e.g., the Alberta Infant Motor Scales; observational coding of infant posture) have found evidence of differences in GM development among HR infants (Bhat et al. 2012; Heathcock et al., 2015), especially those later diagnosed with ASD (Nickel et al., 2013).
Conclusions
In sum, our findings are generally consistent with a growing number of studies indicating the presence of delayed motor development among HR infants, regardless of developmental outcome. While this body of work makes a strong case that HR infants are likely to exhibit early motor delays, to date it has provided relatively limited information about the nature of these delays. Along these lines, one limitation of the present study is that, as noted above, the MSEL only yields information about the presence/absence of motor behaviors, as defined by item-specific criteria. We do not know, for example, what behaviors were demonstrated when infants failed an item. It would be particularly informative in future work to review videotaped administrations of the MSEL to examine behavior on failed items and how it differed from the targeted behavior. Similarly, more detailed coding of passed items could reveal potential differences in how gross and fine motor behaviors are organized. Although a given motor behavior may be observed in several infants, there may be critical variation across infants in its organization, quality, frequency of occurrence, or how it is utilized. Differences along these dimensions may be more useful than simple presence/absence measures in distinguishing profiles of motor development among HR infants and in linking these to developmental outcomes. Indeed, while these dimensions have not yet been well studied, the few studies that have examined motor behaviors at this level of detail have revealed striking differences between HR and LR infants (e.g., Srinivasan & Bhat, 2016), and particularly HR-ASD infants (e.g., Sacrey et al., 2013; West, Leezenbaum, Northrup, & Iverson, in press).
Finally, the present study underscores the importance of continued developmental surveillance of HR infants, especially those exhibiting delayed motor development from early in infancy (Harris, 2017). Vulnerabilities in infants’ fine and gross motor skills may have significant developmental consequences—exerting cascading effects on later outcomes and in domains other than motor skill (Thelen, 2004; Massand & Karmiloff-Smith, 2015). Over the course of the first year of life, infants acquire and refine an array of new motor skills that vastly alter and enhance their interactions with objects and people and create new opportunities for exploration (see Iverson, 2010, for a review). Thus, for example, fine motor skills support the object exploration that provides infants with the rich perceptual-motor information foundational for learning about and categorizing objects and their properties. To the extent that object manipulation and exploration permit the incorporation of objects into interactions with social partners, they also play a role in the shift from dyadic to triadic interaction and the development of joint attention. In addition, there is research indicating that motor exploratory competence (defined as motor maturity and active exploration) in infancy is positively associated with expressive vocabulary at 1, 2, and 3.5 years, cognitive abilities in toddlerhood (Ruddy & Bornstein, 1989; Siegel, 1981) and childhood (e.g., Broman, 1989), and even with later academic achievement (Bornstein, Hahn, & Suwalsky, 2013).
For infants exhibiting clinically significant delays in motor abilities early in development, intervention efforts should focus on foundational motor skills that develop in the first year (e.g., grasping, upright postural control). There is evidence supporting the utility of developmentally appropriate, parent-delivered interventions for both of these skills (Libertus & Landa, 2014; Lobo & Galloway, 2008), and additional evidence that they have positive effects on skills in other domains (e.g., face processing, means-end understanding). Early identification and intervention for infants with motor delays may help prevent the potentially negative cascading effects of these delays on development.
Acknowledgments
Autism Speaks and Autism Science Foundation provided support for the creation and maintenance of the Baby Siblings Research Consortium (BSRC) database. Data collection, analyses, and manuscript preparation were supported by the National Institutes of Health (Bookheimer P50 HD55784; Chawarska R01 MH087554; Iverson R01 HD54979; Landa R01 MH059630; Messinger R01GM105004; Ozonoff R01 MH068398; Piven HD055741 Shic K01 MH104739; Sigman U54 MH68172; Stone/Messinger R01 HD057284; Tager-Flusberg/Nelson R21 DC08637, R01 DC10290; Vouloumanos/Curtin R01 HD072018), Autism Speaks (Piven, Tager-Flusberg/Nelson), The Simons Foundation (Piven, Tager-Flusberg/Nelson), and the Alberta Centre for Child, Family, and Community Research Grant 060909-TOP (Curtin). Portions of the research were presented at the 2015 International Meeting for Autism Research, Salt Lake City, UT. We thank Robert H. Wozniak for extensive comments on previous versions of the manuscript and the families and infants who participated in the research.
Footnotes
15 LR children obtained an elevated ADOS score at 36 months and were excluded from the sample given their small number. Five received an ASD diagnosis. An additional LR child who had a low ADOS score but received a CBE ASD diagnosis was also excluded.
The difference in available sample sizes for the GM and FM analyses may have impacted our ability to detect significant group differences in GM vs. FM scores. While not all participants with FM scores had GM scores, all infants with GM scores also had FM scores. We therefore conducted a followup analysis of between group differences in the subset of infants with both FM and GM scores (N = 478), so that the statistical power of the FM and GM analyses would be comparable. As expected, there was no change in the GM results. Importantly, the new analysis of FM performance in this reduced subset of participants did not change statistical interpretations (F(3,474) = 6.550, p < .001; with all posthoc tests significant or nonsignificant in the same pattern as the original, expanded FM dataset).
References
- Adolph KE & Berger SE (2015). Physical and motor development. In Bornstein MH & Lamb ME (Eds.), Developmental science: An advanced textbook, (7th ed., pp. 261–333). New York: Psychology Press/Taylor & Francis. [Google Scholar]
- Ament K, Mejia A, Buhlman R, Erklin S, Caffo B, Mostofsky S, & Wodka E (2015). Evidence for specificity of motor impairments in catching and balance in children with autism. Journal of Autism and Developmental Disorders, 45(3), 742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders-text revision (DSM-IV-TR). Washington, DC: American Psychiatric Association. [Google Scholar]
- Baranek GT (1999). Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders, 29(3), 213–224. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, & Landa RJ (2012). Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior and Development, 35(4), 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boin Choi A, Leech KA, Tager-Flusberg H, & Nelson CA (2018). Development of fine motor skills is associated with expressive language outcomes in infants at high and low risk for autism spectrum disorder. Journal of Neurodevelopmental Disorders, 10(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Hahn CS, & Suwalsky JT (2013). Physically developed and exploratory young infants contribute to their own long-term academic achievement. Psychological Science, 24(10), 1906–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, Szatmari P, & Zwaigenbaum L (2008). Clinical assessment of autism in high-risk 18-month-olds. Autism, 12(5), 433–456. [DOI] [PubMed] [Google Scholar]
- Broman SH (1989). Infant physical status and later cognitive development. In Bornstein MH & Krasnegor NA, (Eds.), Stability and continuity in mental development (pp. 45–62). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Estes A, Zwaigenbaum L, Gu H, John TS, Paterson S, Elison JT, … & Kostopoulos P (2015). Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. Journal of Neurodevelopmental Disorders, 7(1), 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JE, Landa RL, Bhat AN, & Bauman M (2012). Head lag in infants at risk for autism: A preliminary study. American Journal of Occupational Therapy, 66, 577–585. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Naik SK, Hass CJ, Lodha N, & Cauraugh JH (2010). Motor coordination in Autism Spectrum Disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders, 40, 1227–1240. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of autism and developmental disorders, 39(5), 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SR (2017). Early motor delays as diagnostic clues in autism spectrum disorder. European Journal of Pediatrics, 176(9), 1259–1262. [DOI] [PubMed] [Google Scholar]
- Heathcock JC, Tanner K, Robson D, Young R, & Lane AE (2015). Retrospective analysis of motor development in infants at high and low risk for autism spectrum disorder. American Journal of Occupational Therapy, 69(5), 6905185070p1–6905185070p9. [DOI] [PubMed] [Google Scholar]
- Hilton CL, Zhang Y, Whilte MR, Klohr CL, & Constantino J (2012). Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism, 16(4), 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking DR, & Caeyenberghs K (2017). What is the nature of motor impairments in autism, are they diagnostically useful, and what are the implications for intervention? Current Developmental Disorders Reports, 1–9.
- Iverson JM (2010). Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language, 37, 229–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, & Wozniak RH (2007). Variation in vocal-motor development in infant siblings of children with autism. Journal of Autism and Developmental Disorders, 37, 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EJ, Gliga T, Bedford R, Charman T, & Johnson MH (2014). Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience & Biobehavioral Reviews, 39, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Srinivasan SM, & Bhat AN (2015). Atypical object exploration in infants at-risk for autism during the first year of life. Frontiers in Psychology, 6, 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, & Garrett-Meyer E (2006). Development in infants with autism spectrum disorder: A prospective study. Journal of Child Psychology and Psychiatry, 47, 627–638. [DOI] [PubMed] [Google Scholar]
- LeBarton ES, & Iverson JM (2013). Fine motor skill predicts expressive language in infant siblings of children with autism. Developmental science, 16(6), 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard HC, Elsabbagh M, Hill EL, & BASIS team. (2014). Early and persistent motor difficulties in infants at-risk of developing autism spectrum disorder: A prospective study. European Journal of Developmental Psychology, 11(1), 18–35. [Google Scholar]
- Libertus K, & Landa RJ (2014). Scaffolded reaching experiences encourage grasping activity in infants at high risk for autism. Frontiers in Psychology, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertus K, Sheperd KA, Ross SW, & Landa RJ (2014). Limited fine motor and grasping skills in 6-month-old infants at high risk for autism. Child development, 85(6), 2218–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MA, & Galloway JC (2008). Postural and object-oriented experiences advance early reaching, object exploration, and means–end behavior. Child Development, 79(6), 1869–1890. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, … & Rutter M (2000). The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, & Lecouteur A (1994). Autism diagnostic interview-revised—a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Massand E, & Karmiloff-Smith A (2015). Cascading genetic and environmental effects on development: Implications for intervention. In Mitchell KJ (Ed). The genetics of neurodevelopmental disorders (pp. 275–288). New York, NY: John Wiley & Sons. [Google Scholar]
- Messinger D, Young GS, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, … & Hutman T (2013). Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child & Adolescent Psychiatry, 52(3), 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger DS, Young GS, Webb SJ, Ozonoff S, Bryson SE, Carter A, … & Dobkins K (2015). Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Molecular Autism, 6(1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen Scales of Early Learning Circle Pines, MN: American Guidance Service. [Google Scholar]
- Nickel LR, Thatcher. AR, Keller F, Wozniak RH, & Iverson JM (2013). Postural development in infants at heightened vs. low risk for Autism Spectrum Disorder. Infancy, 18, 639–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, & Rogers SJ (2008). Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism, 12, 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost B, Lopez BR, & Heimerl S (2007). A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders, 37(2), 321–328. [DOI] [PubMed] [Google Scholar]
- Rochat P (1989). Object manipulation and exploration in 2- to 5-month old infants. Developmental Psychology, 25, 871–884. [Google Scholar]
- Ruddy MG & Bornstein MH (1984). Cognitive correlates of infant attention and maternal stimulation over the first year of life. Child Development,53, 183–188. [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The social communication questionnaire: Manual Western Psychological Services. [Google Scholar]
- Sacrey LAR, Karl JM, & Whishaw IQ (2012). Development of rotational movements, hand shaping, and accuracy in advance and withdrawal for the reach-to-eat movement in human infants aged 6–12 months. Infant Behavior and Development, 35(3), 543–560. [DOI] [PubMed] [Google Scholar]
- Sacrey LAR, Bryson SE, & Zwaigenbaum L (2013). Prospective examination of visual attention during play in infants at high-risk for autism spectrum disorder: A longitudinal study from 6 to 36 months of age. Behavioural Brain Research, 256, 441–450. [DOI] [PubMed] [Google Scholar]
- Sacrey LAR, Zwaigenbaum L, Bryson S, Brian J, Smith IM, Roberts W, … & Vaillancourt T (2015). Can parents’ concerns predict autism spectrum disorder? A prospective study of high-risk siblings from 6 to 36 months of age. Journal of the American Academy of Child & Adolescent Psychiatry, 54(6), 470–478. [DOI] [PubMed] [Google Scholar]
- Siegel LS (1981). Infant tests as predictors of cognitive and language development at two years. Child Development, 545–557.
- Srinivasan SM, & Bhat AN (2016). Differences in object sharing between infants at risk for autism and typically developing infants from 9 to 15 months of age. Infant Behavior and Development, 42, 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E (2004). The central role of action in typical and atypical development: A dynamic systems perspective. In Stockman IJ (Ed.), Movement and action in learning and development: Clinical implications for pervasive developmental disorders (pp. 49–73). San Diego, CA: Elsevier Academic Press. [Google Scholar]
- West KL (in press). Infant motor development in Autism Spectrum Disorder: A synthesis and meta-analysis. Child Development [DOI] [PubMed]
- West KL, Leezenbaum NB, Northrup JB & Iverson JM (in press). Relation between walking and language in infant siblings of children with autism spectrum disorder. Child Development [DOI] [PMC free article] [PubMed]