Unmet medical need
Despite significant progress in pharmacological management of patients with primary hypertension, treatment-resistant hypertension (TRH) is still relatively prevalent among them (1). This condition is defined as blood pressure that remains above goal despite concurrent use of optimal doses of three anti-hypertensive drugs of different classes and exclusion of secondary causes of hypertension (2). Current evidence-based pharmacological approach to TRH targets 3 well-established pathophysiological mechanisms thought to underlie this condition simultaneously: increased sodium and fluid retention, enhanced activation of renin-angiotensin system, and over-reactive sympathetic nervous system (3, 4). Thus, patients with TRH are presently treated with combined daily oral diuretics, angiotensin converting enzyme (ACE) inhibitors/angiotensin receptor blockers and calcium channel blockers (5). If blood pressure remains persistently elevated, daily oral spironolactone, an antagonist of aldosterone receptors, is then added to the 3-drug regimen, which provides significant benefit to patients with hyperaldosteronism (2). However, this poly-pharmacopeia approach is not only insufficient to adequately control blood pressure in a significant population of TRH patients but may also expose patients with TRH to serious adverse events and drug interactions leading eventually to non-adherence and treatment failure. Hence, despite recent advances in hypertension research and therapeutics, TRH still represents a life-threatening medical problem.
Consequently, there is an urgent need to develop and test novel, safe and efficacious drugs for TRH to improve long-term clinical management and outcomes of patients. Unfortunately, accomplishing this goal is challenging because current drug development programs in primary hypertension revolve around traditional oral administration of active pharmaceutical ingredients (API’s). An important drawback of this approach is that traditional oral formulations of promising drug candidates may still be limited by their low bioavailability, short half-life and unfavorable safety profile that disqualify them from further clinical development and marketing. To begin to address these constraints, harnessing the unique chemical, biophysical, safety and efficacy attributes of nanopharmaceuticals could represent an innovative therapeutic strategy for patients with TRH.
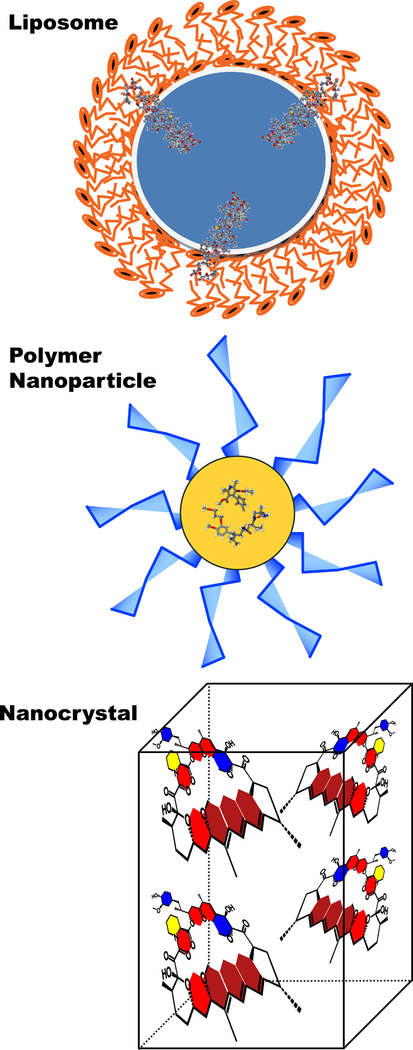
To date, approximately 50 nanodrugs have been approved by the FDA for various indications predominantly cancer, infections and bone substitute (6). However, while it is well established that marketed anti-hypertension medications, such as ACE inhibitors, angiotensin receptor blockers, and calcium channel blockers, have low oral bioavailability and potentially serious adverse effects, no nanodrugs are presently approved for cardiovascular disorders, including TRH. The purpose of this review is, therefore, to provide a snapshot of liposomal, polymeric and nanocrystal nanoparticles, the most-commonly used FDA-approved nanotechnology-based drug delivery platforms since the 1990’s (Figure 1), as potential novel modalities to deliver both marketed and new anti-hypertension API’s to treat patients with TRH. We discuss evidence that these modalities provide a targeted, safe and efficacious delivery approach for anti-hypertensive medications.
Figure 1:
Schematic representation of three most commonly used, FDA-approved nanocarriers for novel anti-TRH nanodrugs.
Currently, several animal models of hypertension have been developed to allow the in vivo investigation of treatment modalities and drug efficacy. In this review, we discuss studies reporting the beneficial effects of nanomedicine in reducing blood pressure and improving bioavailability of standard anti-hypertensives and poorly soluble biomolecules (e.g. superoxide dismutase). We discuss these findings in each respective NP section and have outlined those studies that include FDA-approved anti-hypertensives used in combination with specific NP formulations in Table 1. It is important to note, however, that an in vivo animal model exactly representing TRH is not yet available to directly test the benefits of nanomedicine in treating TRH. Despite this, the present literature details reductions in blood pressure with nanomedicine as compared to free drug through increased and long-lasting drug bioavailability and efficacy. Therefore, the in vivo studies highlight the potential for using previously FDA-approved anti-hypertensives with NP formulations to safely combat TRH independent of a detailed understanding of the underlying mechanisms mediating TRH.
Table 1.
FDA-approved anti-hypertensives that show promising in vivo results for treatment of hypertension when combined with NP formulations
| FDA-approved antihypertensive | NP Formulation | NP delivery method | In vivo model of hypertension | Effect of NP-antihypertensive on BP | Bioavailability | References |
|---|---|---|---|---|---|---|
| SOD | Liposome encapsulation | Daily injections (x8 days) | 5 day infusion of AngII in rats | Reduced MAP by 50 mm Hg | Increased circulation time of 5 hours | 22, 23 |
| Lercanidipine | Proliposome | Oral dose using intragastric tube | *DOCA salt protocol, rats | Immediate and long lasting (24 hrs) reduction of SBP | t½ = 6.95 h (vs. 5.26 h for free drug) |
29 |
| Felodipine | PLGA | Orally (1mg/kg) | *DOCA salt protocol, rats | Long lasting (4 days) reduction of SBP compared to free drug (< 2 days) | Sustained release in vitro (144 hours). In vivo measures N/A |
41 |
| aliskiren | Magnetic poly(D, -lactide) | Oral gavage | Spontaneously hypertensive rats | Reduced SBP ~25 mm Hg compared to aliskiren alone | N/A | 44 |
Abbreviations: FDA, Food and Drug Administration. NP, nanoparticle. BP, blood pressure. SOD, superoxide dismutase. PLGA, polylactide-co-glycolide. PLA, poly-lactic acid. AngII, angiotensin II. DOCA, deoxycorticosterone acetate. MAP, mean arterial pressure. SBP, systolic blood pressure. t½ = half-life.
Subcutaneous injections of DOCA in olive oil administered twice weekly for four weeks. Doses varied from 20–25 mg/kg among studies.
Liposomes
Liposomes are small, self-assembled, spherical vesicles composed of biocompatible and biodegradable unilamellar or multilamellar phospholipid bilayers that surround an aqueous core (7, 8). They can be loaded with hydrophilic, hydrophobic or amphipathic API’s, including peptides, biomolecules and nucleotides, and once administered passively accumulate in target tissues and organs where their payloads are released by various local processes thereby achieving higher drug concentrations locally. For instance, liposomes can be designed to be pH- or temperature-sensitive thereby enabling controlled release of their payloads when exposed to specific environmental conditions in target tissues and organs. Liposomes can also be loaded with several API’s each targeting distinct metabolic pathways that mitigate tissue injury and/or promote repair.
Targeted delivery:
Early studies by Hodis and his colleagues (1990 &1991) (9, 10) demonstrated the potential efficiency of liposome delivery of drugs into the vascular wall in animal models of hypertension and atherosclerosis. Specifically, they showed that passive accumulation of liposomes in arterial wall of hypertensive rabbits is increased by almost 3-fold in comparison to that of normotensive rabbits and that this process may be mediated, in part, by monocyte/macrophage cells, providing the first direct evidence for liposomes escaping the circulation and entering the vascular wall. These data further suggest that circulating liposomes could preferentially target and accumulate in injured vascular beds, most likely due to the disruption of the endothelial barrier and accumulation of monocytes/macrophages, thus providing a mechanism for the targeted delivery of liposomes loaded with anti-TRH drugs into the diseased areas of the vascular beds of patients with TRH. Another strategy for the targeted delivery is decorating liposome surface with ligands that are specific to receptors expressed on target cells (aka cellular zip codes), which may enable active targeting and selective delivery of high concentrations of liposome-loaded anti-TRH drugs to these cells. This, in turn, circumvents collateral damage to bystander cells and tissues and lessens adverse events.
Long circulation time:
Liposome delivery systems also allow prolonging circulation time of the drugs by evading their recognition, uptake and clearance by the human body’s reticuloendothelial system. This is accomplished by attaching pre-defined molecular weight and amount of biocompatible and biodegradable poly(ethylene glycol) chains to the surface of liposomes (11). With FDA approval of PEGylated liposomal doxorubicin in 1995, liposomes became since then the most commonly FDA-approved, lipid-based drug delivery platform for anticancer, antifungal and anti-pain drugs (6).
Antihypertensive liposomal drug formulations tested in animal models:
Several liposomal drug products have already been developed and tested successfully in animal models of hypertension. The most prominent example is liposomal preparation of Vasoactive intestinal peptide (VIP), a 28-amino acid pleiotropic, amphipathic, endogenous human peptide with pronounced vasodilatory, immunomodulating and anti-proliferative properties (12, 13). A single intravenous injection of VIP, self-associated with sterically-stabilized liposomes, but not free VIP nor empty liposomes, has been shown to normalize systemic arterial pressure in spontaneously hypertensive hamsters for the entire duration of the observation period (6 hours) (14, 15). Injection of free VIP was ineffective because of rapid degradation and inactivation within minutes in vivo (16). Importantly, anti-hypertensive effects of liposomal VIP are also observed after both subcutaneous and intratracheal administration to spontaneously hypertensive hamsters (17). The salutary effects of liposomal VIP were attributed to its potent vasodilatory properties (14).
Another promising candidate for liposome delivery for TRH is Superoxide dismutase (SOD), an enzyme that converts the superoxide radical, known to have numerous adverse effects on vascular function, into non-toxic molecular oxygen or water (18, 19). As such, SOD is considered a major defense mechanism against oxidative stress (18, 20). Current concepts suggest that reactive oxygen species and oxidative stress play a key role in the development and maintenance of primary hypertension in humans (21). Unfortunately, this metabolic pathway is not targeted directly by currently-approved anti-hypertensive drugs. A major limitation of SOD as a therapeutic drug is its short life time in circulation, as recombinant human superoxide dismutase (rhSOD) was reported to have a half-life of 20 minutes in healthy volunteers (22). It also has low membrane permeability (20). To overcome both problems, several studies developed liposomes with encapsulated recombinant human rh-Cu/Zn-SOD (23–25) and at least one study, Laursen et al (23) showed that liposome-loaded SOD reduced blood pressure significantly in rats with angiotensin II-induced hypertension while having no effects in rats with norepinephrine-induced hypertension or controls. Patel et al (25), however, did not see a beneficial effect of liposomal SOD delivery in spontaneously hypertensive rats, which could be related to different hypertension rat models used in both studies or different biophysical properties of the liposomes, which were not well described. Administration of SOD encapsulated into poly(ethyleneglycol) (PEG)-grafted liposomes was also shown to have significant advantage, as compared to free SOD in a rat model of adjuvant arthritis (26). Notably, the stability of liposomal delivery of rhSOD was also tested in a large animal model. Kaipel et al showed that (27) intravenous delivery of rhSOD loaded liposomes resulted in a pronounced but transient increase in rhSOD in the plasma, which dissipated within 5 hours, which was still significantly longer than earlier studies of free SOD in humans (22), however, free SOD was not tested in the pig study. Most interestingly, aerosolization of the liposomal rhSOD and application via a breathing device led to further significant increase in plasma rhSOD life time (27). The latter observation not only shows promising results in terms of improving SOD delivery for therapeutic use but also indicates that it can be delivered in a non-invasive “needle free” way with improved efficacy. Moreover, topical liposomal recombinant human SOD (rhSOD) manufactured under GMP conditions (Lipoxysan™ Polymun Scientific Immunobiologische Forschung GmbH, Vienna, Austria), was safe and efficacious in a randomized, placebo-controlled, double-blind prospective clinical trial conducted in patients with painful Peyronie’s disease (28).
Recently, a new liposomal preparation was developed for lercanidipine (29), a calcium channel blocker currently used to treat hypertension but is also known to have poor aqueous solubility and low bioavailability (30). Deshpande et al show that liposomal encapsulation and delivery of lercanidipine result in a significant increase in absorption rate, increase in bioavailability and increased efficiency in reducing blood pressure in DOCA-salt induced hypertensive rats as compared to pure lercanidipine (29).
Routes of delivery:
At present, intravenous administration of anti-hypertensive drug-loaded liposomes is the traditional delivery system to use in humans and animal models and this delivery route was used in the studies described above. However, an emerging non-invasive, simple, and more practical administration route is by inhalation where dried anti-hypertensive drug-loaded liposomes of appropriate particle size are deposited in the alveoli, translocate into the bloodstream and/or lymphatics and are then distributed to target tissues (31, 32). In addition, nasal delivery of liposome powders for non-invasive, direct brain targeting of anti-hypertensive drugs through fenestrated capillaries in the olfactory bulbs may be attractive because it enhances efficacy while reducing systemic adverse events (33). Lastly, transdermal and subcutaneous delivery of liposome-encapsulated valsartan and VIP, respectively, have been reported in rats (34, 35). However, efficacy and safety of these liposome delivery routes must first be established in animal models of hypertension before translation to clinical trials.
Clearly, liposomal delivery platforms are an attractive alternative to traditional formulations of anti-hypertensive drugs, as well as possible route to introduce new therapeutic drugs that cannot be used in their pure form because of bio-availability limitations. The advantages of liposomal formulations described above, coupled with recently issued FDA guidance to the pharmaceutical industry for liposome drug products that standardizes drug development and registration (www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation.htm) should promote use of this nanotechnology platform to deliver API’s to patients with TRH by several administration routes outlined above.
Polymeric Nanoparticles
Polymeric NPs (PNPs) represent a broad class of NPs and refer to those composed of any polymer type, from single chain polymers to nanospheres and nanocapsules. Polymeric nanospheres typically refer to a solid nanoparticle which incorporates an active drug and polymeric nanocapsules refer to a particle that contains a liquid, typically oily core that contains the drug encapsulated by a polymeric shell (36). Many of the FDA-approved nanomedicines today are PNPs attached to standard medications to improve circulation through solubility and/or bioavailability (6). The most common “attachment” is the hydrophilic poly-ethylene glycol (PEG) which has been used with a number of FDA approved drugs to increase drug half-life in the circulation (37). PEGylation has a shielding effect on the incorporated drugs which inhibits non-specific interactions and reduces the immune response, known as “stealth behavior” resulting in a slower blood clearance and increased circulation time (38). Indeed, multiple PEGylated NPs were shown to improve the stability of the incorporated protein drugs indicating that this technology is particularly important for the protein-based APIs (37).
The major drawback of PEG polymers, however, is their non-biodegradability and while low molar mass PEG oligomers are easily secreted by renal clearance, they are also found to form toxic oxidative products and not recommended for use in drug delivery (38). Increasing the molar mass on the other hand reduces oxidative degradation but may result in liver accumulation and it is still not clear whether it is completely excreted (38). Growing attention, therefore, is geared to developing bio-degradable polymeric NPs (39), as discussed in more detail below. Nevertheless, as of 2017, 17 PNPs were FDA approved and of these, 13 were drugs that had a PEG-PNP addition alone or in combination with other polymers (6). These PEGylated NPs are currently used to treat cancer, multiple sclerosis and hepatitis C (37) but as with most of the FDA approved NPs, no PNPs currently exist to treat hypertension.
Antihypertensive PNP drug formulations tested in animal models:
Several studies suggested beneficial effects of PNP drug delivery on various cardiovascular diseases. The PNPs that have been studied for treatment of hypertension in animal models focused on a biodegradable polymer polylactide-co-glycolide (PLGA) (40). PLGA is a polymer of lactic and glycolic acids that is easily biodegradable and considered to be non-toxic (41). It has been approved by FDA for drug delivery (42). Moreover, PLGA particles were shown to enhance the bioavailability and improve safety of hydrophobic and enzymatically unstable drugs. Using estradiol as a model drug, it was shown that PLGA particles delivered using oral gavage promote sustained release in plasma (up to 11 days) in Sprague Dawley rats with no observable inflammatory response (43, 44). PLGA PNPs were also shown to enhance the efficacy of atorvastatin, also delivered using oral gavage, in reducing the lipid load in diet-induced dyslipidemic in rats (45). Most importantly, PLGA PNPs were shown recently to improve anti-hypertensive effects of several types of commonly-used anti-hypertensive drugs. Shah et al (2014) (46) showed that encapsulating Felodipine, a dihydropyridine calcium-channel blocker, used alone or in combination with other antihypertensives to treat hypertension, into PLGA PNPs significantly improve its ability to control systolic blood pressure in salt-induced hypertension in rats. The drugs were delivered orally. This was attributed to increased bio-availability of Felodipine, which by itself has low water solubility resulting in poor bioavailability in standard formulations (47).
Consistent with the increased efficacy, Arora et al 2015 (48) showed that encapsulating three commonly-used anti-hypertensive drugs (hydrochlorothiazide, amlodipine, and candesartan) into PLGA PNPs resulted in an increase almost 10-times the free drug circulating time. The study was performed in rats and the drugs were administered by an oral feeding tube. In addition, magnetic poly(D,L-lactide) nanoparticles, were also shown to improve control of blood pressure in spontaneously hypertensive rats by another anti-hypertensive drug, aliskiren, a renin inhibitor (49). The drugs were delivered by oral gavage. Using magnetic particles that consist of a magnetic material and an active chemical component is an interesting technology that allows controlling particle aggregation but this aspect was not explored in the study described above.
Another bio-degradable polymer used on PNPs in experimental studies of hypertension is chitosan. Chitosan, a linear polysaccharide made from the shells of crustaceans, can be formed into spherical biopolymers for the encapsulation of water insoluble drugs, including those to treat hypertension (50–53). As with the other PNPs, chitosan based polymers are suggested to improve oral administration by increasing solubility of the drug. Also like other PNPs, chitosan based polymers exhibit extended-release functionality to improve drug efficacy without increases in drug dosage thereby preventing detrimental side effects (52). In a recent study, Chadha et al demonstrated the therapeutic potential for chitosan PNPs in an in vivo rat model of deoxycorticosterone acetate salt-induced hypertension (53). Chitosan polymers generated to include lecithin, an amphiphilic lipid which renders the PNP both water and lipid soluble, and carrying the water insoluble antihypertensive, Ramipril, decreased systolic blood pressure in hypertensive rats. The emerging interest and relevance for chitosan based PNPs is evidenced by two studies published earlier this year detailing the beneficial effects of using chitosan based PNPs: Auwal et al. showed that chitosan PNPs encapsulating food based antihypertensive biopeptides, a safe and thus potentially attractive source of non-pharmaceutical treatment of TRH, protect the biopeptide from gastrointenstinal degradation (50). In addition, Chinh et al. showed that polylactic acid/chitosan NPs carrying the Ca2+ channel blocker nifedipine reduced blood pressure in mice (51).
Taken together, these studies show that the use of PNPs in essential hypertension could circumvent issues of bioavailability and potentially even prevent off-target effects through active targeting. Furthermore, combining standard antihypertensives with PNP nanomedicines may also be useful in combatting TRH where multiple drugs fail to reduce blood pressure.
Cyclodextrin Nanoparticles
Cyclodextrins (CDs) are cyclic oligosaccharides, which are known to be potent carriers of hydrophobic drugs and are increasingly developed as drug delivery platforms. The key feature of cyclodextrins is that while they are water soluble, they contain a cone-shaped hydrophobic cavity that can encapsulate various hydrophobic compounds (54). Notably, CDs can be crosslinked by polymers and have the ability to form nanoparticles by precipitation (55). Similarly to other nanoparticle technologies, inclusion of a drug in the hydrophobic cavity of CDs protects the drugs from absorption and degradation improving the bioavailability and increasing the circulation time. Furthermore, several types of CD particles received US FDA approval or achieved the status of “Generally Regarded as Safe” (GRAS).
Several studies explored the efficacy of CD nanoparticles in delivery of anti-hypertensive drugs. De Azevedo et al (2011) (56) showed that encapsulation of captopril (CAP), an angiotensin I-converting enzyme inhibitor, into CD nanoparticles had a significant beneficial effect on blood pressure in rats infused with angiotensin-I. The beneficial effects of CD/CAP complexes as compared with CAP alone were more significant at the lower dose. Angiotensin I was delivered by intravenous infusion and the CAP or CD/CAP complexes were delivered by gavage. The CD/CAP complexes (nanoparticles) showed a long-lasting inhibitory activity (up to 22 hours) on the angiotensin I pressor effect. Similarly, valsartan (VAL), an antagonist of angiotensin II, incorporated into β-CD complexes, also resulted in a more efficient decrease in blood pressure in rats (57). More recently, β-CD complexes were also shown to facilitate the effect of hydrochlorothiazide, a diuretic used in clinics to treat arterial hypertension, by improving the biopharmaceutical properties of the drug and protecting the drug from hydrolysis in vivo in rats (58). Thus, cyclodextrin nanoparticles appear to be one of the promising potential delivery systems for anti-hypertensives.
Nanocrystals
Drug nanocrystals are nanoparticles with crystalline structure that are composed solely of the drug molecules with no carrier materials (59). The major advancement of these formulations is their ability to address poor solubility in aqueous solution, which becomes a significant issue for multiple new drugs. It was estimated that 40% or more of new drugs identified by screening approaches are poorly soluble in water, which limits their absorption and decreases bioavailability (60). A conventional method of adding co-solvents or surfactants that is used to increase drug solubility is limited by the toxicity of the additives. Nanocrystals offer an alternative approach to improve solubility by increasing the surface area that is in contact with the aqueous solution and by such to increase the velocity of the dissolution and saturation solubility, which improve bioavailability (59, 60). Typically, optimal effects are achieved with particle sizes of 20–50 nm and the drugs can be administered either orally or suspended in a solution (nanosuspensions) for intravenous or pulmonary delivery route (59). An increase in the bioavailability is associated with significantly improved PK/PD properties of the poorly soluble materials (6, 61, 62). Currently, the nanocrystal technology is used in ~30% of all FDA approved nanoparticle drugs with 15 approved drugs and 2 more in the investigational stages (6). Future studies are needed to explore the potential of this technology in TRH.
Interestingly, the first nanocrystal drug formulation, Rapamune (active component: rapamycin, an inhibitor of mTOR signaling pathway) that is currently used as an immunosuppressant to avoid organ rejection (63) may also be beneficial in different types of hypertension because of its anti-proliferation effects. Specifically, rapamycin was shown to reduce neo-angiogenesis and ameliorate inflammation and fibrosis in mouse and rat models of portal hypertension when delivered both by gavage (64) and intraperitoneally (65). It is also being tested for the treatment of Pulmonary Hypertension using intravenous delivery (66). Emerging evidence that Resistant Hypertension involves vascular remodeling (4) opens an intriguing possibility that Rapamune might be also beneficial for TRH.
Cerium oxide (CeO2) nanoparticles.
These constructs have emerged in the last decade as powerful anti-oxidant materials. CeO2 NPs function as mimetics of superoxide dismutase (SOD) and catalase and as scavengers for free radicals (67). The anti-oxidant property of cerium oxide is based on the ability of cerium to cycle between reduced and oxidized states. They have been proposed, therefore, to provide a therapeutic approach to reduce oxidative stress and inflammation. CeO2 NPs were also shown to have anti-apoptotic activity. Multiple studies explored the beneficial effects of CeO2 NPs in a variety of pathological conditions including radiation damage, cancer, and neurodegenerative diseases (67). A recent study by Oro et al (2016) (68) showed that CeO2 NPs delivered intravenously have a significant protective effect against liver injury and portal hypertension in a rat model of CCl4-induced liver fibrosis.
Clearly, further pre-clinical studies are warranted to determine safety and biocompatibility of these emerging technologies before translating them into clinical practice.
Summary and Perspectives
Collectively, studies presented in our review suggest that nanodrugs could be considered for further development as novel anti-TRH drugs (Table 1 summarizes FDA-approved drugs loaded onto nanoparticles and tested successfully in animal models of hypertension). These nanodrugs may comprise both marketed and investigational API’s loaded onto US Food and Drug Administration (FDA)-approved biocompatible, biodegradable, nontoxic drug delivery platforms. This approach improves formulations of poorly water-soluble, low bioavailability and unstable API’s, promote controlled drug release over an extended period of time, and enable targeted delivery of anti-hypertensive drugs to injured tissues thereby improving both safety and efficacy. Importantly, active targeting of drug-loaded, long-circulating liposomes is accomplished by grafting cell-specific ligands, such as a peptide sequence to the angiotensin II type I receptor (69), onto their PEG moieties resulting in specific binding of these liposomes to complementary cell receptors in target tissues and selective delivery of payloads. Hence, recommended doses of these nanodrugs would be lower than those of bulk drugs resulting in lower pill burden and improved safety, tolerability, and patient adherence. Conceivably, loading FDA-approved drug products could simplify and shorten the duration of liposome-based drug development programs for TRH.
The reasons underlying the lack of FDA-approved nanodrugs formulations for the treatment of hypertension is uncertain but may be multi-factorial (70). Conceivably, they could be related to product instability, polydispersity and toxicity along with technical difficulties in scaling up and maintaining the manufacturing process which then lead to higher production costs (71). An important contributing factor is the lack of standardized FDA-issued written guidance to the pharmaceutical industry for developing and registering nanodrug products, except for liposomes (https://www.fda.gov/downloads/drugs/guidances/ucm070570.pdf). Consequently, each non-liposomal, nanodrug product application is presently reviewed by FDA on a case-by-case basis which then amplifies the sponsor’s assumed financial risk and liability (72).
Furthermore, unlike currently marketed oral medications for primary hypertension, nanodrugs can also be administered by inhalation, nasal, transdermal/dermal and subcutaneous injection routes. Similar to the FDA-approved subcutaneous and inhaled insulin (73), both patient-friendly drug delivery routes of liposomal VIP could be pursued in clinical trials of patients with TRH. Whether pulmonary delivery to the systemic circulation of these nanodrugs is also efficacious in TRH remains to be determined.
It is also possible that because the etiology of TRH is unknown, improving the delivery and efficacy of the existing antihypertensives by nanomedicines will still fail as a treatment. At the very least, however, it will rule out pharmacodynamics/pharmacokinetics as an underlying cause of resistance in this population. If this is the case, then obviously new classes of drugs will have to be developed targeting alternative mechanisms. Based on the current review, it appears that some of the potential alternative mechanisms of TRH can be affected utilizing existing or developing nano-drugs, such as liposomal SOD formulations, rapamycin PNPs, and nanocrystals.
Acknowledgements:
We thank Mr. Gregory Kowalsky and Mr. Nicolas Barbera for designing and rendering chemical structures for Figure 1.
Sources of funding:
This study is supported by NIH grants HL-R01-HL1073965 (IL), R01-HL083298 (IL), and U01-NS083457 (IR) and U01-NS083457-S1 (IR) and T32-HL007829–24 (ISF).
Footnotes
Disclosures:
Ibra S. Fancher – None
Israel Rubinstein – Co-founder EnSol Therpautics, LLC. This entity is not connected to/involved with the subject matter of this review.
Irena Levitan – None
References
- 1.Achelrod D, Wenzel U, & Frey S (2015) Systematic Review and Meta-Analysis of the Prevalence of Resistant Hypertension in Treated Hypertensive Populations. American Journal of Hypertension 28(3):355–361. [DOI] [PubMed] [Google Scholar]
- 2.Wei F- F, Zhang Z- Y, Huang Q- F, & Staessen JA (2018) Diagnosis and management of resistant hypertension: state of the art. Nature Reviews Nephrology 14(7):428–441. [DOI] [PubMed] [Google Scholar]
- 3.Parreira RC, et al. (2017) Decoding resistant hypertension signalling pathways. Clin Sci (Lond) 131(23):2813–2834. [DOI] [PubMed] [Google Scholar]
- 4.Hwang AY, Dietrich E, Pepine CJ, & Smith SM (2017) Resistant Hypertension: Mechanisms and Treatment. Current Hypertension Reports 19(7):56. [DOI] [PubMed] [Google Scholar]
- 5.Mann SJ (2011) Drug therapy for resistant hypertension: simplifying the approach. Journal of clinical hypertension 13(2):120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventola CL (2017) Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T 42(12):742–755. [PMC free article] [PubMed] [Google Scholar]
- 7.Düzgüneş N & Gregoriadis G (2005) Introduction: The Origins of Liposomes: Alec Bangham at Babraham. Methods in Enzymology, (Academic Press; ), Vol 391, pp 1–3. [Google Scholar]
- 8.Betageri GV & Venkatesan N (2011) Liposomes. Nanotechnology in Health Care:164–180. [Google Scholar]
- 9.Hodis HN, Amartey JK, Crawford DW, Wickham E, & Blankenhorn DH (1990) In vivo hypertensive arterial wall uptake of radiolabeled liposomes. Hypertension 15(6 Pt 1):600–605. [DOI] [PubMed] [Google Scholar]
- 10.Hodis HN, et al. (1991) Relationship of arterial wall uptake of radiolabeled liposomes to the presence of monocyte/macrophage cells in the hypertensive and atherosclerotic arterial wall. Atherosclerosis 87(2–3):109–117. [DOI] [PubMed] [Google Scholar]
- 11.Dzieciuch M, et al. (2015) PEGylated Liposomes as Carriers of Hydrophobic Porphyrins. The Journal of Physical Chemistry B 119(22):6646–6657. [DOI] [PubMed] [Google Scholar]
- 12.Sethi V, Önyüksel H, & Rubinstein I (2005) Liposomal Vasoactive Intestinal Peptide. Methods in Enzymology, (Academic Press; ), Vol 391, pp 377–395. [DOI] [PubMed] [Google Scholar]
- 13.Bernhard B, Anna O, Ruth P, Andreas Z, & Wilhelm M (2012) Clinical Potential of VIP by Modified Pharmaco-kinetics and Delivery Mechanisms. Endocrine, Metabolic & Immune Disorders - Drug Targets 12(4):344–350. [DOI] [PubMed] [Google Scholar]
- 14.Ikezaki H, Onyuksel H, & Rubinstein I (1998) Liposomal VIP attenuates phenylephrine- and ANG II-induced vasoconstriction in vivo. Am J Physiol 275(2 Pt 2):R588–595. [DOI] [PubMed] [Google Scholar]
- 15.Önyüksel H, Séjourné F, Suzuki H, & Rubinstein I (2006) Human VIP-α: A long-acting, biocompatible and biodegradable peptide nanomedicine for essential hypertension. Peptides 27(9):2271–2275. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Noda Y, Paul S, Gao X-p, & Rubinstein I (1995) Encapsulation of vasoactive intestinal peptide into liposomes: Effects on vasodilation in vivo. Life Sciences 57(15):1451–1457. [DOI] [PubMed] [Google Scholar]
- 17.Rubinstein I, Ikezaki H, & Önyüksel H (2006) Intratracheal and subcutaneous liposomal VIP normalizes arterial pressure in spontaneously hypertensive hamsters. International Journal of Pharmaceutics 316(1):144–147. [DOI] [PubMed] [Google Scholar]
- 18.McIntyre M, Bohr DF, & Dominiczak AF (1999) Endothelial function in hypertension: the role of superoxide anion. Hypertension 34(4 Pt 1):539–545. [DOI] [PubMed] [Google Scholar]
- 19.Bresciani G, da Cruz IBM, & González-Gallego J (2015) Chapter Four - Manganese Superoxide Dismutase and Oxidative Stress Modulation. Advances in Clinical Chemistry, ed Makowski GS (Elsevier; ), Vol 68, pp 87–130. [DOI] [PubMed] [Google Scholar]
- 20.Dornas WC, et al. (2015) Efficacy of the superoxide dismutase mimetic tempol in animal hypertension models: a meta-analysis. J Hypertens 33(1):14–23. [DOI] [PubMed] [Google Scholar]
- 21.Loperena R & Harrison DG (2017) Oxidative Stress and Hypertensive Diseases. Med Clin North Am 101(1):169–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uematsu T, Nagashima S, Umemura K, Kanamaru M, & Nakashima M (1994) Pharmacokinetics and safety of intravenous recombinant human superoxide dismutase (NK341) in healthy subjects. Int J Clin Pharmacol Ther 32(12):638–641. [PubMed] [Google Scholar]
- 23.Laursen JB, et al. (1997) Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95(3):588–593. [DOI] [PubMed] [Google Scholar]
- 24.Vorauer-Uhl K, Wagner A, & Katinger H (2002) Long term stability of rh-Cu/Zn-superoxide dismutase (SOD)-liposomes prepared by the cross-flow injection technique following International Conference on Harmonisation (ICH)-guidelines. European Journal of Pharmaceutics and Biopharmaceutics 54(1):83–87. [DOI] [PubMed] [Google Scholar]
- 25.Patel K, et al. (2006) Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 290(1):R37–R43. [DOI] [PubMed] [Google Scholar]
- 26.Luisa Corvo M, et al. (2002) Superoxide dismutase entrapped in long-circulating liposomes: formulation design and therapeutic activity in rat adjuvant arthritis. Biochimica et Biophysica Acta (BBA) - Biomembranes 1564(1):227–236. [DOI] [PubMed] [Google Scholar]
- 27.Kaipel M, et al. (2008) Increased Biological Half-Life of Aerosolized Liposomal Recombinant Human Cu/Zn Superoxide Dismutase in Pigs. Journal of Aerosol Medicine and Pulmonary Drug Delivery 21(3):281–290. [DOI] [PubMed] [Google Scholar]
- 28.Riedl CR, et al. (2005) Liposomal Recombinant Human Superoxide Dismutase for the Treatment of Peyronie’s Disease: A Randomized Placebo-Controlled Double-Blind Prospective Clinical Study. European Urology 48(4):656–661. [DOI] [PubMed] [Google Scholar]
- 29.Deshpande PB, et al. (2016) A novel nanoproliposomes of lercanidipine: Development, in vitro and preclinical studies to support its effectiveness in hypertension therapy. Life Sciences 162:125–137. [DOI] [PubMed] [Google Scholar]
- 30.Beckey C, Lundy A, & Lutfi N (2007) Lercanidipine in the Treatment of Hypertension. Annals of Pharmacotherapy 41(3):465–474. [DOI] [PubMed] [Google Scholar]
- 31.Hajos F, Stark B, Hensler S, Prassl R, & Mosgoeller W (2008) Inhalable liposomal formulation for vasoactive intestinal peptide. Int J Pharm 357(1–2):286–294. [DOI] [PubMed] [Google Scholar]
- 32.Elhissi A (2017) Liposomes for Pulmonary Drug Delivery: The Role of Formulation and Inhalation Device Design. Curr Pharm Des 23(3):362–372. [DOI] [PubMed] [Google Scholar]
- 33.Khan AR, Liu M, Khan MW, & Zhai G (2017) Progress in brain targeting drug delivery system by nasal route. J Control Release 268:364–389. [DOI] [PubMed] [Google Scholar]
- 34.Ahad A, et al. (2012) Formulation and optimization of nanotransfersomes using experimental design technique for accentuated transdermal delivery of valsartan. Nanomedicine 8(2):237–249. [DOI] [PubMed] [Google Scholar]
- 35.Rubinstein I, Ikezaki H, & Onyuksel H (2006) Intratracheal and subcutaneous liposomal VIP normalizes arterial pressure in spontaneously hypertensive hamsters. Int J Pharm 316(1–2):144–147. [DOI] [PubMed] [Google Scholar]
- 36.Mora-Huertas CE, Fessi H, & Elaissari A (2010) Polymer-based nanocapsules for drug delivery. Int J Pharm 385(1–2):113–142. [DOI] [PubMed] [Google Scholar]
- 37.Bobo D, Robinson KJ, Islam J, Thurecht KJ, & Corrie SR (2016) Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res 33(10):2373–2387. [DOI] [PubMed] [Google Scholar]
- 38.Knop K, Hoogenboom R, Fischer D, & Schubert US (2010) Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angewandte Chemie International Edition 49(36):6288–6308. [DOI] [PubMed] [Google Scholar]
- 39.Desai PP, Date AA, & Patravale VB (2012) Overcoming poor oral bioavailability using nanoparticle formulations – opportunities and limitations. Drug Discovery Today: Technologies 9(2):e87–e95. [DOI] [PubMed] [Google Scholar]
- 40.Alam T, et al. (2017) Nanocarriers as treatment modalities for hypertension. Drug Deliv 24(1):358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danhier F, et al. (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161(2):505–522. [DOI] [PubMed] [Google Scholar]
- 42.Jain RA (2000) The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 21(23):2475–2490. [DOI] [PubMed] [Google Scholar]
- 43.Mittal G, Sahana DK, Bhardwaj V, & Ravi Kumar MN (2007) Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release 119(1):77–85. [DOI] [PubMed] [Google Scholar]
- 44.Sahana DK, Mittal G, Bhardwaj V, & Kumar MN (2008) PLGA nanoparticles for oral delivery of hydrophobic drugs: influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. J Pharm Sci 97(4):1530–1542. [DOI] [PubMed] [Google Scholar]
- 45.Meena AK, et al. (2008) Oral nanoparticulate atorvastatin calcium is more efficient and safe in comparison to Lipicure in treating hyperlipidemia. Lipids 43(3):231–241. [DOI] [PubMed] [Google Scholar]
- 46.Shah U, Joshi G, & Sawant K (2014) Improvement in antihypertensive and antianginal effects of felodipine by enhanced absorption from PLGA nanoparticles optimized by factorial design. Materials Science and Engineering: C 35:153–163. [DOI] [PubMed] [Google Scholar]
- 47.Todd PA & Faulds D (1992) Felodipine. A review of the pharmacology and therapeutic use of the extended release formulation in cardiovascular disorders. Drugs 44(2):251–277. [DOI] [PubMed] [Google Scholar]
- 48.Arora A, et al. (2015) Development of Sustained Release “NanoFDC (Fixed Dose Combination)” for Hypertension - An Experimental Study. PLoS One 10(6):e0128208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antal I, et al. (2015) Magnetic poly(d,l-lactide) nanoparticles loaded with aliskiren: A promising tool for hypertension treatment. Journal of Magnetism and Magnetic Materials 380:280–284. [Google Scholar]
- 50.Auwal SM, Zarei M, Tan CP, Basri M, & Saari N (2018) Enhanced physicochemical stability and efficacy of angiotensin I-converting enzyme (ACE) - inhibitory biopeptides by chitosan nanoparticles optimized using Box-Behnken design. Sci Rep 8(1):10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chinh NT, et al. (2018) Polylactic Acid/Chitosan Nanoparticles Loading Nifedipine: Characterization Findings and In Vivo Investigation in Animal. J Nanosci Nanotechnol 18(4):2294–2303. [DOI] [PubMed] [Google Scholar]
- 52.Niaz T, et al. (2016) Antihypertensive nano-ceuticales based on chitosan biopolymer: Physico-chemical evaluation and release kinetics. Carbohydr Polym 142:268–274. [DOI] [PubMed] [Google Scholar]
- 53.Chadha R, Bhandari S, Kataria D, & Gupta S (2013) Exploring Lecithin/Chitosan Nanoparticles of Ramipril for Improved Antihypertensive Efficacy. Journal of Nanopharmaceutics and Drug Delivery 1:173–118. [Google Scholar]
- 54.Zidovetzki R & Levitan I (2007) Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochimica et Biophysica Acta (BBA) - Biomembranes 1768(6):1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakkakula JR & Macedo Krause RW (2014) A vision for cyclodextrin nanoparticles in drug delivery systems and pharmaceutical applications. Nanomedicine (Lond) 9(6):877–894. [DOI] [PubMed] [Google Scholar]
- 56.de Azevedo Mde B, et al. (2011) New formulation of an old drug in hypertension treatment: the sustained release of captopril from cyclodextrin nanoparticles. Int J Nanomedicine 6:1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen CE, et al. (2010) Pharmaceutical composition of valsartan: beta-cyclodextrin: physico-chemical characterization and anti-hypertensive evaluation. Molecules 15(6):4067–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mendes C, et al. (2016) Inclusion complexes of hydrochlorothiazide and β-cyclodextrin: Physicochemical characteristics, in vitro and in vivo studies. European Journal of Pharmaceutical Sciences 83:71–78. [DOI] [PubMed] [Google Scholar]
- 59.Junghanns J- UAH & Müller RH (2008) Nanocrystal technology, drug delivery and clinical applications. International Journal of Nanomedicine 3(3):295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merisko-Liversidge E, Liversidge GG, & Cooper ER (2003) Nanosizing: a formulation approach for poorly-water-soluble compounds. European Journal of Pharmaceutical Sciences 18(2):113–120. [DOI] [PubMed] [Google Scholar]
- 61.Havel HA (2016) Where Are the Nanodrugs? An Industry Perspective on Development of Drug Products Containing Nanomaterials. The AAPS Journal 18(6):1351–1353. [DOI] [PubMed] [Google Scholar]
- 62.Gao L, et al. (2013) Application of Drug Nanocrystal Technologies on Oral Drug Delivery of Poorly Soluble Drugs. Pharmaceutical Research 30(2):307–324. [DOI] [PubMed] [Google Scholar]
- 63.Junghanns JU & Muller RH (2008) Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine 3(3):295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geerts AM, et al. (2008) Rapamycin prevents mesenteric neo-angiogenesis and reduces splanchnic blood flow in portal hypertensive mice. Hepatology Research 38(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, et al. (2014) Rapamycin ameliorates inflammation and fibrosis in the early phase of cirrhotic portal hypertension in rats through inhibition of mTORC1 but not mTORC2. PLoS One 9(1):e83908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Segura-Ibarra V, et al. (2017) Rapamycin nanoparticles localize in diseased lung vasculature and prevent pulmonary arterial hypertension. Int J Pharm 524(1–2):257–267. [DOI] [PubMed] [Google Scholar]
- 67.Das S, et al. (2013) Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine (Lond) 8(9):1483–1508. [DOI] [PubMed] [Google Scholar]
- 68.Oro D, et al. (2016) Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J Hepatol 64(3):691–698. [DOI] [PubMed] [Google Scholar]
- 69.Dvir T, et al. (2011) Nanoparticles targeting the infarcted heart. Nano Lett 11(10):4411–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hua S, de Matos MBC, Metselaar JM, & Storm G (2018) Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Frontiers in Pharmacology 9(790). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sartain F, Greco F, Hill K, Rannard S, & Owen A (2016) Emerging nanomedicine applications and manufacturing: progress and challenges. Nanomedicine (Lond) 11(6):577–580. [DOI] [PubMed] [Google Scholar]
- 72.Muhlebach S (2018) Regulatory challenges of nanomedicines and their follow-on versions: A generic or similar approach? Adv Drug Deliv Rev 131:122–131. [DOI] [PubMed] [Google Scholar]
- 73.Kim ES & Plosker GL (2015) AFREZZA® (insulin human) Inhalation Powder: A Review in Diabetes Mellitus. Drugs 75(14):1679–1686. [DOI] [PubMed] [Google Scholar]