Abstract
Five new 8-O-4’ type neolignans, named myrifralignan A-E (1–5), together with five known analogues (6-10), were isolated from the seeds of Myristica fragrans Houtt. Their chemical structures were determined using several spectroscopic methods. Compounds 3–10 exhibited potent inhibitory activity against the production of nitric oxide (NO) in the RAW264.7 cell line stimulated by lipopolysaccaride. Myrislignan (7) and machilin D (10) were the most potent inhibitors of NO production amongst these compounds. The IC50 values of myrislignan and machilin D were 21.2 and 18.5 μM. And, their inhibitory activity was more than L-N6-(1-iminoethyl)-lysine, a selective inhibitor of inducible nitric oxide synthase (IC50 = 27.1 μM). Furthermore, real-time PCR analysis revealed that these neolignans could significantly suppress the expression of inducible nitric oxide synthase mRNA. These results demonstrated that the 8-O-4’ type neolignans are promising candidates as anti-inflammatory agents.
Keywords: Myristica fragrans, Neolignans, Nitric oxide, Inhibition, iNOS mRNA
1. Introduction
Myristica fragrans Houtt. (Myristicaceae) is an aromatic evergreen tree indigenous to the Maluku Province of Indonesia, formerly known as the Spice Islands (van Gils & Cox, 1994). The seeds of M. fragrans are known as nutmeg and the scarlet aril surrounding the seed is named mace. Nutmeg was introduced into Europe during the 12th century by Arab merchants. Nutmeg has been used as the spice in sweet and savoury cooking as well as a medicine. Studies reported that nutmeg exhibits a broad range of pharmacological properties, including anti-inflammatory (Olajide et al., 1999), antibacterial (Narasimhan & Dhake, 2006), antioxidant, antiangiogenic (Piaru, Mahmud, Abdul Majid, & Mahmoud Nassar, 2012), anticarcinogenic (Lee et al., 2006), antidiarrhoeal (Lima et al., 2000) and antiplatelet aggregation (Janssen et al., 1990) activities. Nutmeg is added to the prescriptions or individually used for the treatment of stomach cramps, diarrhoea, rheumatism, psychosis, nausea and flatulence (van Gils & Cox, 1994). Also, nutmeg has been used as an aphrodisiac and an abortifacient. Lignans are the major active components in M. fragrans and possess various bioactivities, such as anti-inflammation (Cao, Yang, Xu, & Li, 2013), antioxidant, anti-cytotoxicity (Duan, Tao, Hao, Gu, & Zhu, 2009), inhibition of protein tyrosine phosphatase 1B (Yang et al., 2006), anti-platelet (Kang, Min, & Lee, 2013) and antifungal activities (Cho et al., 2007).
The discovery that mammalian cells can produce the free radical nitric oxide (NO) has drawn the attentions of investigators in all the fields of biology and medicine (Rubbo, Darley-Usmar, & Freeman, 1996). NO regulates many critical aspects of cellular function (Soloviev, Lehen’kyi, Zelensky, & Hellstrand, 2004). However, excessive production of NO by nitric oxide synthase (NOS) is involved in many diseases, as well as inflammation that can ultimately cause tissue injury. Several studies reported that excessive NO generation is associated with shock (Nava, Palmer, & Moncada, 1991), inflammatory diseases (Molero et al., 1995), liver cirrhosis (Soderman, Leone, Furst, & Persson, 1997), asthma (Stirling et al., 1998), juvenile parkinsonism (Hyun et al., 2002). Thus, the discovery of inhibitors of NO production from natural products is an active area of interest around the world.
A previous study reported that some dihydrobenzofuran type neolignans isolated from nutmeg showed inhibitory activity on NO production induced by lipopolysaccharide (LPS) (Cao et al., 2013). In the current study, eight 8-O-4’ type neolignans (three of them of which are novel) isolated from nutmeg exhibited potent inhibitory effects against NO production, and suppressed the expression level of inducible nitric oxide synthase.
2. Materials and methods
2.1. General
Optical rotation was measured on an Autopol III polarimeter (Rudolph Research Analytical, Flanders, NJ, USA) with chloroform (CHCl3) as a solvent. IR spectra were recorded on a Nicolet™ 470 FT-IR spectrometer (Thermo Nicolet, Inc., Madison, WI, USA) with KBr discs. Ultraviolet (UV) data were recorded on a Varian Cary 300 ultraviolet-visible spectrophotometer (Varian Inc., Palo Alto, CA, USA) in methanol (MeOH). Circular dichroism (CD) spectra were recorded with MeOH as the solvent on a JASCO J-810 spectro-polarimeter (Jasco, Hachioji, Tokyo, Japan). Electron ionisation mass spectrometry (EI-MS) data were obtained using a TRACE 2000 mass spectrometer (Finnigan, Silicon Valley, CA, USA). High-resolution electron spray ionisation mass spectrometry (HR-ESI-MS) data were obtained using a Daltonics APEX IV Fourier Transform ICR high-resolution mass spectrometer (Bruker, Karlsruhe, Baden-Wuerttemberg, Germany). NMR data were acquired on a Bruker AV400 spectrometer (Bruker, Karlsruhe, Baden-Wuerttemberg, Germany); 400 MHz for 1H NMR and 100 MHz for 13C NMR) using deuterated chloroform (CDCl3) as the solvent, with TMS as an internal standard. Open column chromatography (CC) separation was carried out on silica gel (200–300 mesh; Qingdao Marine Chemical Co., Qingdao, China). Thin layer chromatography (TLC) was conducted on silica gel GF254 plates (Merck, Darmstadt, Germany). Spots were visualised under UV light or by spraying with 10% H2SO4 in 95% ethanol followed by heating. Reversed phase semi-preparative HPLC (RP-SP-HPLC) was carried out on a instrument including an LC P600 pump, a UV600 UV-Vis detector and Labtech Chromsoftware (LabTech Co., Beijing, China), equipped with a Phenomenex Luna 10 C18 column (21.2 mm ×250 mm, 10 μm, Phenomenex Inc., Torrance, CA, USA) at a flow rate of 5 ml/min and all UV detection was set up at 210 nm. cDNA synthesis was carried out using a MyCycler PCR instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA). A MX3005P Real Time PCR instrument was used to amplify and detect DNA (Agilent Technologies Inc., Wilmington, DE, USA).
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), Griess reagent, phosphate buffered saline (PBS), lipopolysaccharide (LPS), L-N6-(1-iminoethyl)-lysine (L-NIL), indomethacin (IND), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco™ (Grand Island, NY, USA). 96-Well plates were obtained from Corning Costar (Corning Costar, Cambridge, MA, USA). Chromatographic grade MeOH was purchased from Tianjin Xihua Special Type Reagent Factory (Tianjin, China). Deionised water (H2O) was purchased from Wahaha Co., Ltd. (Hangzhou, China). Milli-Q grade H2O was used for the bio-assay. Other analytical grade reagents were purchased from Beijing Chemical Works (Beijing, China). Trizol reagent kit was purchased from Life Technologies Co (San Diego, CA, USA). Reverse Transcription System kit and GoTaq® qPCR Master Mix kit were purchased from Promega Co (Madison, Wisconsin, USA). All of the primers were synthesised by Beijing Liuhe Genomics Technology Co. Ltd. (Beijing, China).
The murine macrophage cell line RAW264.7 was obtained from the Cell Resource Center, IBMS, CAMS/PUMC (Beijing, China). Cell culture was carried out in a Sanyo MCO-15 AC carbon dioxide (CO2) incubator (Sanyo Electric Co., Ltd., Osaka, Japan), and MTT assay was taken on Thermo Multiskan MK 3 Automated Microplate Reader (Thermo-Labsystems, Franklin, MA, USA).
2.2. Plant material
The dried ripe seeds of M. fragrans (nutmeg) were purchased from Indonesia in 2011 and identified by Professor Xiu-Wei Yang of School of Pharmaceutical Sciences, Peking University Health Science Center, Peking University. A voucher specimen (No. 6396121RDK) was deposited in State Key Laboratory of Natural and Biomimetic Drugs (Peking University).
2.3. Extraction and isolation
The extraction of nutmeg (24.00 kg) was performed using CO2 supercritical extraction at 20 Mpa and 50 °C for 2 h under CO2 with a flow rate of 280 kg h-1. The separation pressure was 8 Mpa and separation temperature was 50 °C. 8.02 kg of CO2 extract was obtained and 4.00 kg of the extract (4.00 kg) was dissolved in MeOH (13 l). After six times extraction using microwaves, it resulted in a red-brown viscous oil of 1450 g and an insoluble residue. The oil (797 g) was subjected to a silica gel CC, eluted with a gradient solvent system of cyclohexane (CHA)-ethyl acetate (EtOAc) (60:1 → 1:1, ν/v), EtOAc, and MeOH to give fractions A to M. The fraction D (126 g) was purified by medium pressure CC over silica gel H using a gradient solvent system of CHA-acetone (ACE) (1:0 → 50:1, ν/v) to yield nine fractions (Fr.D1 to Fr.D9). The Fr.D4 (30.8 g) was separated by RP-SP-HPLC (80% aqueous MeOH) to give twenty-five (Fr.D4-1 to Fr.D4-25). By further purification of RP-SP-HPLC, compounds 6 (5.0 mg, 70% aqueous MeOH, tR = 61 min) from the Fr.D4-5 (18.6 mg) and 1 (1.1 mg, 58% aqueous CH3CN, tR = 120 min) from the Fr.D4-11 (73.1 mg) were afforded. Fr.G (39.3 g) was separated by CC on a silica gel and eluted with petroleum ether (PE)-ACE (5:1, ν/v) to yield nine subfractions. Fr.G1 to Fr.G9, and Fr.G4 (15.2 g) was further separated by RP-SP-HPLC to give compound 2 (0.6 mg, 55% CH3CN, tR = 38 min). The Fr.I (43 g) was separated by CC on a silica gel eluted with PE-ACE (5:1, ν/v) to give eleven fractions (Fr.I8 to Fr.I11). By further purification of RP-SP- HPLC, compound 7 (14.2 mg, 65% aqueous MeOH, tR = 60 min) was purified from a part of Fr.I8 (4.7 g) and compound 8 (7.8 mg, 56% MeOH, tR = 56 min) was separated from Fr.I9 (12.1 g). Fr.K (6.0 g) was subjected to CC over silica gel and eluted with PE-ACE (9:2, ν/v). The eluate was collected in portions of 100 ml and eluates containing similar components by TLC detection were combined to yield 11 fractions (Fr.K1 to Fr.K11). Then Fr.K7 (1.0 g) was separated by RP-SP-HPLC (60% MeOH) to yield a residue (72.2 mg), which was further purified by RP-SP-HPLC (45% CH3CN) to give compounds 3 (3.8 mg, tR = 48 min) and 4 (3.5 mg, tR = 58 min). Fr.L (35.1 g) was chromatographed over silica gel and eluted with PE-ACE (4:1, ν/v) to generate Fr.L1 to Fr.L11, and Fr.L10 (9.0 g) was further separated through RP-SP-HPLC (70% aqueous MeOH) to generate eight subfractions, Fr.L10-1 to Fr.L10-8. Subfractions Fr.L10-4 (123.1 mg), Fr.L10-5 (39.3 mg) and Fr.L10-7 (13.0 mg) were further purified by RP-SP-HPLC resulted in the isolation of compounds 9 (18.1 mg, 63% aqueous MeOH for Fr.L10-4, tR = 58 min), 10 (8.2 mg, 63% aqueous MeOH for Fr.L10-5, tR = 85 min) and 5 (0.5 mg, 70% aqueous MeOH for Fr.L10-7, tR = 43 min).
2.3.1. Myrifralignan A (1)
(7R,8S)-2-(4-propenyl-2,6-dimethoxyphenoxy)-1-(3,4-methylenedioxyphenyl)-propan-1-ol. Yellowish oil; (c 1.0, CHCl3); UV λmax (MeOH) nm (log ε): 203 (4.59), 277 (3.87); CD (c 3.2 × 10−4 M, MeOH): λmax (Δε): 222 (−1.59), 234 (−1.f16), 289 (−2.93); IR (KBr) νmax 3440, 2921, 1637, 1508, 1452, 1432, 1384, 1237,1144,1124,1101,1038,931, 827, 721 cm−1; 1H NMR(CDCl3, 400 MHz) data: see Table 1; 13C NMR (CDCl3, 100 MHz) data: see Table 2; HR-ESI-MS (+) m/z 395.1467 ([M+Na]+, calcd for C21H24- O6Na, 395.1471).
Table 1.
1H NMR (400 MHz, CDCl3; δH, J in Hz) data for compounds 1–5.
| Positions | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | 6.85 (d, 1.3) | 6.91 (d, 1.8) | 6.58 (br s) | 6.58 (br s) | 6.54 (br s) |
| 5 | 6.75 (d, 7.6) | 6.80 (d, 8.3) | |||
| 6 | 6.72 (dd, 7.6, 1.3) | 6.86 (1H, dd, 8.3, 1.8) | 6.58 (br s) | 6.58 (br s) | 6.54 (br s) |
| 7 | 4.76 (d, 2.7) | 5.85 (d, 3.5) | 4.81 (d, 3.2) | 4.58 (d, 8.5) | 4.78 (d, 2.9) |
| 8 | 4.33 (dq, 6.4, 2.1) | 4.60 (dq, 6.4, 3.5) | 4.32 (dq, 6.4, 3.2) | 3.93 (dq, 8.5, 6.3) | 4.42 (dq, 6.4, 2.9) |
| 9 | 1.12 (d, 6.4) | 1.30 (d, 6.4) | 1.17 (d, 6.4) | 1.19 (d, 6.3) | 1.13 (d, 6.4) |
| 3′ | 6.60 (br s) | 6.78 (br s) | 6.91 (d, 1.8) | 6.44 (br s) | 6.85 (br s) |
| 5′ | 6.60 (br s) | 6.78 (br s) | 6.88 (dd, 8.2, 1.8) | 6.44 (br s) | 6.85 (br s) |
| 6′ | 6.94 (d, 8.2) | ||||
| 7′ | 6.35 (dd, 15.6, 1.5) | 7.39 (d, 15.8) | 6.35 (dd, 15.7, 1.6) | 3.35 (d, 6.8) | 7.42 (d, 15.8) |
| 8′ | 6.19 (dq, 15.6, 6.5) | 6.64 (dd, 15.8, 7.7) | 6.15 (dq, 15.7, 6.6) | 5.96 (ddt, 16.9, 10.1, 6.8) | 6.61 (dd, 15.8, 1.6) |
| 9′ | 1.89 (dd, 6.5, 1.5) | 9.68 (d, 7.7) | 1.87 (dd, 6.6, 1.6) | 5.12 (dd, 16.9, 1.7 Hz), 5.10 (dd, 10.1, 1.7) | 9.69 (d, 1.6) |
| OCH2O | 5.92 (s) | ||||
| COCH3 | 2.16 (s) | ||||
| 2′-OMe | 3.89 (s) | 3.84 | 3.87 (s) | 3.867 (s) | 3.93 (s) |
| 6′-OMe | 3.89 (s) | 3.84 | 3.867 (s) | 3.93 (s) | |
| 3-OMe | 3.85 | 3.85 (s) | 3.871 (s) | 3.87 (s) | |
| 4-OMe/OH | 3.86 | 5.56 (br s) | 5.49 (br s) | 5.48 (br s) | |
| 5-OMe | 3.85 (s) | 3.871(s) | 3.87 (s) | ||
Table 2.
13C NMR (100 MHz, CDCl3) data for compounds 1–5.
| Positions | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 134.0 s | 130.0 s | 131.0 s | 131.8 s | 130.6 s |
| 2 | 106.7 d | 110.4 d | 102.9 d | 104.1 d | 102.7 d |
| 3 | 147.5 s | 148.8 s | 146.8 s | 146.9 s | 146.9 s |
| 4 | 146.4 s | 148.7 s | 133.8 s | 134.3 s | 133.8 s |
| 5 | 107.9 d | 110.8 d | 146.8 s | 146.9 s | 146.9 s |
| 6 | 119.1 d | 119.6 d | 102.9 d | 104.1 d | 102.7 d |
| 7 | 72.9 d | 77.2 d | 73.7 d | 79.4 d | 73.4 d |
| 8 | 82.4 d | 80.4 d | 82.2 d | 86.6 d | 83.1 d |
| 9 | 12.7 q | 14.8 q | 13.3 q | 17.6 q | 12.7 q |
| 1′ | 133.8 s | 138.6 s | 145.5 s | 135.3 s | 137.8 s |
| 2′ | 153.6 s | 153.8 s | 151.3 s | 152.7 s | 154.0 s |
| 3′ | 102.9 d | 105.7 d | 109.2 d | 105.5 d | 105.6 d |
| 4′ | 134.1 s | 129.5 s | 133.5 s | 135.9 s | 129.9 s |
| 5′ | 102.9 d | 105.7 d | 118.9 d | 105.5 d | 105.6 d |
| 6′ | 153.6 s | 153.8 s | 119.5 d | 152.7 s | 154.0 s |
| 7′ | 130.8 d | 152.7 d | 130.4 d | 40.2 t | 152.3 d |
| 8′ | 125.7 d | 127.9 d | 124.9 d | 137.0 d | 128.2 d |
| 9′ | 18.4 q | 193.4 d | 18.3 q | 116.2 t | 193.3 d |
| OCH2O | 100.8 t | ||||
| COCH3 | 170.1 s | ||||
| 21.2 q | |||||
| 2′-OMe | 56.1 q | 56.1 q | 55.7 q | 56.0 q | 56.33 q |
| 6′-OMe | 56.1 q | 56.1 q | 56.0 q | 56.33 q | |
| 3-OMe | 55.91 q | 56.2 q | 56.3 q | 56.29 q | |
| 4-OMe | 55.89 q | ||||
| 5-OMe | 56.2 q | 56.3 q | 56.29 q | ||
C-multiplicities were established by a HSQC experiment. s: C; d: CH; t: CH2; q: CH3.
2.3.2. Myrifralignan B (2)
(7R,8S)-2-(4-acroloyl-2,6-dimethoxyphenoxy)-1-(3,4-dimethoxy-phenyl)-propan-1-ol acetate. Yellowish oil; (c 1.0, CHCl3); UV λmax (MeOH) nm (logε): 201 (4.45), 318 (3.65); CD (c 3.2 × 10−4 M, MeOH): λmax(Δε): 221 (−1.84), 237 (−1.05), 306 (−3.57); IR (KBr) νmax 2994, 1770, 1758, 1674, 1622, 1580, 1519, 1501, 1464, 1423, 1373, 1335, 1243, 1126, 1051, 926, 848, 804 cm−1; 1H NMR (CDCl3, 400 MHz) data: see Table 1; 13C NMR (CDCl3, 100 MHz) data: see Table 2; HR-ESI-MS (+) m/z 467.1674 ([M+Na]+, calcd for C24H28O8- Na, 467.1676).
2.3.3. Myrifralignan C (3)
(7R,8S)-2-(4-propenyl-2-methoxyphenoxy)-1-(4-hydroxy-3,5-dimethoxyphenyl)-propan-1-ol. Yellowish oil; (c 1.0, CHCl3); UV λmax (MeOH) nm (logε): 207 (4.97), 260 (3.96); CD (c 5.9 × 10−4 M, MeOH): λmax (Δε): 222 (−1.02), 238 (−0.13), 263 (−1.87), 282 (−1.76), 296 (−1.84); IR (KBr) νmax 3465, 2979, 2938, 2840, 1734, 1671, 1612, 1601, 1509, 1463, 1425, 1374, 1326, 1261, 1240, 1219, 1116, 1045, 966, 912, 860, 825, 787, 759, 717 cm−1; 1H NMR (CDCl3, 400 MHz) data: see Table 1; 13C NMR (CDCl3, 100 MHz) data: see Table 2; HR-ESI-MS (+) m/z 397.1633 ([M+Na]+, calcd for C21H26O6Na, 397.1627).
2.3.4. Myrifralignan D (4)
(7S,8S)-2-(4-allyl-2,6-dimethoxyphenoxy)-1-(4-hydroxy-3,5-dimethoxyphenyl)-propan-1-ol. Yellowish oil; (c 1.0, CHCl3); UV λmax (MeOH) nm (logε): 206 (4.51), 305 (3.61); CD (c 2.6 × 10−4 M, MeOH): λmax (Δε): 222 (−1.66), 237 (−1.07), 298 (−2.86); IR (KBr) νmax 3464, 2983, 2939, 2841, 1734, 1671, 1611, 1589, 1518, 1503, 1461, 1423, 1373, 1330, 1241, 1124, 1044, 917, 832, 791, 702 cm−1; 1H NMR (CDCl3, 400 MHz) data: see Table 1; 13C NMR (CDCl3, 100 MHz) data: see Table 2; HR- ESI-MS (+) m/z 427.1725 ([M+Na]+, calcd for C22H28O7Na, 427.1733).
2.3.5. Myrifralignan E (5)
(7R,8S)-2-(4-acroloyl-2,6-dimethoxyphenoxy)-1-(4-hydroxy-3,5-dimethoxyphenyl)-propan-1-ol. Yellowish oil; (c 1.0, CHCl3); UV λmax (MeOH) nm (logε): 205 (4.42), 316 (3.72); CD (c 4.4 × 10−4 M, MeOH): λmax (Δε): 212 (−2.62), 238 (0.52), 281 (−2.57); IR (KBr) νmax 3494, 2983, 2939, 2841, 1734, 1672, 1614, 1583, 1521, 1503, 1461, 1423, 1374, 1332, 1242, 1123, 1047, 1001, 971, 917, 827, 716 cm−1; 1H NMR (CDCl3, 400 MHz) data: see Table 1; 13C NMR (CDCl3, 100 MHz) data: see Table 2; HR-ESI-MS (+) m/z 419.1712 ([M+H]+, calcd for C22H27O8, 419.1706).
2.4. Biological study
2.4.1. Assay for cell viability
Cell viability of RAW264.7 was measured as described previously using MTT assay (Mosmann, 1983). RAW264.7 cells were grown in DMEM with 10% FBS at 37 °C in a humidified atmosphere of 5% CO2 air. In brief, RAW264.7 cells were seeded into a 96 well plate (1.2 × 105 cells/well) for 12 h under the above conditions, followed by treatment with LPS (1 μg/ml) in the presence of various concentrations (12.5–100 μM) of the tested compound, and further incubated for 20 h under the same conditions. Then 20 μl of MTT stock solution (5 mg/ml) was added to each well after the supernatant was removed. After incubation for 4 h, 100 μl of a dissolving solution (10% sodium dodecyl sulphate, 5% isopropanol and 0.012 M HCl) (Zhou, Yue, Han, & Yang, 1993) was added to each well. The absorbance was determined on a Multiskan MK 3 Automated Microplate Reader at 492 nm.
2.4.2. NO inhibition assay
RAW264.7 cells were cultivated at 1.2 × 105 cells/well in a 96 well plate for 12 h and incubated for another 20 h with or without LPS (1 μg/ml) in the absence or presence of various concentrations (12.5–100 μM) of assayed compound. Nitrite levels in culture media were determined as described previously using the Griess reaction (Green et al., 1982). Briefly, the culture supernatant (100 μl) was transferred into another 96 well plate and reacted with the same volume of standard Griess reagent for 15 min. The absorbance was determined at 540 nm with a Microplate Reader. Fresh culture media were used as blanks in all experiments. The 1C50 values were calculated using the software origin 7.5 and statistical analysis was performed using SPSS 17.0. Statistical differences were determined by one-way analysis of variance followed by Dunnett’s t-test, and a value of p < 0.01 was considered a significant difference.
2.4.3. Evaluation of iNOS mRNA expression levels
PCR primers of iNOS and β-actin, isolation of total RNA, synthesis of cDNA, and quantitative real-time PCR were carried out as described previously (Cao et al., 2013). Briefly, total RNA from cell pellets was extracted using Trizol reagent, cDNA was synthesised with one microgram of RNA using reverse transcriptase. Quantitative PCR amplification was carried out using a MyCycler PCR instrument and GoTaq® qPCR Master Mix kit according to the manufacturer’s recommendations. Relative iNOS mRNA expression was analysed by the 2-ΔΔCT method. β-Actin amplification was used as the control. The data were expressed as mean ± standard deviation of three independent experiments and analysed using one-way analysis of variance with the Dunnett’s t-test. Values of p < 0.05 and p < 0.01 was considered to be statistically significant.
3. Results and discussion
Repeated CC (silica gel and semi-preparative HPLC) of the CO2 extract of nutmeg resulted in the isolation of five new (1–5) and five known (6–10) compounds (Fig. 1). The chemical structures of the known compounds were identified to be (7S,8R)-2-(4-allyl-2,6-dimethoxy-henoxy)-1-(3,4,5-trimethoxyphenyl)-propan-1-ol (6) (Yang, Huang, & Ahmat, 2008), myrislignan (7) (Yang et al., 2008), (7R,8S)-2-(4-propenyl-2-methoxyphenoxy)-1-(3,4,5-trime-thoxyphenyl)-propan-1-ol (8) (Konya, Kiss-Szikszai, Kurtan, & Antus, 2004), (7S,8R)-2-(4-allyl-2,6-dimethoxyphenoxy)-1-(4-hydroxy-3,5-dimethoxyphenyl)-propan-1-ol (9) (Harvey, 1975) and machilin D (10) (Sung, Huh, & Kim, 2001). The spectra data of known compounds are provided in Supplementary Material. All the proton (Table 1) and carbon (Table 2) signals for five new compounds were assigned using 2D NMR spectra, including 1H-1H correlation spectroscopy (1H-1H COSY), heteronuclear single quantum coherence (HSQC) and heteronuclear multiple-bond correlation (HMBC).
Fig. 1.
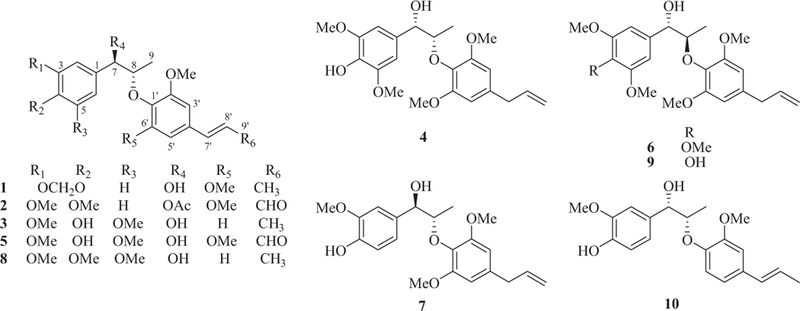
Chemical structures of compounds l-lO.
Compound 1 was obtained as a yellowish oil. Its IR spectrum suggested the presence of hydroxyl (3440 cm−1) and methylenedioxyl (1038, 931 cm−1) groups. The molecular formula of 1 was determined as C21H24O6 by HR-ES1-MS at m/z 395.1467 [M+Na]+ (calcd for C21H24O6Na, 395.1471). The 1H NMR spectrum of 1 showed one methylenedioxyl group at δH 5.92, one benzylic methine substituted by oxygen at δH 4.76, one methine substituted by oxygen at δH 4.33, one sec-methyl group at δH 1.12, and two aromatic methoxyl groups at δH 3.89. In addition, AMX3 type signals at δH 6.35, 6.19, 1.89 (JA,B = 15.6, JA,X = 6.5, JB,X=1.5) due to (1E)-1-propen-1-yl group and five aromatic protons at δH 6.60–6.85 can be observed. The 1H NMR patterns of 1 were very similar to those of erythro-2-(4-allyl-2,6-dimethoxyphenoxy)-1(3,4-methylenedi-oxyphenyl)-propan-1-ol (Forrest, Heacock, & Forrest, 1974), except for the additional signals for a (1E)-1-propen-1-yl group and the absence of signals for a allyl group. 1H-1H COSY allowed the construction of the proton spin systems: H-7/H-8/H-9 and H-7’/H-8’/H-9’. The HMBC correlations from H-7’ (δH 6.35) to C-3’ and C-5’ (δC 102.9) suggested the (1E)-1-propen-1-yl group was linked to C-4’ of 1. The small coupling constant value (J7,8 = 2.7 Hz), and the chemical shifts of the H-7 and H-9 protons, as well as those of the C-7 and C-9 carbons agreed with an erythro configuration (Besombes, Robert, Utille, Taravel, & Mazeau, 2003; Kingsbury, 1970; Yang et al., 2008). The absolute configuration was determined as 7R,8S on the basis of the specific rotation (c 1.0, CHCl3)] (Zacchino & Badano, 1988, 1991), whilst the positive Cotton effect at 222–250 nm in the CD spectrum of 1 further supported the above inference (Arnoldi & Merlini, 1985; Greca, Molinaro, Monaco, & Previtera, 1994). Accordingly, the chemical structure of 1 was unambiguously established as (7R,8S)-2-(4-propenyl-2,6-dimethoxyphenoxy)-1-(3,4-methylenedioxyphenyl)-propan-1-ol, trivially named myrifralignan A.
Compound 2 was isolated as a yellowish oil, gave the positive HR-ES1-MS at m/z 467.1674 [M+Na]+ (calcd for C24H28O8Na, 467.1676), consistent with the molecular formula C24H28O8. The 1R spectrum of 2 suggested the presence of a benzene ring (1622, 1580, 1501 cm−1) and a conjugation carboxaldehyde group (1674 cm−1). The 1H NMR spectroscopic data of 2 were found to be similar to those of erythro-2-(4-allyl-2,6-dimethoxyphenoxy)-1-(3,4-dimethoxyphenyl)-propan-1-ol acetate (Yang et al., 2008) except for the appearance of three additional signals at δH 9.68, 7.39 and 6.64 and the absence of signals for allyl group. The larger coupling constant between the two olefinic protons at δH 7.39 (1H, J7’,8’ = 15.8 Hz) and 6.64 (1H, dd, J8’,7’ = 15.8 Hz, J8’,9’ = 7.7 Hz) indicated the presence of trans double bond, and the later one was coupled with a carboxaldehyde group at δH 9.68 (J9’,8’ = 7.7 Hz) indicated the presence of the —CH=CHCHO group. This inference was confirmed by the analysis of 13C NMR (δC 193.4, 152.7, 127.9) (El-Feraly & Hoffstetter, 1980) and 1H-1H COSY spectrum of 2. The linkage position of —CH=CHCHO group on the C-4’ was confirmed from the HMBC correlation between δH 7.39 (H-7’) and δC 105.7 (C-3’, C-5’). The erythyo configuration was established by comparison the J7,8 value with that of compound 1, whilst the absolute configuration was established as 7R,8S on the basis of the positive Cotton effect at 237 nm in its CD spectrum and the value (Arnoldi & Merlini, 1985; Greca et al., 1994; Zacchino & Badano, 1988, 1991). Thus, the chemical structure of 2 was concluded to be (7R,8S)-2-(4-acro- loyl-2,6-dimethoxyphenoxy)-1-(3,4-dimethoxyphenyl)-propan-1-ol acetate and given the trivial name myrifralignan B.
Compound 3 was isolated as a yellowish oil. Its molecular formula was deduced as C21H26O6 based on the quasi-molecular ion peak at m/z 397.1633 ([M+Na]+, calcd for C21H26O6Na, 397.1627) in its positive HR-ESI-MS spectrum. The IR spectrum of 3 showed absorption bands for hydroxyl (3465 cm−1) and aromatic (1601 and 1509 cm−1) functional groups. The NMR spectroscopic data of 3 was found to be similar to those of erythro-1-(4’-hydroxy-3’- methoxyphenyl)-2-[2”-methoxy-4”-( (E)-propenyl)-phenoxy]-propan-1-ol (Hada, Hattori, Tezuka, Kikuchi, & Namba, 1998), except for the signal assignable to an additional methoxyl group on a benzene ring. In the 13C NMR spectra, C-5 was shifted toward downfield whilst C-4 and C-6 were upfield in comparison to ery- thro-1-(4’-hydroxy-3’-methoxyphenyl)-2-[2”-methoxy-4”-( (E)-propenyl)-phenoxy]-propan-1-ol, suggesting that the methoxyl group to be at C-5 position. This inference was further confirmed by HSQC correlation between δH 6.58 (2H, br s, H-2 and H-6) with δC 102.9 (C-2 and C-6) and HMBC correlation between δH 3.85 (6H, s, 3-OMe and 5-OMe) with δC 146.8 (C-3 and C-5). The small coupling constant between H-7 and H-8, and the chemical shifts of the H-7 and H-9, C-7 and C-9 indicated the erythyo configuration (Besombes et al., 2003; Kingsbury, 1970; Yang et al., 2008). The value and the positive CD maxima justified the 7R,8S configuration (Arnoldi & Merlini, 1985; Greca et al., 1994; Zacchino & Badano, 1988,1991). These findings consequently led us to characterise the chemical structure of 3 as (7R,8S)-1-(4-hydroxy-3,5- dimethoxyphenyl)-2-(4-propenyl-2-methoxyphenoxy)-propan-1-ol and named myrifralignan C.
Compound 4 was also isolated as a yellowish oil, whose molecular formula was deduced to be C22H28O7 by positive HR- ESI-MS data. Its IR spectrum showed the presence of hydroxyl group (3464 cm−1). The 1H and 13C NMR data of 4 were quite similar to those of threo-3,4,5-trimethoxy-7-hydroxy-1’-allyl-3’,5’-dimethoxy-8-O-4’-neolignan (Zacchino et al., 1997), except for the appearance of the signal assignable to an additional hydroxyl group on a benzene ring at δH 5.49 (1H, br s). The difference between them suggest the presence of the 4-hydroxy-3,5- dimethoxy group present in place of the 3,4,5-trimethoxy group in threo-3,4,5-trimethoxy-7-hydroxy-1’-allyl-3’,5’-dimethoxy-8-O-4’-neolignan, which could be confirmed by HMBC correlation at δH 5.49 (4-OH) with δC 146.9 (C-3, C-5). The coupling constant of 8.5 Hz between H-7 and H-8 was similar to that of threo-3,4,5-trimethoxy-7-hydroxy-1’-allyl-3’,5’-dimethoxy-8-O-4’-neolignan, larger than that of 1, indicating the relative threo-configuration of 4. The positive Cotton effect at 237 nm in its CD spectrum and the value allowed the determination of the absolute configuration of 4 to be 7S,8S (Arnoldi & Merlini, 1985; Greca et al., 1994). The chemical structure of 4 was thus concluded to be (7S,8S)-1-(4-hydroxy-3,5-dimethoxyphenyl)-2-(4-allyl-2,6-dime- thoxyphenoxy)-propan-1-ol and given the trivial name myrifralignan D.
Compound 5 was isolated as a yellowish oil. Its molecular formula was established as C22H26O8 on the basis of its HR-ESI-MS data. The IR spectrum of 5 suggested the presence of hydroxyl group at 3494 cm-1. The 1H NMR spectrum showed signals for one benzylic methine substituted by oxygen at δH 4.78, one methine substituted by oxygen at δH 4.42, one sec-methyl group at δH 1.13, four aromatic methoxyl groups, one hydroxyl group at δH 5.48 and two couples of equivalent aromatic protons at δH 6.85 and 6.54, along with those for one —CH=CHCHO group at δH 9.69, 7.42 and 6.67 (Table 1). The 1H NMR data reveal the presence of 4-acroloyl-allyl-2,6-dimethoxyphenoxyl group and 1-(4- hydroxy-3,5-dimethoxy-phenyl)-propan-1-ol group, which was confirmed by comparing the 1H and 13C NMR data with those of compounds 2 and 3. The small coupling constant value (J7,8 = 2.9 Hz), and the chemical shifts of the H-7 and H-9, as well as those of the C-7 and C-9 suggested that 5 belonged to an erythro series (Besombes et al., 2003; Kingsbury, 1970; Yang et al., 2008). The value (Zacchino & Badano, 1988, 1991) and the positive CD maxima (Arnoldi & Merlini, 1985; Greca et al., 1994) suggested that 5 had an absolute configuration of 7R and 8S. The proton and carbon signals of 5 were all assigned based on analysis of its HSQC and HMBC spectra. Finally, the chemical structure of 5 was characterised as (7R,8S)-1-(4-hydroxy-3,5-dimethoxyphenyl)-2-(4-acroloyl-2,6-dimethoxyphenoxy)-propan-1-ol and given a trivial name myrifralignan E.
NO, a diatomic free radical, was reported to be involved in numerous regulatory functions. Some studies have shown that NO shows cytotoxic properties and is involved in inflammatory conditions that can led to tissue injury (Hibbs, Taintor, & Vavrin, 1987). Increased NO production is a typical phenomenon that occurs in LPS-stimulated macrophages. In this study, compounds 3–10 were first tested on their cytotoxic activities against RAW264.7 macrophages by an MTT method and then tested for inhibitory activities against LPS-induced NO production in this cell line under the concentration range from 12.5 to 100 μM. The MTT assay demonstrated that the cell viability of compounds 3, 5, 6, 8, and 9 was above 95% at the treated concentrations (12.5–100 μM), whilst some compounds showed cytotoxic effects at higher concentrations (100 μM for compound 4 and, 75 μM and 100 μM for compounds 7 and 10) (see Supplementary Data Table S1). Therefore, 12.5–75 μM was selected for compound 4 and 12.5–50 μM for compounds 7 and 10. The assay of compounds 1 and 2 could not be carried out due to their insufficient yields. L-NIL, a selective inhibitor of iNOS, and IND, a nonselective cyclooxygenase inhibitor, were used as positive controls.
The half maximal inhibitory concentration (IC50) values indicated that compounds 3–10 significantly inhibited NO production with IC50 values in the range of 18.5–49.8 μM (Table 3). Specifically, compounds 7 and 10 showed most significant inhibitory effect with IC50 of 21.2 μM and 18.5 μM, respectively. Their inhibitory activity was higher than that of both positive controls, L-NIL and IND. The inhibitory activity of the other compounds was more than that of IND, but slightly weaker than L-NIL.
Table 3.
Inhibition of compounds 3–10 on NO production.
| Compound | IC50 (μM) |
|---|---|
| 3 | 47.2 ±1.1 |
| 4 | 49.0 ± 1.0 |
| 5 | 32.8 ± 2.7 |
| 6 | 48.3 ± 1.4 |
| 7 | 21.2 ±0.8** |
| 8 | 48.0 ± 1.2 |
| 9 | 49.8 ± 1.9 |
| 10 | 18.5 ±0.5*** |
| L-NIL | 27.1 ± 2.2 |
| IND | 65.3 ± 6.7 |
L-NIL: L-N6-(1-iminoethyl)-lysine.
IND: indomethacin.
p < 0.01
p < 0.001 indicate significant differences from L-NIL.
Comparison with the IC50 values of compounds 4 and 9, a pair of epimers, suggested that the change of C-8 configuration does not affect NO production. Amongst these, compounds 6 and 8, 7 and 10, 3 and 9 have the same substitution pattern in ring A, with IC50 values of 48.3 and 48.0, 21.2 and 18.5, 47.2 and 49.8 μM, respectively. This indicated that the methoxyl functional group at C-6’ and allyl or propenyl at C-4’ in the ring B do not influence NO production. The substitution pattern of ring A plays an important role in the inhibitory effect of NO production in LPS-activated RAW264.7. The inhibitory activity of 8-O-4’ neolignans with methoxyl group at C-3 and hydroxyl group at C-4 (7 and 10) was more potent than that of compounds 6 and 8 (3,4,5-trimethoxyl function), 3 and 9 (3,5-dimethoxy-4-hydroxyl function). The present results suggest that the 3-methoxyl-4-hydroxyl functional group may be the most important structure in 8-O-4’ neolignans involved in the inhibition NO production.
In order to elucidate the mechanism of these neolignans inhibiting NO production in LPS-stimulated RAW264.7 cells, the expression of the iNOS mRNA was measured by quantitative real-time PCR analysis. Compound 10 was used in this assay due to its high inhibitory activity. iNOS mRNA levels in RAW264.7 cells were increased markedly after 20 h of LPS stimulation (Fig. 2). With the treatment of compound 10 (12.5–50 μM), a dose-dependent inhibition of iNOS mRNA expression was observed (Fig. 2), indicating that compound 10 modulates iNOS mRNA expression. This suggested that these neolignans may effectively inhibit NO overproduction via inhibition of the iNOS mRNA expression. However, the anti-inflammatory properties in vivo need to be further examined.
Fig. 2.
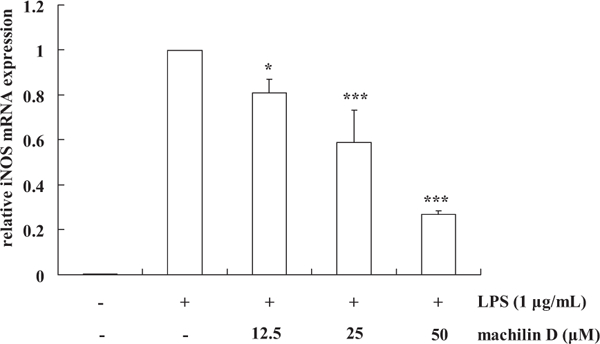
The inhibitory effects of machilin D (10) on iNOS mRNA expression in LPSstimulated RAW264.7 cells. RAW264.7 cells were incubated with LPS (1 μg/ml) and various concentrations (12.5–50 μM) of machilin D for 20 h and the expression of iNOS mRNA measured using real-time PCR. β-Actin mRNA was used as a control. Each column represents the mean ± S.D. of three independent experiments. *p < 0.05 and ***p < 0.001 compared to the LPS alone treated group.
Myrislignan (7) is one of the main compounds (more than 3.5 mg/g crude drug) isolated from nutmeg (Wang & Yang, 2008). It was discovered in 1973, and has attracted increasing interest because of its biological activities, especially anti-inflammatory effects, which were mediated by inhibiting the activation of the NF-ĸB and P65 nuclear translocation (Jin et al., 2012). In addition, myrislignan could be well transported in the Caco-2 cell monolayer model (Yang, Huang, Ma, Wu, & Xu, 2010). It is also distributed to different tissues after intravenous administration to rats (Wang, Liu, Zhang, Li, & Yang, 2012). The current study also indicated that myrislignan is a potent inhibitor of NO production. Therefore, myrislignan and other compounds are viable candidates as inhibitors of NO production, and might be developed as anti-inflammatory and chemopreventive agents.
4. Conclusion
Five new (1–5) and five known (6–10) 8-O-4’ neolignans were isolated from nutmeg. Their effect on the production of NO was measured in LPS-stimulated murine macrophage using the Griess reaction. All the tested compounds (3–10) showed inhibition on NO synthesis and production. Notably, iNOS mRNA expression was significantly suppressed by machilin D (10) in LPS-stimulated RAW264.7 cells. Therefore, these results strongly suggest that the 8-O-4’ neolignans may be used for the treatment of inflammation related to excessive production of NO. In addition, the results also provide a structure-activity relationship that would be used to design anti-inflammatory agents in the future.
Supplementary Material
Acknowledgements
This research was partly supported by National Key Technologies R&D Program of China (2011BAI07B08) and the National Natural Science Foundation of China (30973863; 81161120429).
Abbreviations:
- CC
column chromatography
- RP-SP-HPLC
reversed phase semipreparative high-performance liquid chromatography
- tR
retention time
- MeOH
methanol
- DMSO
dimethyl sulfoxide
- CDCl3
deuterated chloroform
- TMS
tetramethylsilane
- IR
infrared
- UV
ultraviolet
- CD
circular dichroism
- EI-MS
electron ionisation mass spectrometry
- HR-ESI-MS
high-resolution electron spray ionisation mass spectrometry
- NMR
nuclear magnetic resonance
- 1H-1H COSY
1H-1H correlation spectroscopy
- HSQC
heteronuclear single quantum coherence
- HMBC
heteronuclear multiple bond correlation
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphe-nyl-2H-tetrazolium bromide
- LPS
lipopolysaccharide
- L-NIL
L-N6-(1-iminoethyl)- lysine
- IND
indomethacin
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- RT-PCR
reverse transcription-polymerase chain reaction
- IC50
half maximal inhibitory concentration
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.foodchem.2014.09.170.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Arnoldi A, & Merlini L (1985). Asymmetric synthesis of 3-methyl-2-phenyl-1,4- benzodioxanes. Absolute configuration of the neolignans eusiderin and eusiderin C and D. Journal of the Chemical Society, Perkin Transactions, 1(12), 2555–2557. [Google Scholar]
- Besombes S, Robert D, Utille JP, Taravel FR, & Mazeau K (2003). Molecular modeling of syringyl and p-hydroxyphenyl β-O-4 dimers. Comparative study of the computed and experimental conformational properties of lignin β-O-4 model compounds. Journal of Agricultural and Food Chemistry, 51, 34–42. [DOI] [PubMed] [Google Scholar]
- Cao GY, Yang XW, Xu W, & Li F (2013). New inhibitors of nitric oxide production from the seeds of Myristica fragrans. Food and Chemical Toxicology, 62, 167–171. [DOI] [PubMed] [Google Scholar]
- Cho JY, Choi GJ, Son SW, Jang KS, Lim HK, Lee SO, et al. (2007). Isolation and antifungal activity of lignans from Myristica fragrans against various plant pathogenic fungi. Pest Management Science, 63, 935–940. [DOI] [PubMed] [Google Scholar]
- Duan L, Tao HW, Hao XJ, Gu QQ, & Zhu WM (2009). Cytotoxic and antioxidative phenolic compounds from the traditional Chinese medicinal plant, Myristica fragrans. Planta Medica, 75,1241–1245. [DOI] [PubMed] [Google Scholar]
- El-Feraly FS, & Hoffstetter MD (1980). Isolation, characterization and synthesis of 3-methoxy-4,5-methylenedioxycinnamaldehyde: A novel constituent from Canella winterana. Journal of Natural Products, 43, 407–410. [Google Scholar]
- Forrest JE, Heacock RA, & Forrest TP (1974). Diarylpropanoids from nutmeg and mace (Myristica fragrans Houtt.). Journal of the Chemical Society, Perkin Transactions, 1(2), 205–209. [DOI] [PubMed] [Google Scholar]
- Greca MD, Molinaro A, Monaco P, & Previtera L (1994). Neolignans from Arum italicum. Phytochemistry, 35, 777–779. [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, & Tannenbaum SR (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry, 126,131–138. [DOI] [PubMed] [Google Scholar]
- Hada S, Hattori M, Tezuka Y, Kikuchi T, & Namba T (1988). Constituents of mace. Part III. New neolignans and lignans from the aril of Myristica fragrans. Phytochemistry, 27, 563–568. [Google Scholar]
- Harvey DJ (1975). Examination of the diphenylpropanoids of nutmeg as their trimethylsilyl, triethylsilyl, and tripropylsilyl derivatives using combined gas chromatography and mass spectrometry. Journal of Chromatography, 110, 91–102. [DOI] [PubMed] [Google Scholar]
- Hibbs JB Jr., Taintor RR, & Vavrin Z (1987). Macrophage cytotoxicity: role for Larginine deiminase and imino nitrogen oxidation to nitrite. Science, 235, 437–476. [DOI] [PubMed] [Google Scholar]
- Hyun DH, Lee M, Hattori N, Kubo SI, Mizuno Y, Halliwell B, et al. (2002). Effect of wild-type or mutant Parkin on oxidative damage, nitric oxide, antioxidant defenses, and the proteasome. Journal of Biological Chemistry, 277, 28572–28577. [DOI] [PubMed] [Google Scholar]
- Janssen J, Laekeman GM, Pieters LAC, Totte J, Herman AG, & Vlietinck AJ (1990). Nutmeg oil: identification and quantitation of its most active constituents as inhibitors of platelet aggregation. Journal of Ethnopharmacology, 29, 179–188. [DOI] [PubMed] [Google Scholar]
- Jin H, Zhu Z-G, Yu P-J, Wang G-F, Zhang J-Y, Li J-R, et al. (2012). Myrislignan attenuates lipopolysaccharide-induced inflammation reaction in murine macrophage cells through inhibition of NF-kB signalling pathway activation. Phytotherapy Research, 26,1320–1326. [DOI] [PubMed] [Google Scholar]
- Kang JW, Min BS, & Lee JH (2013). Anti-platelet activity of erythro-(7S,8R)-7- acetoxy-3,4,3’,5’-tetramethoxy-8-O-4’-neolignan from Myristica fragrans. Phytotherapy Research, 27,1694–1699. [DOI] [PubMed] [Google Scholar]
- Kingsbury CA (1970). Conformational preferences in diastereomers. V. Hydrogenbonding systems. Journal of Organic Chemistry, 35,1319–1323. [Google Scholar]
- Konya K, Kiss-Szikszai A, Kurtan T, & Antus S (2004). Enantiomeric separation of racemic neolignans on chiralcel OD and determination of their absolute configuration with online circular dichroism. Journal of Chromatographic Science, 42, 478–483. [DOI] [PubMed] [Google Scholar]
- Lee JW, Choi YH, Yoo MY, Choi SU, Hong KS, Lee BH, et al. (2006). Inhibitory effects of the seed extract of Myristicaceae semen on the proliferation of human tumor cell lines. (II). Korean Journal of Pharmacognosy, 37, 206–211. [Google Scholar]
- Lima CC, Criddle DN, Coelho-de-souza AN, Monte FJQ, Jaffar M, & Leal- cardoso JH (2000). Relaxant and antispasmodic actions of methyleugenol on guinea-pig isolated ileum. Planta Medica, 66, 408–411. [DOI] [PubMed] [Google Scholar]
- Molero X, Guarner F, Salas A, Mourelle M, Puig V, & Malagelada JR (1995). Nitric oxide modulates pancreatic basal secretion and response to caerulein in the rat: effects in acute pancreatitis. Gastroenterology, 108,1855–1862. [DOI] [PubMed] [Google Scholar]
- Mosmann T (1983). Rapid colorimetric assay for the cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Narasimhan B, & Dhake AS (2006). Antibacterial principles from Myristica fragrans seeds. Journal of Medicinal Food, 9, 395–399. [DOI] [PubMed] [Google Scholar]
- Nava E, Palmer RM, & Moncada S (1991). Inhibition of nitric oxide synthesis in septic shock: how much is beneficial? Lancet, 338,1555–1557. [DOI] [PubMed] [Google Scholar]
- Olajide OA, Ajayi FF, Ekhelar AI, Awe SO, Makinde JM, & Alada ARA (1999). Biological effects of Myristica fragrans (nutmeg) extract. Phytotherapy Research, 13, 344–345. [DOI] [PubMed] [Google Scholar]
- Piaru SP, Mahmud R, Abdul Majid AMS, & Mahmoud Nassar ZD (2012). Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pacific Journal of Tropical Medicine, 5, 294–298. [DOI] [PubMed] [Google Scholar]
- Rubbo H, Darley-Usmar V, & Freeman BA (1996). Nitric oxide regulation of tissue free radical injury. Chemical Research in Toxicology, 9, 809–820. [DOI] [PubMed] [Google Scholar]
- Soderman C, Leone A, Furst V, & Persson MG (1997). Endogenous nitric oxide in exhaled air from patients with liver cirrhosis. Scandinavian Journal of Gastroenterology, 32, 591–597. [DOI] [PubMed] [Google Scholar]
- Soloviev A, Lehen’kyi V, Zelensky S, & Hellstrand P (2004). Nitric oxide relaxes rat tail artery smooth muscle by cyclic GMP-independent decrease in calcium sensitivity of myofilaments. Cell Calcium, 36,165–173. [DOI] [PubMed] [Google Scholar]
- Stirling RG, Kharitonov SA, Campbell D, Robinson DS, Durham SR, Chung KF, et al. (1998). Increase in exhaled nitric oxide levels in patients with difficult asthma and correlation with symptoms and disease severity despite treatment with oral and inhaled corticosteroids. Asthma and Allergy Group. Thorax, 53, 1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SH, Huh MS, & Kim YC (2001). New tetrahydrofuran-type sesquilignans of Saururus chinensis root. Chemical & Pharmaceutical Bulletin, 49, 1192–1194. [DOI] [PubMed] [Google Scholar]
- van Gils C, & Cox PA (1994). Ethnobotany of nutmeg in the Spice Islands. Journal of Ethnopharmacology, 42,117–124. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu JX, Zhang YB, Li F, & Yang XW (2012). Determination and distribution study of myrislignan in rat tissues by RP-HPLC. Chromatographia, 75, 541–549. [Google Scholar]
- Wang Y, & Yang XW (2008). Quantitative determination of neolignanoids in the seeds of Myristica fragrans. Modern Chinese Medicine, 10,10–13. [Google Scholar]
- Yang XW, Huang X, & Ahmat M (2008). New neolignan from seed of Myristica fragrans. China Journal of Chinese Materia Medica, 33, 397–402. [PubMed] [Google Scholar]
- Yang XW, Huang X, Ma L, Wu Q, & Xu W (2010). The intestinal permeability of neolignans from the seeds of Myristica fragrans in the Caco-2 cell monolayer model. Planta Medica, 76, 1587–1591. [DOI] [PubMed] [Google Scholar]
- Yang S, Na MK, Jang JP, Kim KA, Kim BY, Sung NJ, et al. (2006). Inhibition of protein tyrosine phosphatase 1B by lignans from Myristica fragrans. Phytotherapy Research, 20, 680–682. [DOI] [PubMed] [Google Scholar]
- Zacchino SA, & Badano H (1988). Enantioselective synthesis and absolute configuration assignment of erythro-(3,4,5-trimethoxy-7-hydroxy-1’-allyl-2’,6’- dimethoxy)-8.O.4′-neolignan, isolated from mace (Myristica fragrans). Journal of Natural Products, 51, 1261–1265. [Google Scholar]
- Zacchino SA, & Badano H (1991). Enantioselective synthesis and absolute configuration assignment of erythro-(3,4-methylenedioxy-7-hydroxy-1’-allyl- 3’,5’-dimethoxy)-8-O-4’-neolignan and its acetate, isolated from nutmeg (Myristica fragrans). Journal of Natural Products, 54,155–160. [Google Scholar]
- Zacchino S, Rodriguez G, Pezzenati G, Orellana G, Enriz R, & Gonzalez Sierra M (1997). In vitro evaluation of antifungal properties of 8.O.4’-neolignans. Journal of Natural Products, 60, 659–662. [DOI] [PubMed] [Google Scholar]
- Zhou JJ, Yue XF, Han JX, & Yang WY (1993). Improved MTT assay for activity of antitumor agents. Chinese Journal of Pharmaceuticals, 24, 455–457. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


