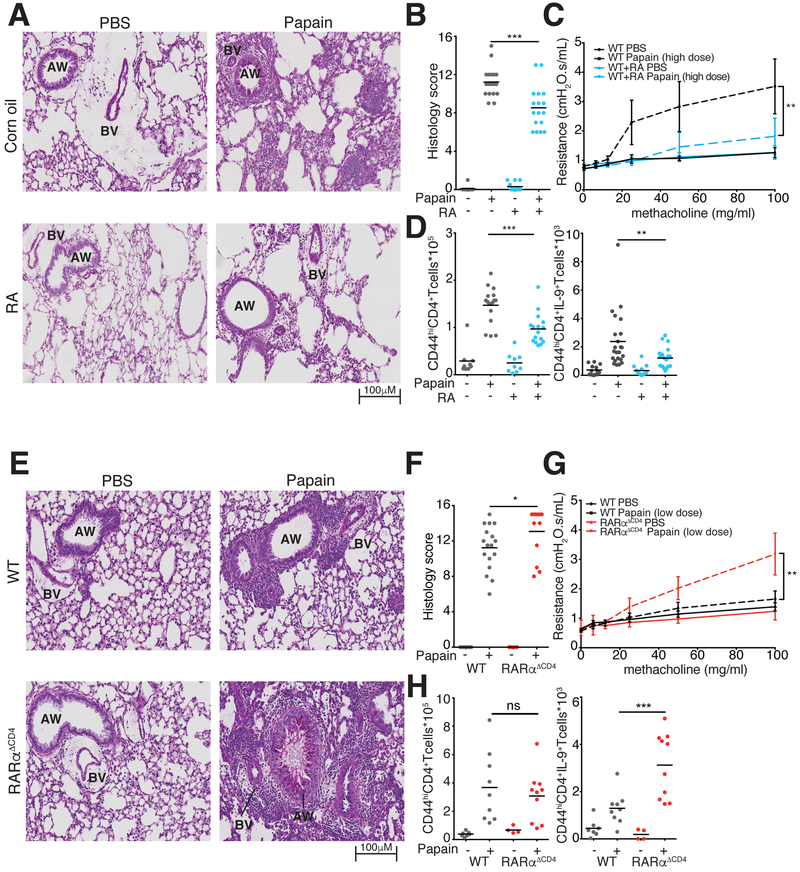
Figure 7. RA is essential to control pathology in allergic lung disease.
A-C. RA effect on lung inflammation and airway resistance. A. Representative images of Periodic Acid Schiff (PAS) stained lung tissue demonstrate reduced mucus production and lymphocytic infiltration in RA-treated vs. vehicle-treated mice with papain-induced asthma. B. Pulmonary histology scores in mice treated with RA and vehicle (3 replicates, n=3–6 per replicate). C. Airway resistance in mice exposed to escalating doses of intratracheal methacholine to induce bronchospasm. (2 replicates, n=1–3 per replicate) D. Flow cytometric analysis of IL-9 producing T cells in lung tissue at d14 of papain-induced asthma. Graphs show total numbers of Lin−TCRβ+CD4+CD44hi cells and of IL-9+ Lin−TCRβ+CD4+CD44hi T cells extracted from lung tissue of mice treated with vehicle control or RA. (4 replicates, n=3–5 per replicate) E-G. Lung inflammation and airway resistance in WT and RARα−/− mice at d14 of papain-induced asthma. E. Representative images of PAS stained slides demonstrate increased lymphocytic infiltration in RARα−/− vs. WT mice. F. Pulmonary histology scores in WT and RARα−/− mice. (3 replicates, n=3–5 per replicate) G. Airway resistance in mice exposed to escalating doses of intratracheal methacholine to induce bronchospasm. (3 replicates, n=1–3 per experiment) H. Flow cytometric analysis of IL-9 producing T cells in lung tissue at d14 of papain-induced asthma. Graphs show total numbers of Lin−TCRβ+CD4+CD44hi cells and of IL9+ Lin−TCRβ+CD4+CD44hi T cells extracted from lung tissue of WT and RARα−/− mice (2 replicates, n=3–5 per replicate). *p<0.05,**p<0.01,***p<0.005, Mann-Whitney; AW, Airway; BV, blood vessel. See also Figure S5–S7.