Abstract
Context
Defective pancreatic β-cell adaptation in pregnancy plays an important role in the pathophysiology of gestational diabetes mellitus (GDM), but the molecular basis remains unclear. Objectives of this study were to determine if circulating levels of adrenomedullin (ADM) in women with GDM are elevated and to assess the effects of ADM on insulin synthesis and secretion by human pancreatic β-cells.
Design
A stable gene product of ADM precursor, midregional pro-adrenomedullin (MR-proADM), was measured in plasma of pregnant women with normal glucose tolerance (NGT, n = 10) or GDM (n = 11). The β-Lox5 cell line, derived from human pancreatic β-cells, was transduced with homeodomain transcription factor pancreatic-duodenal homeobox (PDX) factor 1 (PDX1) encoding lentiviral vector and treated with different doses of ADM. mRNA for insulin, ADM, and its receptor components in β-Lox5 cells and insulin in media were measured.
Results
Plasma MR-proADM levels were significantly higher in GDM compared with patients with NGT. Pancreatic β-Lox5 cells express mRNA for insulin, ADM, and its receptor components. PDX1 transduction and cell-cell contact synergistically promote β-Lox5 cells insulin mRNA and secretion. Furthermore, ADM dose-dependently inhibited mRNA and secretion of insulin in β-Lox5 cell aggregates. These inhibitory effects were blocked by ADM antagonist ADM22-52, cAMP-dependent protein kinase A inhibitor KT5720, and Erk inhibitor PD98059, but not by PI-3K the inhibitor wortmannin.
Conclusions
Circulating ADM concentrations were elevated in pregnant women with GDM. ADM suppresses insulin synthesis and secretion by pancreatic β-cells in vitro. Thus, increased circulating ADM may contribute to the defective adaptation of β-cells in diabetic pregnancies, and blockade of ADM actions with its antagonists may improve β-cell functions.
Knowledge of GDM pathogenesis remains incomplete. We studied if serum ADM levels are elevated in subjects with GDM and assessed its possible inhibitory effects on insulin secretion.
In pregnant women, maternal insulin secretion is increased together with marked insulin resistance (1). This increase in insulin resistance plays an important role in the facilitation of the net flow of glucose and other nutrients toward the fetus. However, in gestational diabetes mellitus (GDM), which is a rapidly growing public health concern with the global prevalence of up to 18% (2), the endocrine pancreas, and in particular β-cells, have a reduced capacity to synthesize and secrete insulin (3). Thus, reduced adaptation of the pancreatic β-cells to the increased demands during pregnancy has been proposed as a major causal factor for GDM (4). GDM increases the incidence of complications in both the mother and the fetus, including gestational hypertension and preeclampsia for the mother and macrosomia and caesarian delivery for the infant (5). In addition, the diabetic environment in the uterus may induce a defective adaptation of the β-cells in the fetus (6), resulting in the transgenerational effect of metabolic disorders. In spite of vigorous research in this area, the molecular basis for the defective adaptation of the β-cells in GDM remains unclear.
Adrenomedullin (ADM) is a potent vasodilator peptide with 52 amino acid residues originally isolated from human pheochromocytoma (7). ADM is a member of the calcitonin peptide superfamily and signals through its receptor components calcitonin receptor–like receptor (CRLR) and receptor activity–modifying protein (RAMP)2 or RAMP3. ADM22-52 is an antagonist of ADM that blocks the functions of CRLR/RAMP2. CGRP8-37 is an antagonist that blocks ADM actions through ADM when it functions through CRLR/RAMP3 (8). ADM is expressed by a variety of cell types and is considered to have pleiotropic effects, including cell growth (9), inflammation (10), hormone secretion (11), pregnancy-related vascular adaptations (12), and fetal growth (13). It has been reported that pancreatic islets express ADM (14), and ADM receptors have been identified and well characterized in insulin-producing cells (15). It is thus possible that ADM may be involved in the physiological regulation of insulin secretion, and changes in ADM levels during pregnancy may contribute to the defective β-cell adaptation seen in women with GDM.
The development of an expanded population of human pancreatic β-cells that can be used for in vitro studies and cell transplantation is a major goal for diabetes research. Derived from the purified human pancreatic β-cell line, β-Lox5 has been created by the expression of dominant oncogenes (16). The cell lines grow indefinitely but lose differentiated function. Pancreatic-duodenal homeobox (PDX) factor-1 (PDX1), a homeodomain protein, is the earliest known molecular signpost of the developing pancreas (17). Disruption of the PDX1 gene in mice and spontaneous mutation in humans with pancreatic agenesis led to elucidation of its critical role in pancreas formation (18, 19). Conditional knockout of PDX1 in β-cells leads to diabetes, demonstrating a further role of PDX1 in maintaining β-cell function (20). Induction of β-cell differentiation has been achieved by stimulating the signaling pathways downstream of the transcription factor PDX1, cell-cell contact, and the glucagon-like peptide (GLP)-1 receptor. Synergistic activation of those pathways resulted in differentiation into functional β-cells exhibiting glucose-responsive insulin secretion in vitro. However, the expression of ADM and its receptors in these cells and its effects on the insulin expression and production by pancreatic β-cells remain unknown. Therefore, the objectives of this study were to determine if circulating levels of ADM in pregnant women with diabetes are elevated and to determine whether ADM inhibits human pancreatic β-cell insulin production and, if so, to identify the signaling molecules involved in ADM actions.
Materials and Methods
Subjects
This study was approved by Baylor College of Medicine Institutional Review Board (IRB # H28527) and was conducted according to Declaration of Helsinki Principles. Informed consent was obtained from all participating subjects admitted to the Pavilions for Women at Texas Children’s Hospital and Ben Taub Hospital. Blood samples were collected during admission for ceasarean delivery or labor from women who had normal glucose tolerance (NGT, n = 10) or who had been diagnosed with GDM (n = 11). Plasma was separated from blood samples and stored in PeriBank. Oral glucose tolerance test was performed during mid-gestation. Women with GDM had glucose intolerance, whereas women in the NGT group had negative screening results. The 1-hour glucose screen cutoff for GDM was 140 mg/dL. The 3-hour glucose tolerance test values are fasting >95 mg/dL, 1 h >180 mg/dL, 2 h >155 mg/dL, and 3 h >140 mg/dL. Patients with GDM included in this study were either under diet control or insulin treatment. Exclusion criteria included preexisting diabetes, fetal anomalies, multifetal pregnancy, hypertension, preeclampsia, immunosuppressive treatment, or clinical evidence of maternal or fetal infection. Prepregnancy and early pregnancy height and weight from each subject were obtained from the records. Body mass index was calculated based on the recorded height and the weight measured at the first visit during early pregnancy or using self-reported prepregnancy weight. The relevant clinical details of the subjects are shown in Table 1.
Table 1.
Patient Characteristics
| Group | NGT | GDM | P |
|---|---|---|---|
| Number | 10 | 11 | |
| Ethnicity (non-Hispanic/Hispanic) | 3/7 | 2/9 | |
| Maternal age, y | 28.6 ± 1.1 | 32.3± 1.3 | 0.053 |
| Gestational age, wk | 39.5 ± 0.2 | 39.2 ± 0.2 | 0.338 |
| Fetal sex, M/F | 6/4 | 5/6 | |
| Birth weight, g | 3495 ± 29 | 3503 ± 41 | 0.884 |
| BMI, kg/m2 | 28.4 ± 2.3 | 29.0 ± 1.6 | 0.830 |
| Gestational age when BMI was recorded, wk | 10.3 ± 0.1.7 | 10.1 ± 1.1 | 0.889 |
| MR-pro-ADM, pg/mL | 79.80 ± 1.17 | 114.60 ± 7.92 | 0.0004 |
Abbreviation: BMI, body mass index.
Midregional pro-adrenomedullin determination
Previous studies measuring ADM levels in the human circulation were inconsistent, perhaps due to the short half-life of ADM (21) and its interaction with a binding protein (22). Recently an assay was developed to detect midregional pro-adrenomedullin (MR-proADM), a stable cleaved product of the prehormone that is in direct correlation with ADM (23). Because the quantification of ADM in plasma was hampered by its short half-life and the existence of a binding protein (22, 24), we measured MR-proADM using a Human MR-proADM ELISA kit (MyBiosource, San Diego, CA) according to the manufacturer’s instructions. Briefly, 100 µL of plasma were added to the wells of a micro-ELISA plate, followed by incubation for 90 minutes at 37°C. After the liquid was removed from each well, 100 µL of biotinylated detection antibody working solution was added to each well, followed by incubation for another 1 hour at 37°C. Then, 100 µL of horseradish peroxidase conjugate working solution was added, followed by incubation for 30 minutes at 37°C. Substrate solution (90 µL) was added, and samples were kept for 15 minutes at 37°C. The absorbance was read at 450 nm and analyzed using a Spectrophotometer CLARIO STAR (BMG Labtech, Inc., Cary, NC). According to the Certificate of Analysis provided by the manufacturer, this kit recognized natural and recombinant human MR-proADM. The coefficient of variation was calculated as the ratio of standard deviation to the mean based on duplicate measurements and expressed as percentage value; for MR-proADM in our laboratory, this was 3.31%. The regression coefficient for linearity calculated by software of CLARIO STAR was 0.998.
β-Lox5 cell transduction with PDX1 and aggregation
The β-Lox5 cell line, derived from human pancreatic β-cells, was kindly provided by Dr. Clayton Mathews from the University of Florida College of Medicine. β-Lox5 cells were maintained in low glucose (1 mg/mL) DMEM (Gibco, Life Technology, Gaithersburg, MD) supplemented with 10% fetal bovine serum (HyClone; Fisher Scientific, Pittsburgh, PA), 1% MEM nonessential amino acids (Gibco), 1% penicillin-streptomycin (Gemini Bio-Products, West Sacramento, CA), 0.02% BSA (Sigma, St. Louis, MO), and 15 mM HEPES (Gibco).
The insulin producing islet-like aggregates of PDX1-containing human β-Lox5 cells were generated as described previously (16, 25). Human PDX1 cDNA was inserted into an optimized HIV-based vector backbone used for constitutive expression (pWPT) (Addgene, Cambridge, MA). Stocks of pWPT-PDX lentiviral vectors were generated by transient cotransfection of HEK293T cells with pWPT-PDX, trans-lentiviral packaging mix (Dharmacon, Lafayette, CO) according to the manufacturer’s instructions. Viral particles were collected and concentrated by ultracentrifugation at 23,000 × g for 2 hours, and pellets were resuspended in DMEM. For human pancreatic-duodenal homeobox factor-1 transduction, 1.2 × 106β-Lox5 cells were transduced with 5 µL of viral particle suspension in DMEM 10% fetal bovine serum for 24 hours. Islet-like aggregates of PDX1–β-Lox5 cells were induced by removal from monolayer culture with Cell Dissociation Media (Sigma, Saint Louis, MO), pelleting, and shaking in a cryovial at 70 cycles/min for 1 hour in the presence of 10 nM Exendin-4 (Sigma-Aldrich, MO). Contents of the cryovial were removed without resuspension and released dropwise into media-filled wells of ultra-low adhesion tissue culture plates (Corning SPD, Acton, MA) prepared according to the manufacturer’s instructions. After growth for 5 to 10 days, with media changes every 48 h, cell aggregates were treated with ADM in the presence or absence of ADM antagonist, ADM22-52, or signaling pathway inhibitors. Cell growth and aggregate formation were viewed daily and photographed under an Olympus BX51 microscope (Olympus Scientific, Waltham, MA).
Real-time quantitative PCR
Total RNA was isolated from β-Lox5 cells using TRIzol (Life Technologies, Grand Island, NY), and reverse transcription was performed as previously described (26). Real-time quantitative PCR was performed using TaqMan probes for insulin, ADM, CRLR, and RAMP2 (Life Technologies, Grand Island, NY). Amplification of housekeeping GAPDH (forward primer: 5′-GGTCTCCTCTGACTTCAACA-3′; reverse primer: 5′-AGCCAAATTCGTTGTCATAC-3′) served as an endogenous control. PCR conditions for TaqMan® gene expression were 2 min at 50°C and 10 min at 95°C for 1 cycle, then 15 seconds at 95°C and 1 min at 60°C for 40 cycles. All experiments were performed in triplicate. Results were calculated using the 2-ΔΔCT method and expressed in fold increase/decrease of the gene of interest.
Determination of insulin secretion by β-Lox5 cell aggregates
The secretion of insulin by β-Lox5 cell aggregates in culture medium were determined using the insulin ELISA kit for humans (Invitrogen, Waltham, MA) according to the manufacturer’s instructions. Briefly, 50 µL of samples and 50 µL of anti-insulin horseradish peroxidase conjugate were added to the wells of a micro-ELISA plate and incubated for 30 minutes at room temperature. After removing the liquid from each well, cells were washed, 100 µL of stabilized chromogen was added, and cells were incubated for another 15 minutes at room temperature in the dark. Then, 100 µL of stop solution was added, and cells were incubated for 30 minutes at room temperature. The absorbance at 450 nm was read and calculated using a spectrophotometer (CLARIO STAR; BMG Labtech, Inc., Cary, NC).
Statistics
All data are presented as mean ± SEM. Data were calculated and analyzed by Prism software (GraphPad, La Jolla, CA). Repeated measures ANOVA (treatment and time as factors) with a Bonferroni post hoc test were used for comparisons between groups. mRNA expression and ELISA data were compared between groups using unpaired Student t test. Statistical significance was defined as P < 0.05.
Results
Characteristics of human subjects studied
There were three non-Hispanic and seven Hispanic women in the group with NGT and two non-Hispanic and nine Hispanic women in the group with GDM. There were no significant differences in maternal age, gestational age, and birthweight between groups (Table 1). In addition, there were no significant differences in body mass index between subjects with NGT and subjects with GDM in this study.
Maternal plasma MR-proADM concentrations are significantly higher in pregnancies with GDM (114.6 ± 7.9 pg/mL) compared with women with NGT (79.80 ± 1.17 pg/mL, P = 0.0004), suggesting that elevated circulating ADM may be involved in the pathogenesis of GDM.
PDX1–β-Lox5 cells can be induced to form islet-like cell clusters
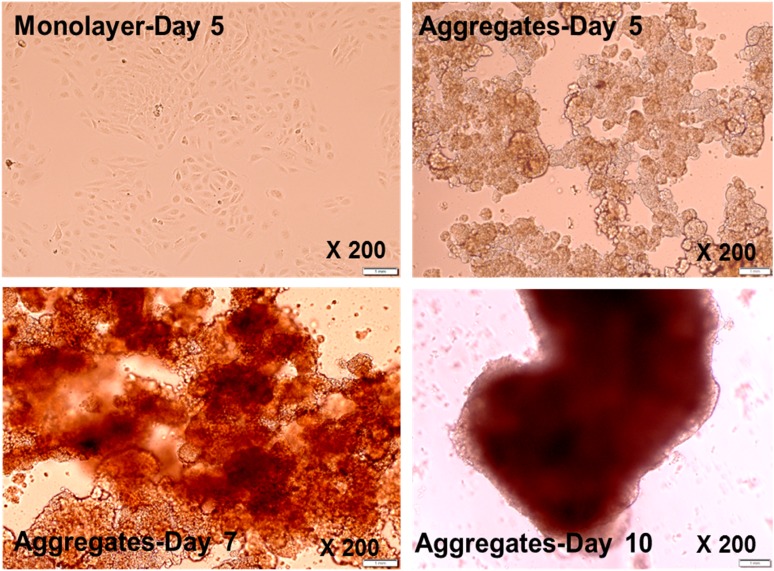
To determine whether PDX1–β-Lox5 cells respond to the physiological cues induced by cell-cell contact, we assessed whether PDX1–β-Lox5 cells were amenable to culture as islet-like aggregates. Here we showed that, without constant shaking, PDX1–β-Lox5 cells remained in monolayer fashion after 5 days in culture (Fig. 1). However, constant shaking at 70 rpm for 1 hour at 37°C and culturing for 5 days promotes cell-cell contact, and the cells become more compact, round, and dense and form three-dimensional islet-like clusters. This structure can be maintained in suspension for 7 to 12 days with no apparent change in viability.
Figure 1.
Islet-like aggregate formation of PDX1-transduced β-Lox-5 cells. PDX1-transduced β-Lox-5 cells were grown on glass slides and photographed under a microscope. Photographs of monolayer culture on day 5 and islet-like aggregates upon processing (see Materials and Methods) on day 5, day 7, and day 10 are shown. Magnification ×200.
PDX1 does not induce ADM and its receptor component expression
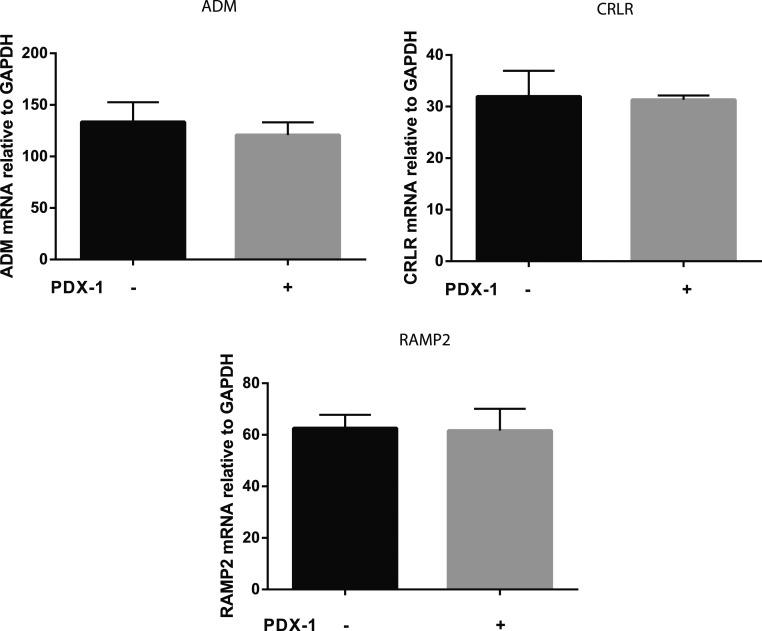
Because PDX1 is reportedly expressed in nearly all β-cells (27), we investigated the effects of PDX1 transduction on the mRNA expression for ADM and its receptor components in β-Lox5 cells. Here we showed that mRNA for ADM and its receptor components CRLR and RAMP2 were expressed in β-Lox5 cells (Fig. 2). However, PDX1 transduction did not significantly affect the mRNA for ADM and its receptor component expression, suggesting that PDX1 transduction is not sufficient to stimulate ADM and its receptor component gene expression in the β-Lox5 cell line.
Figure 2.
ADM and its receptor component mRNA in β-Lox5 cell aggregates. The mRNA levels of ADM, CRLR, and RAMP2 in β-Lox5 cells transduced with or without PDX1 were measured using real-time PCR and normalized to GAPDH mRNA expression. Data are displayed as mean ± SEM of the results from three separate experiments. There were no significant differences between groups (P > 0.05).
Cell-cell contact acts synergistically with PDX1 to promote insulin expression
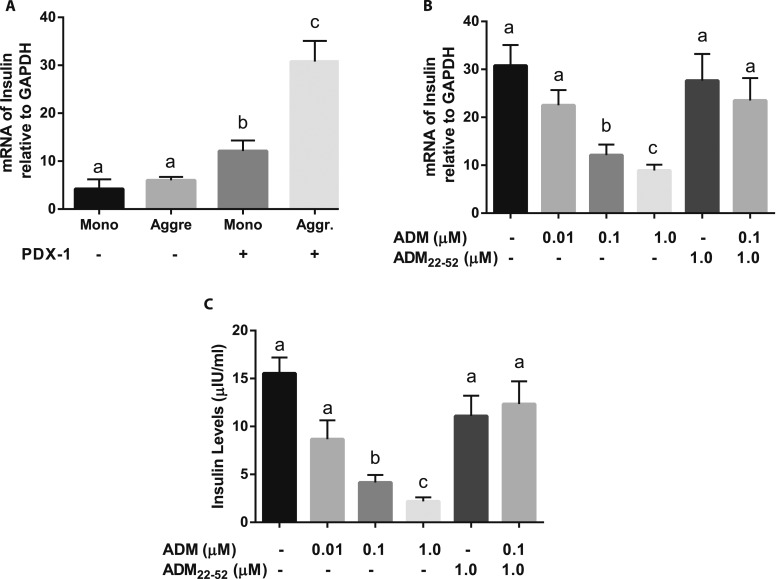
To determine the effects of cell-cell contact on β-Lox5 cell insulin expression, we measured the mRNA expression of insulin in β-Lox5 cells by quantitative PCR. We found that mRNA for insulin was expressed at lower levels by monolayer cells and cell aggregates without PDX1 transduction, but the expression was significantly increased by PDX1-transduced β-Lox5 cells in monolayer, with a further increase in cell aggregates (Fig. 3A), suggesting that promotion of cell-cell contact by aggregation of PDX1–β-Lox5 cells into islet-like clusters resulted in a significant increase in insulin mRNA expression. Thus, transduction of PDX1 and cell-cell contact act synergistically to activate the endogenous insulin gene expression in these β-cells.
Figure 3.
ADM inhibits insulin mRNA and secretion in PDX1–β-Lox5 cell aggregates. (A) mRNA for insulin in monolayer (Mono) or aggregated (Aggr) β-Lox-5 cells transduced with or without PDX1 on day 7 of culture in DMEM containing 4 mg/mL glucose was determined using real-time quantitative PCR and normalized to GAPDH mRNA expression. (B) Aggregated β-Lox-5 cells transduced with PDX1 on day 7 of culture in DMEM containing 4 mg/mL glucose were treated with ADM in the presence or absence of ADM22-52 for 24 h, and mRNA for insulin in the cells was determined using real-time quantitative PCR and normalized to GAPDH mRNA expression. (C) Insulin concentration in cell culture medium from (B) was determined using a human insulin ELISA kit. Data are displayed as mean ± SEM of the results from three separate experiments. Different letters indicate significant differences between groups (P > 0.05).
ADM inhibits insulin mRNA expression and secretion by PDX1–β-Lox5 cell aggregates
To determine the effects of ADM on insulin mRNA expression by PDX1–β-Lox5 cell aggregates, we stimulated the cells with DMEM containing 4 mg/mL of glucose followed by treatments with ADM for 24 hours. The results showed that insulin mRNA expression in these cells was inhibited by ADM in a dose-dependent manner (Fig. 3B). This inhibition was reversed by ADM antagonist ADM22-52, indicating that the inhibitory effect of ADM was mediated through ADM receptor components. Insulin secretion was assessed by measuring insulin levels in cell culture medium using an ELISA kit. Insulin secretion was dose-dependently suppressed by ADM (Fig. 3C). This ADM-induced inhibition was reversed by ADM22-52, confirming that the inhibitory action of ADM on insulin production by PDX1–β-Lox5 cells is mediated through ADM receptors.
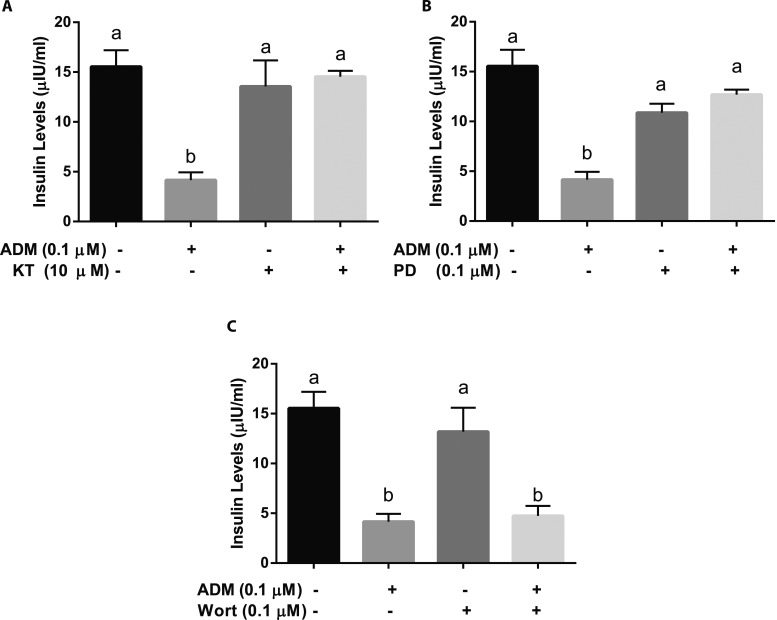
cAMP-dependent protein kinase A and Erk pathways are involved in the inhibitory actions of ADM on β-Lox5 cell insulin secretion
To determine the underlying mechanisms of ADM actions, human β-Lox5 cell aggregates were treated with a cAMP-dependent protein kinase A (PKA) inhibitor KT5720 for 30 minutes prior to the addition of ADM. The inhibitory action of ADM on insulin production was abolished by KT5720 (Fig. 4A), implying that cAMP-dependent PKA is involved in the ADM signaling pathway. In addition, the inhibition of insulin secretion by ADM was reversed by the Erk pathway inhibitor PD98059 (Fig. 4B), indicating that the Erk pathway is involved in ADM actions. However, ADM-induced reductions in insulin secretions were not blocked by wortmannin, a PI-3K inhibitor (Fig. 4C), suggesting that PI-3K is not involved in ADM signaling.
Figure 4.
Signaling molecules involved in ADM actions on PDX1–β-Lox5 cell aggregates. Aggregated β-Lox-5 cells transduced with PDX1 on day 7 of culture in DMEM containing 4 mg/mL glucose were treated with (A) KT5720 (KT, 10 µM), (B) PD98059 (PD, 10 µM), or (C) wortmannin (Wort, 0.1 µM) for 30 min and then ADM (0.1 µM) for 24 h, and insulin concentrations in the cell culture media were determined using human insulin ELISA kit. Data are displayed as mean ± SEM of the results from three separate experiments. Different letters indicate significant differences between groups (P > 0.05).
Discussion
GDM is considered a status of insufficient insulin production by pancreatic β-cells in an insulin resistance setting, but the pathogenesis of defective β-cell function remains unclear. The current study demonstrates that plasma MR-proADM levels are increased in patients with GDM compared with patients with NGT. Human pancreatic β-Lox5 cells express mRNA for insulin, ADM, and the ADM receptor components CRLR and RAMP2. ADM dose-dependently inhibits mRNA expression and secretion of insulin in β-Lox5 cell aggregates, and these inhibitory effects were blocked by ADM antagonist ADM22-52, cAMP-dependent PKA inhibitor KT5720, and Erk inhibitor PD98059 but not by wortmannin, a PI-3K inhibitor. Therefore, we propose that increased circulating ADM levels may contribute to the defective adaptation of β-cells in diabetic pregnancies and that blockade of ADM actions with its antagonists may improve β-cell functions.
ADM is derived from a common larger precursor peptide. Elevation of circulating levels of this peptide has been described in human pregnancy (28), and feto-placental tissues produce ADM (29). We have previously demonstrated that human adipose tissues produce ADM, and elevated ADM mRNA expression was observed in omental adipose tissues from women with GDM compared with women with NGT (30). Here we measured MR-proADM, the peptide derived from the ADM precursor, and showed that maternal plasma MR-proADM concentrations are significantly higher in women with GDM compared with women with NGT (Table 1), implying that elevated circulating ADM may be associated with the pathogenesis of GDM. ADM concentration in maternal and fetal plasma and in amniotic fluid has been assessed by specific radioimmunoassay (31). The overall mean amniotic fluid ADM concentration was higher in pregnant women with diabetes than in women with uncomplicated pregnancies, whereas no differences were present in maternal and fetal plasma ADM levels between women with GDM and women with uncomplicated pregnancies. Because plasma ADM levels measured using radioimmunoassay were not different between women with GDM and pregnant control subjects (32), it is possible that the reliable quantification of ADM in plasma may have been hampered by its short half-life and by the existence of a binding protein, as suggested by other studies (22, 24), and MR-proADM appears to be a reliable measure of ADM levels as reported in previous studies (33–35). Our ability to reliably measure MR-proADM in plasma and that we were able to detect significant differences related to GDM further validates the use of MR-proADM measurements.
It has been demonstrated that PDX1 is sufficient to stimulate transcription from the endogenous somatostatin gene in β-Lox5 cells (17) and that signals initiated by cell-cell contact enhance PDX1–induced gene activation to the level of islets (16). Cell-cell contact, acting in synergy with PDX1 to activate somatostatin expression in β-Lox5 cells, reveals a direct signaling pathway between the cell surface and nuclear factors in pancreatic endocrine cells. In the current study, we found that mRNA for ADM and its receptor components CRLR and RAMP2 were expressed in β-Lox5 cells (Fig. 2). However, PDX1 transduction did not significantly affect the mRNA for ADM and its receptor component expression, suggesting that PDX1 transduction is not sufficient to stimulate ADM and its receptor component gene expression in the β-Lox5 cell line. Further, mRNA for insulin was expressed at lower levels by monolayer cells and cell aggregates without PDX1 transduction, but the expression of insulin was significantly increased after the β-Lox5 cells were transduced with PDX1 and the formation of three-dimensional islet-like clusters (Fig. 3A), suggesting that transduction of PDX1 and cell-cell contact act synergistically to activate endogenous insulin gene expression. Thus, the potent induction of insulin gene expression by synergistic interactions of a transcription factor and cell-cell contact that occurs in β-Lox5 cells is likely to be similar to interactions that take place during the process of normal endocrine cell development. Thus, the β-Lox5 cell line provides a powerful model system for studying human pancreatic endocrine cell development and enabled us to determine the effects of ADM on human pancreatic β-cell function and the underlying mechanisms by which ADM regulates pancreatic insulin mRNA expression and production.
ADM-immunoreactive cells are widely distributed in human tissues, including pancreatic islets, adipocytes, placenta, and uterus (36). A homogeneous expression of ADM receptor has been found throughout the rat pancreatic islet using in situ hybridization (37). Six different insulin-producing cell lines have been analyzed by reverse transcription PCR, demonstrating expression of both ADM and its receptor (14). Further, ADM is reported to inhibit insulin release from isolated rat islet cells, and ADM antibody increases insulin release from these cells in the absence of exogenous ADM (14), suggesting a potential involvement of ADM in the insulin regulatory system. However, the effects of ADM on pancreatic β-cell insulin mRNA and secretion in human remain unknown. The current study demonstrates that human β-Lox5 cells express mRNA for ADM and its receptor components CRLR and RAMP2 and that expression of insulin mRNA and secretion in human β-Lox5 cell aggregates are inhibited by ADM in a dose-dependent manner (Fig. 3B and 3C); this inhibition was reversed by the ADM antagonist ADM22-52. This study implicates a role for ADM on insulin synthesis and secretion, and the elevated circulating ADM in pregnant women with diabetes may contribute to defective pancreatic β-cell adaptation. The influence of CGRP8-37 on insulin production in human β-Lox5 cells warrants further investigation. Elevated circulating MR-proADM levels in patients with GDM, despite attempts to improve patient status by diet control or insulin treatment, may be suggestive of a continued β-cell dysfunction.
ADM stimulates cAMP accumulation in cultured rat vascular smooth muscle cells (38) and increases Ca2+ mobilization in bovine aortic endothelial cells (39). These results suggest that ADM elicits the hypotensive effect through at least two different mechanisms: a direct action on vascular smooth muscle cells to increase intracellular cAMP and an action on endothelial cells to stimulate nitric oxide release, with both leading to vascular relaxation. In addition, insulin release induced by cAMP-raising agents (forskolin plus 3-isobutyl-1-methylxanthine or the calcium ionophore ionomycin) was significantly inhibited by 10 and 100 pM ADM in rat β-cells using the cell line INS-1 (40). This effect is likely due to the inhibition of insulin exocytosis through the activation of PTX-sensitive G proteins. However, the signaling molecules involved in ADM actions on human pancreatic β-cells remain unclear. The current study demonstrates that the inhibitory actions of ADM on insulin production by human pancreatic β-cells are abolished by KT5720 (Fig. 4A), implying that the cAMP-dependent PKA is involved in the ADM signaling pathway. In addition, the inhibition of insulin secretion by ADM was reversed by PD98059 (Fig. 4B), indicating that the Erk pathway is also involved in ADM actions. However, ADM-inhibited insulin secretions were not blocked by wortmannin (Fig. 4C), suggesting that PI-3K is not involved in ADM signaling. The effect of ADM on cAMP production and the crosstalk between PKA and ERK in regulating insulin expression and secretion in human β-Lox5 cells was not assessed in the current study, but we will use cAMP mimics, such as 8CPT-2ME-cAMP or DB-cAMP, in the future to examine its impact on insulin mRNA and secretion by human β-Lox5 cells and the interaction between PKA and ERK signaling molecules.
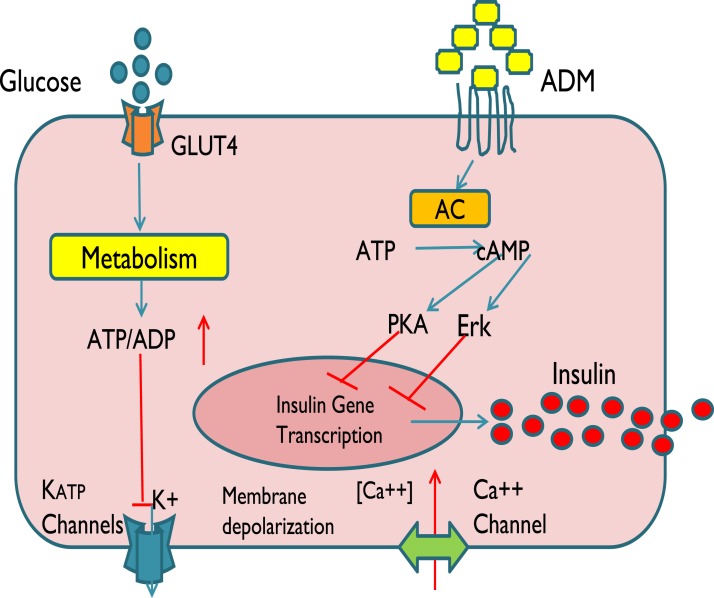
From a clinical perspective, the current study provides evidence that ADM could be involved in the physiological regulation of insulin secretion as well as the pathogenesis of diabetes of impaired insulin secretion. Based on the data from this study and published reports, we propose that glucose enters β-cells via GLUT4 on the cell membrane and undergoes metabolization in mitochondria to increase cytosolic ATP or the ATP-to-ADP ratio, leading to the closure of ATP-sensitive K+ channels and causing membrane depolarization and Ca2+ influx to facilitate exocytosis of insulin (Fig. 5). This study demonstrates that ADM inhibits synthesis and the release of insulin through a PKA- and Erk-dependent pathway and that these effects are mediated through ADM receptors on the β-cell membrane. However, it is unknown whether the elevated circulating ADM in GDM is a causative or compensatory event of metabolic dysfunction and whether elevated circulating ADM and the signaling pathways of ADM on pancreatic β-cells are specific for GDM or in general for diabetes mellitus type II. These questions warrant further investigation. A weakness in this study is the limited number of patients. We believe that functional characterization of ADM controlling both β-cell proliferation and survival might not only lead to the development of successful therapeutic strategies to enhance the response of the β-cell to increased metabolic loads but may also improve islet transplantation regimens in diabetes treatments.
Figure 5.
Schematic illustration of ADM actions in human β-cells. We propose that glucose enters β-cells via GLUT4 on the cell membrane and is metabolized in mitochondria, which increase cytosolic ATP or the ATP-to-ADP ratio, leading to the closure of ATP-sensitive K+ channels (KATP) channels, causing membrane depolarization resulting in Ca2+ influx and thereby facilitating exocytosis of insulin. ADM is proposed to inhibit insulin mRNA and exocytosis through its receptors on β-cell membrane in a PKA- and Erk-dependent manner.
Acknowledgments
The authors thank Dr. Clayton Mathews from University of Florida College of Medicine for providing the Lox-5 cell line, Dr. Fred Levine from University of California San Diego Cancer Center for technical advice, and Ms. Sandra Dale for assistance in reagent ordering and manuscript preparation.
Financial Support: This work was supported by National Institutes of Health Grants HD091503 and HL58144 (to C.Y.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ADM
adrenomedullin
- CRLR
calcitonin receptor–like receptor
- GDM
gestational diabetes mellitus
- GLP
glucagon-like peptide
- MR-proADM
midregional pro-adrenomedullin
- NGT
normal glucose tolerance
- PDX
pancreatic-duodenal homeobox
- pWPT
vector backbone used for constitutive expression
- PKA
protein kinase A
- RAMP
receptor activity–modifying protein
References
- 1. Bellmann O, Hartmann E. Influence of pregnancy on the kinetics of insulin. Am J Obstet Gynecol. 1975;122(7):829–833. [DOI] [PubMed] [Google Scholar]
- 2. Cundy T, Ackermann E, Ryan EA. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ. 2014;348(March 11):g1567. [DOI] [PubMed] [Google Scholar]
- 3. Imam K. Gestational diabetes mellitus. Adv Exp Med Biol. 2012;771:24–34. [DOI] [PubMed] [Google Scholar]
- 4. Devlieger R, Casteels K, Van Assche FA. Reduced adaptation of the pancreatic B cells during pregnancy is the major causal factor for gestational diabetes: current knowledge and metabolic effects on the offspring. Acta Obstet Gynecol Scand. 2008;87(12):1266–1270. [DOI] [PubMed] [Google Scholar]
- 5. Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208–2211. [DOI] [PubMed] [Google Scholar]
- 6. Chavey A, Ah Kioon MD, Bailbé D, Movassat J, Portha B. Maternal diabetes, programming of beta-cell disorders and intergenerational risk of type 2 diabetes. Diabetes Metab. 2014;40(5):323–330. [DOI] [PubMed] [Google Scholar]
- 7. Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192(2):553–560. [DOI] [PubMed] [Google Scholar]
- 8. McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. [DOI] [PubMed] [Google Scholar]
- 9. Iwasaki H, Eguchi S, Shichiri M, Marumo F, Hirata Y. Adrenomedullin as a novel growth-promoting factor for cultured vascular smooth muscle cells: role of tyrosine kinase-mediated mitogen-activated protein kinase activation. Endocrinology. 1998;139(8):3432–3441. [DOI] [PubMed] [Google Scholar]
- 10. Hayashi M, Shimosawa T, Isaka M, Yamada S, Fujita R, Fujita T. Plasma adrenomedullin in diabetes. Lancet. 1997;350(9089):1449–1450. [DOI] [PubMed] [Google Scholar]
- 11. Nussdorfer GG, Rossi GP, Mazzocchi G. Role of adrenomedullin and related peptides in the regulation of the hypothalamo-pituitary-adrenal axis. Peptides. 1997;18(7):1079–1089. [DOI] [PubMed] [Google Scholar]
- 12. Yallampalli C, Chauhan M, Sathishkumar K. Calcitonin gene-related family peptides in vascular adaptations, uteroplacental circulation, and fetal growth. Curr Vasc Pharmacol. 2013;11(5):641–654. [DOI] [PubMed] [Google Scholar]
- 13. Witlin AG, Li ZY, Wimalawansa SJ, Grady JJ, Grafe MR, Yallampalli C. Placental and fetal growth and development in late rat gestation is dependent on adrenomedullin. Biol Reprod. 2002;67(3):1025–1031. [DOI] [PubMed] [Google Scholar]
- 14. Martínez A, Weaver C, López J, Bhathena SJ, Elsasser TH, Miller MJ, Moody TW, Unsworth EJ, Cuttitta F. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology. 1996;137(6):2626–2632. [DOI] [PubMed] [Google Scholar]
- 15. Martínez A, Kapas S, Miller MJ, Ward Y, Cuttitta F. Coexpression of receptors for adrenomedullin, calcitonin gene-related peptide, and amylin in pancreatic beta-cells. Endocrinology. 2000;141(1):406–411. [DOI] [PubMed] [Google Scholar]
- 16. de la Tour D, Halvorsen T, Demeterco C, Tyrberg B, Itkin-Ansari P, Loy M, Yoo SJ, Hao E, Bossie S, Levine F. Beta-cell differentiation from a human pancreatic cell line in vitro and in vivo. Mol Endocrinol. 2001;15(3):476–483. [DOI] [PubMed] [Google Scholar]
- 17. Itkin-Ansari P, Demeterco C, Bossie S, de la Tour DD, Beattie GM, Movassat J, Mally MI, Hayek A, Levine F. PDX-1 and cell-cell contact act in synergy to promote delta-cell development in a human pancreatic endocrine precursor cell line. Mol Endocrinol. 2000;14(6):814–822. [DOI] [PubMed] [Google Scholar]
- 18. Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371(6498):606–609. [DOI] [PubMed] [Google Scholar]
- 19. Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15(1):106–110. [DOI] [PubMed] [Google Scholar]
- 20. Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. β-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the β-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12(12):1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meeran K, O’Shea D, Upton PD, Small CJ, Ghatei MA, Byfield PH, Bloom SR. Circulating adrenomedullin does not regulate systemic blood pressure but increases plasma prolactin after intravenous infusion in humans: a pharmacokinetic study. J Clin Endocrinol Metab. 1997;82(1):95–100. [DOI] [PubMed] [Google Scholar]
- 22. Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, Elsasser TH, Cuttitta F. Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276(15):12292–12300. [DOI] [PubMed] [Google Scholar]
- 23. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823–1829. [DOI] [PubMed] [Google Scholar]
- 24. Lewis LK, Smith MW, Yandle TG, Richards AM, Nicholls MG. Adrenomedullin(1-52) measured in human plasma by radioimmunoassay: plasma concentration, adsorption, and storage. Clin Chem. 1998;44(3):571–577. [PubMed] [Google Scholar]
- 25. Ritz-Laser B, Gauthier BR, Estreicher A, Mamin A, Brun T, Ris F, Salmon P, Halban PA, Trono D, Philippe J. Ectopic expression of the beta-cell specific transcription factor Pdx1 inhibits glucagon gene transcription. Diabetologia. 2003;46(6):810–821. [DOI] [PubMed] [Google Scholar]
- 26. Dong Y, Betancourt A, Chauhan M, Balakrishnan M, Lugo F, Anderson ML, Espinoza J, Fox K, Belfort M, Yallampalli C. Pregnancy increases relaxation in human omental arteries to the CGRP family of peptides. Biol Reprod. 2015;93(6):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12(11):4251–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Iorio R, Marinoni E, Scavo D, Letizia C, Cosmi EV. Adrenomedullin in pregnancy. Lancet. 1997;349(9048):328. [DOI] [PubMed] [Google Scholar]
- 29. Marinoni E, Di Iorio R, Letizia C, Villaccio B, Scucchi L, Cosmi EV. Immunoreactive adrenomedullin in human fetoplacental tissues. Am J Obstet Gynecol. 1998;179(3 Pt 1):784–787. [DOI] [PubMed] [Google Scholar]
- 30. Dong Y, Betancourt A, Belfort M, Yallampalli C. Targeting adrenomedullin to improve lipid homeostasis in diabetic pregnancies. J Clin Endocrinol Metab. 2017;102(9):3425–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Iorio R, Marinoni E, Urban G, Costantini A, Cosmi EV, Letizia C. Fetomaternal adrenomedullin levels in diabetic pregnancy. Horm Metab Res. 2001;33(8):486–490. [DOI] [PubMed] [Google Scholar]
- 32. Pöyhönen-Alho M, Viitasalo M, Nicholls MG, Lindström BM, Väänänen H, Kaaja R. Imbalance of the autonomic nervous system at night in women with gestational diabetes. Diabet Med. 2010;27(9):988–994. [DOI] [PubMed] [Google Scholar]
- 33. Lopes D, Menezes Falcão L. Mid-regional pro-adrenomedullin and ST2 in heart failure: Contributions to diagnosis and prognosis. Rev Port Cardiol. 2017;36(6):465–472. [DOI] [PubMed] [Google Scholar]
- 34. Krintus M, Kozinski M, Braga F, Kubica J, Sypniewska G, Panteghini M.. Plasma midregional proadrenomedullin (MR-proADM) concentrations and their biological determinants in a reference population. Clin Chem Lab Med. 2018;56(7):1161–1168. [DOI] [PubMed] [Google Scholar]
- 35. Lorubbio M, Conti AA, Ognibene A. Midregional pro-adrenomedullin (MR-ProADM) reference values in serum. Clin Biochem. 2018;53:173–174. [DOI] [PubMed] [Google Scholar]
- 36. Ichiki Y, Kitamura K, Kangawa K, Kamoto M, Matsuo H, Eto T. doi: 10.1016/0014-5793(94)80106-1. Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett. 1994;338:6–10. [DOI] [PubMed] [Google Scholar]
- 37. Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21(2):138–167. [DOI] [PubMed] [Google Scholar]
- 38. Eguchi S, Hirata Y, Iwasaki H, Sato K, Watanabe TX, Inui T, Nakajima K, Sakakibara S, Marumo F. Structure-activity relationship of adrenomedullin, a novel vasodilatory peptide, in cultured rat vascular smooth muscle cells. Endocrinology. 1994;135(6):2454–2458. [DOI] [PubMed] [Google Scholar]
- 39. Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, Eto T, Kangawa K, Matsuo H. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J Biol Chem. 1995;270(9):4412–4417. [DOI] [PubMed] [Google Scholar]
- 40. Sekine N, Takano K, Kimata-Hayashi N, Kadowaki T, Fujita T. Adrenomedullin inhibits insulin exocytosis via pertussis toxin-sensitive G protein-coupled mechanism. Am J Physiol Endocrinol Metab. 2006;291(1):E9–E14. [DOI] [PubMed] [Google Scholar]