Abstract
Objective:
To determine the association between dual-energy x-ray absorptiometry (DXA) testing for osteoporosis and subsequent fractures in US male veterans without a previous fracture.
Patients and Methods:
This is a propensity score—matched observational study using Centers for Medicare and Medicaid Services and Veterans Affairs (VA) data from January 1, 2000, through December 31, 2010, with a mean follow-up time of 4.7 years (range, 0-10 years). Men receiving VA primary care aged 65 to 99 years without a previous fracture (N=2,539,812) were included. Men undergoing DXA testing were propensity score matched with untested controls in a 1:3 ratio, indicating the probability of DXA testing within the next year. Time to first clinical fracture was the primary outcome. Comorbidities, demographic characteristics, medications, DXA results, and osteoporosis treatment were defined using administrative data and natural language processing. A landmark analysis contingent on surviving to 12 months after screening was completed, accounting for competing risk of mortality.
Results:
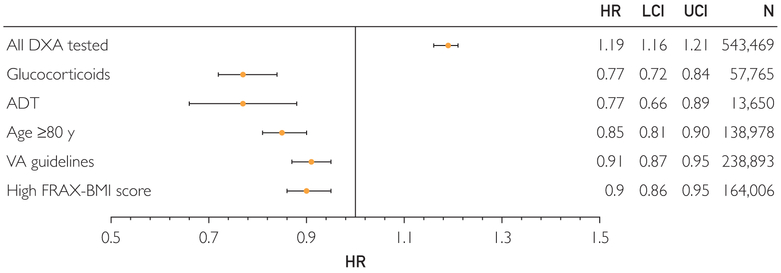
During follow-up of 153,311 men tested by DXA and 390,158 controls, 56,083 (10.3%) had sustained a fracture and 111,774 (20.6%) died. Overall, DXA testing was not associated with a decrease in fractures; conclusions are limited by unmeasured confounders and low medication initiation and adherence in those meeting treatment thresholds (12% of follow-up time). In contrast, DXA testing in prespecified subgroups was associated with a lower risk of fracture in comparison to the overall population who underwent DXA testing: androgen deprivation therapy (hazard ratio [HR], 0.77; 95% CI, 0.66-0.89), glucocorticoids (HR, 0.77; 95% CI, 0.72-0.84), age 80 years and older (HR, 0.85; 0.81-0.90), 1 or more VA guideline risk factors (HR, 0.91; 95% CI, 0.87-0.95), and high Fracture Risk Assessment Tool using body mass index score (HR, 0.90; 95% CI, 0.86-0.95).
Conclusion:
Current VA DXA testing practices are ineffective overall; interventions to improve treatment adherence are needed. Targeted DXA testing in higher-risk men was associated with a lower fracture risk.
Osteoporosis is underrecognized in older men. At the age of 50, 13% to 25% of white men can expect to suffer a hip, vertebral, or forearm fracture in their remaining lifetime, comparable to the risk of prostate cancer.1 Men are more than twice as likely as women to experience complications after an osteoporotic fracture and have higher mortality after hip fracture.2 It is estimated that hip fractures result in $43 million of additional cost to the Veterans Health Administration (VA) annually.3 Despite this high burden, men are rarely diagnosed and treated for osteoporosis before a fracture occurs.4
Low diagnosis and treatment rates are related in part to uncertainty about testing for osteoporosis in men. Although guidelines uniformly endorse dual-energy x-ray absorptiometry (DXA) testing in men older than 50 years after a low-trauma fracture,5 there is no consensus on testing men who have not had a previous fracture.6 Current practice guideline recommendations vary widely and include DXA testing for all older men7-9 or selecting men based on fracture risk factors or clinical risk scores.10-12 However, the US Preventive Services Task Force and VA National Center for Disease Prevention concluded that there was insufficient evidence to recommend for or against DXA testing in men without a previous fracture,13 citing a lack of direct evidence that DXA testing reduces fractures and recommending longitudinal studies. As a result of these conflicting recommendations and resulting Medicare reimbursement policies, the recent primary screening rate in high-risk men in managed care was reported to be 11%14 and is likely lower in other settings.
We sought to examine the effect of DXA testing in men without a previous fracture as it is currently operationalized in the largest US health care system. We analyzed administrative and electronic health record data for all US male veterans receiving primary care within the VA health care system from January 1, 2000, through December 31, 2010. Using propensity score matching, we examined the association between DXA testing and subsequent fractures in the overall cohort and in prespecified high-risk groups in whom DXA is recommended by at least 1 practice guideline. Although such an analysis will not directly determine the effectiveness of DXA testing in men, it will prioritize the groups most likely to benefit and inform practice redesign.
PATIENTS AND METHODS
This is a propensity score—matched observational study using the cohort of 5,869,668 men with any VA outpatient encounters from January 1, 2000, through December 31, 2010. We hypothesized that DXA testing would be associated with lower fracture rates relative to untested men with a similar probability of being tested, especially when followed by osteoporosis treatment for those meeting recommended treatment thresholds.7 Ethical approval was obtained from the Durham and Richmond VA Medical Centers and the Department of Defense.
Data Sources
Veterans Affairs inpatient treatment file, outpatient care file, fee basis file, pharmacy treatment file, and laboratory files were merged with Centers for Medicare and Medicaid Services (CMS) Part A and B claims. Death was determined from the Vital Status File.
Patients and Controls
Men aged 65 to 99 years who were eligible for primary DXA testing (no previous DXA, fracture, osteoporosis diagnosis, or osteoporosis medication in the 3 years before their start date) were included in this study. Only men with at least 2 VA primary care visits within 2 consecutive years were included to improve ascertainment of comorbidities and medications.15
Observation Period and Follow-Up
Each subject’s start date was their first primary care visit in the first calendar year after DXA testing became available at their medical center. Relevant diagnoses and medications were determined for the 3 years before their start date and updated on January 1 of each year thereafter. Patients and controls were followed until the end of the study or death. Because CMS records were available for all patients and controls, we did not censor for loss to VA follow-up.
Dual-Energy X-Ray Absorptiometry Testing, Results, and Treatment
Current Procedural Terminology codes for DXA were identified from VA and CMS. To supplement this, natural language processing identified bone density test results in radiology reports, primary care, and consultation notes. Natural language processing extracted femoral neck T-scores and lowest T-scores at any site; validation suggested excellent accuracy.16 Only DXA testing done before a fracture or osteoporosis medication prescription was considered.
Treatment was defined as at least 1 prescription for a bisphosphonate, calcitonin, or teriparatide. Using US treatment thresholds (osteoporosis by bone mineral density [BMD] measurement at any site or 10-year Fracture Risk Assessment Tool [FRAX]-BMD score of ≥20% for major osteoporotic fracture or ≥3% for hip fracture),17 subjects’ treatment status was defined 12 months after DXA testing as appropriately treated, appropriately untreated, overtreated, or undertreated. If DXA results were not available, FRAX using the body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) instead of BMD was used for classification; a previous study has reported that few patients are classified differently with FRAX-BMI compared with FRAX-BMD.18 Treatment adherence was estimated using the continuous measure of medication acquisition.19
Comorbidities and Other Variables
Comorbid conditions, medications, and other factors that increase fracture risk were identified using International Classification of Disease, Ninth Revision codes, laboratory values (hemoglobin A1C level and estimated glomerular filtration rate), and medication prescriptions (glucocorticoids, androgen deprivation therapy (ADT), hypoglycemic agents, dopaminergic medications, cholinesterase inhibitors, selective serotonin reuptake inhibitors, opiates, and proton pump inhibitors). Charlson comorbidity scores were calculated using a validated approach.20,21 Fall risk factors (neuropathy, stroke, seizure disorder, dementia, and psychoactive medications) were included. The 10-year risk of hip and major osteoporotic fractures was calculated at baseline for all patients and controls using FRAX-BMI and updated for those with documented femoral neck T-scores within the 365 days of DXA testing. Other social and health system factors likely to influence receipt of DXA testing included marital status, co-pay level (financial status), homelessness, rural zip code, receipt of colorectal cancer screening, and number of outpatient visits.
Propensity Score Development
We used propensity scores to match DXA tested “cases” with untested “controls” with a similar probability of undergoing DXA testing in the next year based on comorbidities, medications, demographic characteristics, health system, and social factors (Table). The propensity score contains information on a large number of measured confounders and has a property of balancing them between tested and untested patients.22 For this analysis, a 3-year look-back period from each subject’s start date was used to identify baseline risk factors; then, new propensity scores were calculated on January 1 of each year, incorporating new diagnoses and medications from the previous year. Because DXA testing practices vary by region and medical center’s complexity level, 80 separate logistic models stratified by year, region, and complexity level were used. Logistic models included all the potential risk factors for DXA testing listed in the Table.
TABLE.
Characteristics of the Overall Cohort at Baseline and Matched Subset on January 1 of Year in Which the Screened Patient Underwent DXA Testinga,b,c
| Matched patients (as of January 1 of the matching year) |
|||
|---|---|---|---|
| Characteristic | Overall cohort (at baseline) (N=2,539,812) |
DXA tested (n=183,943) |
Untested (n=475,449) |
| Demographic, social, and system factors | |||
| Age (y) | 74.5±6.2 | 77.0±5.7 | 76.7±5.9 |
| Body mass index (kg/m2) | 28.2±4.7 | 28.0±4.4 | 27.9 ±4.3 |
| Race | |||
| Black, non-Hispanic | 176,903 (7.0) | 13,100 (7.1) | 33,610 (7.1) |
| Other | 90,869 (3.6) | 8040 (4.4) | 20,538 (4.3) |
| Unknown | 519,216 (20.4) | 33,046 (18.0) | 83,572 (17.6) |
| White, non-Hispanic | 1,752,824 (69.0) | 129,757 (70.5) | 337,729 (71.0) |
| No co-pay (low income/disability) | 1,628,545 (64.1) | 113,703 (61.8) | 292,091 (61.4) |
| Medicaid | 152,670 (6.0) | 11,381 (6.2) | 29,826 (6.3) |
| Married | 1,814,547 (71.4) | 137,421 (74.7) | 355,461 (74.8) |
| Rural | 170,478 (6.7) | 10,228 (5.6) | 26,281 (5.5) |
| Homeless | 17,953 (0.7) | 1059 (0.6) | 2574 (0.5) |
| Prisoner of war | 25,626 (1.0) | 2478 (1.3) | 6297 (1.3) |
| Colorectal cancer screened | 295,027 (11.6) | 102,578 (55.8) | 263,640 (55.5) |
| No. of outpatient visits | 5.0 (6.0) | 8.0 (10.0) | 8.0 (11.0) |
| Fracture risk factors | |||
| Chronic lung disease | 442,017 (17.4) | 68,023 (37.0) | 171,166 (36.0) |
| Diabetes | 414,548 (16.3) | 63,475 (34.5) | 158,970 (33.4) |
| Hyperthyroidism, Cushing syndrome, and hypogonadism | 20,057 (0.8) | 7519 (4.1) | 16,987 (3.6) |
| Parkinson disease | 27,527 (1.1) | 3992 (2.2) | 10,012 (2.1) |
| Prostate cancer | 212,223 (8.4) | 42,682 (23.2) | 108,382 (22.8) |
| Rheumatoid arthritis | 11,843 (0.5) | 5331 (2.9) | 8597 (1.8) |
| Gastrectomy and malabsorption | 6606 (0.3) | 2209 (1.2) | 5041 (1.1) |
| Alcohol abuse | 407,335 (16.0) | 32,487 (17.7) | 82,985 (17.5) |
| Smoking | 506,936 (20.0) | 33,172 (18.0) | 86,505 (18.2) |
| 10-y major fracture risk calculated using FRAX-BMI (%) | 6.4±3.0 | 6.6±3.0 | 6.5±3.0 |
| Medications | |||
| Androgen deprivation therapy | 11,078 (0.4) | 6393 (3.5) | 13,197 (2.8) |
| Glucocorticoids | 57,448 (2.3) | 25,304 (13.8) | 58,799 (12.4) |
| Proton pump inhibitors | 187,410 (7.4) | 57,674 (31.4) | 143,556 (30.2) |
| SSRIs | 119,391 (4.7) | 27,444 (14.9) | 67,921 (14.3) |
| Traditional antiepileptic drugs | 53,670 (2.1) | 17,269 (9.4) | 41,732 (8.8) |
| Other diagnoses | |||
| Mood disorder | 345,200 (13.6) | 61,210 (33.3) | 152,551 (32.1) |
| Other psychiatric diagnosis | 175,646 (6.9) | 30,162 (16.4) | 74,535 (15.7) |
| Dementia | 84,154 (3.3) | 15,663 (8.5) | 36,580 (7.7) |
| Chronic liver disease | 54,162 (2.1) | 14,358 (7.8) | 34,505 (7.3) |
| Nonskin cancer | 491,142 (19.3) | 87,892 (47.8) | 224,772 (47.3) |
| Neuropathy | 165,038 (6.5) | 38,148 (20.7) | 94,287 (19.8) |
| Congestive heart failure | 256,692 (10.1) | 23,295 (12.7) | 58,154 (12.2) |
| Chronic kidney disease | 340,785 (13.4) | 97,068 (52.8) | 242,350 (51.0) |
| Stroke | 10,174 (0.4) | 2317 (1.3) | 5341 (1.1) |
| Charlson comorbidity score | 1.0 (3.0) | 0.0 (3.0) | 1.0 (3.0) |
DXA = dual-energy x-ray absorptiometry; FRAX-BMI = Fracture Risk Assessment Tool using body mass index; SSRI = selective serotonin reuptake inhibitor.
Data are presented as mean ± SD, or No. (percentage).
Because of the large sample size, nearly all comparisons are statistically significant. Clinically important trends are noted in the text. The following variables had missing values (overall, screened, unscreened): body mass index (132,909; 8239; 20,381), co-pay (18,328; 1080; 2710), married (8681; 598; 1669), and rural (40,365; 3770; 9509).
Matching
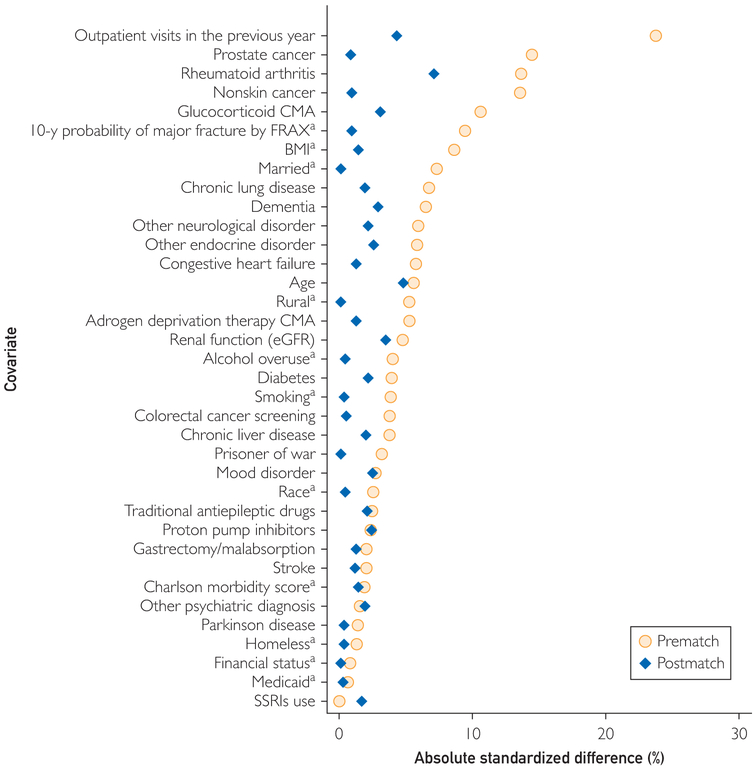
A greedy matching algorithm identified up to 3 untested controls with the propensity score closest to that of the DXA tested case as of January 1 of the case’s DXA testing year. Some controls were excluded after matching because they had fracture, death, or first osteoporosis medication use in the year they were matched but before their case’s DXA testing date. Therefore, not all cases had 3 controls in the analysis. In greedy matching, cases are matched sequentially with controls with the closest propensity score; once matched, controls are no longer available to match with subsequent cases. Patients tested by DXA were eligible to serve as controls before their testing year, at which time they became cases. Matching effectiveness was assessed by ensuring that standardized difference was less than 0.1 for all fracture risk factors (Figure 1). Propensity score distributions were examined graphically.
FIGURE 1.
Plot of standardized differences between dual x-ray absorptiometry tested and untested individuals (difference in mean values divided by pooled SD) before and after propensity score matching.
aindicates variables were ascertained at baseline only. Other variables were updated annually. BMI = body mass index; CMA = continuous medication adherence; FRAX = Fracture Risk Assessment Tool; eGFR = estimated glomerular filtration rate; SSRI = selective serotonin reuptake inhibitor.
Analysis
Cox proportional hazards models, stratified by the matched groups, assessed the hazard of fracture in DXA tested vs untested men. Because death could confound the observed effects, competing risk analyses implemented in the Fine and Gray methods23 were estimated. To allow for the lag time to benefit from an osteoporosis medication,24 we set a landmark of 12 months after testing (ie, patients and controls who died or fractured within 12 months of their DXA testing/match date were censored). The use of this landmark had several additional analytical benefits. First, the proportional hazards assumption for fracture risk was not met, with curves crossing around 12 months. Second, we observed a spike in fractures immediately after the first DXA testing date, suggesting that these were likely postfracture DXA tests with errors in coding dates. Setting the 1-year landmark removed these misclassified events.
Patients and controls were censored at death or the end of the study; in addition, controls were censored if they underwent DXA testing (became a case) or began taking an osteoporosis medication. Interaction terms for prespecified subgroups were examined, including age 80 years and older, glucocorticoids, ADT, high baseline FRAX-BMI score (≥20% major osteoporotic fracture or ≥3% hip fracture), and 1 or more fracture risk factor per the VA Undersecretary guidelines (glucocorticoids, ADT, traditional antiepileptic drugs, rheumatoid arthritis, alcohol abuse, smoking, BMI < 25 kg/m2, hyperthyroidism, hyperparathyroidism, chronic lung disease, chronic liver disease, stroke, parkinsonism, and gastrectomy). All hypothesis tests were 2-sided with a significance level less than .05. Data were analyzed by authors C.F.P. and J.M.G. using SAS 9.4 (SAS Institute, Inc.).
RESULTS
Participant flow and baseline characteristics are presented in Figure 2 and Table, respectively. As expected, because they were selected for DXA testing in routine clinical care, matched cases and controls had more fracture risk factors than did the overall cohort, but both groups presented similar proportions for smoking, alcohol abuse, and mean FRAX-BMI scores. Cases and controls were older, were more likely to be married, were living in nonrural areas, had higher income, had previous colorectal cancer DXA testing, and had more outpatient visits in the testing year than did the overall cohort. After matching, the standardized difference for all risk factors used in calculating the propensity score was less than 0.10 (Figure 1), indicating good balance of known fracture risk factors.
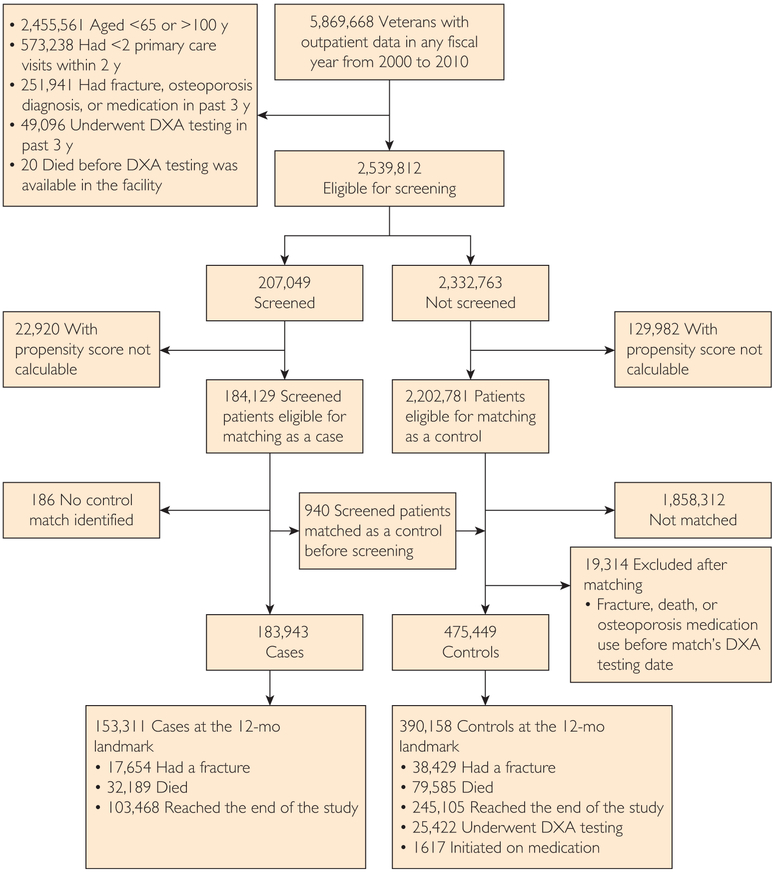
FIGURE 2.
CONSORT diagram showing subject flow through the analysis. Exclusion or censoring criteria are given as bullet points. DXA = dual x-ray absorptiometry.
Osteoporosis DXA Testing, DXA Results, and Follow-Up in the Overall Cohort
Dual-energy x-ray absorptiometry testing was uncommon (8.2% overall; 10.3% in men older than 70 years) and varied by region and over time. During a mean follow-up time of 4.7 years (range, 0-10 years) after DXA testing, 10.3% sustained a fracture and 20.6% died. Among the subset of individuals for which DXA results were available (n=81,479 [39.4%]), 26% had osteoporosis and 46% had osteopenia at any site.
Association of DXA Testing With Fracture Risk in the Matched Cohort
Dual-energy x-ray absorptiometry testing was associated with a higher fracture risk in the overall cohort (hazard ratio [HR], 1.19; 95% CI, 1.16-1.21) (Figures 3 and 4), suggesting the presence of unmeasured fracture risk factors that prompted physicians to order DXA testing for cases but were unavailable in administrative data. Assuming a prevalence of 10% in the untested group, these risk factors would have to be fully independent of the 32 risk factors in our model, would increase fracture risk by 100%, and would be 2.5 times as prevalent in the tested group to have resulted in missing a 10% decrease in the hazard of fracture attributable to DXA testing.
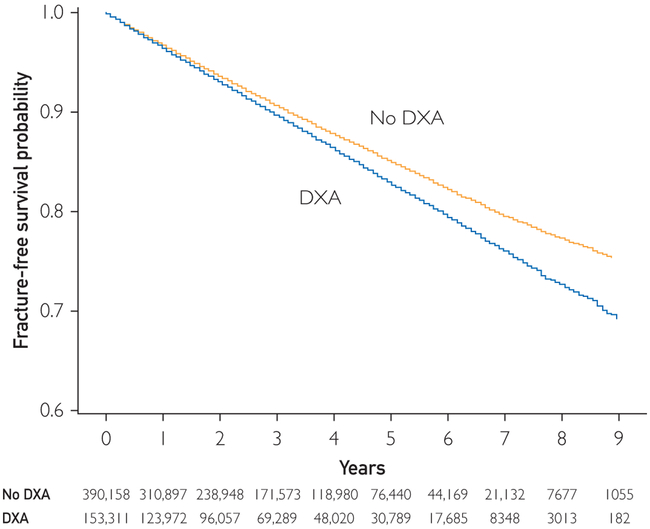
FIGURE 3.
Fracture-free survival for screened and unscreened men. DXA = dual-energy x-ray absorptiometry.
FIGURE 4.
Hazard ratio for fracture in screened vs unscreened men. Hazard ratios for the prespecified subgroups are relative to the DXA testing risk in the overall population. High FRAX-BMI score was defined as 10-year fracture risk ≥3% hip fracture or 20% major osteoporotic fracture before DXA testing. ADT = androgen deprivation therapy; DXA = dual-energy x-ray absorptiometry; FRAX-BMI = Fracture Risk Assessment Tool using body mass index; HR = hazard ratio; LCI = lower 95% CI; UCI = upper 95% CI; VA = Veterans Affairs.
Examining osteoporosis medication use in DXA tested individuals, we found that 43% of those meeting treatment thresholds were not initiated on medications during follow-up. For those with at least 1 prescription for an osteoporosis medication, the median percentage of follow-up time during which they received medication after the 1-year landmark was 12%. More than 60% of osteoporosis medication use occurred within 90 days of testing, with less than 10% of prescriptions dispensed 1 year after testing.
The effectiveness of DXA testing improved over time (3.3% reduction in the hazard of fracture in tested individuals per year; 95% CI, 2.3%-4.2%; interaction, P<.001). The proportion of cases who were classified as receiving guideline-recommended care was similar during the study period (77%-82%).
Prespecified Subgroups
Interaction terms suggested higher DXA testing effectiveness in all prespecified groups (P<.001), and therefore separate analyses were completed (Figure 4). Compared with the risk of fracture in the overall screened cohort, DXA was associated with a 23% lower hazard of fracture relative to untested controls for patients receiving ADT (HR, 0.77; 95% CI, 0.66-0.89) and glucocorticoids (HR, 0.77; 95% CI, 0.72-0.84). Smaller but significant benefits associated with DXA were observed in men 80 years and older (HR, 0.85; 95% CI, 0.81-0.90), those with 1 or more VA guideline risk factors (HR, 0.91; 95% CI, 0.87-0.95), and those with high 10-year fracture risk calculated using FRAX-BMI (HR, 0.90; 95% CI, 0.86-0.95).
DISCUSSION
Practitioners and policymakers have little data to inform decisions about DXA testing for osteoporosis in men. In the Cardiovascular Health Study, 654 men from 2 counties underwent DXA testing whereas those in 2 other counties received “usual care”; there was a trend toward a lower hazard of hip fracture (HR, 0.68; 95% CI, 0.32-1.42),25 which was likely affected by geographic differences in hip fracture rates.26 Other studies have modeled osteoporosis prevalence rates from cohort studies and treatment benefits from clinical trials to estimate the cost-effectiveness of DXA testing.27,28 Prospective studies such as Osteoporotic Fractures in Men Study29 have been crucial in establishing osteoporosis prevalence and risk factors but lack a comparison group. Although a randomized controlled trial would be required to establish unbiased estimates of the true effectiveness of DXA testing under optimal conditions, such a study would require a large sample size and long follow-up duration; indeed, there is a paucity of data supporting DXA testing even in women.30 A single pragmatic trial of more than 12,000 women in the United Kingdom reported a decrease in hip, but not all clinical, fractures when women at high risk calculated using FRAX-BMI were offered DXA testing.31In the absence of clinical trial data, well-done observational studies can provide important insights to guide practice in men.
Our findings suggest that DXA testing as it is currently operationalized in the largest health system in the United States is associated with a lower risk of subsequent fractures in subgroups of men at high fracture risk. However, we were unable to find a reduction in fractures from DXA testing in the overall cohort in the setting of low treatment and adherence rates, suggesting that major changes in the DXA testing program are needed to avoid waste. Our results are influenced by unmeasured risk factors; the counterintuitive higher fracture rates associated with DXA testing overall suggest that ordering clinicians are identifying fracture risk factors that were unavailable in administrative data. However, the effect of these risk factors would have to be very large to have resulted in our missing even a modest decrease in fractures with general DXA testing. These results have important clinical implications for DXA selection and osteoporosis care delivery.
The selection of the most appropriate group of men to undergo DXA has been controversial. In this cohort, 75% of those tested in routine clinical care did not ultimately meet osteoporosis treatment thresholds and therefore did not benefit from DXA. This figure is higher than for women undergoing DXA testing,32 although it compares favorably with the result of colon cancer, aortic aneurysm, and lung cancer DXA testing.33 However, DXA was associated with a lower hazard of fracture in all subgroups at higher risk relative to the overall population. Therefore, more targeted selection of men at highest risk of fracture would improve the effect of DXA. Variability in guideline recommendations may be contributing to inefficient selection of DXA testing. Our results support the VA Undersecretary12 and American College of Physicians10 guidelines, which select older men with 1 or more fracture risk factors. This recommendation is further supported by recent cohort studies reporting substantially increased hip fracture incidence in men with these fracture risk factors, with the incidence increasing exponentially in those with multiple risk factors.34 Several guidelines recommend age-based DXA testing, selecting all men older than 65 or 70 years.8,9 Although it remains unclear what the effect of general DXA testing would be on patients and controls aged 65 to 79 years without other risk factors if treatment and adherence rates were improved, our results do support general DXA testing for men older than 80 years, the age at which fracture rates increase exponentially. Fracture Risk Assessment Tool-BMI could also be used to focus on DXA testing efforts because those with high baseline FRAX-BMI score in our sample also benefited from DXA testing; in the United Kingdom, only intermediate- or high-risk individuals are referred for DXA.11,31
In addition to informing selection for DXA testing, the results highlight the urgent need to improve treatment rates and promote longterm adherence. Among tested men who met treatment thresholds in our study, 43% were never treated and 90% of those who received an osteoporosis medication discontinued it before it is likely to have provided a benefit. Previous studies outside the VA suggest that this is a widespread issue internationally; 45% of patients taking osteoporosis medications discontinue them within 1 year, with men more likely to discontinue than women.35 Poor medication persistence is associated with a higher fracture rate,36 with no evidence of a healthy adherer effect.37
Challenges with both DXA testing selection and treatment monitoring indicate a need for system level approaches. Validated algorithms selecting men for DXA testing based on risk factors found in the electronic health record have been developed,38 but have not been widely adopted owing to concerns about provider alert fatigue. Quality improvement interventions targeting primary care clinicians and patients have had limited success in improving osteoporosis treatment and adherence,39 and these tasks may be best accomplished by a centralized team focused on fracture prevention. Such “fracture liaison service” teams have been effectively deployed worldwide (including within VA)40 to improve secondary osteoporosis testing and treatment after a fragility fracture and are associated with reductions in fracture rates and costs.41 However, this model is not widely used for primary DXA testing before a fracture has occurred.
It is important to emphasize that we considered DXA testing only in men who have not had a previous fracture; there is broad consensus that DXA testing or osteoporosis treatment for osteoporosis is indicated for those with a low-trauma fracture. Recent studies have documented alarming international trends of declining treatment rates after fragility fracture.4
Although our cohort was large with 10 years of follow-up, the accuracy and completeness of administrative data are limited. Coding for fracture is both sensitive and specific,42 but we could not distinguish between fragility fractures and high-trauma fractures. Administrative data algorithms for other chronic conditions generally have good specificity but poor sensitivity,43resulting in unrecognized fracture risk factors. We used mitigating strategies such as including pharmacy records and laboratory results, combining VA and CMS records, and updating propensity scores annually; however, unmeasured risk factors clearly remain. We lacked Medicare D prescription drug program data and likely underestimated treatment outside the VA, though studies suggest that primary prevention osteoporosis treatment is uncommon in male Medicare beneficiaries.44 Our use of a propensity score—matched cohort selects a population who is more likely to be tested. The generalizability of our findings to nonveterans should also be considered; because risk factors are common in veterans, it is estimated that the rates of osteopenia and osteoporosis are nearly double those of nonveterans and therefore they represent a population more likely to benefit from DXA testing, but with additional adherence challenges.45
CONCLUSION
Dual-energy x-ray absorptiometry testing in high-risk subgroups of men is associated with clinically important reductions in fractures. However, DXA testing across the entire VA population was not associated with decreased fractures because of low treatment initiation and adherence rates. System interventions that assist clinicians in identifying men at risk and support treatment decision making and adherence are needed.
ACKNOWLEDGMENTS
We acknowledge excellent analytical support from Lynn VanScoyoc, MS, and Merritt Schnell, MS, and logistical support from Megan Pearson, MA.
Grant Support: The work was supported by the Department of Defense (grant nos. W81XWH-12-2-0093, NIA K24 AG049077-01A1, and NIA 2P30AG028716-06) and the Claude D. Pepper Older Americans Independence Center.
Abbreviations and Acronyms:
- ADT
androgen deprivation therapy
- BMD
bone mineral density
- BMI
body mass index
- CMS
Centers for Medicare and Medicaid Services
- DXA
dual-energy x-ray absorptiometry
- FRAX
Fracture Risk Assessment Tool
- HR
hazard ratio
- VA and VHA
Veterans Affairs
Footnotes
Potential Competing Interests: Dr Colón-Emeric is a consultant to Novartis and Amgen and receives research support from Amgen. Dr Lyles is a consultant to Novartis, Amgen, and UCB and receives research support from Amgen, Novartis, and Kirin Pharmaceuticals. Dr Lyles is a coinventor of US patent applications 20050272707 “Methods for preventing or reducing secondary fractures after hip fracture” and 12532285 “Medication kits and formulations for preventing, treating or reducing secondary fractures after previous fracture.” Drs Colón-Emeric and Lyles are coinventors of US patent applications 20104717 “Bisphosphonate compositions and methods for treating heart failure” and 61560328 “Bisphosphonate compositions and methods for treating and/or reducing cardiac dysfunction.” They are equity owners of BisCardia, Inc. The rest of the authors report no competing interests.
Contributor Information
Cathleen S. Colón-Emeric, Center for the Study of Aging and Human Development, Duke University School of Medicine, Durham, NC; Durham VA Geriatric Research, Education and Clinical Center, Durham, NC.
Carl F. Pieper, Center for the Study of Aging and Human Development, Duke University School of Medicine, Durham, NC.
Courtney H. Van Houtven, Center for the Study of Aging and Human Development, Duke University School of Medicine, Durham, NC; Durham VA Health Services Research and Development Center of Innovation, Durham, NC.
Janet M. Grubber, Durham VA Health Services Research and Development Center of Innovation, Durham, NC.
Kenneth W. Lyles, Center for the Study of Aging and Human Development, Duke University School of Medicine, Durham, NC; Durham VA Geriatric Research, Education and Clinical Center, Durham, NC.
Joanne Lafleur, University of Utah, Salt Lake City, UT.
Robert A. Adler, Hunter Holmes McGuire VA Medical Center, Richmond, VA; Department of Medicine, Virginia Commonwealth University, Richmond, VA.
REFERENCES
- 1.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):l76l–l767. [DOI] [PubMed] [Google Scholar]
- 2.Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010;152(6):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohldin A, Floyd J. Unrecognized risks among Veterans with hip fractures: opportunities for improvements. J South Orthop Assoc 2003;12(1):18–22. [PubMed] [Google Scholar]
- 4.Khosla S, Shane E. A crisis in the treatment of osteoporosis. J Bone Miner Res 2016;31(8):1485–1487. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Forciea M, McLean RM, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians [published correction appears in Ann Intern Med. 2017 (Correction: Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women)]. Ann Intern Med 2017; 166(11):8l8–839. [DOI] [PubMed] [Google Scholar]
- 6.Shekelle P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-Based Synthesis Program. Washington, DC: U.S. Department of Veterans Affairs; 2007. [PubMed] [Google Scholar]
- 7.Cosman F, de Beur SJ, LeBoff MS, et al. ; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis [published correction appears in Osteoporos Int. 2015 (Clinician’s guide to prevention and treatment of osteoporosis)]. Osteoporos Int 2014;25(10):2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watts NB, Adler RA, Bilezikian JP, et al. ; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–1822. [DOI] [PubMed] [Google Scholar]
- 9.Papaioannou A, Morin S, Cheung AM, et al. ; Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines forthe diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182(17):1864–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qaseem A, Snow V, Shekelle P, Hopkins R Jr, Forciea MA, Owens DK; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians [published correction appears in Ann Intern Med. 2008;148(11):888]. Ann Intern Med 2008;148(9):680–684. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A; National Osteoporosis Guideline Group. Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK [published correction appears in Osteoporos Int. 2009;20(3):499-502]. Osteoporos Int 2008;19(10):1395–1408. [DOI] [PubMed] [Google Scholar]
- 12.Adler R, Semla T, Cunningham F, Pogach L. The VHA male osteoporosis program: a national model for bone health. Fed Practitioner 2012;29:31–37. [Google Scholar]
- 13.Nelson HD, Haney EM, Dana T, Bougatsos C, Chou R. Screening for osteoporosis: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2010;153(2):99–111. [DOI] [PubMed] [Google Scholar]
- 14.Jain S, Bilori B, Gupta A, Spanos P, Singh M. Are men at high risk for osteoporosis underscreened? A quality improvement project. Perm J. 2016;20(1):60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan RO, Petersen LA, Hasche JC, et al. VHA pharmacy use in veterans with Medicare drug coverage. Am J Manag Care. 2009;15(3):e1–e8. [PubMed] [Google Scholar]
- 16.LaFleur J, Ginter T, Haroldsen C, DuVall SL, Adler R, Nebeker J. Natural language processing (NLP) technology for extracting bone mineral density (BMD) results from radiology reports in the Veterans Affairs (VA) healthcare system Paper presented at: Annual Meeting of the American Society for Bone and Mineral Research; September 2011; San Diego, CA. [Google Scholar]
- 17.Kanis JA, Johansson H, Oden A, Dawson-Hughes B, Melton LJ III, McCloskey EV. The effects of a FRAX revision for the USA. Osteoporos Int 2010;21(1):35–40. [DOI] [PubMed] [Google Scholar]
- 18.Gadam RK, Schlauch K, Izuora KE. FRAX prediction without BMD for assessment of osteoporotic fracture risk. Endocr Pract 2013;19(5):780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann. Pharmac-other. 2006;40(7-8): 1280–1288. [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 21.Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res 2003;38(4):1103–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Pieper CF, Ahmed A, Colón-Emeric CS. Use and interpretation of propensity scores in aging research: a guide for clinical researchers. J Am Geriatr Soc 2016;64(10):2065–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94(446): 496–509. [Google Scholar]
- 24.van de Glind EM, Willems HC, Eslami S, et al. Estimating the time to benefit for preventive drugs with the statistical process control method: an example with alendronate. Drugs Aging. 2016;33(5):347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kern LM, Powe NR, Levine MA, et al. Association between screening for osteoporosis and the incidence of hip fracture. Ann Intern Med 2005;142(3):173–181. [DOI] [PubMed] [Google Scholar]
- 26.Cummings SR. Bone density screening: a new level of evidence? Ann Intern Med 2005;142(3):217–219. [DOI] [PubMed] [Google Scholar]
- 27.Nayak S, Greenspan SL. Cost-effectiveness of osteoporosis screening strategies for men. J Bone Miner Res 2016;31(6): 1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schousboe JT, Taylor BC, Fink HA, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA. 2007;298(6):629–637. [DOI] [PubMed] [Google Scholar]
- 29.Cummings SR, Cawthon PM, Ensrud KE, Cauley JA, Fink HA, Orwoll ES; Osteoporotic Fractures in Men (MrOS) Research Groups; Study of Osteoporotic Fractures Research Groups. BMD and risk of hip and nonvertebral fractures in older men: a prospective study and comparison with older women. J Bone Miner Res 2006;21(10):1550–1556. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Preventive Services Task Force. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med 2011;154(5):356–364. [DOI] [PubMed] [Google Scholar]
- 31.Shepstone L, Lenaghan E, Cooper C, et al. ; SCOOP Study Team. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet. 2018; 391(10122):741–747. [DOI] [PubMed] [Google Scholar]
- 32.Richy F, Ethgen O, Bruyere O, Mawet A, Reginster JY. Primary prevention of osteoporosis: mass screening scenario or prescreening with questionnaires? An economic perspective. J Bone Miner Res 2004;19(12):1955–1960. [DOI] [PubMed] [Google Scholar]
- 33.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137(2):132–141. [DOI] [PubMed] [Google Scholar]
- 34.Cauley JA, Cawthon PM, Peters KE, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group. Risk factors for hip fracture in older men: the Osteoporotic Fractures in Men Study (MrOS). J Bone Miner Res 2016;31(10):1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med 2005;165(20):2414–2419. [DOI] [PubMed] [Google Scholar]
- 36.Landfeldt E, Ström O, Robbins S, Borgstrom F. Adherence to treatment of primary osteoporosis and its association to fractures—the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2012;23(2):433–443. [DOI] [PubMed] [Google Scholar]
- 37.Ström O, Landfeldt E, Garellick G. Residual effect after oral bisphosphonate treatment and healthy adherer effects—the Swedish Adherence Register Analysis (SARA). Osteoporos Int 2015;26(1):315–325. [DOI] [PubMed] [Google Scholar]
- 38.LaFleur J, Nelson RE, Yao Y, Adler RA, Nebeker JR. Validated risk rule using computerized data to identify males at high risk for fracture. Osteoporos Int 2012;23(3): 1017–1027. [DOI] [PubMed] [Google Scholar]
- 39.Gleeson T, Iversen MD, Avorn J, et al. Interventions to improve adherence and persistence with osteoporosis medications: a systematic literature review. Osteoporos Int 2009;20(12):2127–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee RH, Pearson M, Lyles KW, Jenkins PW, Colon-Emeric C. Geographic scope and accessibility of a centralized, electronic consult program for patients with recent fracture. Rural Remote Health. 2016;16(1):3440. [PMC free article] [PubMed] [Google Scholar]
- 41.Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 2013;24(2):393–406. [DOI] [PubMed] [Google Scholar]
- 42.Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S. The validity of administrative data to identify hip fractures is high—a systematic review. J Clin Epidemiol 2013; 66(3):278–285. [DOI] [PubMed] [Google Scholar]
- 43.Quan H, Li B, Duncan Saunders LD, et al. ; IMECCHI Investigators. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008;43(4):1424–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon DH, Morris C, Cheng H, et al. Medication use patterns for osteoporosis: an assessment of guidelines, treatment rates, and quality improvement interventions. Mayo Clin Proc 2005;80(2):194–202. [DOI] [PubMed] [Google Scholar]
- 45.Krall E, Miller D, Watkins B, Rourke A. Prevalence of osteoporosis and osteopenia in male veterans Paper presented at: 128th Annual Meeting of APHA; November l2-l6, 2000; Boston, MA. [Google Scholar]