Abstract
Background:
In most research studies and clinical trials, Alzheimer’s disease (AD) has been diagnosed using the criteria developed by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) work group in 1984. Developments over the last 27 years have lead to the need for new diagnostic criteria.
Review Summary:
Four articles in the journal Alzheimer’s & Dementia in 2011 describe new criteria for AD dementia and mild cognitive impairment (MCI) due to the AD pathophysiological process (MCI due to AD) and the underlying rationale for them. These new criteria emphasize that the AD pathophysiological process starts years and perhaps decades prior to clinical symptoms, and that biomarkers can be used to detect amyloid beta (Aβ) deposition and the effects of neurodegeneration in the brain.
Conclusion:
These new criteria are immediately helpful to the practicing clinician, providing more accurate and specific guidelines for the diagnosis of AD dementia and MCI due to AD. As new diagnostic tools and new treatments for AD become available, diagnosis using these criteria will enable patients with this disorder to receive the best possible care.
Keywords: Alzheimer’s disease, AD, Mild Cognitive Impairment, MCI, Criteria, Preclinical
Rationale for new criteria
Updating the prior criteria developed by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) work group in 1984, new clinical criteria have recently been published for Alzheimer’s disease (AD) and mild cognitive impairment (MCI) due to the AD pathophysiological process (MCI due to AD). Four articles in the journal Alzheimer’s & Dementia in 2011 describe these criteria and the underlying rationale for them. The first is an introduction that provides the background of why new criteria are needed.1 The second details the hypothesis that the AD pathophysiological process starts years and perhaps decades prior to the onset of clinical symptoms and dementia. It also discusses up the concept of “preclinical” AD.2 The third considers both clinical and research criteria for MCI due to AD.3 Finally, the fourth describes new clinical and research criteria for AD.4 We will focus on new clinical criteria, the area that will be of greatest use to practicing clinicians, and briefly touch on the research criteria and theoretical issues.
As discussed in these articles, the new criteria were developed due to the recognition of a number of important factors that have changed since 1984:
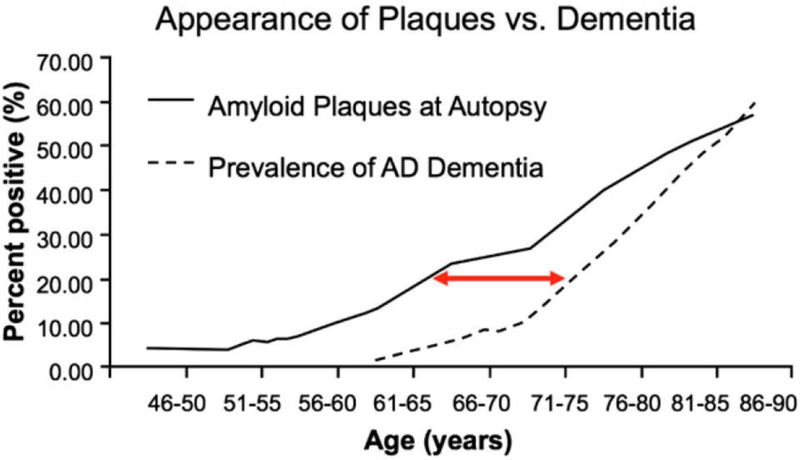
(1) It is now believed that the AD pathophysiological process starts years prior to detectable cognitive changes and perhaps decades prior the onset of clinical dementia (Figure 1). The concept of the “AD pathophysiological process” is thus separated from “AD dementia.”
(2) There are many patients whose cognition is not normal for age but also do not meet criteria for dementia.
(3) Other causes of dementia such as frontotemporal dementia, vascular dementia, and dementia with Lewy bodies are now understood to be more likely mistaken for AD than are thyroid disorders and vitamin B12 deficiency.
(4) The genetics of AD are now better understood.
(5) Putative biomarkers of AD are now available in some centers.
(6) New criteria are needed for clinical trials and other research.
(7) New treatments are being developed that are specific for the AD pathophysiological process, making it more important that patients truly have that process prior to the initiation of this type of specific treatment.
Figure 1.
Postulated temporal lag between the deposition of Aβ in amyloid plaques from an autopsy series and the development of clinical AD dementia based upon three epidemiological studies (From Sperling et al.2).
The overall purpose of these changes to the criteria are to help the clinician identify and treat the disease as early as possible—ultimately before the onset of decline with the goal of enabling individuals to live long and productive lives free of the dementia caused by AD. Recent data from an Alzheimer’s Association report suggest that a delay in the onset of symptoms of AD dementia by 5 years would result in a 45% reduction in the number of people with the disorder by the year 2050 and reduce projected Medicare costs of disease from 627 to 344 billion dollars.5
In general, the new criteria differ from the original criteria along two important dimensions that have both immediate and future implications for the practicing clinician. One is the recognition of three stages of Alzheimer’s disease progression, with the first (Preclinical AD) occurring before changes in cognition and everyday activities are detected, the second (MCI due to AD) occurring when cognitive symptoms emerge but function is still relatively unimpaired, and the third (AD dementia) diagnosed when day-to-day function is impaired. By contrast, the original guidelines only diagnosed AD dementia. Another is the incorporation of biomarkers into the diagnosis, whereas the original criteria were based primarily on clinical judgment.
The three stages of AD discussed are:
Preclinical Alzheimer’s disease. Preceding any clinical changes, this stage is defined by measureable changes in biomarkers and poor performance on challenging cognitive tests. The biomarkers may be detectable by brain imaging or by molecular changes in CSF. There are currently no clinical criteria to make the diagnosis of preclinical AD. The primary purpose of the designation of preclinical Alzheimer’s disease is to provide a framework for researchers to better define this stage of AD.
Mild Cognitive Impairment due to Alzheimer’s disease. This stage is characterized by the first clinical changes. Mild changes in memory and other cognitive abilities that are noticeable to patients and families and can be detected through careful evaluation, but are not sufficient to interfere with day-to-day activities.
Dementia due to Alzheimer’s disease. This stage is characterized by changes in two or more aspects of cognition and behavior that interfere with the ability to function in everyday life.
Preclinical Alzheimer’s disease & biomarkers.
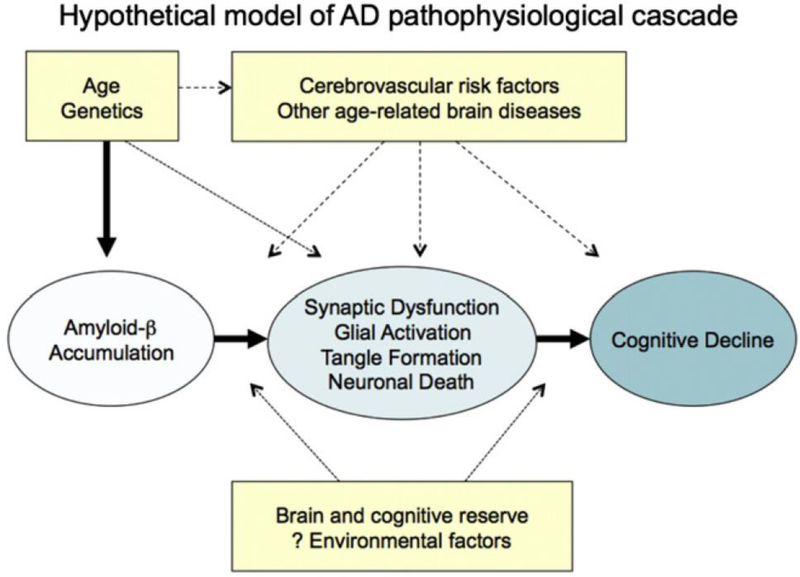
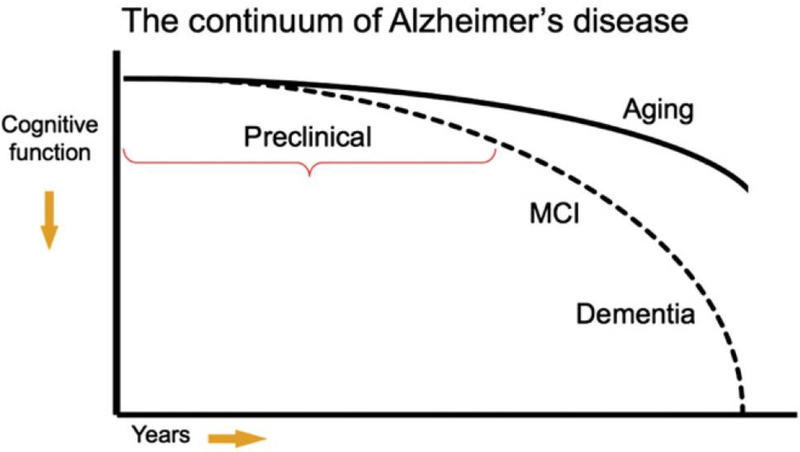
As described by Sperling et al.,2 one commonly used model of AD is that many factors including age, genetics, the environment, and others combine to lead to the accumulation of amyloid beta (Aβ) in the brain, which in turn produces synaptic dysfunction, neurofibrillary tangle formation, and neuronal death, all of which ultimately lead to cognitive decline (Figure 2). Given that the events in this pathological cascade sequence likely take years or decades from the accumulation of Aβ to the development of dementia or even MCI, there must be a prior “preclinical” stage of AD (Figure 3).
Figure 2.
Hypothetical model of AD pathophysiological cascade sequence. (From Sperling et al.2)
Figure 3.
Model of the clinical course of AD. (From Sperling et al.2)
The concept of a preclinical phase of a disease should not be new to practicing clinicians, nor should the concept of diagnosing a disease in the absence of symptoms. Cancer is routinely detected at the stage of “carcinoma in situ” and atherosclerosis may cause detectable narrowing of the arteries prior to myocardial infarct. Similarly, many diseases including Type II diabetes, osteoporosis, and hypertension are routinely detected and treated before symptoms emerge. The goal is to move AD to a similar status.
These guidelines are primarily aimed at helping researchers determine which signature biological changes (i.e. biomarkers) occur years before the onset of clinical symptoms. Currently AD biomarkers have minimal impact on clinical practice, although it is likely that these biomarkers will be important to the clinician in the near future.
Table 1 presents several biomarkers of Aβ deposition or neurodegeneration that are currently in use. Markers of Aβ deposition include low CSF Aβ42 and positive PET amyloid imaging. Markers of neurodegneration include elevated CSF tau (both total and hyperphosphorylated tau (p-tau)), decreased metabolism in temporal and parietal cortex on 18flurodeoxyglucose (FDG) PET, and atrophy on MRI in temporal (medial, basal, and lateral) and medial parietal cortex. Although it makes the most sense scientifically to divide the biomarkers into those measuring either Aβ deposition or neurodegeneration, in clinical practice one tends to divide the biomarkers by how they are obtained: structural MRI, PET, and CSF studies.
Table 1:
Putative biomarkers for the AD pathophysiologic process currently being used
| (1) Markers of amyloid-beta (Aβ) protein deposition in the brain |
| a. low CSF Aβ42 |
| b. positive PET amyloid imaging |
| (2) Markers of downstream neurodegeneration |
| a. elevated cerebrospinal fluid tau (total and phosphorylated) |
| b. decreased metabolism in temporal and parietal cortex on 18flurodeoxyglucose (FDG) positron emission tomography |
| c. atrophy on MRI in temporal (medial, basal, and lateral) and medial parietal cortex |
Structural MRI, when analyzed using a quantitative protocol, can provide whole brain volume and volume in anatomically distinct areas of the brain. Patients in whom the AD pathophysiologic process has caused neurodegeneration should have less brain tissue in temporal (medial, basal, and lateral) and medial parietal cortex—and they should lose tissue faster—than healthy individuals.6 Volumetric MRI is currently being used in a number of ongoing clinical trials to evaluate the efficacy of disease-modifying drugs in AD. Additionally, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) also includes serial volumetric MRIs in AD and MCI patients and healthy controls.7 The ADNI data should ultimately provide a normative database that would be an important step in allowing volumetric MRI to be used clinically. Volumetric MRI analyses are not routinely available, although we encourage all clinicians to look for qualitative patterns of atrophy in temporal (medial, basal, and lateral) and medial parietal cortex, and progressive atrophy when serial scans are available.
There are two types of PET biomarkers: those that show amyloid deposition, and those that show neurodegeneration. Brain neurodegeneration can be identified by measuring glucose metabolism in the brain with FDG PET. These scans show which areas of the brain are and are not functioning well. Decreased metabolism is observed in temporal and parietal cortex when the AD pathophysiologic process has caused neurodegeneration.8 FDG PET scans are available to the clinician now, and are covered by Medicare. We do not, however, recommend using these scans routinely when the history, physical examination, cognitive testing, and structural imaging are all consistent with AD—it simply is not necessary.8 In situations, however, in which one suspects an atypical neurodegenerative disease, an FDG PET scan can help distinguish AD from another disorder (such as dementia with Lewy bodies or frontotemporal dementia). Another reason to obtain an FDG PET scan is if one is suspecting AD in a patient younger than 66 years of age. At such a young age the prevalence of AD is similar to that of many other etiologies (such as frontotemporal dementia),9 making it more critical to be sure that the diagnosis is AD before treatment is initiated.
Several compounds that can identify β-amyloid deposition using PET are currently being used for research and/or are under commercial development. The two most widely used are the Pittsburgh compound B (PiB) and florbetapir. Both have been shown to bind to brain β-amyloid.10,11 Because amyloid accumulation is thought to be present years before the onset of clinical symptoms, PET identification of β-amyloid raises the possibility of identifying and treating patients with AD years before symptoms emerge—once a drug has been proven to be disease modifying. Amyloid PET imaging is currently being used in several clinical trials both to help identify individuals with preclinical AD and to measure the effects of anti-amyloid treatments. Moreover, florbetapir has recently been submitted to the FDA for approval in clinical practice. Detection of β-amyloid by PET could be available to clinicians soon.
Standard CSF biomarkers for AD are Aβ42, total tau, and p-tau. Studies have shown that when all three markers are combined, the accuracy of the diagnosis is highest, with sensitivity and specificity of 85–90%.12 Although CSF analysis is already commercially available (www.athenadiagnostics.com) and used by some clinicians to aid in diagnosis, we view this test as highly promising but not yet ready for routine clinical practice.8
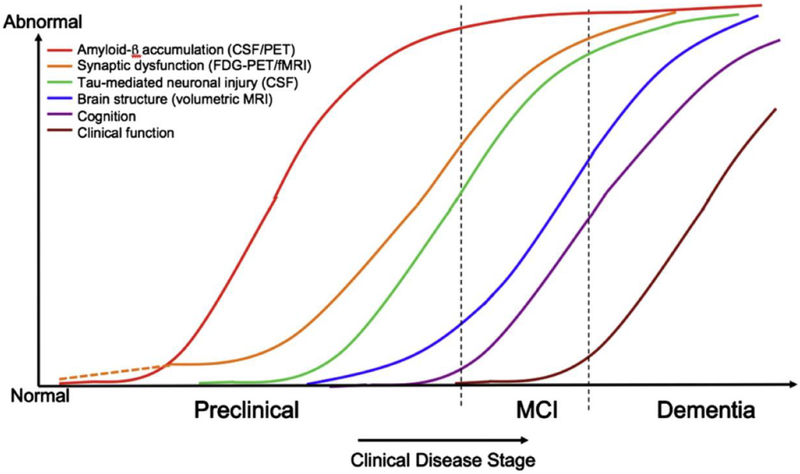
Based upon the current scientific model regarding when different events occur in the AD pathophysiological process (Figure 4), Sperling et al.2 discussed three stages of preclinical AD to be considered for research, based upon biomarkers and cognitive change. Stage 1 is asymptomatic cerebral amyloidosis, which could be determined by the presence of a biomarker sensitive to Aβ, in the absence of any marker of neurodegeneration or evidence of subtle cognitive change. Stage 2 is asymptomatic cerebral amyloidosis plus neurodegeneration in the absence of subtle cognitive change. Finally, stage 3 is amyloidosis plus neurodegeneration plus subtle cognitive or behavioral decline. Guided by these theoretical underpinnings, the new clinical and research criteria are next presented for MCI due to AD and AD dementia.
Figure 4.
Model of how the different stages of AD may be detected by changes in various biological, cognitive, and clinical markers. (From Sperling et al.2)
Criteria for Mild Cognitive Impairment due to the Alzheimer’s disease pathophysiological process
Based upon guidelines currently in use at memory centers and in research studies, Albert et al.3 present the criteria for MCI due to AD. These criteria, aimed both at clinicians and researchers, refer to the symptomatic phase of the AD pathophysiological process but before the individual develops the functional impairment that defines dementia. Clinicians are increasingly making a diagnosis of MCI and the new diagnostic guidelines will formalize this process. The guidelines recognize that everyone who eventually develops AD will go through a transitional period of mild but detectable cognitive impairment. Importantly, however, not everyone who is diagnosed with MCI will go on to develop AD. MCI can be due to a variety of disorders including, vascular dementia, frontotemporal dementia, dementia with Lewy bodies, and others. Both core clinical criteria for the diagnosis of MCI due to AD and clinical research criteria are presented. As the authors start their discussion on the core clinical criteria they point out that, in addition to these criteria, clinical judgment must be used to distinguish between normal cognition and MCI, and between MCI and dementia.
The new guidelines specify the criteria for making a diagnosis of MCI due to AD. The criteria include:
Excluding patients who have other causes of MCI including extensive vascular disease, frontotemporal dementia, and dementia with Lewy bodies. These exclusions will likely be accomplished through a combination of the clinical evaluation and imaging studies.
Including patients who show increasing cognitive decline over time. This decline could be detected historically or via serial evaluations over time.
Including patients who have the very rare mutations associated with early onset familial AD (amyloid precursor protein (APP), presenilin 1 (PSEN1) or presenilin 1 (PSEN2)). These mutations can be identified via commercially available genotyping (www.athenadiagnostics.com).
Because patients with MCI due to AD may actually be considered very early AD patients, it will be important for practicing clinicians to identify this condition. There may be at least as many patients with MCI due to AD as there are with dementia due to AD.13,14 Although there is not substantial evidence that current treatments with FDA approved medications improve patients with MCI due to AD (there is ongoing research aimed at answering this question), patients with this condition may benefit from referrals to centers conducting studies with potential disease modifying treatments such as vaccines that are aimed at slowing the loss of neurons and thus the progression of the disease. It is becoming increasingly clear that disease-modifying drugs will be most beneficial in the earliest stages of the disease. There are now several ongoing studies with hypothesized disease modifying agents (e.g., vaccines) for patients with MCI due to AD, and other studies are being planned.
The clinical criteria are divided into two parts (Table 2). First, criteria are presented for the clinical and cognitive syndrome of MCI, and second, criteria are presented regarding the etiology of the MCI syndrome being consistent with AD.
Table 2:
Clinical and Cognitive Evaluation for MCI due to AD
| Step 1 : Establish clinical and cognitive criteria: Determine that the clinical and cognitive syndrome is consistent with MCI and the patient is not demented | |
|---|---|
| Guideline | Procedures |
| Concern regarding a change in cognition | History & Observation – |
| • Concern of a change in cognition from prior level | |
| • Reported by patient and/or informant, or observed by clinician | |
| Objective evidence of impairment in one of more areas of cognition (e.g, memory, attention, language, visuospatial skills, executive function) | Neurocognitive Testing |
| • Impairment in episodic memory (learning and retention of new information such as word lists), the most common symptom. | |
| • Deficit in episodic memory is the best predictor of progression to Alzheimer’s dementia | |
| • Other cognitive areas should also be evaluated. | |
| • Sample battery: Rey Auditory Verbal Learning Test (memory), the Trail Making Test Parts A & B (executive function), the Boston Naming Test, letter and category fluency (language), figure copying (spatial skills), and digit span forward (attention). (See Budson & Solomon,8 for discussion of these & other tests.) | |
| • Patients with MCI typically score 1 to 1.5 standard deviations below the mean on cognitive tests. | |
| • Note that cognitive assessments are influenced by age, education, motivation, and cultural variation. Not all tests provide normative data taking these factors into account. | |
| • Evaluation by a neuropsychologist is appropriate & helpful in these patients with mild deficits. Brief or informal office testing may not be sensitive enough to detect deficits. | |
| Preservation of independence in functional abilities | History, Questionnaires |
| • MCI patients maintain independence of function in daily life although they may experience more difficulty or take longer in carrying out complex tasks (e.g., balancing the checkbook, household projects, meal planning & preparation). | |
| • Interviews with friends or family will usually detect these changes | |
| • Standardized and validated scales completed by family or friends can be helpful (see Budson & Solomon,8 for a discussion specific scales) | |
| Not demented | History, Observation, Questionnaires |
| • There is no significant impairment in occupational or social function | |
| Step 2: Examine etiology of MCI consistent with AD pathophysiological process: Determine the likely primary cause of signs & symptoms. | |
|---|---|
| Guideline | Procedures |
| Rule out other possible causes of cognitive decline. | History, neurocognitive testing, imaging, & laboratory studies |
| Possibilities include: vascular, Lewy body, other degenerative disease, traumatic, depression, medical comorbidities, mixed dementia, other (see Budson & Solomon,8 for complete list & description of the various disorders). | • History & testing may be consistent with various clinical phenotypes |
| • CT & MRI may show vascular infarcts & patterns of atrophy | |
| • Laboratory studies (e.g., B12, TSH, Lyme titer) may find other causes of cognitive deficits | |
| Provide evidence of longitudinal decline in cognition | History, serial neuropsychological testing |
| • Documentation of progressive cognitive decline increases the probability of MCI due to AD | |
| • Decline can be determined by history and / or neuropsychological testing | |
| Report history consistent with AD genetic factors | Genotyping |
| • Although genotyping is not part of the routine workup for MCI or AD, if an autosomal dominant form of the gene is known to be present (i.e. mutation in APP, PS1, PS2), then the development of MCI is highly likely to be the prodrome of AD | |
| • The vast majority of these cases develop early onset AD in the patient’s 40s or 50s. | |
| • The presence of one or two ε4 alleles in the apolipoprotein protein E increases the risk for late onset AD. | |
| Evaluate for atrophy of temporal (medial, basal, and lateral) and medial parietal cortex and other biomarkers when available and clinically useful. | Biomarkers |
| • Although the use of biomarkers is not recommended routinely, they are available to the clinician when desired. | |
| • There are two categories of biomarkers, those associated with Aβ protein deposition and those associated with downstream neurodegeneration (see Table 1) | |
| • We recommend routine review of CT & MRI patterns of atrophy, a marker of downstream neurodegeneration. | |
| • Presence of one biomarker category makes the “biomarker probability of AD etiology” “intermediate;” both categories must be positive for the “highest” probability. The “lowest” probability is present if both categories are negative. | |
Research criteria for MCI due to AD incorporating biomarkers are next presented. The biomarkers discussed include those in Table 1, above. Two valuable reasons to add biomarkers is that they may help determine (1) the underlying etiology of the MCI and (2) the likelihood and time course of an individual with MCI becoming more impaired or demented. An important point discussed is that biomarkers that detect Aβ protein deposition are most useful in determining underlying etiology, whereas biomarkers that detect neuronal degeneration and injury are most useful in determining prognosis and progression. Adding each of these categories of biomarkers to the core clinical diagnosis of MCI due to AD leads to the following probabilities: If one of these two biomarker categories is positive, the “biomarker probability of AD etiology” rises to “intermediate,” and both categories must be positive for the “highest” probability. The “lowest” probability is present if both categories are negative.
Criteria for all-cause dementia & Alzheimer’s disease
The new criteria for AD clarify existing guidelines and are appropriate for use today by practicing physicians. The new guidelines propose four possible classifications of dementia caused by AD: (1) probable AD dementia, (2) probable AD dementia with increased level of certainty, (3) possible AD dementia, and (4) probable or possible AD dementia with evidence of AD pathophysiological process. The last criteria propose a system for providing additional diagnostic evidence through the use of biomarkers, and are currently intended for research purposes only, although it is likely that it will be increasingly used in future clinical practice. The hope is that the use of these new clinical criteria coupled with the judicious addition of biomarkers will eventually improve diagnostic accuracy and help make accurate diagnoses earlier in the disease process when intervention can be most beneficial. The new guidelines do not suggest any additional diagnostic procedures for patients already diagnosed with AD.
The new criteria suggest a four-step approach to diagnosis of dementia due to AD (Table 3). Step 1 determines that dementia is present, Step 2 determines that the dementia is due to AD, Step 3 provides an increased level of certainty to the diagnosis, and Step 4 evaluates the biomarker probability of AD etiology.
Table 3.
Clinical and Cognitive Evaluation for all cause dementia and AD
| Step 1 – Criteria for “All Cause Dementia” | |
|---|---|
| Guideline | Procedures |
| • Interferes with the ability to function at work or with usual abilities and | History & observation – |
| • Represents a decline from previous ability and | • Evidence of changes in functioning reported by either patient and / or informant, or observed by clinician |
| • Cannot be explained by delirium or major psychiatric disorder | |
| Presence of cognitive impairment | History, observation, neuropsychological testings |
| • History-taking from a knowledgeable informant | |
| • Objective mental status testing and / or neuropsychological testing | |
| • Neuropsychological testing is recommended when history and mental status testing cannot provide a confident diagnosis | |
| The cognitive or behavioral impairment involves a minimum of two domains | History, observation, neuropsychological testing |
| • Impaired ability to acquire / remember new information (e.g., repeating questions, forgetting events or appointments, becoming lost in familiar places). | |
| • Impaired reasoning and handling of complex tasks, poor judgment (e.g., inability to handle finances, poor decision making) | |
| • Impaired visuospatial abilities (e.g., difficulty recognizing faces or common objects) | |
| • Impaired language function (speaking, reading, writing; e.g., difficulty thinking of common words while speaking, hesitations in speech) | |
| • Changes in personality, behavior, comportment (e.g.,agitation, apathy, social withdrawal) | |
| Difference between MCI and dementia | History & observation, |
| • The fundamental difference between a diagnosis of dementia versus MCI depends upon whether or not there is a significant change in the ability to function at work or in daily activities. This will necessarily require clinical judgment based upon the information provided by the patient and a knowledgeable informant. | |
| Step 2: – Criteria for “Probable AD Dementia” | |
|---|---|
| Guideline | Procedures |
| Meets criteria for dementia | See criteria above for dementia, Step 1 |
| Insidious onset – symptoms have a gradual onset over months or years, not sudden over hours or days. | History |
| • from patient and knowledgeable informant | |
| Clear cut history of worsening of cognition | History, serial neuropsychological testing |
| • from patient and knowledgeable informant | |
| Initial cognitive deficits are evident and most prominent in one of the following categories | History, neuropsychological testing |
| • Amnestic presentation – the most common presentation | Amnestic Presentation |
| • Non-amnestic presentations: | • Impairment of learning and recall of recently learned information |
| (1) Language presentation, | • Deficit in at least one other cognitive area) |
| (2) Visuospatial presentation, | Nonamnestic presentations |
| (3) executive dysfunction | • Language – most prominent deficits are word finding, but should also be deficits in other cognitive areas |
| • Visuospatial -- most prominent deficits are spatial cognition, but should also be deficits in other cognitive areas | |
| • Executive -- most prominent deficits are reasoning, judgment and problem solving, but should also be deficits in other cognitive areas | |
| Diagnosis of AD should not be made when there is evidence of another dementing illness | History, neuropsychological testing, imaging studies, laboratory studies |
| • Disorders to rule out include: | |
| • Vascular Cognitive Impairment / Vascular Dementia | |
| • Dementia with Lewy bodies | |
| • Frontal-Temporal dementia – Behavioral variant | |
| • Primary Progressive Aphasia | |
| • Evidence of neurological disease or non-neurological condition or medication that could have a substantial effect on cognition | |
| Step 3: – Criteria for “Probable AD Dementia with increased level of certainty” | |
|---|---|
| Guideline | Procedures |
| Meets criteria for AD dementia | See criteria above for AD dementia, Step 2 |
| Probable AD dementia with documented decline | History, serial neuropsychological testing |
| evidence of progressive cognitive decline on subsequent evaluations from | |
| • knowledgeable informant or | |
| • cognitive testing (either formal neuropsychological evaluation or standardized mental status examinations) | |
| Probable AD dementia in a carrier of a causative AD genetic mutation | Laboratory studies |
| Presence of an early-onset familial genetic mutation | |
| • APP, PSEN1, or PSEN2 | |
| (Note that the apolipoprotein E ε4 allele was not considered specific enough to meet criteria.) | |
| Step 4: – Evaluate the “Biomarker probability of AD etiology” | |
|---|---|
| Evaluate for atrophy of temporal (medial, basal, and lateral) and medial parietal cortex and other biomarkers when available and clinically useful. | Biomarkers |
| • Although the use of biomarkers is not recommended routinely, they are available to the clinician when desired. | |
| • There are two categories of biomarkers, those associated with Aβ protein deposition and those associated with downstream neurodegeneration (see Table 1) | |
| • We recommend routine review of CT & MRI patterns of atrophy, a marker of downstream neurodegeneration. | |
| • Presence of one biomarker category makes the “biomarker probability of AD etiology” “intermediate;” both categories must be positive for a “high” probability. The “lowest” probability is present if both categories are negative. | |
Note: patients who would have met criteria under the 1984 guidelines would also meet criteria under the current guidelines.
The next idea that is introduced is that of research criteria for “probable AD dementia with evidence of the AD pathophysiological process.” These research criteria increase the certainty that the patient’s clinical dementia is due the AD pathophysiological process by adding biomarker evidence to the probable AD dementia criteria. The biomarkers fall into two categories, markers of amyloid-beta (Aβ) protein deposition in the brain and markers of downstream neurodegeneration, (see Table 1, above).
If one of these two biomarker categories is positive, the “biomarker probability of AD etiology” rises to “intermediate,” and if both categories are positive the probability becomes “high.” The authors are specific that they do not advocate obtaining AD biomarkers for routine clinical purposes at the present time, although they do note that they may be used when they are available and deemed appropriate by the clinician.
The clinical criteria for possible AD dementia can be found in Table 4. The term “possible” is used instead of “probable” in two scenarios. One is if the cognitive deficits look like AD but there is an atypical course of the disease: either sudden onset or no definite decline. The second is if there is evidence for a mixed etiology of the dementia, such that the patient meets criteria for probable AD dementia but there is also evidence of significant vascular disease, features of dementia with Lewy bodies, or other disease or condition that could be contributing to the patient’s dementia.
Table 4:
Clinical and Cognitive Evaluation for Possible AD
| Criteria for “Possible AD Dementia” | |
|---|---|
| Guideline | Procedures |
| Atypical course | History, neuropsychological testing, imaging studies, laboratory studies |
| meets the core clinical criteria in terms of the nature of the cognitive deficits for AD dementia, but either | |
| • has a sudden onset of cognitive impairment or | |
| • demonstrates insufficient historical detail or objective cognitive documentation of progressive decline | |
| Etiologically mixed presentation | History, neuropsychological testing, imaging studies, laboratory studies |
| meets all core clinical criteria for AD dementia but has evidence of | |
| • (a) concomitant cerebrovascular disease, defined by a history of stroke temporally related to the onset or worsening of cognitive impairment; or the presence of multiple or extensive infarcts or severe white matter hyperintensity burden; or | |
| • (b) features of Dementia with Lewy bodies other than the dementia itself; or | |
| • (c) evidence for another neurological disease or a non-neurological medical comorbidity or medication use that could have a substantial effect on cognition | |
Pathophysiologically proven AD is next discussed, consisting simply of the unchanged criteria of patients meeting both the clinical and neuropathological criteria for AD. Finally the criteria for dementia unlikely to be due to AD is discussed (Table 5).
Table 5:
Criteria for Dementia Unlikely to be due to AD
| (1) Does not meet clinical criteria for AD dementia |
| (2) Regardless of meeting clinical criteria for probable or possible AD dementia |
| a. There is sufficient evidence for an alternative diagnosis such as HIV dementia, dementia of Huntington’s disease, or others that rarely overlap with AD |
| b. Biomarkers for both Aβ and neuronal degeneration are negative. |
| (adapted from McKhann et al.4) |
Implications for the Practicing Clinician
These new guidelines do not suggest a fundamental difference in the way clinicians will diagnose AD dementia or MCI due to AD. For now, diagnosis of these disorders will continue to be primarily clinical. The new guidelines also do not recommend any specific treatment strategies. Implicitly or explicitly, however, they do encourage clinicians to:
Recognize that AD is the end of a long process, spanning years or perhaps decades, leading to death of brain cells, cognitive loss, and ultimately dementia.
Diagnose (and perhaps treat) AD at the earliest possible stage. At present this would be the MCI due to AD stage, but eventually will include preclinical AD.
Begin to consider using biomarkers in the diagnosis of all stages of AD. For now, most biomarkers will only be used in research studies (both for diagnostic purposes and to evaluate the efficacy of disease modifying treatments), but some either are available to clinicians now (e.g., FDG PET scans, CSF Aβ42 and tau) or will be in the near future (e.g., quantitative MRI, amyloid PET scans).
Evaluate patients with dementia to determine the cause of the dementia with special attention paid to amnestic and nonamnestic presentations of AD.
Remember that they will soon see a significant increase in patients in their practices with AD. There are currently 5.4 million patients in the US with AD dementia and perhaps an equal amount with MCI. Because of the aging population, these numbers could triple in the next 50 years.
Conclusion
Today, these new criteria allow the practicing clinician to diagnose AD dementia and MCI due to AD with more clarity, providing greater certainty of the diagnosis for patients and families. In the future, these criteria will provide practitioners with the tools they need to know which patients are appropriate for the disease-modifying medications which are being developed to slow or even stop the AD pathophysiologic process.
Acknowledgements
This work was supported by National Institute on Aging grant P30 AG13846. This material is also the result of work supported with resources and the use of facilities at the VA Boston Healthcare System.
References
- 1.Jack CR Jr, Albert M, Knopman D, et al. Introduction to the revised criteria for the diagnosis of Alzheimer’s disease: National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7:280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert M, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: report of the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement 2011; 7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011; 7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association, “Changing the Trajectory of Alzheimer’s disease: A National Imperative,” 2010; http://www.alz.org/documents_custom/trajectory.pdf, accessed 10/3/11.
- 6.Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Hyman BT, Blacker D, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickerson BC, Wolk DA; Alzheimer’s Disease Neuroimaging Initiative. Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011. 82:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budson & Solomon Memory Loss: A Practical Guide for Clinicians, Philadelphia: Elsevier Inc., 2011. [Google Scholar]
- 9.Brunnstrom H, Gustafson L, Passant U, & Englund E Prevalence of dementia subtypes: a 30-year retrospective survey of neuropathological reports. Arch.Gerontol.Geriatr 2009;49, 146–149. [DOI] [PubMed] [Google Scholar]
- 10.Grimmer T, Henriksen G, Wester HJ, Forstl H, Klunk WE, Mathis CA, Kurz A, & Drzezga A Clinical severity of Alzheimer’s disease is associated with PIB uptake in PET. Neurobiol.Aging. 2009; 30:1902–1909. [DOI] [PubMed] [Google Scholar]
- 11.Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, Joshi AD, Clark CM, Mintun MA, Pontecorvo MJ, Doraiswamy PM, Johnson KA, Skovronsky DM, Reiman EM. Using Positron Emission Tomography and Florbetapir F 18 to Image Cortical Amyloid in Patients With Mild Cognitive Impairment or Dementia Due to Alzheimer Disease. Arch Neurol. 2011. July 11 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008;4:38–48. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry. 2010;18:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]