Abstract
Objective:
At the present, it is unclear whether association of basal forebrain
cholinergic system (BFCS) volume with cognitive performance exists in healthy as well as in cognitively impaired elderly subjects. Whereas one small study reported an association of BFCS volume with general cognitive ability ‘g’ in healthy ageing, effects on specific cognitive domains have only been found in subjects with cognitive decline. Here we aim to clarify whether an association of BFCS volume and ‘g’ is present in a larger sample of elderly subjects without obvious symptoms of dementia and whether similar associations can also be observed in specific cognitive domains.
Methods:
282 pre-surgical patients from the BioCog study (aged 72.7 ± 4.9 years with a range of 65–87 years, 110 women) with a median MMSE score of 29 points (range 24–30) were investigated. BFCS and brain volume as well as brain parenchymal fraction were assessed in T1-weighted MR images using SPM12 and a probabilistic map of the BFCS. Neuropsychological assessment comprised the CANTAB cognitive battery and paper-and-pencil based tests. For data analysis, generalised linear models and quantile regression were applied.
Results:
Significant associations of BFCS volume with ‘g’ and several cognitive domains were found, with the strongest association found for ‘g’. BFCS volume explained less variance in cognitive performance than brain volume. The association was not confounded by brain parenchymal fraction. Furthermore, the association of BFCS volume and ‘g’ was similar in high- and low-performers.
Conclusion:
Our results extend previous study findings on BFCS volume associations with cognition in elderly subjects. Despite the observed associations of BFCS volume and cognitive performance, this association seems to reflect a more general association of brain volume and cognition. Accordingly, a specific association of BFCS volume and cognition in non-demented elderly subjects is questionable.
1. Introduction
The basal forebrain is the main source of acetylcholine (ACh) for hippocampal and neocortical structures. The basal forebrain cholinergic system (BFCS) comprises the medial septum nuclei (Ch1), Broca's diagonal (Ch2) and horizontal nuclei (Ch3) as well as the Nucleus basalis of Meynert (Ch4) and the Nucleus subputaminalis of Ayala (Mesulam et al., 1983; Simić et al., 1999). Its main neurotransmitter acetylcholine is well known to play a role in cognition which has been shown in several neurophysiological experiments and lesion studies of the BFCS (Parikh et al., 2007; Harati et al., 2008; Cai et al., 2012).
In patients with Alzheimer's Dementia (AD), the number of cholinergic neurons in the human brain is diminished (White et al., 1977; Kilimann et al., 2014). Furthermore, MRI studies have shown that cholinergic atrophy parallels cognitive decline as the disease process is progressing (Grothe et al., 2013). Current treatment strategies of Alzheimer's dementia aim at compensation for the cholinergic deficit by administration of ACh-esterase inhibitors (Birks 2006; Winblad et al., 2001). These drugs increase neurotransmitter persistence in the synaptic cleft by pharmacological inhibition of its degradation, ameliorating cognitive symptoms (Anand and Singh, 2013).
Atrophy of cholinergic cells in the basal forebrain is also seen in healthy elderly subjects without clinical evidence of dementia (Grothe et al., 2012, 2013; Teipel et al., 2015). Post mortem studies reported a decrease of histological markers of ACh-synthesis and cholinergic synapses during normal ageing which is accelerated in AD patients (Sparks et al., 1992; Perry et al., 1992). However, it is still controversial whether cholinergic deficits are also associated with age-associated cognitive decline in subjects without evidence of dementia development. Wolf et al. (2014) recently reported an association of BFCS volume with cognitive performance in a small group of healthy elderly subjects without evidence of dementia. On the other hand, ACh-esterase-inhibitors have not been proven effective in elderly patients with memory complaints without dementia (mild cognitive impairment) and cholinergic cell loss seems to occur at advanced stages of AD (Russ and Morling, 2012; Gilmor et al., 1999). Furthermore, histological studies suggested that 30% of cholinergic neurons in the basal forebrain need to be lost until relevant cognitive deficits manifest (Mini-Mental State Examination/MMSE < 24) which is questioning an association of cognition and basal forebrain volume in healthy ageing (Schliebs and Arendt, 2006).
Associations of BFCS subregion volumes and cognitive domains (episodic memory, executive functions) were reported for patients with mild cognitive impairment (MCI). This association was not observed in the healthy control group (Grothe et al., 2010, 2016). Since these studies were designed to investigate differences between healthy elderly and patients with mild cognitive impairment, one needs to take into account that the control group was strictly defined as a highly functional (“hypernormal”) control group without the slightest suspicion for cognitive impairment. Thus, Grothe et al. (2016) used a control group from the Alzheimer's Disease Neuroimaging Initiative (ADNI-2) comprising individuals without any memory complaints, normal memory function according to the Wechsler Memory Scale-R and a Clinical Dementia Rating Scale (CDR) score of 0. In addition, subjects with significant systemic illness or unstable medical condition were not included in the control group. Since multimorbidity has been shown to be associated with age and cognitive impairment, exclusion of participants due to non-neurocognitive medical conditions affects the prevalence of age-associated cognitive decline in the sample (Barnett et al., 2012; Vassilaki et al., 2015).
The aim of the present study is the analysis of BFCS volume associations with cognitive performance in a natural rather than a “hypernormal” cohort of elderly pre-surgical patients (≥ 65 years) without obvious symptoms of clinical dementia (MMSE score median 29 points, range 24–30, Folstein et al., 1975).
2. Methods
2.1. Participants
Participants were recruited as part of the BioCog project (Biomarker Development for Postoperative Cognitive Impairment in the Elderly study, www.biocog.eu), which is a prospective multicentre cohort study with the aim to develop a biomarker-based algorithm for risk prediction of post-operative cognitive disorders. Only patients ≥ 65 years of age presenting for an elective major surgery were recruited (for further inclusion and exclusion criteria see Table 1).
Table 1.
Inclusion and exclusion criteria of the BioCog study.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Age ≥65 years • European descent (Caucasian) • Major elective surgery with planned operation time ≥ 60 min • Ability to give informed consent |
• MMSE score ≤ 23 points at inclusion • Neuropsychiatric morbidity (including addiction), which limits the conduction of the neurocognitive testing • Anacusis or hypacusis, which limits the conduction of the neurocognitive testing • Centrally acting medication (e.g. antidepressants or tranquillizers) • Homelessness or inability to reach patient for follow-up • Participation in another prospective interventional clinical study during hospital stay • Accommodation in an institution due to an offcial or judicial order • Missing informed consent for saving and handing out pseudonymous data |
All patients gave written informed consent to participate in the study. The study protocol was approved by the local ethics committees and conducted in accordance with the declaration of Helsinki. The study was registered at clinicaltrials.gov (NCT02832193).
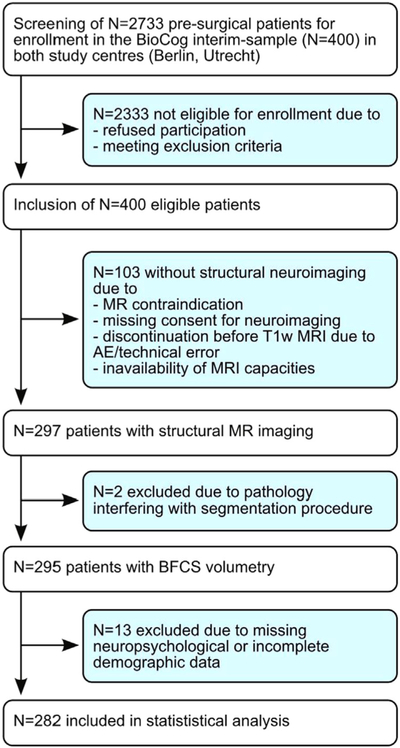
In line with the study protocol, the first 400 out of 1033 participants recruited at two study centres in Berlin, Germany (N = 291) and Utrecht, Netherlands (N = 109) were selected for this interim analysis. In total, 2733 patients were screened for inclusion in the interim sample in both study centres. 297 out of 400 patients underwent anatomical neuroimaging. Two patients were excluded due to brain pathology interfering with the segmentation procedure. Thirteen datasets were incomplete with regard to demographic data or patients did not perform cognitive testing at all. Finally, data from 282 participants are included in the analysis (N = 204 from Berlin, N = 78 from Utrecht). Neuropsychological data were incomplete for several participants reducing the final sample size for test analysis.
2.2. Neuropsychological assessments
One day before surgery, all participants underwent a comprehensive computerised neuropsychological test battery (CANTAB, Cambridge Cognition Ltd., UK), comprising the Paired Associate Learning (PAL), Verbal Recognition Memory (VRM), Spatial Span Length (SSP) and Simple Reaction Time (SRT). Additionally, pen-and-paper versions of the Trail-Making-Test (TMT, Parts A and B) and a manual dexterity test called the Grooved Pegboard Task (GPT) were conducted. Testing was performed by trained doctoral students and study nurses based on a standard operating procedure which was consented with two neuropsychologists.
PAL: The participant is shown different patterns in fixed locations on a screen in a randomised order. Subsequently, the patterns are obscured and the participant is asked to indicate the location where a particular pattern has been shown previously. In case of an error, the participant is asked to repeat the task for a maximum of ten trials. The task is then repeated on another stage with increased diffculty level. The first trial memory score is calculated as the number of patterns correctly allocated at first attempt for all completed stages. The PAL memory score assesses explicit visuospatial memory and has been found to indicate dementia, especially of Alzheimer's type (Lee et al., 2003).
VRM: The participant is shown a list of twelve words and asked to recall freely as many items as possible. Subsequently, the participant is shown a second list including the previously presented words as well as distractors and is asked to choose the items he or she recognises. The recognition procedure is repeated after twenty minutes. The number of items recognised after delay is analysed, since recognition memory has been shown to be associated with medial septal volume in the BFCS in younger participants (Butler et al., 2012). Furthermore, two studies suggest associations of BFCS volume with delayed recall in memory tasks (Grothe et al., 2010, 2016).
SSP: The participant is shown colour changing boxes on a screen. He or she is then asked to indicate the correct sequence by which the boxes have changed colours. The parameter of interest analysed is the longest sequence of boxes recalled (span length), which is thought to represent visual working memory (Monaco et al., 2013).
SRT: The participant is shown a square on a computer screen. He or she is asked to respond to this stimulus by selecting a button as fast as possible. Mean response latency (reaction time) is the parameter of interest. Reaction time is considered to be a marker of fluid intelligence and age-associated decline in cognitive ability (Der and Deary, 2018).
TMT: The participant is asked to connect dots marked with ascending numbers in Part A or alternating numbers and letters (e.g. 1-A-2-B-3…) in Part B. When a mistake is made, the participant is asked to correct it. Time for completion of both parts is the parameter of interest. Values above commonly used cut-off; thresholds (180s for TMT-A, 300s for TMT-B) were excluded during a plausibility check before data analysis. Part A is considered to be a measure of visual search and motor speed, whereas part B measures executive functions including cognitive alternation, working memory and attention (Bowie and Harvey, 2006; Crowe, 1998).
GPT: The participant is asked to insert 25 pegs with a key alongside into wholes in a board. The key slots are rotated randomly, demanding visual-motor coordination skills and manual dexterity (Otten et al., 2012). Test parameter of interest is the time for completion with the dominant hand. Data from participants who exceeded a limit of 300 s for this task were excluded during the plausibility check.
Global cognitive ability ‘g’: The global cognitive component is derived from performance on the neurocognitive battery (above) using Principal Component Analysis (PCA) in line with previous literature (Staff et al., 2006, Shenkin et al., 2009). The first unrotated factor was extracted from data of 303 participants enrolled at both study centres in Berlin and Utrecht with complete neurocognitive assessment. The first factor had an eigenvalue of 2.3 and explained 38.33% of the variance in test performance and is considered the global cognitive ability. In our study the factor is derived from TMT-B (factor loading 0.76), PAL (factor loading: 0.71), GPT (factor loading: 0.68), SSP (factor loading: 0.55), SRT (factor loading: 0.49) and VRM (factor loading: 0.46). Due skewed distributions, time for completion of TMT-B and GPT as well as SRT mean latency were reversed and underwent logarithmic transformation prior to PCA. The test outcomes used for extraction of 'g' are those stated above except for the VRM: The number of words correctly remembered in the free recall has been used for calculation of g, but based on existing literature, the delayed recognition task seemed more relevant for analysis of BFCS volume associations with VRM performance (Butler et al., 2012; Grothe et al., 2010, 2016).
2.3. MR image acquisition
MR imaging was conducted on the same day the neurocognitive assessments took place. In Berlin, data were collected at the Berlin Center for Advanced Neuroimaging using a 3 T Magnetom Trio MR scanner (Siemens) with a 32-channel head coil. T1-weighted 3D structural brain scans were acquired using a MPRAGE sequence (magnetisation prepared rapid gradient echo in 192 sagittal slices, FOV: 256·256 mm2, voxel size: 1·1 mm2 at 1 mm slice thickness, TR: 2500 ms, TE: 4.77 ms, 7° flip angle). In Utrecht, data were collected with an Achieva 3 T MRI scanner (Phillips) equipped with an 8-channel head coil. For technical reasons, the scanner at this study site had to be exchanged with an identical machine equipped with a 32-channel head coil during the study. A similar T1w sequence was recorded here (192 sagittal slices, FOV: 256·232 mm2, voxel size 1·1 mm3; at 1 mm slice thickness, TR: 7.9 ms, TE: 4.5 ms, 8° flip angle).
A board-certified neuroradiologist screened all MR images for incidental findings with clinical relevance.
2.4. MRI processing
2.4.1. Segmentation of brain volume and brain parenchyma fraction
T1-weighted MR images were partitioned into grey and white matter as well as cerebrospinal fluid using the standard SPM12 segmentation routine (http://www.fil.ion.ucl.ac.uk/spm/software/ spm12/) in a MATLAB environment (The Mathworks, Inc., Natick, MA). Partition volume was defined as the sum of all voxels with at least 50% probability to correspond to the respective partition. Total brain volume is calculated as the sum of grey and white matter. Intracranial volume (ICV) is defined as the sum of brain volume and cerebrospinal fluid volume. Brain parenchyma fraction (BPF) is defined as the ratio of brain volume and intracranial volume which is a global marker for age related neurodegeneration. BPF decreases during ageing and has been associated with disease related cognitive impairment in Multiple Sclerosis (Blatter et al., 1995; Hohol et al., 1997; Matsumae et al., 1996; Smith et al., 2008). ICV is thought to be driven by brain growth during childhood and does not undergo shrinkage in ageing (Davis and Wright, 1977; Pfefferbaum et al., 1994). We thus consider ICV a biomarker for archaeological maximum brain size and the BPF a marker of preserved brain volume and the degree of age related atrophy (Shenkin et al., 2009).
2.4.2. Segmentation of the BFCS
We have previously described the segmentation method we used for the volumetry of the BFCS (Lammers et al., 2016). Butler et al. (2014) described a similar method and they were able to show that the results are comparable to manual segmentation techniques.
Segmentation of the BFCS was performed using a probabilistic map of the basal forebrain presented by Zaborszky et al. (2008) which has been used in several previous imaging studies (Grothe et al., 2010; Butler et al., 2012, 2013, 2014; Li et al., 2014a; Kline et al., 2016; Cantero et al., 2017). Zaborszky and colleagues used histological slices and corresponding MR images of ten post-mortem body-donor brains (five female, age 37–75 years) without known neurological or psychiatric disease to define four cholinergic subregions in the basal forebrain. Brain sections were stained for cell bodies using a modified silver method of Gallyas. A comprehensive description of the BFCS delineation is found in the original publication (Zaborszky et al., 2008). The probabilistic map uses a modified version of the Mesulam nomenclature. Furthermore, apart from dense cell aggregates, also scattered magnocellular neurons in adjacent structures (e.g. the basal ganglia) were included. Studies identified these neurons as cholinergic, suggesting that these are displaced cells of the Nucleus basalis Meynert (Saper and Chelimsky, 1984; Mesulam and Geula, 1988). It is a point of discussion whether researchers should refer to these scattered neurons as a part of the Ch1-Ch4 system (Mesulam and Geula, 1988; Halliday et al., 1993).
A voxel in this map was assigned to one of the subregions which it represented in at least four out of ten brains. The final map was referenced to the anatomical MNI (Montreal Neurological Institute) space (Holmes et al., 1998).
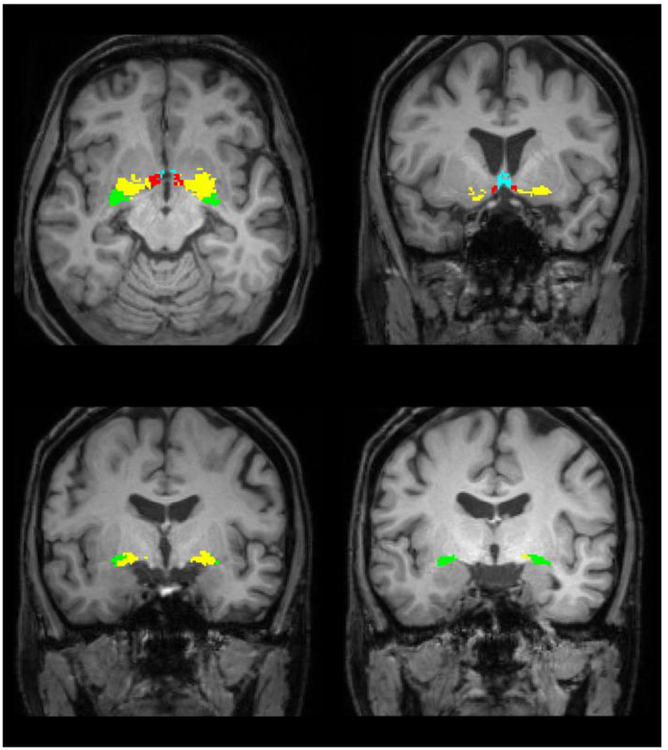
SPM12 was used to segment MR images as well as the anatomical MNI reference brain. The DARTEL (Diffeomorphic Anatomical Registration using Exponentiated Lie algebra) toolbox was then used to create DARTEL flow fields describing deformations from the MNI reference brain to a participant's individual brain (Ashburner, 2007). These deformations were applied to the probabilistic map of the basal forebrain resulting in individual labelling of cholinergic subregions in a participant's brain scan. Volumes of cholinergic subregions in the basal forebrain were determined by counting all voxels labelled as corresponding to this regions. Fig. 1 shows the segmented regions in MR images of one subject.
Fig. 1.
Location of the BFCS in one individual. The Ch1/2 region is displayed in blue, Ch3 in red. Ch4 is shown in yellow and Ch4p corresponds to the green area. We refer to a modified version of the Mesulam-nomenclature (Zaborszky et al., 2008). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.5. Statistical analysis
2.5.1. Analysis pipeline
We first analysed the association of BFCS volume with the global cognitive component ‘g’ (primary parameter of interest). We report unadjusted and adjusted associations (for age, sex and education) in generalised linear models. To assess confounding effects of global volumes, the goodness of fit of a model including BFCS volume was compared with models including brain volume and BPF. We further analysed the associations of BFCS volume with ‘g’ after addition of these confounder variables into the model. We repeated the analysis correcting for scanner and centre effects. Since adjustment for these did not change the results, nor significant effects were found, the variables were dropped for post-hoc analysis of test performance analysis.
In addition, subsequent descriptive analyses were conducted to assess associations of BFCS volume and performance on each of the individual tests of the neurocognitive battery (secondary, post-hoc parameters of interest). If test performance was significantly associated with BFCS volume without adjustment, it was considered for further analysis of adjusted effects. To further assess if associations of BFCS volume with test performance are specific for a cognitive domain, we adjusted the linear model for ‘g’.
In a final step, cognitive performance was analysed for quantile-dependent effects of BFCS on ‘g’ and performance on individual cognitive tests. This allows assessment of associations of BFCS volume with cognitive functions which are specific for low- and high-performers.
All statistical analyses were run in R3.4.1 (https://www.R-project.org/, R Core Team, 2017). The level of significance was set at p < 0.05. No corrections for multiple comparisons were made.
2.5.2. Descriptive analysis
We report test performance including median performance, interquartile range and minimum-maximum range. Demographic and neuropsychological data have been compared between centres. Neuroimaging data have also been compared between MR scanners. We used the ANOVA F-test for continuous normal, the Kruskal-Wallis-test for continuous non-normal distributions and the χ2-test for nominal data. Correlations among independent variables are reported as Pearson's correlation coeffcient R.
2.5.3. Generalised linear model
We analysed the associations of BFCS volume and its subregions (in mm3 and per one standard deviation/SD) with cognitive performance. If a significant association of BFCS volume with subtest performance was found, we adjusted the generalised linear model (GLM) for education (classified according to ISCED/International standard classification of education in three categories), age and sex. For ‘g’, TMT, SRT latency, PAL memory score and GPT a Gaussian distribution was assumed, for SSP and VRM a quasi-Poisson distribution was selected. TMT, SRT and GPT scores were log-transformed before analysis. For Gaussian-distributed data, R2 is reported and for quasi-Poisson distributed tests, D2 is reported (Guisan and Zimmermann, 2000): D2 =(Null deviance – Residual Deviance)/Null Deviance and partial D2= (Residual DevianceReduced Model – Residual DevianceFull Model)/Residual DevianceReduced Model.
2.5.4. Quantile regression analysis
In a second approach, cognitive performance was analysed using quantile regression (Koenker and Hallock, 2001). The rationale for quantile regression is robustness against heteroscedasticity and outliers in neuropsychological data. Rather than analysing the conditional mean as it is done with ordinary least squares (OLS) regression in GLM, quantile regression calculates conditional quantiles. E.g. quantile regression at the 0.5 quantile yields the median of a distribution of the dependent variable for a given value of the independent variable, whereas OLS regression returns the corresponding mean. Furthermore, the analysis of quantiles other than the 0.5th allows assessment of effects at the upper or lower boundaries of a distribution. This approach has appealing properties, when relevant covariates exit, but cannot be included in the regression model (Cade and Noon, 2003). On the other hand, quantile regression allows analysis of associations in high- and low-performing strata of the study sample.
Regression coefficients for different quantiles can be compared and tested for a statistically significant difference (referred to as heterogeneity of slopes). Heterogeneity of slopes suggests that volume affects the shape of a distribution rather than a central shift.
No transformations were applied to these variables prior to quantile regression analysis, but since count data are not eligible for quantile regression, span length was jittered to approximate a continuous distribution (Machado and Silva, 2005).
The Barrodale and Roberts algorithm implemented in the quantreg package was used for computation of quantile regression coefficients (https://CRAN.R-project.org/package=quantreg, Koenker, 2017). We report quantile regression results in scatter plots with quantile regression lines and quantile-coefficent plots (Q-C plots) for nine even spaced quantiles (0.1–0.9) with 95% confidence intervals (CI). Heterogeneity of slopes between the 0.5th and the lowest performing quantile (0.1th or 0.9th, respectively) was analysed using ANOVA. CI and p-values are based on the assumption of non-identical standard errors (called “nid” in the quantreg code).
3. Results
Fig 2 is the Consort Diagram for the sample. Demographic information on the study participants is provided in Table 2. Neuropsychological test performance including the respective number of analysed datasets is reported in Table 3. Only TMT-B performance (median [IQR]: Berlin 101.98 s [86.25–136.5 s], Utrecht 104.1 s [75–118.8 s], χ2 = 6.35, p = 0.0118) and educational status (ISCED1–2 and ≥5: Berlin 21.1% and 34.3%, Utrecht: 28.2% and 44.9%, χ2 = 6.64, p = 0.0361) differ significantly between centres. Significant between-centre differences are found for BFCS volume (F1,279 = 8.65, p = 0.0036, Δ = 93 mm3), total brain volume (F1,279 =5.37, p = 0.0212, Δ= 43 cm3) and BPF (F1,279 = 6.69, p = 0.0102, Δ =0.014, Table 4). Mean volumes are higher in the Utrecht sample. Between-scanner effects for the study centre in Utrecht are not significant.
Fig. 2.
Consort diagram. For this cross-sectional analysis, datasets have been included which have been defined as drop-outs for longitudinal analysis due to missing primary endpoints (assessment of post-operative delirium). AE refers to adverse event.
Table 2.
Demographic description of the study sample. ISCED refers to the International standard classification of education.
| Median | Interquartile Range | Range | ||
|---|---|---|---|---|
| Age | (years) | 72 | 68–76 | 65–87 |
| MMSE | (points) | 29 | 28–30 | 24–30 |
| N | % | |||
| Sex | Female | 110 | 39% | |
| Education | ISCED 1 and 2 | 65 | 23% | |
| ISCED 3 and 4 | 110 | 39% | ||
| ISCED 5 and higher | 107 | 38% |
Table 3.
Summary of cognitive performance: Number of available datasets (N), median, full and interquartile range of performance.
| Test | Parameter of interest | N | Median | Interquartile Range | Range |
|---|---|---|---|---|---|
| SRT | Mean correct latency (ms) | 282 | 290.2 | 252.1–348.6 | 200.8–801.4 |
| TMT-A | Time for completion (s) | 267 | 45 | 36.02–57.50 | 19–132 |
| TMT-B | Time for completion (s) | 250 | 100 | 81–113.04 | 25.39–298 |
| GPT | Time for completion (s) | 270 | 93 | 80–110 | 50–254.27 |
| SSP | Span length | 280 | 5 | 4–5 | 3–8 |
| PAL | Memory score | 280 | 13 | 11–17 | 0–25 |
| VRM | Number of correctly recognised items after delay | 221 | 22 | 20–23 | 0–24 |
| Global cognition | ‘g’ | 243 | 0.10 | −0.50–0.74 | −2.70–2.35 |
Table 4.
Summary of volumetric data: Median, full and interquartile range.
| Median | Interquartile Range | Range | |
|---|---|---|---|
| BFCS volume (mm3) | 2201 | 2035–2350 | 1600–2875 |
| Brain volume (cm3) | 999.9 | 921.7–1077.4 | 720.6–1280.7 |
| BPF (fraction) | 0.68 | 0.65–0.71 | 0.47–0.81 |
BFCS volume correlates with age (R = −0.26, p < 0.0001), total brain volume (R=0.83, p < 0.0001) and BPF (R = 0.25, p < 0.0001). BFCS volume (F1,280 = 75.69, p < 0.0001), total brain volume (F1,280 = 77.06, p < 0.0001) and BPF (F1,280 =11.02, p = 0.0010) differ by sex. BFCS and brain volume are larger in men, while BPF is higher in women. Differences by education are found for BFCS volume (F2,279 =3.27, p = 0.0397), total brain volume (F2,279 =7.95, p = 0.0004), but not for BPF (F2,279 < 0.01, p = 0.85). BFCS and brain volumes are larger in more educated subjects.
3.1. Associations of BFCS volume with global cognitive ability
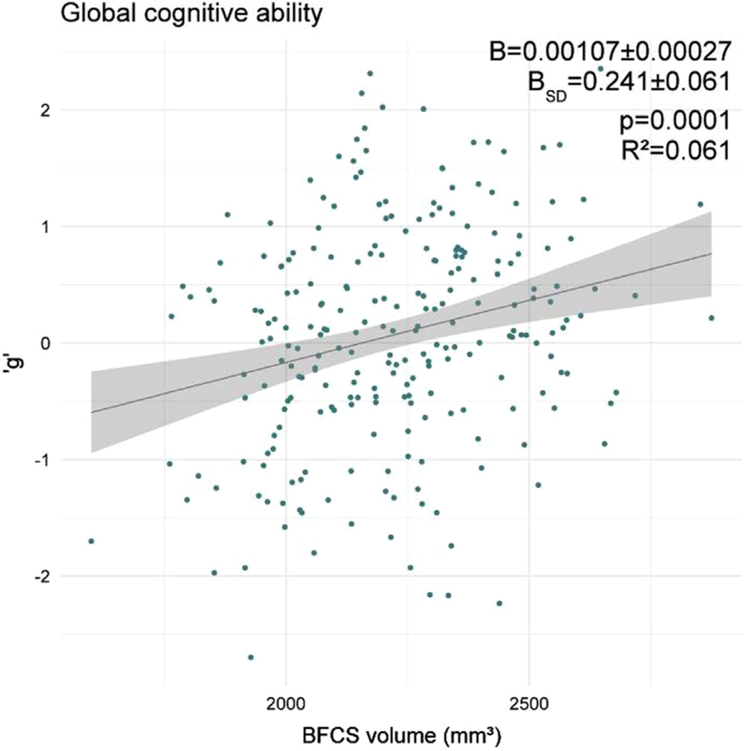
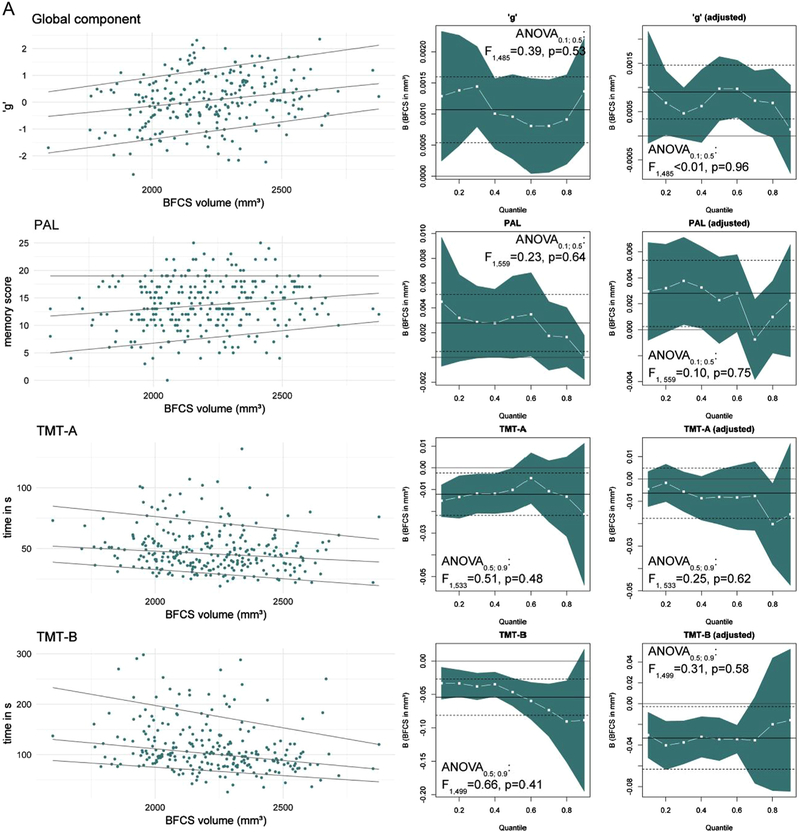
Our primary hypothesis is an association of BFCS volume with global cognitive ability: BFCS volume is significantly associated with ‘g’ (R2 = 0.061, p = 0.0001, see Fig 3). This association is largely independent of age, education and sex and remains significant after adjustment for these variables (R2 = 0.287, partial R2 = 0.041, B = 0.204 ± 0.064 per SD, p = 0.0017, see Table 5).
Fig. 3.
Association of BFCS volume with global cognitive ability. B indicates the change in 'g' per 1 mm3 and one standard deviation (B-SD) change in BFCS volume. Regression coefficients (B), p-value and R2 correspond to the unadjusted model.
Table 5.
General linear model (GLM) describing BFCS volume associations with global cognitive abilities. Regression coefficients are indicated as B with standard error (SE) and the corresponding p-value. SD refers to standard deviation.
| Independent variable | B ± SE | p |
|---|---|---|
| BFCS volume in mm3 (change per SD) | 0.00090 ± 0.00028 (0.204 ± 0.064) |
0.0017 |
| Age in years | −0.059 ± 0.011 | < 0.0001 |
| Male sex | −0.347 ± 0.129 | 0.0075 |
| ISCED level 1 + 2 | − 0.449 ± 0.138 | 0.0013 |
| ISCED level 5 and higher | 0.497 ± 0.122 | < 0.0001 |
Association of BFCS subregions (CH1/2, CH3, CH4, CH4p) with global cognition are also assessed. After correction for age, sex and education, all subregions are significantly associated with ‘g’, and the association of BFCS with general cognition was slightly inferior to the association of ‘g’ and the most posterior subregion CH4p. According to R2, CH4p (R2 =0.290, partial R2 =0.045, p = 0.0009) is slightly more relevant for ‘g’ than more rostral regions CH1/2 (R2 =0.281, partial R2 =0.032, p = 0.0057), CH3 (R2 =0.271, partial R2 =0.019, p = 0.0352) and CH4 (R2 =0.276, partial R2 =0.026, p = 0.0127).
Associations of confounding global anatomical volumes (brain volume, BPF) and global cognitive abilities are tested in general linear models adjusting for age, sex and education. A considerable improvement is achieved by using a model which includes total brain volume (R2 =0.311, partial R2 =0.073, B=0.280 ± 0.065 per SD, p < 0.0001). The association of global cognitive ability and BPF is significant, but was inferior to the model including brain volume (R2 =0.300, partial R2 =0.058, B=0.235 ± 0.062 per SD, p = 0.0002).
BFCS volume and each one of the potentially confounding volume measures are then added concurrently into two further general linear models of ‘g’. When total brain volume and BFCS are included in the same model alongside with age, sex and educational status, only the association of total brain volume with global cognitive ability remains significant (partial R2 =0.033, B=0.274 ± 0.096 per SD, p = 0.0047), but not for BFCS (partial R2 < 0.001, B=0.008 ± 0.093 per SD, p = 0.93). A model including BFCS volume and BPF shows that BFCS and BPF are independently associated with global cognitive ability (partial R2BFCS=0.023, BBFCS=0.154 ± 0.065 per SD, pBFCS=0.0191; partial R2BPF=0.040, BBPF=0.198 ± 0.063 per SD, pBPF=0.0019).
There is no significant effect of MR scanner or study centre, nor does adjustment for these alter the results.
3.2. Associations of BFCS volume with test performance
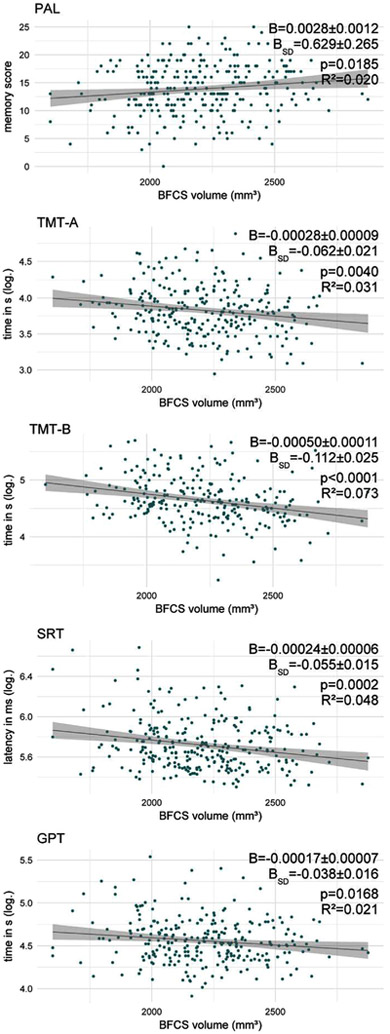
In the unadjusted analyses, BFCS volume is significantly and positively associated with performance in all tests, except delayed recognition in the VRM.
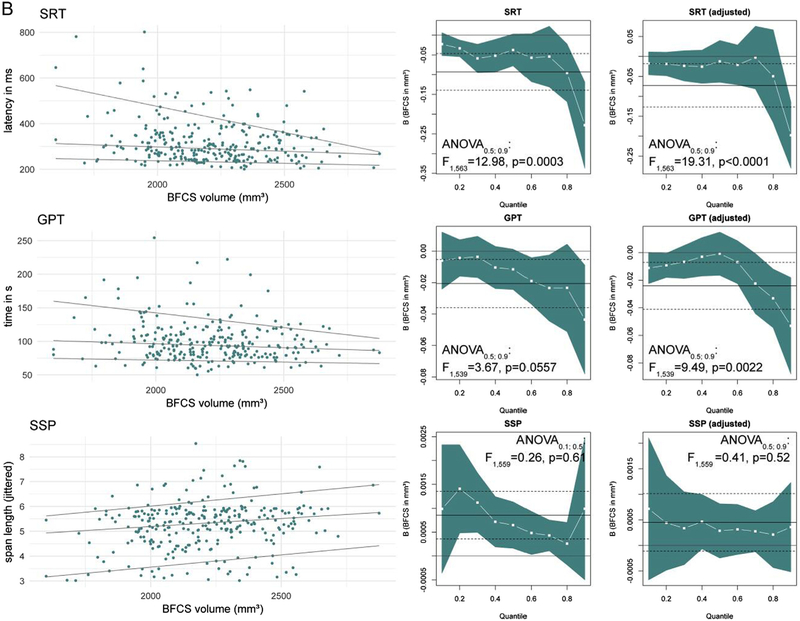
The strongest associations are seen for TMT-B and SRT latency (R2 =0.073 and 0.048, respectively). Somewhat weaker associations are also found for PAL memory score, TMT-A, GPT (see Fig 4) and the SSP (D2 =0.030, B=0.00015 ± 0.00005, BSD=0.034 ± 0.012, p = 0.0034, not presented in Fig 4). Using a Bonferroni-corrected level of significance p′ to account for testing associations with seven cognitive tests (p′=0.05/7 ≈0.0071), only the unadjusted associations of BFCS volume with TMT-A and -B, SRT as well as SSP remained significant. Adjusting for age, education and sex yields similar associations of BFCS volume with PAL memory score, SRT latency, TMT-B and GPT. R2 for these models was lower than for the model of ‘g’. Associations with TMT-A performance and span length are no longer significant after adjustment for age, sex and education (see Table 6).
Fig. 4.
Association of BFCS volume with test performance. B indicates the change in the test parameter per 1 mm3 and one standard deviation (B-SD) change in BFCS volume. Regression coefficients (B), p-value and R2 correspond to the unadjusted model.
Table 6.
Adjusted GLM for associations of BFCS volume with test parameters. All GLM are adjusted for age, sex and education. Associations are listed as regression coefficients (B) with standard errors per one SD (standard deviation) change of BFCS volume. P-values, R2 and partial R2 for BFCS volume are indicated, except for the SSP: These values marked with an asterisk (*) correspond to D2. p-values have not been corrected for multiple tests. p-values marked with a double asterisk (**) are significant after Bonferroni correction for independent tests (p′ < 0.0071).
| Test | B ± SE for BFCS volume per SD |
(p) | Partial R2
(D2) |
R2 (D2) |
|---|---|---|---|---|
| PAL | 0.632 ± 0.294 | (0.0325) | 0.017 | 0.146 |
| TMT-A (log) | −0.030 ± 0.024 | (0.22) | 0.006 | 0.129 |
| TMT-B (log) | −0.084 ± 0.028 | (0.0030**) | 0.036 | 0.241 |
| SRT (log) | −0.039 ± 0.017 | (0.0245) | 0.018 | 0.075 |
| GPT (log) | −0.046 ± 0.017 | (0.0085) | 0.026 | 0.192 |
| SSP | 0.016 ± 0.013 | (0.22) | 0.005* | 0.115* |
VRM is the only test without significant association with BFCS volume (D2 = 0.005, BSD=0.011 ± 0.009, p = 0.22). The result for the VRM does not change after rejection of two outlying subjects (D2 = 0.003, BSD=0.0060 ± 0.0068, p = 0.38).
After adjustment for global cognitive abilities, only TMT-B performance was significantly associated with BFCS volume (partial R2 = 0.018, p = 0.0378). This association was no longer significant after further adjustment for age, sex and education (partial R2 = 0.007, p = 0.19).
3.3. Quantile regression analysis
Fig 5A and B are a graphical summary of quantile regression results of global cognitive ability and test performance. ANOVA for heterogeneity of slopes indicates that differences between associations of BFCS volume and cognitive performance between 0.5th and 0.9th quantile exist for SRT latency and GPT, but neither for the other tests nor ‘g’.
Fig. 5.
A: Quantile regression results of ‘g’ and test performance. The left column shows scatter plots with regression lines for the 0.1th, 0.5th (median) and 0.9th quantile. The columns on the right display quantile-coeffcient plots for simple (unadjusted, middle) and multiple quantile regression adjusting for age, sex and education (right). The regression coefficient for BFCS volume (white –■–) are displayed for each quantile (x-axis) with 95% CI (blue area). Solid and dashed lines mark the OLS regressions result with 95% CI. ANOVA results correspond to analysis for slope heterogeneity between median and lowest-performing quantile. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For the global component, results from the GLM are reproduced in quantile regression. The association of BFCS volume and 'g' showed no quantile-dependent trend, with significance found for all quantiles in the unadjusted model. After adjustment, significance was restricted to quantiles close to the median (B=0.0010 ± 0.0003, BSD=0.220 ± 0.076, p = 0.0043).
For TMT-A and -B, PAL and SSP median regression coefficients were acceptably close to the OLS estimate. Neither ANOVA nor inspection of plots suggested systematic quantile-dependent associations, especially not after adjustment for age, sex and education. With regard to significance, differences between the analysis methods occurred. On the one hand, parametric methods can be more efficient than alternative methods when data are normally distributed, explaining the more optimistic results of the GLM for the association with PAL (Kitchen, 2009). On the other hand, GLM standard errors are sensitive to outliers and skewed distributions, yielding more favourable results of quantile regression (Wilcox, 2012).
Results for the SRT and GPT differ from GLM results. For these tests, BFCS volume association with test performance differs significantly between the 0.5th and 0.9th quantile with a stronger association for the low-performance quantiles (0.9th, see Fig 5B). In the adjusted models regression coefficients for the 0.1th to the 0.7th quantile are close to zero, but significant effects are found at higher quantiles for SRT latency (0.9th quantile: B=−0.198 ± 0.042, B=−44.6 ± 9.5 per SD, p < 0.0001) and GPT completion time (0.9th quantile: B=−0.053 ± 0.018, B=−12.0 ± 4.0 per SD, p = 0.0031).
4. Discussion
In the current study, we analysed the associations of BFCS volume with global cognitive ability and performance on several age-sensitive cognitive tests in a sample of elderly pre-surgical patients. We found significant positive associations of BFCS volume with global cognition and tests of visual memory, manual dexterity, processing speed and executive functions. BFCS volume explains more variance in the global component than in any of the cognitive test outcomes. E.g., independent of age, sex and educational status, BFCS volume explains 4.1% of the variance in ‘g’, but only 3.6% or less variance in test performance. This result suggests that BFCS volume is associated with better overall cognitive function in ageing rather than affecting a specific cognitive domain. This conclusion is further supported by our finding that performance in cognitive tests was no longer independently associated with BFCS volume when analyses were adjusted for ‘g’, suggesting that global cognitive abilities mediate the associations with test performance. However, the association of BFCS volume with global cognitive ability is driven by a more general association of total brain volume and cognition. BPF and BFCS volume are independently associated with cognition, suggesting BFCS volume has a beneficial function for cognitive reserve in atrophy-related cognitive decline. We further found that whereas the association of BFCS volume and global cognition is stable for all levels of performance, the association with SRT and GPT performance is only found for low-performing participants.
Our study needs to be discussed in the context of results reported by Wolf et al. (2014) and Grothe et al. (2016). The former work analysed associations of BFCS subregion volumes with global cognition and test performance in 43 healthy adults aged 60–85 years with a very high IQ. After correction for intracranial volume, age and education they reported a significant association with global cognitive ability, but not with performance in single cognitive domains. Wolf's results support our findings with regard to an association of BFCS volume with global cognition rather than single test performance. Furthermore, we found a similar pattern of BFCS subregion contribution to global cognition: Total BFCS volume and posterior compartments (Ch4p) seem to be more relevant than anterior sections. In our study, the association of BFCS volume with ‘g’ was stronger than with performance in any cognitive test. Thus it is likely that this study missed associations with test performance due to lower statistical power.
Grothe et al. (2016) analysed the association of BFCS volume and test performance in 132 MCI subjects and 177 “hypernormal” healthy controls enrolled in the ADNI project. After controlling for age, sex and education, no significant associations with subtest performance were found in the healthy control group. Instead, significant associations with memory and attentional control were only found in the MCI group. Although the authors did not report analyses of global cognitive ability, our positive findings on BFCS volume associations with performance in single tests from the neurocognitive battery disagree with the results by Grothe and colleagues. We think that to some extent these differences can be explained by clinical differences in the analysed groups. Whereas the respective studies analysed unusually high performing elderly individuals, cognitive performance in our cohort had greater variance and most likely includes subjects with preclinical cognitive impairment. For instance, comparison of TMT performance, which is reported in all aforementioned studies, suggests that Wolf et al. (TMT mean time: A 36.6 s, B 91.1 s) and Grothe et al. (TMT mean time: A 33.7 s, B 80.3 s) analysed subject groups with better performance compared to the BioCog cohort (TMT mean time: A 48.8 s, B 113.0 s). Tombaugh (2004) presented age-stratified normative data for the TMT. The average performance in Wolf's and Grothe's samples were better than 50% and 70% of the reference group (70–74 years) in Tombaugh's data, whereas the BioCog cohort mean corresponds to the 30th percentile of this reference group. We further compared TMT performance in our sample to reference data stratified by prevalence and future incidence of dementia in a sample of patients aged over 70 years (Holtzer et al., 2008). Although TMT performance of 75% of the BioCog cohort is close to the reference value of the group without prevalent dementia or future conversion (TMT mean time: A 56.7 s, B128.8 s), the TMT completion time in the worst performing quartile of our sample is closer to the groups with incident (TMT mean time: A 87 s, B 186 s), or prevalent dementia (TMT mean time: A 112 s, B 208 s) in that study.
Both the sample analysed by Wolf as well as the control subjects from the ADNI project included in the study by Grothe underwent additional dementia screening (Clinical Dementia Rating Scale, Stamm Screening Questionnaire and International Checklist of ICD-10 and DSM-IV). Dementia screening in the BioCog study is restricted to consultation of medical records and patient interview for neuropsychiatric diseases as well as MMSE assessment. The MMSE score was designed originally as a bedside screening tool for dementia, but is not generally recommended as a diagnostic criterion for dementia (Folstein et al., 1975; Tombaugh and McIntyre, 1992). Comparisons of the MMSE score with the Clinical Dementia Rating Scale (CDR) in a memory clinic suggested that among patients with score of 26–29 points, about 40% have a CDR score of 0.5 or 1, corresponding to probable or mild dementia (Perneczky et al., 2006). The authors further reported CDR scores of at least 0.5 (probable dementia) for all patients with MMSE scores below 26. This study reports the limited ability of the MMSE score to exclude patients with pre-existing cognitive impairment, which might also apply to the BioCog study (MMSE cut-off at 24 points). Furthermore, the BioCog study only includes elderly pre-operative patients with an indication for surgery and the overall morbidity of the cohort is probably higher compared to the groups analysed by Grothe et al. (2016) and Wolf et al. (2014). Vassilaki et al. (2015) reported associations of multimorbidity and mild cognitive impairment which implies that pre-clinical cognitive decline might occur more often in the clinical sample presented here. Overall, our considerations suggest that a larger proportion of patients with (mild) cognitive impairments in the BioCog group contributes to the significant associations of cognitive performance with BFCS volume compared to the group analysed by Grothe et al. (2016).
To further test this assumption in our data, we used quantile regression analysis to assess the association of BFCS volume with test parameters for different levels of performance. Although in this analysis statistical significance was found only for subjects with median global cognitive ability, we found no quantile-dependent association of BFCS and global cognition, suggesting that BFCS volume is associated with better cognitive performance at all stages of age-associated cognitive decline. Since the association seems to be very stable also for high-performing subjects we suggest that this might contribute to the findings by Wolf et al. (2014). Further analysis of SRT and GPT, which are tests of reaction time and visual-motor control, revealed significantly different associations for the 0.5th and the lowest performing quantile. Independent associations have only been found in poorly achieving subjects. These findings suggest that their association with BFCS volume seems to be specific for individuals with low task performance. It is possible that BFCS volume limits minimum performance in these participants, rather than improving performance in average- to high-performers. This might point to a function of BFCS volume as a structural cognitive reserve which is recruited in low performing subjects, but with regard to the different results of the studies mentioned above, it shows that the association of BFCS volume with test performance indeed depends on the overall performance level of the study group. Whereas Wolf and Grothe might have missed significant associations by recruiting only high performing participants, our results might be driven by a higher fraction of subjects with relevant age-related cognitive decline.
As pointed out above, most differences between studies may arise from differences in sample composition. Even so, some additional differences between these studies need to be taken into account. The BFCS volumes we report are considerably larger than those reported by Wolf and Grothe. Furthermore, the MRI derived volumes in our study are larger than the work by Zaborszky et al. (2008) suggest. Thus, it seems unlikely that these differences are caused by varying degrees of BFCS atrophy between the studies. Instead, we need to take into account that we used a probabilistic map of the BFCS which was derived from histological sections of ten brains. The map summarises brain regions with at least 40% overlap from these brains. In contrast to this, Wolf and Grothe report to have used a BFCS map based on one single brain (Kilimann et al., 2014). Thus, our method overestimates absolute BFCS volume. On the other hand, the location of cholinergic cells in the basal forebrain has high inter-individual variability, limiting the generalisability of single-subject based maps for larger populations (Zaborszky et al., 2008). The choice of the appropriate method is thus a point of discussion and the lack of a final recommendation reinforces the need for different studies using complementing approaches.
Our study has greater statistical power by including more participants than Wolf and colleagues and by refusing to correct for multiple independent tests. Especially after a significant association between ‘g’ and BFCS volume has been shown, it is not reasonable to assume independence of the various tests ultimately constituting the basis of global cognitive ability (Johnson et al., 2004, 2008; Deary et al., 2010).
Notably, we found no association of BFCS volume and episodic memory in VRM. Thus our results could not reproduce findings from studies on younger samples and MCI patients (Butler et al., 2012; Grothe et al., 2010, 2016). Apart from differences in sample composition, methodological differences also need to be taken into account to explain these findings. First, only one of these studies has reported a sample size comparable to the dataset presented here. Second, Butler and colleagues reported associations with source memory accuracy and discriminability rather than recognition memory. Finally, the association of BFCS volume and episodic memory seems to be specific for subjects with impairment in the memory domain. For instance, Grothe et al. (2016) suggested that BFCS atrophy effects on cognition are mediated by disease-related cortical dysfunction.
4.1. Limitations
In our study, ‘g’ has been calculated from several cognitive tests using principal component analysis. Thus, analysis of global cognitive abilities and subtest performance is to some extent redundant. We cannot exclude the possibility that our ‘g’ is biased towards measuring cognitive subdomains, e.g. with over-representation of short term memory (PAL, SSP, VRM free recall) and under-representation of long-term memory. As a consequence, we also might overestimate the association of volumes and ‘g’ by including tests of cognitive domains which might have an unusual strong association with BFCS volume. This is of particular relevance, since in the unadjusted models, the association of BFCS volume with TMT-B performance was stronger than the association with ‘g’, which nevertheless was confounded by age, education and sex.
Segmentation of cholinergic cell groups in the basal forebrain cannot be based on visual features of the cells in MR images (Teipel et al., 2015; Wolf et al., 2014). Therefore, we have to assume that volumetric data of the BFCS have relatively more noise contamination than brain volume which can be segmented by voxel intensity. Thus, associations of cognition and brain volume are a priori stronger than associations of cognition and BFCS volume due to noise distribution. Finally, our results are to some extent inconclusive with respect to the relative relevance of BFCS and total brain volume for cognition.
5. Conclusion
We were able to extend previous reports of BFCS volume association with global cognitive performance in a large sample of older adults. Our results suggest that contradictory findings of BFCS volume effects on subtest performance reflect a specific association for low-performing individuals, although these findings need further evidence.
Acknowledgments
Funding
The research leading to these results has received the funding from the European Community's FP7 under Grant agreement no. 602461. Additional (internal) funding was obtained from the Berlin Institute of Health (BIH, Berlin, Germany). Laszlo Zaborszky was supported by the NIH (USA) Grant NIH/NINDS NS023945 for his work on the maximum probability map of the basal forebrain cholinergic system.
Footnotes
Conflict of interest
This publication is part of Florian Lammers' doctorate. Prof. Winterer is coordinator of the BioCog Consortium and chief executive of the company Pharmaimage Biomarker Solutions GmbH. The company is one of the partners of the BioCog Consortium. None of the other authors declares a conflict of interest.
References
- Anand P, Singh B, 2013. A review on cholinesterase inhibitors for Alzheimer's disease. Arch. Pharm. Res. 36, 375–399. 10.1007/s12272-013-0036-3. [DOI] [PubMed] [Google Scholar]
- Ashburner J, 2007. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B, 2012. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43. 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn SD, 1995. Quantitative volumetric analysis of brain MR: nor-mative database spanning 5 decades of life. AJNR Am. J. Neuroradiol. 16, 241–251. [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD, 2006. Administration and interpretation of the Trail Making Test. Nat. Protoc. 1, 2277. 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- Butler T, Blackmon K, Zaborszky L, Wang X, DuBois J, Carlson C, Barr WB, French J, Devinsky O, Kuzniecky R, Halgren E, Thesen T, 2012. Volume of the human septal forebrain region is a predictor of source memory accuracy. J. Int. Neuropsychol. Soc. 18, 157–161. 10.1017/S1355617711001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Zaborszky L, Pirraglia E, Li J, Wang XH, Li Y, Tsui W, Talos D, Devinsky O, Kuchna I, Nowicki K, French J, Kuzniecky R, Wegiel J, Glodzik L, Rusinek H, deLeon MJ, Thesen T, 2014. Comparison of human septal nuclei MRI measurements using automated segmentation and a new manual protocol based on histology. Neuroimage 97, 245–251. 10.1016/j.neuroimage.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Zaborszky L, Wang X, McDonald CR, Blackmon K, Quinn BT, DuBois J, Carlson C, Barr WB, French J, Kuzniecky R, Halgren E, Devinsky O, Thesen T, 2013. Septal nuclei enlargement in human temporal lobe epilepsy without mesial temporal sclerosis. Neurology 80, 487–491. 10.1212/WNL.0b013e31827f0ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade BS, Noon BR, 2003. A gentle introduction to quantile regression for ecologists. Front. Ecol. Environ. 1, 412–420. 10.1890/1540-9295(2003)001[0412:AGITQR]2.0.CO;2. [DOI] [Google Scholar]
- Cai L, Gibbs RB, Johnson DA, 2012. Recognition of novel objects and their location in rats with selective cholinergic lesion of the medial septum. Neurosci. Lett. 506, 261–265. 10.1016/j.neulet.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero JL, Zaborszky L, Atienza M, 2017. Volume loss of the nucleus basalis of meynert is associated with atrophy of innervated regions in mild cognitive impairment. Cereb. Cortex 27, 3881–3889. 10.1093/cercor/bhw195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe SF, 1998. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the Trail Making Test. J. Clin. Psychol. 54, 585–591. [DOI] [PubMed] [Google Scholar]
- Davis PJM, Wright EA, 1977. A new method for measuring cranial cavity volume and its application to the assessment of cerebral atrophy at autopsy. Neuropathol. Appl. Neurobiol. 3, 341–358. 10.1111/j.1365-2990.1977.tb00595.x. [DOI] [Google Scholar]
- Deary IJ, Penke L, Johnson W, 2010. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 11, 201. 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Der G, Deary IJ, 2018. Reaction times match IQ for major causes of mortality: Evidence from a population based prospective cohort study. Intelligence 69, 134–145. 10.1016/j.intell.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI, 1999. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer's disease. J. Comp. Neurol. 411, 693–704. . [DOI] [PubMed] [Google Scholar]
- Grothe M, Heinsen H, Teipel S, 2013. Longitudinal measures of cholinergic forebrain atrophy in the transition from healthy aging to Alzheimer's disease. Neurobiol. Aging 34, 1210–1220. 10.1016/j.neurobiolaging.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M, Heinsen H, Teipel SJ, 2012. . Atrophy of the cholinergic basal forebrain over the adult age range and in early stages of Alzheimer′s disease. Biol. Psychiatry 71, 805–813. 10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe M, Zaborszky L, Atienza M, Gil-Neciga E, Rodriguez-Romero R, Teipel SJ, Amunts K, Suarez-Gonzalez A, Cantero JL, 2010. Reduction of basal forebrain cholinergic system parallels cognitive impairment in patients at high risk of developing Alzheimer's disease. Cereb. Cortex 20, 1685–1695. 10.1093/cercor/bhp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe MJ, Heinsen H, Amaro E, Grinberg LT, Teipel SJ, 2016. Cognitive Correlates of Basal Forebrain Atrophy and Associated Cortical Hypometabolism in Mild Cognitive Impairment. Cereb. Cortex 26, 2411–2426. 10.1093/cercor/bhv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisan A, Zimmermann NE, 2000. Predictive habitat distribution models in ecology. Ecol. Model. 135, 147–186. 10.1016/S0304-3800(00)00354-9. [DOI] [Google Scholar]
- Halliday GM, Cullen K, Cairns MJ, 1993. Quantitation and three-dimensional reconstruction of Ch4 nucleus in the human basal forebrain. Synapse 15, 1–16. 10.1002/syn.890150102. [DOI] [PubMed] [Google Scholar]
- Harati H, Barbelivien A, Cosquer B, Majchrzak M, Cassel J-C, 2008. Selective cholinergic lesions in the rat nucleus basalis magnocellularis with limited damage in the medial septum specifically alter attention performance in the five-choice serial reaction time task. Neuroscience 153, 72–83. 10.1016/j.neuroscience.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Hohol MJ, Guttmann CRG, Orav J, Mackin GA, Kikinis R, Khoury SJ, Jolesz FA, Weiner HL, 1997. Serial neuropsychological assessment and magnetic resonance imaging analysis in multiple sclerosis. Arch. Neurol. 54, 1018–1025. 10.1001/archneur.1997.00550200074013. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC, 1998. Enhancement of MR images using registration for signal averaging. J. Comput. Assist Tomogr. 22, 324–333. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Goldin Y, Zimmerman M, Katz M, Buschke H, Lipton RB, 2008. Robust norms for selected neuropsychological tests in older adults. Arch. Clin. Neuropsychol. 23, 531–541. 10.1016/j.acn.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, Bouchard TJ, Krueger RF, McGue M, Gottesman II, 2004. Just one g: consistent results from three test batteries. Intelligence 32, 95–107. 10.1016/S0160-2896(03)00062-X. [DOI] [Google Scholar]
- Johnson W, te,Nijenhuis J, Bouchard TJ, 2008. Still just 1 g: consistent results from five test batteries. Intelligence 36, 81–95. 10.1016/j.intell.2007.06.001. [DOI] [Google Scholar]
- Kilimann I, Grothe M, Heinsen H, Alho EJL, Grinberg L, Amaro E, Dos Santos GAB, da Silva RE, Mitchell AJ, Frisoni GB, Bokde ALW, Fellgiebel A, Filippi M, Hampel H, Klöppel S, Teipel SJ, 2014. Subregional basal forebrain atrophy in Alzheimer's disease: a multicenter study. J. Alzheimers Dis. 40, 687–700. 10.3233/JAD-132345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen CMR, 2009. Nonparametric versus parametric tests of location in biomedical research. Am. J. Ophthalmol. 147, 571–572. 10.1016/j.ajo.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RL, Zhang S, Farr OM, Hu S, Zaborszky L, Samanez-Larkin GR, Li C-SR, 2016. The effects of methylphenidate on resting-state functional connectivity of the Basal Nucleus of Meynert, Locus Coeruleus, and ventral tegmental area in healthy adults. Front. Hum. Neurosci. 10. 10.3389/fnhum.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenker R, 2017. quantreg: Quantile Regression. R package version 5.34. 〈https://CRAN.R-project.org/package=quantreg〉. (Accessed 25 January 2018). [Google Scholar]
- Koenker R, Hallock KF, 2001. Quantile regression. J. Econ. Perspect. 15, 143–156. 10.1257/jep.15.4.143. [DOI] [Google Scholar]
- Lammers F, Mobascher A, Musso F, Shah NJ, Warbrick T, Zaborszky L, Winterer G, 2016. Effects of Ncl. Basalis Meynert volume on the Trail-Making-Test are restricted to the left hemisphere. Brain Behav. 6 10.1002/brb3.421. (n/a–n/a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ACH, Rahman S, Hodges JR, Sahakian BJ, Graham KS, 2003. Associative and recognition memory for novel objects in dementia: implications for diagnosis. Eur. J. Neurosci. 18, 1660–1670. 10.1046/j.1460-9568.2003.02883.x. [DOI] [PubMed] [Google Scholar]
- Li CR, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L, 2014a. Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. Neuroimage 97, 321–332. 10.1016/j.neuroimage.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado JAF, Silva JMCS, 2005. Quantiles for counts. J. Am. Stat. Assoc. 100, 1226–1237. 10.1198/016214505000000330. [DOI] [Google Scholar]
- Matsumae M, Kikinis R, Mórocz IA, Lorenzo AV, Sándor T, Albert MS, Black PM, Jolesz FA, 1996. Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. J. Neurosurg. 84, 982–991. 10.3171/jns.1996.84.6.0982. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C, 1988. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J. Comp. Neurol. 275, 216–240. 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH, 1983. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 214, 170–197. 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Monaco M, Costa A, Caltagirone C, Carlesimo GA, 2013. Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol. Sci. 34, 749–754. 10.1007/s10072-012-1130-x. [DOI] [PubMed] [Google Scholar]
- Otten ML, Mikell CB, Youngerman BE, Liston C, Sisti MB, Bruce JN, Small SA, McKhann GM, 2012. Motor deficits correlate with resting state motor network connectivity in patients with brain tumours. Brain 135, 1017–1026. 10.1093/brain/aws041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M, 2007. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56, 141–154. 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A, 2006. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am. J. Geriatr. Psychiatry 14, 139–144. 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- Perry EK, Johnson M, Kerwin JM, Piggott MA, Court JA, Shaw PJ, Ince PG, Brown A, Perry RH, 1992. Convergent cholinergic activities in aging and Alzheimer's disease. Neurobiol. Aging 13, 393–400. 10.1016/0197-4580(92)90113-C. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO, 1994. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 51, 874–887. [DOI] [PubMed] [Google Scholar]
- R:The R Project for Statistical Computing [WWW Document], 2017. URL 〈https://www.r-project.org/〉 (Accessed 25 January 2018) [Google Scholar]
- Russ TC, Morling JR, 2012. Cholinesterase inhibitors for mild cognitive impairment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Database Syst. Rev. CD009132. 10.1002/14651858.CD009132.pub2. [DOI] [Google Scholar]
- Saper CB, Chelimsky TC, 1984. A cytoarchitectonic and histochemical study of nucleus basalis and associated cell groups in the normal human brain. Neuroscience 13, 1023–1037. 10.1016/0306-4522(84)90286-0. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T, 2006. The significance of the cholinergic system in the brain during aging and in Alzheimer's disease. J. Neural Transm. 113, 1625–1644. 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Rivers CS, Deary IJ, Starr JM, Wardlaw JM, 2009. Maximum (prior) brain size, not atrophy, correlates with cognition in community-dwelling older people: a cross-sectional neuroimaging study. BMC Geriatr. 9, 12. 10.1186/1471-2318-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simić G, Mrzljak L, Fucić A, Winblad B, Lovrić H, Kostović I, 1999. Nucleus subputaminalis (Ayala): the still disregarded magnocellular component of the basal forebrain may be human specific and connected with the cortical speech area. Neuroscience 89, 73–89. [DOI] [PubMed] [Google Scholar]
- Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, Tanzi RE, Albert MS, Greenberg SM, Guttmann CRG, 2008. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch. Neurol. 65, 94–100. 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hunsaker JC, Slevin JT, DeKosky ST, Kryscio RJ, Markesbery WR, 1992. Monoaminergic and cholinergic synaptic markers in the nucleus basalis of Meynert (nbM): normal age-related changes and the effect of heart disease and Alzheimer's disease. Ann. Neurol. 31, 611–620. 10.1002/ana.410310608. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Deary IJ, Whalley LJ, 2006. Generality and specificity in cognitive aging: a volumetric brain analysis. Neuroimage 30, 1433–1440. 10.1016/j.neuroimage.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Grothe MJ, Wittfeld K, Hoffmann W, Hegenscheid K, Völzke H, Homuth G, Grabe HJ, 2015. Association of a neurokinin 3 receptor polymorphism with the anterior basal forebrain. Neurobiol. Aging 36, 2060–2067. 10.1016/j.neurobiolaging.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, 2004. Trail Making Test A and B: normative data stratified by age and education. Arch. Clin. Neuropsychol. 19, 203–214. 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ, 1992. The mini-mental state examination: a comprehensive review. J. Am. Geriatr. Soc. 40, 922–935. [DOI] [PubMed] [Google Scholar]
- Vassilaki M, Aakre JA, Cha RH, Kremers WK, St. Sauver JL, Mielke MM, Geda YE, Machulda MM, Knopman DS, Petersen RC, Roberts RO, 2015. Multimorbidity and Risk of Mild Cognitive Impairment. J. Am. Geriatr. Soc. 63, 1783–1790. 10.1111/jgs.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P, Hiley CR, Goodhardt MJ, Carrasco LH, Keet JP, Williams IE, Bowen DM, 1977. Neocortical cholinergic neurons in elderly people. Lancet 1, 668–671. [DOI] [PubMed] [Google Scholar]
- Wilcox R, 2012. Chapter 1 - Introduction. In: Introduction to Robust Estimation and Hypothesis Testing (Third Edition), Statistical Modeling and Decision Science. Academic Press, Boston, pp. 1–22. 〈 10.1016/B978-0-12-386983-8.00001-9〉. [DOI] [Google Scholar]
- Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm AL, Zhang R, Haglund A, Subbiah P, Donepezil Nordic Study Group, 2001. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 57, 489–495. [DOI] [PubMed] [Google Scholar]
- Wolf D, Grothe M, Fischer FU, Heinsen H, Kilimann I, Teipel S, Fellgiebel A, 2014. Association of basal forebrain volumes and cognition in normal aging. Neuropsychologia 53, 54–63. 10.1016/j.neuropsychologia.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Hoemke L, Mohlberg H, Schleicher A, Amunts K, Zilles K, 2008. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage 42, 1127–1141. 10.1016/j.neuroimage.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]