Abstract
Background
The most common etiological agents of infections in onco-hematological patients are Gram-negative rods resistant to many antimicrobials, including carbapenems. Recently, ceftolozane combined with tazobactam became a novel therapeutic option. The aim of the present study was to analyze the susceptibility to ceftolozane/tazobactam of the clinical strains of these bacteria.
Material/Methods
Material comprised rectal swabs, urine, and bronchoalveolar lavage fluid obtained from onco-hematological patients hospitalized in a clinical hospital (1050 beds) in Poland. Identification of the isolated bacteria was done by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) using the MALDI Biotyper (Bruker). Ceftolozane/tazobactam susceptibility of the isolates was assessed using antimicrobial gradient strips (E-test, BioMérieux). Antimicrobial susceptibility testing and interpretation of the results was done according to the current recommendations of the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
Results
In total, 281 rectal swabs and 116 urine samples were tested for the presence of Gram-negative rods producing ESBL, and 531 rectal swabs and 8 bronchoalveolar lavage fluid samples were tested for the presence of Gram-negative rods resistant to carbapenems. In the analyzed period, 69 non-repetitive strains of bacteria were isolated that were in the spectrum of activity of ceftolozane/tazobactam. Among 44 clinical strains of ESBL(+) Enterobacteriaceae rods, 76% were susceptible to ceftolozane/tazobactam. All 9 strains of non-carbapenemase-producing P. aeruginosa resistant or with decreased susceptibility to carbapenems were susceptible to ceftolozane/tazobactam.
Conclusions
Ceftolozane/tazobactam may be an option in the therapy of infections caused by ESBL(+) strains of Enterobacteriaceae as well as non-carbapenemase-producing carbapenem-resistant strains of P. aeruginosa.
MeSH Keywords: Carbapenems, Enterobacteriaceae, Pseudomonas aeruginosa
Background
Onco-hematological patients comprise a very important group particularly prone to infections caused by exogenous and endogenous microflora because of the course of their primary malignancy and prolonged and repetitive use of antibiotics. Among bacteria causing colonization or infection of these patients, the most common are Gram-negative rods classified in the family Enterobacteriaceae (as well as other Gram-negative rods at present classified in the order Enterobacterales). Apart from enteric rods, Gram-negative nonfermenting bacilli such as Pseudomonas aeruginosa also play a significant role in the etiology of healthcare-associated infections (HAIs) in this group of patients. In healthcare institutions, multidrug-resistant (MDR) or extensively drug-resistant (XDR) strains of Gram-positive and Gram-negative bacteria are cultured from clinical samples, often posing a significant therapeutic challenge in the management of the infections they cause [1,2].
Worldwide, the most commonly isolated strains of Gram-negative rods of the family Enterobacteriaceae are ESBL (extended-spectrum β-lactamases) – producers, particularly Klebsiella pneumoniae. Therefore, in recent years carbapenems have been increasingly used in the treatment of infections caused by ESBL-positive strains, as so called “life-saving drugs” or “last-line agents”. This has certainly contributed to the selection of bacterial strains resistant to even this group of antibiotics by production of carbapenemases and/or changes in cell wall permeability, as well as activation of efflux pumps [3].
According to the data of the European Antimicrobial Resistance Surveillance System (EARS-Net), in 2016 the highest percentage of K. pneumoniae CPE (carbapenemase-producing Enterobacteriaceae) strains was reported in Italy (33.9%) and Romania (31.4%). In Portugal it amounted to 5.2%. In contrast to these data, in the United Kingdom, Germany, France, and the Scandinavian countries, the strains of K. pneumoniae CPE comprised <1%. In Poland, the percentage of K. pneumoniae CPE strains increased from 0.5% in 2015 to 2.1% in 2016, while the percentage of E. coli CPE strains isolated from blood samples rose from 0.1% to 0.2%, respectively.
Recently, a novel therapeutic option became available in the form of a new cephalosporin – ceftolozane (representing the third-generation cephalosporins) – combined with tazobactam (an inhibitor of many β-lactamases) in the ratio of 2: 1. The ceftolozane molecule binds to the penicillin-binding proteins (PBPs) localized in the bacterial cell membrane, thus causing inhibition of synthesis of the bacterial cell wall.
Extended-spectrum β-lactamases (ESBL)
Extended-spectrum β-lactamases (ESBL) are the enzymes which are able to hydrolyze all penicillins, cephalosporins (except cephamycin), and monobactams. According to the classification based on the chemical structure, they belong to class A (subgroup 2be) and class D (subgroup 2de) of these enzymes [4]. In the literature, the rate of infections caused by ESBL-producing bacterial strains vary from 25% to even >90%, depending on the type of ward/hospital and geographical location [5,6]. The genes encoding ESBL enzymes are often located on conjugative plasmids and within the specific mobile genetic elements (e.g., transposons, integrons, and resistance islands), which contribute to their rapid spread and high rate of expression. At present, an increase in the number of infections caused by ST13 strain of E. coli producing a β-lactamase type CTX-M is observed (mainly urinary tract infections and complicated infections within the abdominal cavity) [7]. The presence of CTX-M β-lactamases means resistance to cephalosporins, and – in case of hyperproduction of this enzyme or the inoculum effect – resistance to β-lactam/β-lactamase inhibitor combinations such as amoxicillin/clavulanate, ampicillin/sulbactam, ticarcillin/clavulanate, or piperacillin/tazobactam. However, in most cases CTX-M β-lactamase is fully inhibited by tazobactam [8].
Ceftolozane/tazobactam
The chemical structure of the ceftolozane molecule was described for the first time in 2014 by Zhanel et al. [9]. It consists of 2 side-chains: R1 and R2. Chain R1 contains a molecule similar to ceftazidime, while the chain R2 is responsible for resistance of ceftolozane to hydrolytic activity of AmpC β-lactamase of P. aeruginosa [9]. The mechanism of antibacterial activity of ceftolozane depends on its binding to the penicillin-binding proteins (PBPs) of the cell membrane (mainly PBP1b and PBP3), which leads to the inhibition of the cell wall synthesis, and subsequently to the death of the bacterial cell.
Tazobactam is an inhibitor of all β-lactamases classified in class A and also several enzymes in class C. By its binding to the active center of the β-lactamase, it blocks hydrolysis of ceftolozane [10,11].
Ceftolozane/tazobactam is registered for use in the therapy of complicated urinary tract infections and acute pyelonephritis caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa. Combined with metronidazole, this drug may also be used in the therapy of complicated infections within the abdominal cavity caused by both Gram-negative bacteria (Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Proteus mirabilis, Pseudomonas aeruginosa), Gram-positive bacteria (Streptococcus anginosus, Streptococcus constellatus, Streptococcus salivarius), and strict anaerobes (Bacteroides fragilis) [12,13]. Ceftolozane/tazobactam is active against strains of the Enterobacteriaceae family, producing β-lactamases of the ESBL type (and several class A β-lactamases, including TEM-1, TEM-2, SHV-1, SHV-2, and CTX-M 14/15), and against Pseudomonas aeruginosa, including the strains which are multidrug-resistant due to the loss of porin proteins in cell membrane, overproduction of the chromosomal AmpC enzymes (mutation in PBP4), or overexpression of the efflux pumps [14–17]. However, ceftolozane/tazobactam has limited activity against Acinetobacter spp., Stenotrophomonas maltophilia, Gram-positive cocci, and Gram-negative rods of the family Enterobacteriaceae which produce β-lactamases type AmpC, carbapenemases, or metallo-β-lactamases [18].
The excellent activity of ceftolozane/tazobactam against P. aeruginosa strains is due to the fact that this combined drug is not a substrate for AmpC β-lactamases and it is not removed from the bacterial cells by the efflux pumps. Ceftolozane/tazobactam also has a higher affinity to PBPs in comparison to other antibiotics such as ceftazidime. Furthermore, the activity of ceftolozane is not diminished by a decreased permeability of the cell membranes resulting from the loss of OprD porin proteins (specific for carbapenems), which are present in P. aeruginosa [19,20].
Aim of the study
The aim of the study was to analyze the susceptibility to ceftolozane/tazobactam of the clinical strains of bacteria isolated from rectal swabs, urine, and bronchoalveolar lavage fluid (BALF) samples obtained from onco-hematological patients hospitalized in a tertiary care university-affiliated hospital.
Material and Methods
Material used included rectal swabs, urine, and bronchoalveolar lavage fluid (BALF) samples (total n=936) obtained from the hematological patients hospitalized in the Department of Internal Medicine, Hematology and Oncology (Central Clinical Hospital in Warsaw, Poland) from 01.07.2017 to 21.09.2017. The study group comprised mainly patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), or multiple myeloma (MM). In total, 281 rectal swabs and 116 urine samples were tested for the presence of Gram-negative rods producing ESBL, as well as 531 rectal swabs and 8 BALF samples, for the presence of Gram-negative rods resistant to carbapenems (Figure 1).
Figure 1.
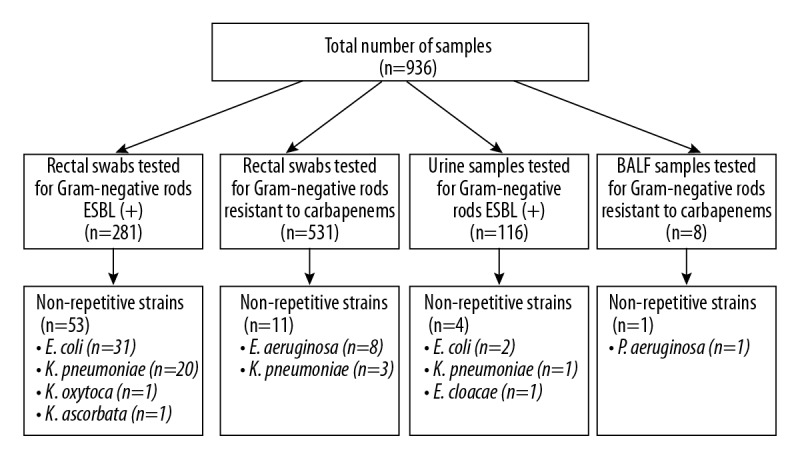
Study design.
The clinical samples were inoculated on the selective chromogenic media for preliminary isolation and identification of ESBL-producing strains (BioMaxima) and strains resistant to carbapenems due to non-enzymatic mechanism (Graso). Identification of the isolated bacteria was done by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) using the MALDI Biotyper Microflex LT (Bruker) analyzer.
Thereafter, each of the isolated strains was tested for ESBL production by the phenotypic method – the DDST (double-disk synergy test), using the following disks: ceftazidime (30 μg), cefotaxime (30 μg), and amoxicillin/clavulanic acid (20/10 μg). To increase the sensitivity of the test, disks containing aztreonam (30 μg) and cefepime (30 μg) were added.
Ceftolozane/tazobactam susceptibility of the isolates was assessed using antimicrobial gradient strips (E-test, BioMérieux). Antimicrobial susceptibility testing and interpretation of the results was done according to the current recommendations of EUCAST (the European Committee on Antimicrobial Susceptibility Testing) and KORLD (the National Reference Center for Antibiotic Resistance and Surveillance).
Results
In the analyzed period, a total of 69 non-repetitive strains of bacteria were isolated, which were in the spectrum of activity of ceftolozane/tazobactam (Figure 1). Gram-negative rods of the family Enterobacteriaceae were dominant, comprising almost 87% (n=60) of all isolates. The remaining 9 strains (13%) were identified as P. aeruginosa with resistance or decreased susceptibility to carbapenems, cultured mainly from rectal swabs (n=8) and 1 strain was isolated from the bronchoalveolar lavage fluid; all these strains were susceptible to ceftolozane/tazobactam. Among Enterobacteriaceae, ESBL production was detected in 57 strains. Among them, 33 strains (55%) were E. coli (31 strains from rectal swabs and 2 from urine) and 21 strains (35%) were K. pneumoniae, out of which 20 isolates were cultured from rectal swabs and 1 strain from urine, 1 strain (1.67%) of K. oxytoca from a rectal swab, 1 strain (1.67%) of K. ascorbata from a rectal swab, and 1 strain (1.67%) of E. cloacae from a urine sample. Regarding susceptibility to ceftolozane/tazobactam of the strains classified in the family Enterobacteriaceae, isolated from urine, 2 strains of E. coli were susceptible, while 1 strain of K. pneumoniae and 1 strain of E. cloacae were resistant to this combined formulation. Furthermore, 3 strains (5%) of K. pneumoniae were cultured from rectal swabs, which were characterized by resistance or decreased susceptibility to carbapenems. All these strains were resistant to ceftolozane/tazobactam. Among the ESBL(+) strains of Enterobacteriaceae, susceptible strains comprised 81% (n=46/57 strains), while resistance was detected in 19% (n=11/57 strains) of all isolates. Among ESBL(+) strains of E. coli, susceptibility was detected in 94% (31/33 strains), while ESBL(+) strains of K. pneumoniae were detected in 62% (13/21 strains) (Figure 2). Of note, all strains of Pseudomonas aeruginosa (n=9) that were resistant or with decreased susceptibility to carbapenems were susceptible to ceftolozane/tazobactam (Figure 3). The profile of susceptibility to ceftolozane/tazobactam of strains of the family Enterobacteriaceae and Pseudomonas aeruginosa isolated from rectal swabs is shown in Table 1.
Figure 2.
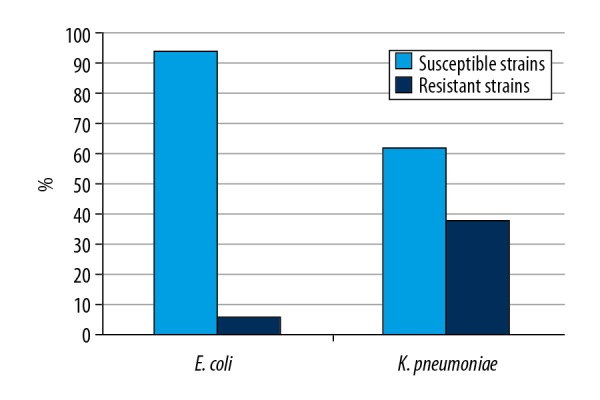
Profile of susceptibility to ceftolozane/tazobactam (%) of ESBL(+) strains of E. coli (n=33) and K. pneumoniae (n=21).
Figure 3.

Profile of susceptibility to ceftolozane/tazobactam (%) of carbapenem-resistant (non-enzymatic mechanism) clinical strains of K. pneumoniae (n=3) and P. aeruginosa (n=9).
Table 1.
Profile of susceptibility to ceftolozane/tazobactam of strains of the family Enterobacteriaceae and Pseudomonas aeruginosa isolated from rectal swabs.
| Susceptible strains (S) | Resistant strains (R) | |
|---|---|---|
| E. coli ESBL(+) | 29 (93.5%) | 2 (6.5%) |
| K. pneumoniae ESBL(+) | 13 (65.0%) | 7 (35.0%) |
| K. oxytoca ESBL(+) | 1 | 0 |
| K. pneumoniae (resistance or decreased susceptibility to carbapenems) | 0 | 3 (100.0%) |
| Kluyvera ascorbata ESBL(+) | 1 | 0 |
| P. aeruginosa (resistance or decreased susceptibility to carbapenems) | 8 (100.0%) | 0 |
Discussion
The high frequency of multidrug-resistance of Gram-negative rods to antibiotics, which spreads mainly in the hospital environment, endangers patients and may lead to many complications during the therapy of infections, including even death. Onco-hematological patients are at particularly high risk of infections, including those caused by endogenous microflora. This group comprises mainly patients with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) or multiple myeloma (MM), chronic myelomonocytic leukemia (CMML), and acute lymphoblastic leukemia (ALL), as well as different types of lymphomas. In the course of hospitalization these patients are subjected to many risk factors predisposing them to infections, including chemotherapy, hematopoietic stem cell transplantation (HSCT) and retransplantation, the use of broad-spectrum antibiotics and glucocorticoids, prolonged neutropenia, the use of central venous catheters, and graft-versus-host disease (GVHD).
Availability on the market of new antimicrobials opens new possibilities in combating microorganisms, including MDR strains. New antimicrobials that are a combination of a β-lactam antibiotic with a β-lactamase inhibitor, including ceftolozane/tazobactam, may be an option in the therapy of infections caused by ESBL(+) strains. Their use will also enable protection of carbapenems as “last-line” antimicrobials.
There are many reports in the literature indicating the effectiveness of ceftolozane/tazobactam against Gram-negative rods of the family Enterobacteriaceae. Sorbera et al. showed that ceftolozane/tazobactam has higher activity than piperacillin/tazobactam and cefepime against the strains of Enterobacteriaceae resistant to ceftazidime, while its activity against ESBL(+) strains of P. mirabilis is similar to the activity of piperacillin/tazobactam [21].
Skalweit reported that ceftolozane/tazobactam combined with metronidazole is a comparable alternative therapy in the empiric treatment of complicated infections within the abdominal cavity, similar to carbapenems, and therefore should be used as first-line therapy [12]. It was also documented that the rate of cure was higher in patients with infections caused by ESBL-producing Enterobacteriaceae treated with ceftolozane/tazobactam combined with metronidazole (95.8%) compared to meropenem (88.5%).
Titelman et al. also analyzed the activity of ceftolozane/tazobactam against ESBL-producing strains of E. coli i K. pneumoniae [22]. They showed that only 58% of tested strains were susceptible to piperacillin/tazobactam, while up to 96% of these strains were susceptible to ceftolozane/tazobactam, with an MIC value of ≤1 μg/ml. Similar results were reported by Shortridge et al. [18], who analyzed 966 isolates of E. coli and 369 strains of K. pneumoniae, in which susceptibility to ceftolozane/tazobactam was 92.2% and 5.1%, respectively. In the present study, we confirmed other published reports that in vitro activity of ceftolozane/tazobactam is higher against E. coli ESBL(+) strains than against K. pneumoniae ESBL(+) isolates [23]. Despite in vitro susceptibility of many other enteric rods to ceftolozane/tazobactam, there are no recommendations as to the clinical effectiveness of this combined formulation against Citrobacter freundii, Citrobacter koseri, Enterobacter aerogenes, Morganella morganii, Proteus vulgaris, Serratia liquefaciens, or Serratia marcescens.
In another study, Alatoom et al. compared the activity of ceftolozane/tazobactam and ceftazidime/avibactam. They reported that both these antibacterial formulations have comparable activity against Gram-negative rods of the family Enterobacteriaceae producing ESBL (97% vs. 100%, respectively) and P. aeruginosa (97% vs. 94%, respectively) [24]. Ceftolozane has excellent activity against P. aeruginosa, both alone and combined with tazobactam. This activity is at least 8-fold higher than that of ceftazidime, cefepime, or doripenem [25].
In a multicenter study performed in the USA, Shortridge et al. found that activity of ceftolozane/tazobactam against 3737 non-repetitive strains of P. aeruginosa was excellent, with 97.3% strains susceptible to this combined formulation [18]. Analysis of P. aeruginosa strains resistant to meropenem revealed that up to 88.6% of them are susceptible to ceftolozane/tazobactam. Among the MDR strains resistant to 4 groups of antibiotics (piperacillin/tazobactam, ceftazidime, cefepime and meropenem), 70.5% remained susceptible to ceftolozane/tazobactam.
Walkty et al. examined 2435 strains of P. aeruginosa isolated from patients hospitalized in different hospitals in Canada, and reported an excellent in vitro activity of ceftolozane/tazobactam against this bacterial species, with the MIC90 value of ceftolozane/tazobactam 32-fold lower than that of ceftazidime [26]. This suggests that ceftolozane/tazobactam is useful in therapy of infections caused by P. aeruginosa, including the MDR and XDR strains, resistant to piperacillin/tazobactam, antipseudomonal cephalosporins, and carbapenems [26,27].
These findings were also confirmed by Sorbera et al., who showed that ceftolozane/tazobactam is more active than piperacillin/tazobactam or imipenem against the strains of P. aeruginosa susceptible to imipenem and ceftazidime, and comparable to the activity of doripenem [23]. At the same time, ceftolozane/tazobactam maintained its activity against ceftazidime-resistant (but susceptible to imipenem) strains of P. aeruginosa. Sorbera et al. also reported that ceftolozane/tazobactam inhibited the growth of strictly anaerobic bacteria, mainly Bacteroides spp. and Prevotella spp. [21].
There are recent reports suggesting that ceftolozane/tazobactam should be considered as an alternative antibacterial agent in the empiric combined therapy of infections caused by resistant strains of P. aeruginosa, particularly in patients from the risk group who cannot be treated with aminoglycosides [28].
We found that ceftolozane/tazobactam shows activity against the clinical isolates of P. aeruginosa, including the carbapenem-resistant strains (non-carbapenemase-producing). Pfaller et al. [29] also documented that this combination is the most active β-lactam antibiotic against P. aeruginosa, and the second most powerful (after meropenem) against Gram-negative rods of the family Enterobacteriaceae. However, it was inactive or only slightly active against carbapenem-resistant strains of Enterobacteriaceae rods, which was also confirmed in our study. The limitations of the present study were lack of no analysis of the duration of the malignant disease in our patients or their prior exposure to antibiotics, which may have influenced the rate of multidrug-resistant bacteria isolated from these patients. However, we have confirmed that ceftolozane/tazobactam is highly active against the ESBL(+) clinical isolates of Enterobacteriaceae rods, particularly E. coli, as well as against the clinical strains of non-carbapenemase-producing strains of P. aeruginosa.
Conclusions
In total, 76% of ESBL(+) clinical strains of Enterobacteriaceae rods were susceptible to ceftolozane/tazobactam.
Among the ESBL(+) clinical isolates of Enterobacteriaceae rods, 94% of E. coli strains and 61.9% of K. pneumoniae strains were susceptible to ceftolozane/tazobactam.
Strains of K. pneumoniae showing resistance or decreased susceptibility to carbapenems (non-carbapenemase-producing) comprised 5% of all isolates and were totally resistant to ceftolozane/tazobactam.
In the present study, all clinical strains of non-carbapenemase-producing P. aeruginosa (13%), characterized by resistance or decreased susceptibility to carbapenems, were susceptible to ceftolozane/tazobactam.
Footnotes
Source of support: Departmental sources
Conflict of interests
Beata Sulik-Tyszka, Grzegorz W. Basak, and Marta M. Wróblewska participated in the lectures and conferences sponsored by MSD Poland.
References
- 1.Kollef MH, Golan Y, Micek ST, et al. Appraising contemporary strategies to combat multidrug resistant Gram-negative bacterial infections: Proceedings and data from the Gram-negative Resistance Summit. Clin Infect Dis. 2011;53(Suppl 2):S33–55. doi: 10.1093/cid/cir475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pan-drug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 3.Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–54. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54(3):969–76. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen DS, Schumacher H, Hansen F, et al. Extended-spectrum β-lactamase (ESBL) in Danish clinical isolates of Escherichia coli and Klebsiella pneumoniae: Prevalence, β-lactamase distribution, phylogroups, and co-resistance. Scand J Inf Dis. 2012;44(3):174–81. doi: 10.3109/00365548.2011.632642. [DOI] [PubMed] [Google Scholar]
- 6.Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkey PM. Multidrug-resistant Gram-negative bacteria: A product of globalization. J Hosp Infect. 2015;89(4):241–47. doi: 10.1016/j.jhin.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Estabrook M, Bussell B, Clugston SL, Bush K. In vitro activity of ceftolozane-tazobactam as determined by broth dilution and agar diffusion assays against recent U.S. Escherichia coli isolates from 2010 to 2011 carrying CTX-M-type extended-spectrum β-lactamases. J Clin Microbiol. 2014;52(11):4049–52. doi: 10.1128/JCM.02357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhanel GG, Chung P, Adam H, et al. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant Gram-negative bacilli. Drugs. 2014;74(1):31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 10.Bush K, Macalintal C, Rasmussen BA, et al. Kinetic interactions of tazobactam with beta-lactamases from all major structural classes. Antimicrob Agents Chemother. 1993;37:851–58. doi: 10.1128/aac.37.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassetti M, Righi E. Ceftolozane/tazobactam for the treatment of complicated urinary tract and intra-abdominal infections. Future Microbiol. 2015;10(2):151–60. doi: 10.2217/fmb.14.112. [DOI] [PubMed] [Google Scholar]
- 12.Skalweit MJ. Profile of ceftolozane/tazobactam and its potential in the treatment of complicated intra-abdominal infections. Drug Des Devel Ther. 2015;9:2919–25. doi: 10.2147/DDDT.S61436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snydman DR, McDermott LA, Jacobus NV. Activity of ceftolozane-tazobactam against a broad spectrum of recent clinical anaerobic isolates. Antimicrob Agents Chemother. 2014;58(2):1218–23. doi: 10.1128/AAC.02253-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: Results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI) Clin Infect Dis. 2015;60:1462–71. doi: 10.1093/cid/civ097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castanheira M, Deshpande LM, Costello A, et al. Epidemiology and carbapenem resistance mechanisms of carbapenem-non-susceptible Pseudomonas aeruginosa collected during 2009–11 in 14 European and Mediterranean countries. J Antimicrob Chemother. 2014;69(7):1804–14. [Google Scholar]
- 16.Sader HS, Farrell DJ, Castanheira M, et al. Antimicrobial activity of ceftolozane/tazobactam tested against Pseudomonas aeruginosa and Enterobacteriaceae with various resistance patterns isolated in European hospitals (2011-12) J Antimicrob Chemother. 2014;69:2713–22. doi: 10.1093/jac/dku184. [DOI] [PubMed] [Google Scholar]
- 17.Giacobbe DR, Bassetti M, De Rosa FG, et al. ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). Ceftolozane/tazobactam: Place in therapy. Expert Rev Anti Infect Ther. 2018;9:1–14. [Google Scholar]
- 18.Shortridge D, Pfaller MA, Castanheira M, Flamm RK. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2013–2016) as part of the surveillance program: Program to assess ceftolozane/tazobactam susceptibility. Microb Drug Resist. 2018;24(5):563–77. doi: 10.1089/mdr.2017.0266. [DOI] [PubMed] [Google Scholar]
- 19.Moya B, Zamorano L, Juan C, et al. Affinity of the new cephalosporin CXA-101 to penicillin-binding proteins of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54:3933–37. doi: 10.1128/AAC.00296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda S, Nakai T, Wakai Y. In vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2007;51:826–30. doi: 10.1128/AAC.00860-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorbera M, Chung E, Ho CW, Marzella N. Ceftolozane/tazobactam: A new option in the treatment of complicated Gram-negative infections. Pharm Ther. 2014;39(12):825, 828–32. [PMC free article] [PubMed] [Google Scholar]
- 22.Titelman E, Karlsson IM, Ge Y, Giske CG. In vitro activity of CXA-101 plus tazobactam (CXA-201) against CTX-M-14- and CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae. Diagn Microbiol Infect Dis. 2011;70:137–41. doi: 10.1016/j.diagmicrobio.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Popejoy MW, Paterson DL, Cloutier D, et al. Efficacy of ceftolozane/tazobactam against urinary tract and intra-abdominal infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae: A pooled analysis of Phase 3 clinical trials. J Antimicrob Chemother. 2017;72:268–72. doi: 10.1093/jac/dkw374. [DOI] [PubMed] [Google Scholar]
- 24.Alatoom A, Hashim E, Lawlor K, et al. Comparison of antimicrobial activity between ceftolozane–tazobactam and ceftazidime–avibactam against multidrug-resistant isolates of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Int J Infect Dis. 2017;62:39–43. doi: 10.1016/j.ijid.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Moya B, Dotsch A, Juan C, et al. Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 2009;5:e1000353. doi: 10.1371/journal.ppat.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walkty A, Karlowsky JA, Adam H, et al. In vitro activity of ceftolozane/tazobactam against Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals in the CANWARD Study, 2007 to 2012. Antimicrob Agents Chemother. 2013;57(11):5707–9. doi: 10.1128/AAC.01404-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodlet KJ, Nicolau DP, Nailor MD. Ceftolozane/tazobactam and ceftazidime/avibactam for the treatment of complicated intra-abdominal infections. Ther Clin Risk Manag. 2016;12:1811–26. doi: 10.2147/TCRM.S120811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodlet KJ, Nicolau DP, Nailor MD. In vitro comparison of ceftolozane/tazobactam to traditional beta-lactams and ceftolozane/tazobactam as an alternative to combination antimicrobial therapy for Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61(12):e01350–17. doi: 10.1128/AAC.01350-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller MA, Bassetti M, Duncan LR, Castaheira M. Ceftolozane/tazobactam activity against drug-resistant Enterobacteriaceae and Pseudomonas aeruginosa causing urinary tract and intraabdominal infections in Europe: Report from an antimicrobial surveillance programme (2012–2015) J Antimicrob Chemother. 2017;72:1386–95. doi: 10.1093/jac/dkx009. [DOI] [PubMed] [Google Scholar]


