ABSTRACT
Background/purpose: Plantar foot pain of neural origin is a challenging diagnosis to identify and treat. The purpose of this paper is to illustrate the novel way in which cupping was utilized in conjunction with neural glides to better diagnose and manage a patient who presented with symptoms of peripheral neuropathic plantar foot pain.
Case description: A 65-year-old male presented to physical therapy with the diagnosis of plantar fasciitis by an orthopedic surgeon. The presentation included a diffuse area of pain toward the medial border of the foot with a peripheral neuropathic pain description. Cupping was used to identify pain in the saphenous nerve distribution and aided in resolving symptoms with the concomitant use of lower quarter neural glides.
Outcome: At discharge and 1-year follow-up, the patient had a full resolution of symptoms and a return to prior level of function. Self-report outcomes included the numeric pain rating scale and the lower extremity functional scale.
Discussion: This case is the first to describe the use of cupping combined with neural glides in the diagnosis and management of peripheral neuropathic pain from the saphenous nerve that was previously diagnosed as plantar fasciitis. The proposed mechanisms behind this treatment are also reviewed.
Conclusion: In patients that present with symptoms of plantar fasciitis, testing neural glides combined with cupping may be warranted to confirm or refute the presence of a peripheral neuropathic pain source. Further studies are necessary to determine the mechanisms and further utility of the combined interventions in well controlled trials.
Level of Evidence: Level IV
KEYWORDS: Cupping, neuropathic, neurodynamics, saphenous, manual, therapy, IASTM, nerve
Plantar foot pain is a common symptom for patients seeking care in a physical therapy setting. Additionally, physical therapists use cupping as a tool in the management of soft tissue dysfunction. However, there is a paucity of evidence regarding the utilization of cupping combined with neural glides to identify and treat peripheral neuropathic plantar foot pain mimicking plantar fasciitis. Therefore, the purpose of this case study is to describe a novel method of identifying and treating neuropathic plantar foot pain from the saphenous nerve utilizing cupping with neural glides. The patient in this case was a 65-year-old male misdiagnosed with plantar fasciitis that presented with pain in a more diffuse area toward the medial border of the foot, and not in the classical distribution of plantar fasciitis. Cupping combined with neural glides was used to identify and resolve pain in the saphenous nerve distribution. The patient had positive outcomes on the Numeric Pain Rating Scale: Initial 9/10 at worst, 2nd visit 2/10 worst, Discharge 0/10 worst, 1-year follow-up 0/10 worst. He also met had outstanding improvements in his Lower Extremity Functional Scale (LEFS): Initial 56/80, Discharge 78/80, 1-year follow-up 80/80, reaching the minimal clinically important difference of 9 points. Importantly, he was able to return to all functional activities. This case highlights the utility of using combined cupping with neural glides to identify and treat peripheral neuropathic pain within the saphenous nerve distribution that was mimicking plantar fasciitis.
Background
Plantar foot pain is a common disorder that may affect up to 2 million Americans per year, with a peak incidence occurring between ages 40 and 60 years, and prevalence of 3.6% to 7% in the general population [1,2]. It is considered the most common foot condition seen by health care providers [3]. In light of this, appropriate diagnosis is needed for optimal management [3]. Additionally, it is important to accurately identify this condition vs. conditions that also produce plantar foot pain, such as heel pad atrophy, Baxter’s nerve entrapment, calcaneal stress fractures, tarsal tunnel syndrome, and saphenous neuropathy [1,4].
In this case study, we focused our attention on a patient who was initially diagnosed with plantar fasciitis but found to have symptoms of neuropathy in the saphenous nerve distribution. The saphenous nerve has a broad distribution that begins at the medial knee and extends to the medial and slightly plantar aspect of the foot anterior to the calcaneus [4] (Figure 1). Symptoms have been described as burning or shooting with saphenous nerve pathology, including swelling or sensitivity to palpation [5,6]. This differs from the symptoms classically found with plantar fasciitis on the plantar surface of the foot and generally has an exquisite point of local tenderness to palpation at the proximal plantar fascia insertion [3]. Additionally, this patient described symptoms that hinted at a peripheral neuropathic dominance in his pain presentation (Table 1) that led to a trial of cupping combined with nerve glides.
Figure 1.
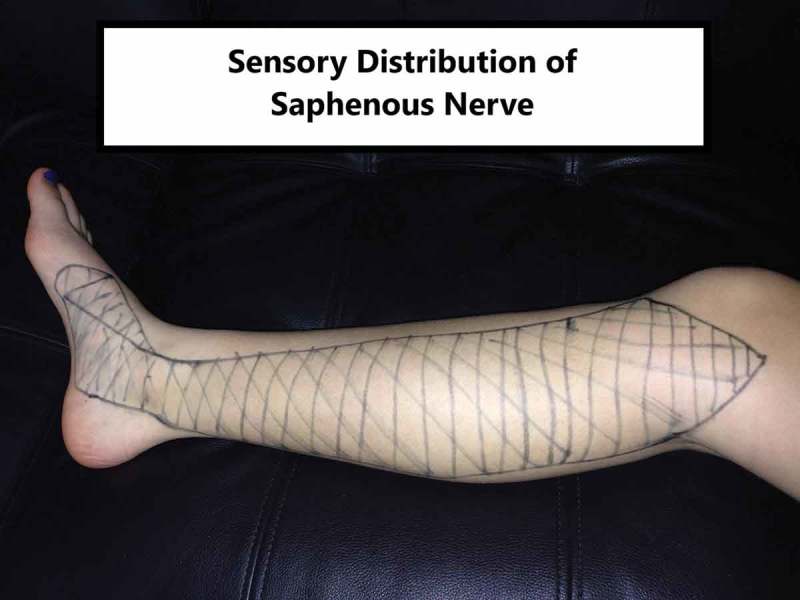
Saphenous nerve distribution.
Table 1.
| Nociceptive category | Peripheral neuropathic category |
|---|---|
| Clear, proportionate mechanical nature to aggravating and easing factors | Pain descriptions of burning, shooting, sharp, or electric-shock-like |
| Pain associated with and in proportion to trauma, pathology, or movement process | Medical history that includes nerve injury |
| Pain localized to the area of dysfunction | Pain also presents with neurological symptoms like pins/needles, numbness, weakness; dermatome distribution |
| Usually resolves in accordance with expected tissue healing time frames | Decreased responsiveness to simple analgesia (NSAIDs), increased responsiveness to anti-epileptic (Neurontin/Lyrica) or anti-depression (amitriptyline) medication |
| Usually intermittent pain and sharp with movement/mechanical provocation; may be dull ache at rest | Presents with high severity and irritability |
| Localized pain on palpation | Aggravating and easing descriptors are associated with movements to neural tissue |
Cupping therapy is a soft tissue treatment method that has been identified as being in use since 3000 BCE in which glass, plastic, or bamboo cups are used [8]. Physical therapists perform ‘dry cupping’, which is a non-invasive procedure used for modulating pain and movement in a patient [8]. This is contrary to ‘wet cupping’ which places small incisions on the treatment area in order to cause bleeding [8]. Cupping treatment by physical therapists is also considered either ‘static’ in which the cup applies a stress over a soft tissue structure, or ‘dynamic’ in which these stresses are applied in a moving fashion over a larger area [9]. Static cupping commonly leaves the suction cups in place for 5–40 min [8].
Cupping therapy has a local biomechanical effect on tissue, in which a negative pressure causes tension under the surface of the cup in the center, while the rim of the cup compresses the soft tissue under it [10]. This pressure is greatest in the center of the cup and tensile stresses have been shown to extend down to the muscle layer of tissue at this area [10]. Additionally, larger cups have been found to exert greater tension force to a deeper area than a smaller cup [10]. This biomechanical effect can sometimes cause erythema, edema, and ecchymosis at the placement site, and the longer the cup is placed on the skin, the more prominent the ecchymosis can become. These bruises have been reported to diminish over the course of several days or weeks depending on the time frame of application and intensity of cupping pressure [8].
With regard to cupping pressure, the intensity of this pressure has been classified by Al-Bedah et al. [9] Categories are described as either light, medium, or strong, and ranges between 100 and less than 300 millibar (1–2 manual pumps), 300 and less than 500 millibar (3–4 manual pumps), and greater than 500 millibar (5 or more manual pumps), respectively [9]. The higher the intensity of the suction and the longer the cups are placed on the skin has been correlated with greater levels of skin discoloration [8].
In certain schools of thought, static cups are placed at areas called acupoints to elicit effects on pain or other aspects of health [8]. Decision-making on why acupoints are utilized are based off of the background of the practitioner, and there is often carryover from areas identified in Traditional Chinese Medicine (TCM) as acupoints, areas known as Myofascial Trigger Points (MTP), and areas of higher neurovascular sensitivity due to local anatomy [8,11]. Placing a suction cup over an acupoint or MTP is theorized to have an analgesic effect locally and globally via several mechanisms [8]. Cupping over an MTP or acupoint can produce stimulation of small diameter nerves in muscles that send impulses to higher centers of pain control to block nociceptive information. Additional mechanisms have been proposed that include local release of endorphins, serotonin or cortisol for pain relief, local changes in vascularity, and immune system response to local stimulation over a painful area [8].
This case utilized static cupping while the patient moved his limb to elicit a remote effect. This method has been found in the primary author’s experience to augment the effects of neurodynamic testing and treatment in a manner that is clearer to the clinician and patient. There is no evidence describing this method in the literature; therefore, it is being described for the current case study.
Case description
The patient was a 65-year-old male referred to physical therapy from an orthopedic surgeon with a diagnosis of right plantar fasciitis and acquired short Achilles tendon. Written consent was received by the patient for participation in this case study. The patient presented with symptoms of medial plantar foot pain with onset 3.5 months prior that had failed conservative physical therapy treatment including heel cord stretching, intrinsic muscle strengthening, and cryotherapy to the plantar surface of the foot. The pain was described as a sharp pain of insidious onset at the medial plantar surface of the foot with weight bearing. This was rated at a 9/10 at worst on the Numeric Pain Rating Scale [12], and 0/10 in sitting during the subjective portion of the examination. Aggravating tasks included walking barefoot, walking long distances with shoes, golf, and stair navigation. Relieving activities included calf stretching in standing with the lower extremity in a grossly externally rotated position. The LEFS [13] was collected at baseline with a score of 56/80 (70%). This scale has been shown to have excellent reliability and validity in patients with lower extremity injuries [13].
The patient reported no history of cardiovascular disease, diabetes, cancer, or sexually transmitted diseases. The patient denied tobacco use and exercise consisted of walking and golf 4 days per week. The patient denied any recent fevers, numbness or tingling, night sweats, unexplained weight change, bowel/bladder changes, or red flags as described by Boissonnault [14]. The patient was screened for depression symptoms with a ‘no’ response for the two-question screen described by Arrol et al. [15] The patient noted that he had been prescribed Naprosyn at a dose of 500 mg daily, and this had minimal effect on his symptoms. Additionally, the patient noted a history of low back pain with right-sided radiculopathy at an unknown level that had resolved several years previously without medical management. His goals were to eliminate pain in the foot, return to walking, and perform asymptomatic golf.
On initial assessment, the patient indicated an area of pain on the plantar surface of the foot. However, when questioned about symptom distribution, the patient indicated a broader area of pain including portions of the medial foot/heel. The objective exam noted mild to moderate mobility & range of motion (ROM) limitations in several areas (Table 2), although these were not correlated with symptom production. The screening examination found neurodynamic testing to have relevant findings (Table 2), but no signs of radiculopathy or referral from proximal structures or familiar pain. Lumbar screening included ROM testing and symptom provocation testing in a posterior–anterior (PA) force direction with the patient in prone. Palpation examination identified specific areas of painful cutaneous mobility deficits along the saphenous nerve distribution, but not in the areas classically known for plantar fasciitis (Table 2 and Figure 1).
Table 2.
Objective examination and findings.
| Examination technique | Finding |
|---|---|
| Talocrural anterior–posterior (AP) mobility | Right = moderate |
| Left = mild | |
| Great toe extension mobility | Mild mobility deficit bilaterally |
| Windlass test | Painless bilaterally |
| Dorsiflexion ROM | Left = 10 |
| Right = 5 | |
| Plantarflexion ROM | Left = 45 |
| Right = 40 | |
| Palpation findings | Superior Medial Border of Tibia Increased Familiar Pain |
| Painless at the Medial Calcaneal Tubercle or Quadratus Plantae | |
| Skin rolling | Tender Along the Medial Shank |
| Myotome examination | Intact and equal for the L2-S1 levels bilaterally |
| Lumbar mobility examination | Posterior–anterior (PA) testing unremarkable |
| Straight leg raise examination | Left = 65° with painless hamstring tension |
| Right = 55° with painless hamstring tension | |
| Prone knee bend examination | Negative pain production bilaterally |
| Slump examination | Left = negative pain production |
| Right = familiar pain production in distal saphenous nerve distribution | |
| Hip internal rotation ROM | Left = 15 |
| Right = 10 | |
| Hip manual muscle examination | Abduction left = 5/5 |
| Abduction right = 4/5 | |
| Single leg stance examination | Compensated Trendelenburg with decreased balance on the left |
At this point, a peripheral neuropathic source of pain was suspected vs. a local nociceptive source due to a lack of responsiveness to non-steroidal anti-inflammatory drugs (NSAIDs), a history of radiculopathy on the same side, abnormal findings with neural tension testing, and a lack of common findings that are classically found with plantar fasciitis [3]. The patient was asked to walk barefoot for a pre-intervention assessment of an aggravating task. He was pain-limited to three steps.
The primary intervention chosen was a neural glide in the sitting position (Figure 2) using a 4.5mm diameter cup placed statically at the superior medial tibia at the proximal distribution of the saphenous nerve, using an intensity known as ‘medium cupping’ per by Al-Bedah et al. [9]. This site is close to a common acupoint called Spleen – 9 (SP-9), (2). A large cup was used at this site in order to produce higher stress at the interface between the fat and muscle layers [10].
Figure 2.
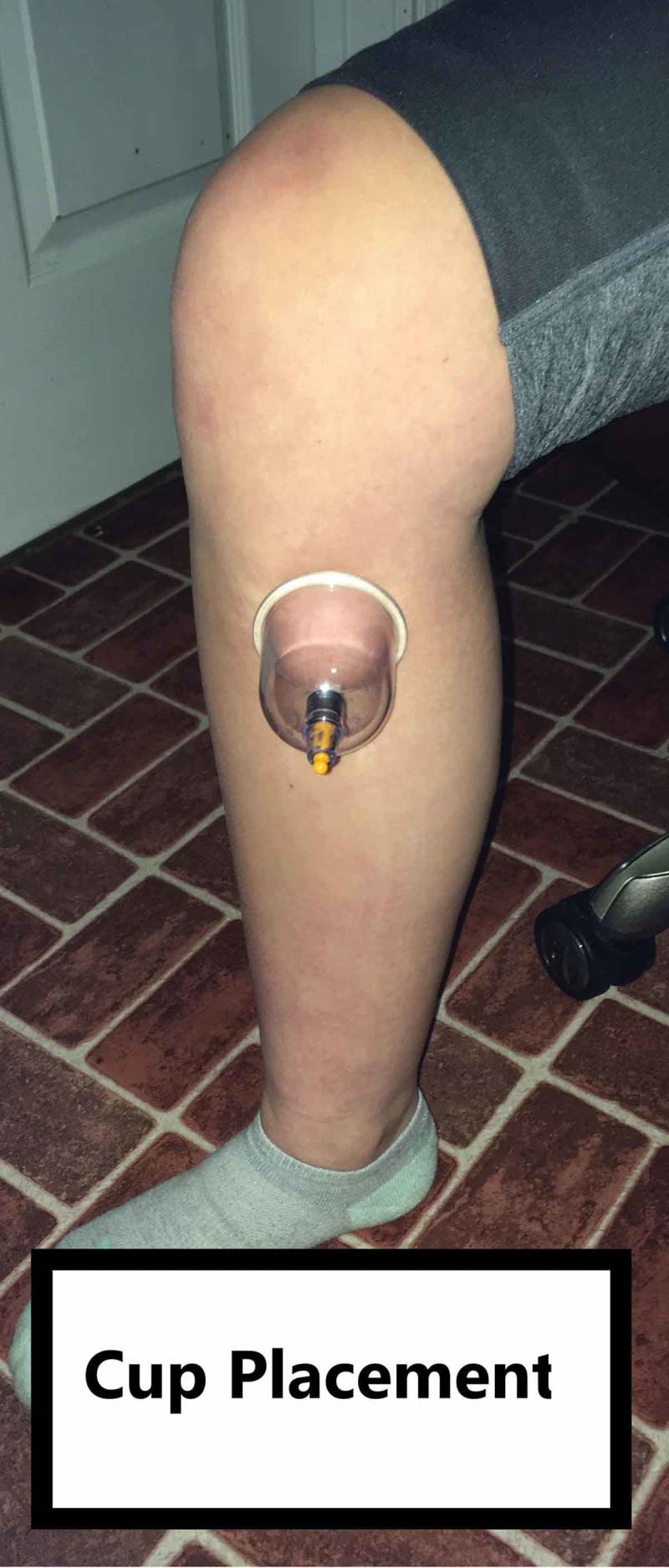
Cup on medial proximal tibia.
The cup produced local tenderness when the suction was applied. The patient was then instructed to extend his knee with ankle dorsiflexion with the cup maintained at SP-9 via negative pressure from the suction. The patient felt a strong pulling sensation in the cup during knee extension with ankle dorsiflexion in sitting that limited his knee extension ROM without causing plantar foot pain. This sensation was also increased when a slump-sitting position was added to the position without cervical flexion. The addition of cervical flexion further added to the pull at the cup. The patient first alternated between plantarflexion and dorsiflexion in sitting with the knee flexed as this reproduced pain under the cup. After this pain resolved, repeated knee extension was performed until the pain under the cup had resolved again. These motions were fully advanced into the end range of a slump-sitting position. Following this activity, the patient was able to gain full slump ROM without pain under the cup.
This treatment was repeated with similar findings with a cup placed on an area 4 fingerbreadths proximal to the medial malleolus, near acupoint Spleen – 6, secondary to local tenderness, and tissue motion restriction in this area. When the activity was again painless, post-test ambulation assessment was performed and the patient reported significant pain improvement during barefoot walking.
Outcomes
The patient was seen for 5 total visits in a period of 3 weeks. At discharge, the patient noted 0/10 pain on the NPRS, full ROM in the foot, equal strength and ROM in both hips, and clinically relevant improvement in his outcome measures from baseline. The LEFS score improved to 66/80 (82.50%) on treatment day 2 and improved to 78/80 (97.50%) at discharge. At the 1-year follow-up, the patient’s pain was rated at 0/10 at worst, and reported an LEFS score of 80/80. See Table 3 for details regarding symptom and functional progression, as well as treatment provided each session.
Table 3.
Numeric pain rating scale, lower extremity functional scale, treatments, and home exercise program for patient visits.
| Visit sequence | NPRS current (sitting position) | NPRS worst | LEFS | Treatment | Home exercise program |
|---|---|---|---|---|---|
| Initial visit | 0/10 | 9/10 | 56/80 | Static cupping at the medial knee at two sites with neural glides | Neural glides in sitting and supine |
| Visit 2 | 0/10 | 2/10 | 66/80 | Static cupping at the medial knee at two sites with heel raises and lateral step-ups | Neural glides, heel raises, and lateral step-ups |
| Visit 3 | 0/10 | 2/10 | 77/80 | Static cupping at the medial knee at two points with neural glides, followed by heel raises and lateral step-ups with static cupping at two sites | Neural glides, heel raises, and lateral step-ups |
| Visit 4 | 0/10 | 10-Feb | 77/80 | Manual therapy directed at hip mobility ipsilaterally followed by CKC hip exercise. Functional position neural mobilization. |
Neural glides, heel raises, and lateral step-ups. Re- introduction of golf swing after home exercise program |
| Visit 5 | 0/10 | 0/10 | 78/80 | Written home exercise review plan for daily activity and pre- golf warm-up routine | Neural glides, calf stretches, hip stretches, hip dynamic warm-up |
| One-year follow-up | 0/10 | 0/10 | 80/80 | N/A | N/A |
Discussion
This report describes a case of recalcitrant plantar foot pain that was misdiagnosed as plantar fasciitis. Several items were helpful in the clinical decision-making process.
The patient noted intermittent sharp pain with mechanical provocation, and clear aggravating activities, which initially could indicate per Smart et al. [16,17] (Table 2) a nociceptive pain source associated with chronic plantar fasciitis. However, pain was not localized to the area of injury classically associated with plantar fasciitis (medial calcaneal tubercle). Though there was a mild restriction in dorsiflexion ROM, the windlass test was negative for reproduction of symptoms or first toe mobility restriction. Per this line of testing, there was a decreased likelihood of plantar fasciitis [3]. Though elements of his testing had nociceptive qualities, the dominant feature appeared to be leaning toward a peripheral neuropathic source of pain.
Moving forward, an important component of the patient’s medical history was a history of lumbar radiculopathy as this can potentially create future susceptibility to injury in the affected nerve distribution [4,18,19]. The patient’s failure of conservative treatment for plantar fasciitis was also noteworthy. According to Aslan et al. [20] ‘if conservative treatments fail, neurologic causes should be considered’ in cases of heel pain. The patient also reported relief in a position that is described by Shacklock as a method to assess the saphenous nerve [21]. In the objective exam, notable findings stood out that guided the next step of treatment. These were a limited ipsilateral ROM in the slump test that produced tension outside of what is considered a normal finding, painful skin traction in the distribution of the saphenous nerve, and an absence of signs that are classically associated with plantar fasciitis [3,21,22]. After considering these findings, there was an increased likelihood of the primary pain presentation to be peripheral neuropathic in origin, and treatment moved forward to target neural tissue vs. the plantar fascia.
The use of neural glides with static cupping was utilized as the primary intervention to improve the effects of treatment and to assist in refuting the original diagnosis of plantar fasciitis. The hypothesis was the saphenous nerve was the source of peripheral neuropathic symptoms, therefore the combination of a neural glide with the static cup was chosen. This intervention can allow for local nerve and soft tissue stimulation during a slump motion that was perceived as threatening by the nervous system to now receive a counter-irritant stimulation to potentially alter previous output & change pain with movement [8,10,23]. As has been discussed previously, static cupping can produce tension force down to the muscle layer [10]. The site of the cupping was noted to have exquisite tenderness when tissue tension was applied & this hyperalgesia may be due to sensitized primary afferent nerve endings or keratinocytes in a heightened stated of abnormal sensory transmission [24,25], The site of cupping chosen is a location in which the sartorial branch of the saphenous nerve is superficial and can be reached by tension from a large cup [26]. The placement of the cup over a site of increased sensitivity can allow for analgesic stimulation with soft tissue tension to occur during gross neural movement [8]. Since the cup did indeed lose painful tension as the neural glide repetitions increased, this seems like a logical hypothesis. The repeated neural glides added to the effect of decreased pain and skin relaxation from their effects on local and systemic neural changes. Sympathetic nervous system and dorsal periaqueductal gray stimulation occurs with neural mobilization, and sequelae such as dermal sudomotor changes, skin conductance, and skin temperature, increase motor movement, and non-opioid analgesia can all occur [27,28]. The combined effect of the neural glide on skin mechanics and central analgesia, with the local effect on the keratinocytes and sensitized primary nerve endings may have been the underlying reasons why the combination of the two treatments can be more effective than either in isolation for the symptom resolution and ultimate confirmatory diagnosis of saphenous neuropathy vs. plantar fasciitis.
Pragmatically, the primary author has found that cupping with neural glides improves the efficiency of this intervention and decision-making process. This is because the tension in the cup will decrease considerably at the point in which the mechanical tissue sensitivity in that nerve distribution decreases. This allows the clinician and patient to know a treatment had its desired effect. Since the use of a neural glide in isolation as a treatment can have variable results, cupping with neural glides improved the chance there would be a positive treatment response from a combined intervention [29,30].
One suspected reason for this type of pain to occur after playing golf may be that the end of the golf swing mimics a position that can increase tension in the saphenous nerve (Figure 3). Additionally, some authors suggest that the foot is involved in pathology of the distal branch of the saphenous nerve [6,31]. This is theorized to be due to repetitive traction, acute traumatic traction, or entrapment in scar tissue. In these scenarios, the injury will not present with motor deficits (similar to the current case) [31,32]. Though this mechanism could be at play for the case described, it is unknown why the golf event in question caused the pain complaints when previous games did not.
Figure 3.
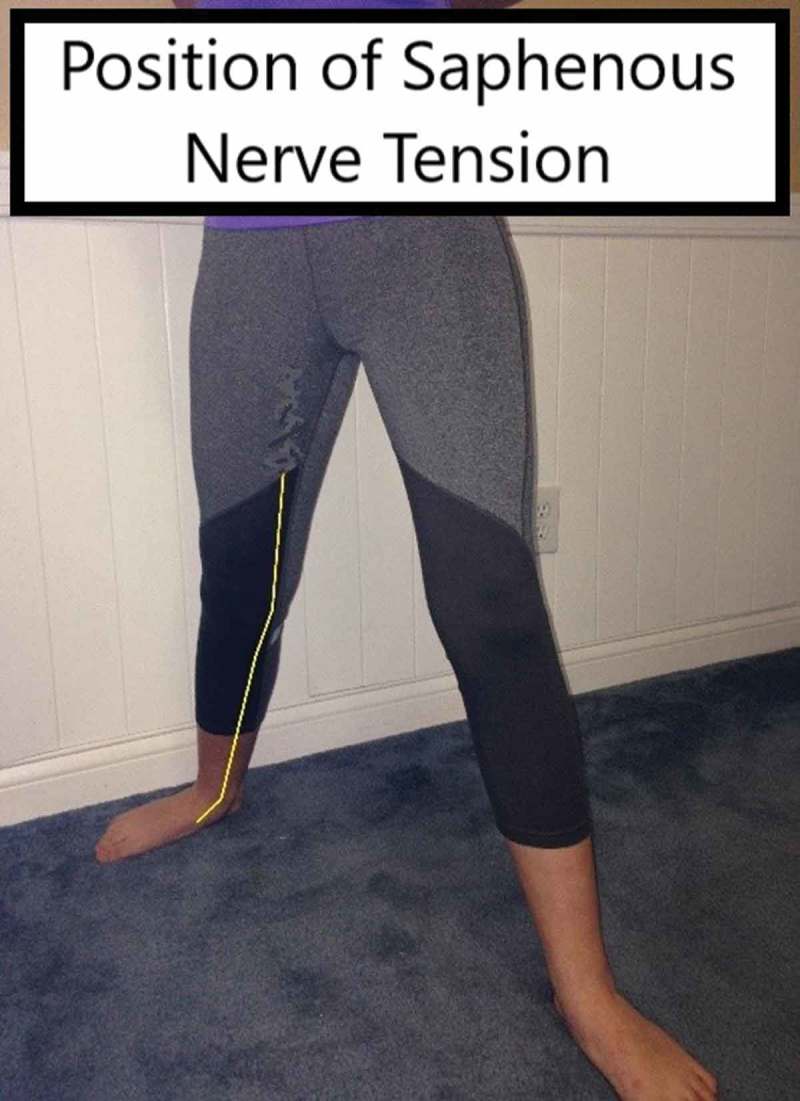
Tension position of saphenous nerve.
Several elements of neural physiology, biomechanics, and pathology stand out in hypothesizing why the neural tissue was in the described state. There is a ‘length-dependent theory’, which states longer nerves are more likely to suffer trauma, peripheral nerve edema can be caused by mild traction injuries, previous injury to a nerve can affect the blood-nerve barrier distal from the site of injury decreasing firing threshold, the endoneurium has a lack of lymphatic vessels to aid with edema resolution, and longer sensory nerves are more vulnerable to traction injuries than motor axons [18,19,33–35]. These factors can potentially lead to fibrosis, adhesions, impaired intrafascicular gliding, and intraneural thickening [36]. Somatosensory changes and nerve sheath inflammation may can occur in the absence of severe axonal damage and significant morphological changes [33]. In a patient with chronic symptoms that limit motion due to pain (as in this case), this could prevent independent resolution of symptoms.
Conclusion
In patients that present with symptoms of plantar fasciitis, a trial of cupping with neural glides may be helpful to confirm or refute the presence of peripheral neuropathic pain stemming from the saphenous nerve distribution. This case can serve as a model for future studies hoping to identify pain referred to the foot from a peripheral nerve source. Ultimately, the results of this case study need to be viewed with caution as this is a case report without a control group. Additionally, with the combination of two interventions it is impossible to know which may have been the more effective treatment or if both were needed simultaneously. This is a limitation to the firm conclusion of the cause and effect relationship of the intervention to the outcome. Lastly, nociceptive and peripheral neuropathic pain types can be present simultaneously, and uncertainty can be present in this decision-making process [20,37]. As the gold standard for small fiber testing is clinically infeasible, it adds further limitation to the conclusions of this case [38]. For more broad recommendations to be made, more robust studies need to be performed to confirm these clinical findings.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Lareau CR, Sawyer GA, Wang JH, et al. Plantar and medial heel pain: diagnosis and management. J Am Acad Orthop Surg. 2014June;22(6):372–380. PubMed PMID: 24860133. [DOI] [PubMed] [Google Scholar]
- [2].Caratun R, Rutkowski N, Finestone H.. Stubborn heel pain: treatment of plantar fasciitis using high-load strength training. Can Fam Physician. 2018;64:1. [PMC free article] [PubMed] [Google Scholar]
- [3].Martin RL, Davenport TE, Reischl SF, et al. Heel pain-plantar fasciitis: revision 2014. J Orthop Sports Phys Ther. 2014November;44(11):A1–33. PubMed PMID: 25361863. [DOI] [PubMed] [Google Scholar]
- [4].McCrory P, Bell S, Bradshaw C.. Nerve entrapments of the lower leg, ankle and foot in sport. Sports Med. 2002;32(6): 371–391. PubMed PMID: 11980501. [DOI] [PubMed] [Google Scholar]
- [5].Morrison C, Dalsing MC. Signs and symptoms of saphenous nerve injury after greater saphenous vein stripping: prevalence, severity, and relevance for modern practice. J Vasc Surg. 2003;38(5):886–890. [DOI] [PubMed] [Google Scholar]
- [6].Brown MN, Pearce BS, Karl HW, et al. Distal saphenous nerve entrapment In: Peripheral nerve entrapments. Cham: Springer. 2016. p. 645–654. [Google Scholar]
- [7].Smart KM, Blake C, Staines A, et al. Clinical indicators of ‘nociceptive’, ‘peripheral neuropathic’ and ‘central’ mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Man Ther. 2010February;15(1):80–87. PubMed PMID: 19679504. [DOI] [PubMed] [Google Scholar]
- [8].Rozenfeld E, Kalichman L. New is the well-forgotten old: the use of dry cupping in musculoskeletal medicine. J Bodyw Mov Ther. 2016January;20(1):173–178. . PubMed PMID: 26891653. [DOI] [PubMed] [Google Scholar]
- [9].Al-Bedah AAT, Alqaed M, Qureshi N, et al. Classification of Cupping Therapy: A tool for modernization and standardization. J Complement Altern Med Res. 2016;1(1):1–10. [Google Scholar]
- [10].Tham LM, Lee HP, Lu C. Cupping: from a biomechanical perspective. PubMed PMID: 16126216J Biomech. 2006;3912:2183–2193. [DOI] [PubMed] [Google Scholar]
- [11].Hong C. Myofascial trigger points: pathophysiology and correlation with acupuncture points. Acupuncture Med. 2000;18:1. [Google Scholar]
- [12].Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986October;27(1):117–126. PubMed PMID: 3785962. [DOI] [PubMed] [Google Scholar]
- [13].Binkley JM, Stratford PW, Lott SA, et al. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther. 1999April;79(4):371–383. PubMed PMID: 10201543. [PubMed] [Google Scholar]
- [14].Boissonnault WG. Primary care for the physical therapist: examination and triage. 2nd ed. St. Louis, MO: Elsevier/Saunders; 2011. [Google Scholar]
- [15].Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. Bmj. 2003November15;327(7424):1144–1146. . PubMed PMID: 14615341; PubMed Central PMCID: PMC261815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Smart KM, Blake C, Staines A, et al. The discriminative validity of “nociceptive,” “peripheral neuropathic,” and “central sensitization” as mechanisms-based classifications of musculoskeletal pain. Clin J Pain. 2011October;27(8):655–663. PubMed PMID: 21471812. [DOI] [PubMed] [Google Scholar]
- [17].Smart KM, Blake C, Staines A, et al. Clinical indicators of ‘nociceptive’,‘peripheral neuropathic’and ‘central’mechanisms of musculoskeletal pain. Delphi Survey Expert Clinicians Manual Therapy. 2010;15(1):80–87. [DOI] [PubMed] [Google Scholar]
- [18].Stewart JD. Peripheral nerve fascicles: anatomy and clinical relevance. Muscle & Nerve. 2003November;28(5):525–541. . PubMed PMID: 14571454. [DOI] [PubMed] [Google Scholar]
- [19].Lim TK, Shi XQ, Martin HC, et al. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. Pain. 2014May;155(5):954–967. PubMed PMID: 24502843. [DOI] [PubMed] [Google Scholar]
- [20].Ferraro F, Jacopetti M, Spallone V, et al. Diagnosis and treatment of pain in plexopathy, radiculopathy, peripheral neuropathy and phantom limb pain. Evidence and recommendations from the Italian Consensus Conference on Pain on Neurorehabilitation. Eur J Phys Rehabil Med. 2016;52(6):855-866. [PubMed] [Google Scholar]
- [21].Shacklock M. Clinical neurodynamics: a new system of musculoskeletal treatment. Edinburgh: Elsevier; 2005. [Google Scholar]
- [22].Majlesi J, Togay H, Unalan H, et al. The sensitivity and specificity of the slump and the straight leg raising tests in patients with lumbar disc herniation. J Clin Rheumatol. 2008April;14(2):87–91. PubMed PMID: 18391677. [DOI] [PubMed] [Google Scholar]
- [23].Sierra-Silvestre E, Bosello F, Fernández-Carnero J, et al. Femoral nerve excursion with knee and neck movements in supine, sitting and side-lying slump: an in vivo study using ultrasound imaging Musculoskeletal Science and Practice. 2018. Oct;37:58–63. [DOI] [PubMed] [Google Scholar]
- [24].Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010August;9(8):807–819. PubMed PMID: 20650402. [DOI] [PubMed] [Google Scholar]
- [25].Baumbauer KM, DeBerry JJ, Adelman PC, et al. Keratinocytes can modulate and directly initiate nociceptive responses. Elife. 2015September02;4:e09674 PubMed PMID: 26329459; PubMed Central PMCID: PMC4576133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Horn J-L, Pitsch T, Salinas F, et al. Anatomic basis to the ultrasound-guided approach for saphenous nerve blockade. Reg Anesth Pain Med. 2009;34(5):486–489. [DOI] [PubMed] [Google Scholar]
- [27].Vicenzino B, Collins D, Wright T. Sudomotor changes induced by neural mobilisation techniques in asymptomatic subjects. J Man Manipulative Ther. 1994;2(2):66–74. [Google Scholar]
- [28].Jowsey P, Perry J. Sympathetic nervous system effects in the hands following a grade III postero-anterior rotatory mobilisation technique applied to T4: a randomised, placebo-controlled trial. Man Ther. 2010June;15(3):248–253. . PubMed PMID: 20093065. [DOI] [PubMed] [Google Scholar]
- [29].Stener-Victorin E, Kruse-Smidje C, Jung K. Comparison between electro-acupuncture and hydrotherapy, both in combination with patient education and patient education alone, on the symptomatic treatment of osteoarthritis of the hip. Clin J Pain. 2004May-Jun;20(3):179–185. PubMed PMID: 15100594. [DOI] [PubMed] [Google Scholar]
- [30].Bialosky J, Bishop M, Price D, et al. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. JOSPT. 2009;39(10):709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hunter LY, Louis DS, Ricciardi JR, et al. The saphenous nerve: its course and importance in medial arthrotomy. Am J Sports Med. 1979Jul-Aug;7(4):227–230. PubMed PMID: 474860. [DOI] [PubMed] [Google Scholar]
- [32].Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014Sep-Oct;13(5):299–306. . PubMed PMID: 25211617. [DOI] [PubMed] [Google Scholar]
- [33].Gilbert KK, Roger James C, Apte G, et al. Effects of simulated neural mobilization on fluid movement in cadaveric peripheral nerve sections: implications for the treatment of neuropathic pain and dysfunction. J Man Manip Ther. 2015September;23(4):219–225. PubMed PMID: 26917940; PubMed Central PMCID: PMC4727735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hofmeijer J, Franssen H, van Schelven LJ, et al. Why are sensory axons more vulnerable for ischemia than motor axons? PLoS One. 2013;8(6):e67113 PubMed PMID: 23840596; PubMed Central PMCID: PMC3688630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mizisin AP, Weerasuriya A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol. 2011March;121(3):291–312. . PubMed PMID: 21136068; PubMed Central PMCID: PMCPMC3038236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lim TK, Shi XQ, Johnson JM, et al. Peripheral nerve injury induces persistent vascular dysfunction and endoneurial hypoxia, contributing to the genesis of neuropathic pain. J Neuroscience: Official Journal Soc Neurosci. 2015February25;35(8):3346–3359. PubMed PMID: 25716835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schmid AB, Hailey L, Tampin B. Entrapment neuropathies: challenging common beliefs with novel evidence. Journal of Orthopaedic & Sports Physical Therapy. 2018;48(2):58–62. [DOI] [PubMed] [Google Scholar]
- [38].Botez SA, Herrmann DN. Pitfalls of diagnostic criteria for small fiber neuropathy. Nat Rev Neurol. 2008;4(11):586. [DOI] [PubMed] [Google Scholar]


