Abstract
Objective
To compare medication adherence, cost, and utilization in Medicare beneficiaries attributed to nurse practitioners (NP) and primary care physicians (PCP).
Data
Medicare Part A, B, and D claims and beneficiary summary file data, years 2009‐2013.
Study Design
We used propensity score‐weighted analyses combined with logistic regression and generalized estimating equations to test differences in good medication adherence (proportion of days covered (PDC >0.8); office‐based and specialty care costs; and ER visits.
Data Extraction
Beneficiaries with prescription claims for anti‐diabetics, renin‐angiotensin system antagonists (RASA), or statins.
Principal Findings
There were no differences in good medication adherence (PDC >0.8) between NP and PCP attributed beneficiaries taking anti‐diabetics or RASA. Beneficiaries taking statins had a slightly higher probability of good adherence when attributed to PCPs (74.6% vs 75.5%; P < 0.05). NP attributed beneficiaries had lower office‐based and specialty care costs and were less likely to experience an ER visit across all three medication cohorts (P < 0.01).
Conclusions
Examining the impact of NP and PCP provided care on outcomes beyond the primary care setting is important to the Medicare program in general but will also help practices seeking to meet benchmarks under alternative payment models that incentivize higher quality and lower costs.
Keywords: health care costs, Medicare, primary care
1. INTRODUCTION
Demand for primary care services is expected to grow. The Medicare program is anticipating an increase from 54 million beneficiaries today to over 80 million beneficiaries in 2030, many of whom will have longer life expectancies, high rates of obesity, and chronic conditions.1 These population changes will challenge a volatile primary care workforce facing major compositional changes in order to meet projected health care demands. This includes slower growth in the number of physicians and faster growth of non‐physician providers, such as nurse practitioners (NPs) and physician assistants (PAs). Data from forecast studies suggest that a serious investment in increasing the numbers of NPs is needed for most states to meet population demands.2, 3, 4 With fewer physicians choosing primary care, workforce analysts have pushed for expanded NP scope of practice (SoP) and changes to payment policies that accommodate team‐based outcomes.
Yet, despite an established body of evidence that NP and physician management of patients yield similar outcomes,5, 6, 7, 8, 9, 10 many states continue to have restrictive SoP, especially for NP prescribing. Legally, NP prescribing is regulated in a separate statue from NP practice. A state allowing NPs to diagnose and manage patients without physician supervision may require oversight of a physician for NP prescribing. A large part of the ongoing concern of expanding SoP regulations may stem from the limited knowledge of NP prescribing outcomes, a neglected consideration in studies comparing NP and PCP care that our study aimed to address.
The purpose of this paper was to add to the quality outcomes literature on NP and physician care by examining medication adherence, cost and utilization. Previous studies have not examined the combination of clinical, cost and utilization outcomes in one sample, providing insights only into a fraction of the patient's health care experience.11, 12, 13, 14 Understanding patient outcomes across the spectrum of health care delivery and understanding the role of primary care providers in meeting desirable goals within and outside of the office‐based setting are increasingly important for alternative payment models rewarding higher quality and lower institutional costs.15 In particular, we need better information on clinical decisions that lead to more efficient care. This could be, for example, low cost efforts to improve adherence that results in less use of specialty or institutional care.
We chose medication adherence as our clinical outcome because of the lack of robust NP prescribing outcomes in prior studies. One study examined the volume, types, and dosages of medications in Medicare beneficiaries attributed to NPs and physicians.13 Another study used quality indicators related to disease management and appropriate medication use in survey data of patient visits.16 Both studies reported comparable prescribing patterns of NPs and physicians. Closest to our study in design is a study that examined the medication possession ratio (MPR) over 1 year of data in a sample of Medicare diabetes patients.17 Rates were comparable between patients who received care from either an NP or physician. However, the MPR can lead to overestimation in adherence and is not recommended by the National Quality Forum (NQF).18, 19 Further, research has shown a strong link between adherence, cost, and utilization for different patient populations,20, 21, 22, 23 making it an ideal outcome for the purpose of this study. Specifically, we examined the effect of being attributed to NP or PCP care on achieving good adherence (proportion of days covered >0.8), costs (Part B and office‐based costs), and utilization (emergency room visits) for three cohorts of Medicare beneficiaries.
1.1. Medication adherence
Medication adherence is a compelling measure because it captures prescribing practices and patient behavior. Good adherence is at least partly driven by selecting tolerable and effective medications along with a patient's commitment, cost of the medication, and willingness to take the drug. Provider communication, trust, and educating the patient on the medication and its side effects are important provider factors associated with good adherence.24, 25, 26, 27, 28 Based on the conceptual underpinnings of nursing care, NPs may be more likely assess socioeconomic and other factors contributing to the medication adherence, such as out‐of‐pocket expenses and medication preferences. We postulated that NPs spend more time assessing these factors. Thus, we hypothesized that beneficiaries attributed to NPs are more likely to achieve good adherence than physician‐attributed beneficiaries.
2. METHODS
2.1. Sample
The sample was designed to be representative of NPs and PCPs treating Medicare beneficiaries in 2009‐2010. This was done in several steps starting with a random sample of NPs (specialty code “50” = nurse practitioner) and PCPs (specialty code “08” = family medicine and “11” = internal medicine) from the universe of clinicians with independent billing numbers (NPIs) in 2009/2010. This resulted in 4,065 NPs and 549 PCPs. As a second step, for each provider, we gathered all beneficiaries they treated and all their claims at a ratio of 4 NP beneficiaries to 1 physician beneficiary until we reached a maximum sample of 1 million beneficiaries. Since Medicare beneficiaries see many providers, this second step resulted in capturing many more NPs and PCPs than in the original random sample (NPs = 51 595; PCPs = 160 000). It also resulted in many specialist clinicians, which we excluded from the analysis.
We selected beneficiaries who were 65 years of age or older at baseline; were continuously enrolled in Part A, B, and D; did not switch Part D plans in any calendar year; had two or more prescription claims in at least one of the drug classes studied; and had no missing observations for all independent variables. Figure 1 illustrates the sample selection process after attribution. The sample eligible for attribution to a primary care provider consisted of 475 291 beneficiaries. To better understand the effect of provider type on patient outcomes over time, the sample was extended to multiple years, creating a longitudinal cohort.
Figure 1.
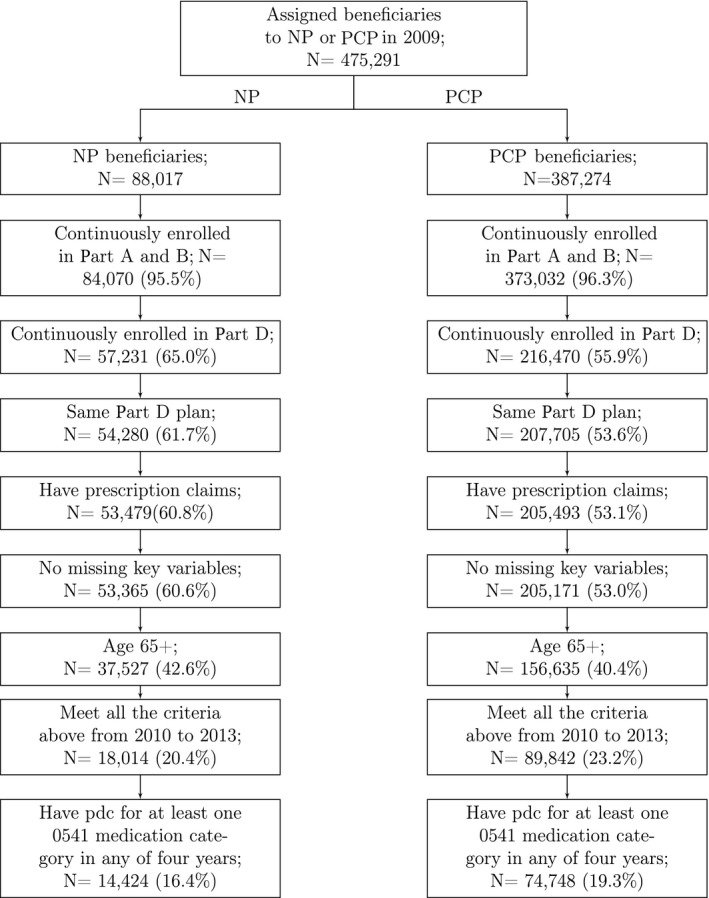
Sample construction
2.2. Data
The primary analysis used two core data files: Part D prescription drug event (PDE) claims to evaluate medication adherence and the Cost and Use segment of the beneficiary summary file to evaluate ER visits and costs of care. The annual PDE data spanned the years 2009‐2013 with the exception of 2011, a decision we made due to financial constraints. To ensure beneficiaries had continued coverage in 2011, we obtained the beneficiary summary file for demographic and plan enrollment characteristics throughout all years, including 2011. Finally, we used Part A and B 2009 claims to obtain beneficiary comorbidity information and Part B claims for provider attribution (described below). Table S1 shows a list of the Medicare data files used in each study year.
2.3. Attribution
Because beneficiaries received care from many different clinicians during a year, a primary care provider type (NP or PCP) needed to be for each beneficiary to test the effect of the type of provider on study outcomes. We followed a validated attribution method described in more detail elsewhere (Perloff et al 2015). This method assigns beneficiaries to either an NP or PCP based on the provider type who (a) provided the highest proportion of evaluation and management (E&M) paid amounts to that beneficiary in 2009 and (b) provided at least 30% of the overall paid services. In the rare case of a tie (<500), the provider type was chosen at random. This analysis excluded beneficiaries who could not be assigned to a dominant provider, for example when no one NPI accounted for 30% of E&M services. Worth noting, incident‐to billing may mean that some beneficiaries with a high volume of NP care could have been assigned to physicians. This may happen when care by an NP is billed under the physician's NPI. This miss‐attribution would lead to less notable differences between the two provider groups.
2.4. Dependent variables
The empirical analysis included medication adherence and measures of health services cost and use, the first was calculated from the PDE data, the latter two were obtained from the Cost and Use segment of the beneficiary summary file.
2.5. Medication adherence
Medication adherence was measured using the proportion of days covered (PDC) method. The PDC is calculated as the number of days in the measurement period covered by medications in a therapeutic class divided by number of days in the measurement period. For example, for a February 1st start date and a 300‐day supply in the same year, the medication adherence rate is 0.9 or 90% (300 divided by 333). The National Quality Forum (NQF) endorsed using PDC for medication adherence and defined high adherence as a PDC of at least 80%.29
We used NQF medication adherence quality measure 0541—Proportion of Days Covered (PDC): 3 Rates by Therapeutic Category.29 This measure calculates the PDC for three medication categories: diabetes medications, renin‐angiotensin system antagonists (RASA), and statins. A number of different medication adherence quality measures have been tested and recommended by the NQF. Our goal was to use a measure developed for evaluating chronic disease management, the foundation of primary care. The three drug class groups, anti‐diabetics, RASA, and statins, formed the cohorts for the analysis.
The measurement period was defined as the beneficiary's date of first fill of medication in one of the classes of interest, and ending on the last day of each calendar year or the beneficiary's date of death if she is not alive throughout that year. For this reason, beneficiaries had different adherence measurement periods, since some experienced a gap year in medication coverage or left the sample before 2013 due to death. To avoid overestimation, we truncated days supplied on the last day of the measurement period. We accounted for generic ingredient overlap of medications and the longitudinal data structure allowed us to adjust for the number of days the medication continued into the next year. We excluded beneficiaries whose first fill was later than the end of March in 2013 to have a measurement period long enough to capture any non‐adherence.
2.6. Costs: general evaluation and management and primacy care management expenditures
Our cost outcomes were the beneficiaries’ general evaluation and management (GE&M) paid amounts, and their primary care evaluation and management paid amounts (PCE&M). Both were treated as actual dollar amounts and were inflation adjusted with 2013 as the base year. Specifically, GE&M costs capture services for the management of a patient in the ER, hospital, nursing home, or specialty care context (M3, M4, M5, and M6). PCE&M, on the other hand, focuses on services provided in an office setting for new or established patients.30 These measures include all services with a BETOS code of “M1A” or “M1B.” To determine these costs, The Centers for Medicaid and Medicare Services (CMS) sums all claims with the relevant procedure code for the beneficiary for the year. The total includes NP services, reimbursed at 85% of the physician rate, and physician services. Because there is no price standardization, we include adjusters for geography in multivariate models to capture variation in reimbursement rates by region.
2.7. Use: emergency department visits
Our utilization outcome was a binary variable measuring whether a beneficiary had any inpatient emergency department (ED) visits, a utilization measure extensively used in the literature, particularly in conjunction with medication adherence.31, 32 This variable captured ED visits using revenue center codes (ie, 0450, 0451, 0452, 0456, or 0459) and was obtained from the Cost and Use segment. It does not measure whether the visit resulted in an inpatient admission.
2.8. Independent variables
To control for geographic variation, we used the beneficiary zip code from the Medicare enrollment file to identify the CMS region in which a given beneficiary resided. A rural flag was added from the Area Health Resource File based on the beneficiary's residence. We included age in years, female sex, and white race as demographic controls, also derived from the enrollment file. Dual Medicaid‐Medicare eligibility status was defined as one or more months of state buy‐in, meaning the state subsidized the Medicare premium for at least one month during the calendar year. This variable serves as a proxy for poverty and identifies approximately 75% of dually eligible beneficiaries.33
Comorbidities for each beneficiary were captured by 29 Elixhauser comorbid conditions,34 identified through diagnostic codes on the beneficiary's institutional and ambulatory 2009 Part A and B claims.
Since Medicare beneficiaries enrolled in Part D can choose from multiple plans, we adjusted for plan type and plan generosity. Following other studies,35 plan generosity was calculated for all beneficiaries who had Part D coverage, including those not included in the study, to obtain a representative measure reflecting average plan out‐of‐pocket expenditures for all beneficiaries in a given plan. Finally, we adjusted for state law differences in NP scope of practice. This variable was collected by the study team using a number of resources for cross‐referencing.36, 37, 38 We used a binary variable indicating whether NPs had full practice and prescribing independence. During our study period, 5 states implemented independent SoP for NPs.
For ER visit and cost analyses, we also included medication adherence as a control variable to capture the relationship between good adherence and positive cost and utilization outcomes.
2.9. Analysis
2.9.1. Propensity score‐weighted analysis
Because the choice of primary care provider (NP vs PCP) can be considered voluntary, beneficiaries assigned to NPs might differ from those assigned to PCPs on characteristics such as health status or preference to see a local provider. To adjust for selection bias due to the observable differences between the two groups, we employed propensity score (PS)‐weighting to balance the characteristics between NP and PCP beneficiaries for each drug class cohorts.
Specifically, we conducted a logistic regression model for choosing an NP as a primary care provider in 2009 and derived the PS of being assigned to the NP group in that year by adjusting for the following study covariates: all available demographics (age, gender, race, dual eligible, and urban versus rural residence), CMS region, Elixhauser comorbidities, and plan characteristics (type of health plan and plan generosity). We then weighted each beneficiary using inverse probability of treatment weighting to adjust for differences between patients assigned to NPs and PCPs. Specifically, the weights are w i = (T i/s i) + ((1−T i)/(1−s i)), where T i is an indicator denoting whether or not the ith beneficiary was assigned to NPs; s i denotes her PS of being assigned to the NP group.39 We carried weights forward and applied them to all 4 years of data. The advantage of PS‐weighting compared to matching is that since there are substantially more beneficiaries assigned to PCPs than NPs, PS‐weighting allows us to use all the individuals in the sample rather than matched cases only.40 We checked the balance of covariates between treated and untreated group for all three cohorts before and after weighting (Table 1) and also checked the overlap assumption (Figure S1).
Table 1.
Weighted and unweighted covariates for nurse practitioners and primary care physician attributed beneficiaries by different drug groups
| Variable | Anti‐diabetics (n = 75,649) | RASA (n = 195,950) | Statins (n = 190 087) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Weighted | Raw | Weighted | Raw | Weighted | |||||||
| NP [%] | PCP [%] | NP [%] | PCP [%] | NP [%] | PCP [%] | NP [%] | PCP [%] | NP [%] | PCP [%] | NP [%] | PCP [%] | |
| Demographics | ||||||||||||
| White | 0.78 | 0.85* | 0.84 | 0.83 | 0.82 | 0.88* | 0.87 | 0.87 | 0.84 | 0.90* | 0.89 | 0.89 |
| Mean age (years) | 74.37 | 74.85* | 74.56 | 74.79 | 75.43 | 75.89* | 75.77 | 75.81 | 74.95 | 75.42* | 75.33 | 75.34 |
| Female | 0.71 | 0.65* | 0.67 | 0.66 | 0.74 | 0.70* | 0.71 | 0.71 | 0.72 | 0.67* | 0.69 | 0.68 |
| Dual eligibility for Medicare/Medicaid | 0.49 | 0.33* | 0.35 | 0.36 | 0.41 | 0.26* | 0.28 | 0.28 | 0.40 | 0.25* | 0.28 | 0.28 |
| Rural | 0.07 | 0.04* | 0.05 | 0.04 | 0.06 | 0.04* | 0.04 | 0.04 | 0.06 | 0.03* | 0.04 | 0.04 |
| Elixhauser Comorbidities | ||||||||||||
| Valve disorder | 0.11 | 0.16* | 0.17 | 0.16 | 0.12 | 0.18 | 0.17 | 0.17 | 0.12 | 0.17* | 0.17 | 0.17 |
| Pulmonary circulation disease | 0.03 | 0.05* | 0.05 | 0.05 | 0.03 | 0.05* | 0.05 | 0.05 | 0.03 | 0.05* | 0.04 | 0.04 |
| Peripheral vascular disease | 0.23 | 0.25 | 0.24 | 0.25 | 0.22 | 0.23 | 0.23 | 0.23 | 0.22 | 0.23 | 0.23 | 0.23 |
| Hypertension | 0.88 | 0.91* | 0.91 | 0.90 | 0.91 | 0.93* | 0.93 | 0.93 | 0.83 | 0.86* | 0.86 | 0.86 |
| Complex hypertension | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 |
| Paralysis | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02* | 0.03 | 0.03 | 0.03 | 0.02* | 0.03 | 0.03 |
| Neurological disorders | 0.18 | 0.16* | 0.16 | 0.16 | 0.17 | 0.15* | 0.15 | 0.15 | 0.17 | 0.15* | 0.15 | 0.15 |
| Chronic pulmonary disease | 0.23 | 0.25* | 0.25 | 0.25 | 0.22 | 0.24* | 0.24 | 0.24 | 0.23 | 0.24* | 0.25 | 0.24 |
| Diabetes w/o chronic complications | 0.97 | 0.97* | 0.97 | 0.97 | 0.44 | 0.43 | 0.44 | 0.43 | 0.44 | 0.42* | 0.44 | 0.42 |
| Diabetes w chronic complications | 0.39 | 0.43* | 0.43 | 0.42 | 0.17 | 0.17 | 0.17 | 0.17 | 0.16 | 0.16 | 0.17 | 0.16 |
| Hypothyroidism | 0.22 | 0.22 | 0.23 | 0.22 | 0.22 | 0.23 | 0.24 | 0.23 | 0.23 | 0.24 | 0.24 | 0.23 |
| Renal failure | 0.16 | 0.17* | 0.17 | 0.17 | 0.13 | 0.14* | 0.14 | 0.14 | 0.13 | 0.13 | 0.14 | 0.13 |
| Liver disease | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 |
| Peptic ulcer Disease x bleeding | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| AIDS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00* | 0.00 | 0.00 | 0.00 | 0.00* | 0.00 | 0.00 |
| Lymphoma | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01* | 0.01 | 0.01 | 0.01 | 0.01* | 0.01 | 0.01 |
| Metastatic cancer | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Solid tumor w/out metastasis | 0.07 | 0.10* | 0.10 | 0.10 | 0.07 | 0.11* | 0.10 | 0.10 | 0.07 | 0.11* | 0.10 | 0.10 |
| Rheumatoid arthritis/collagen vas | 0.04 | 0.06* | 0.05 | 0.05 | 0.04 | 0.06* | 0.06 | 0.06 | 0.04 | 0.06* | 0.06 | 0.05 |
| Coagulopathy | 0.05 | 0.05 | 0.05 | 0.05 | 0.04 | 0.05* | 0.04 | 0.04 | 0.04 | 0.05* | 0.04 | 0.04 |
| Obesity | 0.11 | 0.11 | 0.12 | 0.11 | 0.07 | 0.08 | 0.08 | 0.08 | 0.07 | 0.07 | 0.07 | 0.07 |
| Weight loss | 0.05 | 0.05 | 0.05 | 0.06 | 0.06 | 0.06 | 0.05 | 0.06 | 0.06 | 0.06 | 0.05 | 0.06 |
| Fluid and electrolyte disorders | 0.17 | 0.19* | 0.19 | 0.19 | 0.16 | 0.18* | 0.18 | 0.18 | 0.15 | 0.16* | 0.16 | 0.16 |
| Chronic blood loss anemia | 0.01 | 0.03* | 0.02 | 0.02 | 0.02 | 0.02* | 0.02 | 0.02 | 0.02 | 0.02* | 0.02 | 0.02 |
| Deficiency anemia | 0.29 | 0.30 | 0.30 | 0.30 | 0.26 | 0.27* | 0.26 | 0.27 | 0.24 | 0.25* | 0.25 | 0.25 |
| Alcohol abuse | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Drug abuse | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Psychoses | 0.14 | 0.10* | 0.11 | 0.11 | 0.11 | 0.09* | 0.09 | 0.09 | 0.12 | 0.09* | 0.10 | 0.09 |
| Depression | 0.15 | 0.13* | 0.14 | 0.14 | 0.15 | 0.14* | 0.14 | 0.14 | 0.16 | 0.14* | 0.14 | 0.14 |
| Patient Pay Ratio ‐ Median | 0.18 | 0.22* | 0.21 | 0.21 | 0.20 | 0.24* | 0.23 | 0.23 | 0.21 | 0.24* | 0.24 | 0.24 |
| Plan type | ||||||||||||
| Plan type1 | 0.99 | 0.99* | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Plan type2 | 0.01 | 0.01* | 0.01 | 0.01 | 0.01 | 0.01* | 0.01 | 0.01 | 0.01 | 0.01* | 0.01 | 0.01 |
| CMS region | ||||||||||||
| Region 1 | 0.04 | 0.05 | 0.05 | 0.05 | 0.04 | 0.05* | 0.05 | 0.05 | 0.04 | 0.05* | 0.05 | 0.05 |
| Region 2 | 0.08 | 0.14* | 0.13 | 0.13 | 0.08 | 0.14* | 0.13 | 0.13 | 0.09 | 0.14* | 0.13 | 0.13 |
| Region 3 | 0.30 | 0.28* | 0.27 | 0.28 | 0.29 | 0.27* | 0.26 | 0.27 | 0.27 | 0.26* | 0.25 | 0.26 |
| Region 4 | 0.15 | 0.20* | 0.19 | 0.19 | 0.17 | 0.20* | 0.20 | 0.19 | 0.17 | 0.20* | 0.20 | 0.19 |
| Region 5 | 0.14 | 0.09* | 0.10 | 0.10 | 0.13 | 0.08* | 0.09 | 0.09 | 0.12 | 0.07* | 0.08 | 0.08 |
| Region 6 | 0.10 | 0.08* | 0.08 | 0.08 | 0.11 | 0.08* | 0.08 | 0.09 | 0.11 | 0.08* | 0.09 | 0.09 |
| Region 7 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02* | 0.02 | 0.02 | 0.03 | 0.02* | 0.02 | 0.02 |
| Region 8 | 0.04 | 0.05* | 0.05 | 0.05 | 0.04 | 0.06* | 0.06 | 0.05 | 0.03 | 0.06* | 0.05 | 0.05 |
| Region 9 | 0.06 | 0.04* | 0.04 | 0.04 | 0.07 | 0.04* | 0.04 | 0.04 | 0.07 | 0.04* | 0.05 | 0.04 |
*Indicates differences between NP and PCP beneficiaries are significantly different at p < 0.05. After propensity score weighting no covariates remained statistically different in all three drug cohorts.
2.9.2. Regression analyses
Our regression estimations had three parts: (a) evaluation of good medication adherence, (b) evaluation of office‐based and non‐office‐based costs, and (c) evaluation of inpatient ER visits. The impact of the type of primary care provider on whether a patient had good adherence (PDC ≥ 80%) was estimated using logistic regression since the dependent variable was dichotomous. The probability of having an inpatient ER visit was also estimated using logistic regression. To model the skewed health care costs in our data, we extended the generalized linear model (GLM) to our longitudinal data by fitting a population‐averaged model using generalized estimating equations (GEE), with a link function suggested by a Park test.41 All models included control variables discussed above. Analyses were performed using Stata 14.0 (StataCorp LP, College Station, TX, USA), and we used statistical significance levels at conventional levels.
3. RESULTS
Our sample consisted of 475 291 assigned beneficiaries, of whom 19% were assigned to NPs (N = 88 017) and 81% were assigned to primary care physician providers (N = 387 274). After applying our selection criteria, including continuous enrollment in Part A, B, and D, and defining our sample based on the NQF algorithm, the final analytic samples consisted of 75 649 beneficiaries taking anti‐diabetics, 195 950 beneficiaries taking RASA, and 190 087 beneficiaries taking statins. Approximately, 15% of beneficiaries had observations in all three drug classes, 42% were part of two medication groups, and 43% were taking only one of three classes.
Beneficiaries assigned to NPs were significantly more likely to be non‐white, younger, female, dual eligible for Medicare and Medicaid, and living in rural areas. They were also significantly less likely to have each of the Elixhauser comorbid conditions, with the exception of paralysis, neurological disorders, AIDS, alcohol abuse, drug abuse, and psychoses. This pattern was consistent across the three drug groups. Applying the PS weights balanced the two groups of beneficiaries in all three‐drug cohorts on all demographic and diagnostic characteristics (Table 1).
A crucial assumption that underlies PS analyses is sufficient overlap between treatment and control group in the distribution of covariates. To check whether the overlap assumption was met, we plotted the distribution of the PS for those assigned to the NP group (ie, being treated) or the PCP group (Figure S1). The two PS distributions achieved a high degree of overlap for each drug group, suggesting PS‐weighting analysis significantly mitigated the selection bias by equating groups based on the covariates in our sample. All analyses were restricted to the drug cohort specific regions of common support.
3.1. Medication adherence
Table 2 shows the percent of beneficiaries who had good adherence (PDC >80%) under NP and physician provided care in each of the three drug classes. There was no difference between the share of NP and PCP attributed beneficiaries who had good adherence in two of the drug groups. In the statin group, a slightly larger fraction of PCP attributed beneficiaries had good adherence (NP 76.5% vs PCP 77.3%; P < 0.05).
Table 2.
Unadjusted outcomes for NP and PCP attributed beneficiaries
| NP | PCP | P | |
|---|---|---|---|
| Percent high medication adherent | |||
| Anti‐diabetics (N = 75 649) | 76.46% | 77.34% | 0.050 |
| RASA (N = 195 950) | 77.81% | 77.93% | 0.946 |
| Statins (N = 190 087) | 73.87% | 74.84% | 0.005 |
| Percent with inpatient ER visit | |||
| Anti‐diabetics (N = 75 649) | 15.81% | 19.65% | <0.001 |
| RASA (N = 195 950) | 14.39% | 17.78% | <0.001 |
| Statins (N = 190 087) | 14.65% | 17.45% | <0.001 |
| Average Evaluation & Management, not office based (SD) | |||
| Anti‐diabetics (N = 75 649) | $871.52 (1636.00) | $873.22 (1677.99) | 0.831 |
| RASA (N = 195 950) | $782.68 (1589.53) | $770.86 (1531.70) | 0.674 |
| Statins (N = 190 087) | $796.99 (1629.19) | $770.50 (1538.69) | 0.036 |
| Average Part B cost, office based (SD) | |||
| Anti‐diabetics (N = 75 649) | $552.56 (531.41) | $824.34 (639.58) | <0.001 |
| RASA (N = 195 950) | $527.17 (515.62) | $787.63 (627.56) | <0.001 |
| Statins (N = 190 087) | $538.63 (526.61) | $797.85 (633.21) | <0.001 |
Note: The number of beneficiaries in each drug class differs, as beneficiaries do not necessarily take medications in each therapeutic class we measured.
Table 3 reports the effect of the type of provider on good adherence (PDC >80%) using the PS‐weighted multivariable logistic analysis. There were no differences in the probability of achieving good adherence between NP and PCP attributed beneficiaries in two of the drug classes studied. In the cohort of beneficiaries taking statins, the probability of having good adherence was 1 percentage point lower in the group of beneficiaries attributed to NPs (P < 0.05).
Table 3.
Multivariate regression results for all study outcomes by drug class cohort
| High adherence | Hospital inpatient ER visit | Non‐office based Evaluation & Management Costs | Office Based Part B Costs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes | RASA | Statins | Diabetes | RASA | Statins | Diabetes | RASA | Statins | Diabetes | RASA | Statins | |
| NP | −0.011* | 0.001 | −0.009** | −0.031*** | −0.029*** | −0.026*** | −87.745*** | −100.072*** | −83.173*** | −195.436*** | −193.244*** | −187.077*** |
| (0.006) | (0.003) | (0.004) | (0.004) | (0.002) | (0.003) | (17.842) | (10.398) | (11.236) | (8.995) | (5.611) | (5.736) | |
| Indepentent SOP | −0.009 | 0.013** | 0.013* | −0.000 | 0.009 | 0.009* | −93.465** | −49.225** | −38.952* | 18.424 | 39.234*** | 31.522*** |
| (0.011) | (0.006) | (0.007) | (0.010) | (0.005) | (0.005) | (40.767) | (20.178) | (21.911) | (19.210) | (9.467) | (10.779) | |
| High adherence | −0.094*** | −0.099*** | −0.072*** | −384.209*** | −368.170*** | −294.048*** | −40.369*** | −57.162*** | −33.154*** | |||
| (0.004) | (0.002) | (0.003) | (22.004) | (12.062) | (12.286) | (6.250) | (3.884) | (4.226) | ||||
| N | 75, 649 | 195, 950 | 190, 087 | |||||||||
Notes: All models adjusted for beneficiaries’ sex, race, age, dual eligibility status, provider type, rural region, CMS regions, year, NP independence, and Elixhauser comorbidities.
Cost and utilization models also adjusted for high adherence.
Coefficients for binary outcomes are reported in probabilities.
Results based on an attribution threshold of 30%.
Standard errors in parentheses; *P < 0.10, **P < 0.05, ***P < 0.01.
Propensity score ranges: anti‐diabetics: ≥0.0296622 and ≤0.5590561; RASA: ≥0.0274983 and ≤0.5630087; statins: ≥0.0322372 and ≤0.5937169.
3.2. Hospital emergency department visit
Table 2 shows the share of beneficiaries who experienced an ER visit for each drug cohort. ER visits ranged between 14.4%‐15.8% for NP beneficiaries and between 17.5%‐19.7% for PCP attributed beneficiaries (P < 0.01). Multivariate regression results for the likelihood of a beneficiary to have a hospital ER visit are shown for each cohort in Table 3, reporting probabilities. After adjusting for demographic characteristics, geography, comorbidities, and SoP the propensity to see an NP, and medication adherence, NP beneficiaries were significantly less likely to have an ER visit across all cohorts, with an effect size difference between NP and PCP attributed beneficiaries ranging between 2.6% to 3.1% depending on the cohort (Statin cohort 2.6% (P < 0.01), RASA cohort 2.9% (P < 0.01), diabetes cohort 3.1% (P < 0.01)).
3.3. Costs
In unadjusted results, NP attributed beneficiaries in all three drug groups had significantly lower costs. For non‐office‐based costs, differences between NP and PCP beneficiaries were the largest in the RASA group and the smallest in the statin group. The same pattern emerged for office‐based costs. Table 2 shows the unadjusted differences between NP and PCP attributed beneficiaries for each drug cohort. In multivariate modeling, NP attributed beneficiaries continue to have significantly lower costs, with a greater difference for office‐based Part B care compared to non‐office‐based E&M costs. Non‐office‐based cost differences ranged from −$83 in the Statin group (P < 0.01) to −$86 in the Diabetes group (P < 0.01), and −$100 in the RASA group (P < 0.01). In contrast, office‐based Part B cost differences varied between −$187 in the Statin group (P < 0.01), to −$193 in the RASA group (P < 0.01) and −$195 in the Diabetes group P < 0.01; Table 3).
As expected, good adherence played an important role in all cost and use models across all drug cohorts and was associated with significantly lower costs and lower ER use (Table 2). Detailed regression results for all models are available upon request.
3.4. Robustness analyses
We conducted several robustness analyses to address potential concerns related to attribution, missing data, gaps in medication adherence, and SoP. Because attribution to the primary care provider was carried out using 2009 data, it is possible that a beneficiary may receive the majority of his/her care from a different type of provider in the follow‐up years. To test the stability of attribution, we compared assignments using 2 years of data to assignments using only a single year. We found that 70% of beneficiaries were assigned to the same provider using the 2‐year method compared to the 1‐year method. This suggests that although some beneficiary‐provider relationships change over time, many beneficiaries experience a stable relationship with their dominant provider.
We also examined whether beneficiaries in 2012 and 2013 obtained prescriptions from the same prescribers who prescribed for them in 2009 or 2010. Approximately two‐thirds of our sample received 70% or more of their prescriptions in 2013 from their 2009/2010 prescribers. This suggests a high degree of continuity in how beneficiaries receive primary care for chronic conditions such as heart failure, high blood pressure, and diabetes, which are some of the conditions that the drug classes examined in this paper target. We re‐estimated all regression models on the sample that did have at least 70% of their 2013 prescriptions prescribed by their earlier prescribers and results stayed qualitatively the same.
To assess how results may have been affected by the lack of outcomes data in 2011 (enrollment data in 2011 ensured that access to services did not change for beneficiaries in 2011), we re‐estimated our models without the follow‐up years 2012 and 2013. We essentially obtained the same results, with slightly larger effect sizes on the cost and use measures. This suggests that the missing outcomes in 2011 may dilute our results or that the effect of the type of provider diminishes over time.
To ensure that our analysis was robust to potential differences in beneficiaries who experienced medication gaps, we excluded beneficiaries with gap years. Depending on the drug class, between 9%‐25% of our sample experienced a single gap year, and only 2%‐5% experienced two gap years. Methodologically, this assesses whether imbalance in the panel structure of the samples affected results. If missing observations were frequent and occurred systematically, our results could be biased. In this sample, medication gap years occurred in all years with no apparent pattern to our covariates. Regression results on a balanced 4‐year panel stayed largely the same.
Furthermore, we conducted a follow‐up analysis on a subset of beneficiaries with conditions that are considered ambulatory care sensitive conditions (ACSC). Using chronic condition indicators, we were able to identify six out of ten chronic ACSC, including hypertension, congestive heart failure, chronic obstructive pulmonary disease, anemia, diabetes, and asthma. Subsetting the sample to beneficiaries with ACSCs identified beneficiaries in whom the effect of the primary care provider may be more pronounced. This was reflected in the coefficients, which indicated slightly larger effect sizes on the cost and use measures, supporting that our results are driven by differences in primary care provider type and are not due to unobserved factors systematically affecting beneficiaries with non‐ACSCs.
Finally, because SoP regulations can have a substantial effect on NP (and physician) practice, we estimated models only for those beneficiaries who resided in states in which NPs could practice without physician oversight. Results were directionally and qualitatively similar; however, ER visits and office‐based costs had smaller effect sizes for NP assignment. Potential explanations could be the unexplained geographic variations in costs and utilization, or that the effect of NP care, while remaining robust, might be more modest for the outcomes of ER visits and Part B costs than suggested in Table 3. Results are available in Table S2. All other robustness analyses are available upon request.
4. DISCUSSION
This study evaluated the care of NP attributed beneficiaries in comparison with PCP attributed beneficiaries on three important measures: medication adherence, costs, and ER visits. We found similar results on the outcome of medication adherence for NP and PCP attributed beneficiaries, with no differences between providers for beneficiaries taking anti‐diabetics and RASA medications. NP attributed beneficiaries in the Statin group had a slightly lower likelihood of achieving good adherence compared to PCP beneficiaries. The difference in probabilities was 1%, rather small, and is not likely to have a clinical impact. These results show that good medication adherence is essentially comparable for NP and PCP attributed Medicare beneficiaries.
On all other measures, NP beneficiaries had better outcomes than PCP attributed beneficiaries. NP beneficiaries were less likely to have an emergency department visit and had lower costs across all three cohorts on both office Part B costs and non‐office‐based E&M care. This suggests that provider type may have an impact that extends beyond traditional primary care activities such as screening and managing chronic illnesses. The low rates of ED use and lower specialty care costs for NP attributed patients suggest a different and potentially more efficient practice style. Our results might be particularly relevant for office managers and ACOs when considering the benefits of hiring NPs.
In our study, office‐based costs were lower for beneficiaries attributed to NPs compared to PCP. The size of the effect partly reflects that NPs can only bill Medicare 85% of the physician fee. This reimbursement rule can contribute to NP services being billed “incident‐to” a physician, whereby care provided by an NP is billed under a physician's NPI. This is a well‐known limitation of Medicare claims data,14 and our results should be interpreted with this limitation in mind. Specifically, this means that some PCP billed services may have in fact been carried out by NPs, leading to increases in costs for the system. Practices in states with restrictive scope of practice laws for NPs may be more likely to bill incident‐to. Our study controlled for independent scope of practice for NPs, but other financial factors may also influence the decision to bill incident‐to.
The limitation of incident‐to billing likely would have underestimated the effect of NP care and lower costs. Billing codes used for incident‐to billing are typically at lower costs than actual physician visits; thus, incident‐to physician claims would likely bias down the average physician costs. Similarly, the effect of NP care on ER visits may have been underestimated, as our result of lower rates of ER visits for NP attributed beneficiaries may be even lower than estimated due to some visits to NPs likely being attributed to PCPs. Given the nonsignificant result for the outcome of medication adherence, the effect of incident‐to billing is unclear.
Other limitations of this study are that services provided by NPs who do not have an NPI are not captured in our data. Because NPs without NPIs may practice differently from NPs with NPIs, our results cannot be generalized to all NPs providing services to Medicare beneficiaries. However, results of a recent NP survey indicated that 95% of NPs had an NPI.42 Finally, our analytic approach for addressing selection bias between NP and PCP attributed beneficiaries was a propensity score weighting design. While we had a high degree of propensity scores overlap between the NP and PCP groups, our available set of observable characteristics may not account for all factors affecting whether beneficiaries receive care from NPs or PCPs. For example, there likely remain unobserved differences in severity of illness between the two groups of beneficiaries.
This study also showed that adherence continued to have a significant impact on emergency room use and costs, even after controlling for provider management, demographics, and other factors. Adherence is a complex process with many provider, pharmacy, and beneficiary characteristics coming into play. Our results suggest that other factors beyond provider type are important for achieving good adherence. For example, practice‐wide protocols and attitudes toward prescribing may outweigh clinician training or practice style when it comes to selecting specific medications, educating patients in medication use, and follow‐up. It is not surprising that adherence reduces the expenditures for inpatient and specialist services. This suggests that consistent use of chronic medications may off‐set the need for some visits or services. In contrast, the impact of adherence is much lower when it comes to office‐based care. There is no added effect of having both good adherence and a particular provider type, suggesting that adherence is not the driver of PCP differences.
Overall, our findings on good medication adherence suggest that the pharmaceutical treatment of beneficiaries attributed to NPs and PCPs is comparable for beneficiaries taking antidiabetics, RASA, and Statin medications. This is valuable information for state regulatory bodies considering NP independence in prescribing. Our results support expanding SoP for NP prescribing, especially for medications used for treating chronic conditions. Further, NP and PCP provider type had an independent effect on cost and ED visits, with lower costs and use for NP attributed beneficiaries. This is particularly important for beneficiaries with chronic conditions, such as beneficiaries in the Medicare and Medicaid populations, who can become quite expensive. More research is needed to better understand how NPs affect care beyond the primary care setting. The fact that NP attributed beneficiaries experience a positive NP “spillover” effect on outcomes beyond the office‐based setting is key to workforce composition of primary care practices. It has important implications for trying to meet benchmarks under alternative payment models that incentivize higher quality and lower costs in a primary care delivery system composed of a web of different health care providers each with their unique contributions to care.
Supporting information
ACKNOWLEDGMENTS
Joint Acknowledgment/Disclosure Statement: This work was supported by a grant from the National Council of State Boards of Nursing (NCSBN). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript. We thank Dr. Peter Buerhaus for valuable insights during the early design phase of the study. We would also like to thank three anonymous reviewers for their valuable comments and suggestions.
Muench U, Guo C, Thomas C, Perloff J. Medication adherence, costs, and ER visits of nurse practitioner and primary care physician patients: Evidence from three cohorts of Medicare beneficiaries. Health Serv Res. 2019;54:187–197. 10.1111/1475-6773.13059
REFERENCES
- 1. Medicare Payment Advisory Commission . A Databook: Healthcare Spending and the Medicare Program. Washington, DC: Medicare Payment Advisory Commission; 2016. http://www.medpac.gov/docs/default-source/data-book/June-2016-data-book-health-care-spending-and-the-medicare-program.pdf. [Google Scholar]
- 2. U.S. Department of Health and Human Services , Health Resources and Services Administration , Health Resources and Services Administration , National Center for Health Workforce Analysis . National and Regional Projections of Supply and Demand for Primary Care Practitioners: 2013‐2025. Rockville, Maryland; 2016. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/primary-care-national-projections2013-2025.pdf.
- 3. AAMC . The Complexities of Physician Supply and Demand: Projections from 2013 to 2025. Prepared for the Association of American Medical Colleges. Washington, DC: Association of American Medical Colleges; 2015. https://www.aamc.org/download/426242/data/ihsreportdownload.pdf?cm_mmc=AAMC-_-ScientificAffairs-_-PDF-_-ihsreport. Accessed June 9, 2016.
- 4. Streeter RA, Zangaro GA, Chattopadhyay A. Perspectives: using results from HRSA's Health Workforce simulation model to examine the geography of primary care. Health Serv Res. 2017;52:481‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lenz ER, Mundinger MO, Kane RL, Hopkins SC, Lin SX. Primary care outcomes in patients treated by Nurse Practitioners or Physicians: two‐year follow‐up. Med Care Res Rev. 2004;61(3):332‐351. [DOI] [PubMed] [Google Scholar]
- 6. Mundinger MO, Kane RL, Lenz ER, et al. Primary care outcomes in patients treated by nurse practitioners or physicians. J Am Med Assoc. 2000;283(1):59. [DOI] [PubMed] [Google Scholar]
- 7. Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675‐684. [DOI] [PubMed] [Google Scholar]
- 8. Venning P, Durie A, Roland M, Roberts C, Leese B. Randomised controlled trial comparing cost effectiveness of general practitioners and nurse practitioners in primary care. BMJ. 2000;320(7241):1048‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newhouse RP, Stanik‐Hutt J, White KM, et al. Advanced practice nurse outcomes 1990‐2008: a systematic review. Nurs Econ. 2011;29(5):1. [PubMed] [Google Scholar]
- 10. Buerhaus P, Perloff J, Clarke S, O'Reilly‐Jacob M, Zolotusky G, DesRoches CM. Quality of primary care provided to Medicare beneficiaries by nurse practitioners and physicians. Med Care. 2018;56(6):484. [DOI] [PubMed] [Google Scholar]
- 11. Fisher SE, Vaughan‐Cole B. Similarities and differences in clients treated and in medications prescribed by APRNs and psychiatrists in a CMHC. Arch Psychiatr Nurs. 2003;17(3):101‐107. [DOI] [PubMed] [Google Scholar]
- 12. Jones K, Edwards M, While A. Nurse prescribing roles in acute care: an evaluative case study. J Adv Nurs. 2011;67(1):117‐126. [DOI] [PubMed] [Google Scholar]
- 13. Muench U, Perloff J, Thomas CP, Buerhaus PI. Prescribing practices by nurse practitioners and primary care physicians: a descriptive analysis of Medicare beneficiaries. J Nurs Regul. 2017;8(1):21‐30. [Google Scholar]
- 14. Perloff J, DesRoches CM, Buerhaus P. Comparing the cost of care provided to Medicare beneficiaries assigned to primary care nurse practitioners and physicians. Health Serv Res. 2015;51(4):1407‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muhlestein D, Saunders R, McClellan M. Growth of ACOs and alternative payment models in 2017. https://www.healthaffairs.org/do/10.1377/hblog20170628.060719/full/. Published June 28, 2017. Accessed August 22, 2018
- 16. Jiao S, Murimi IB, Stafford RS, Mojtabai R, Alexander GC. Quality of prescribing by Physicians, Nurse Practitioners, and Physician Assistants in the United States. Pharmacotherapy. 2018;38(4):417‐427. [DOI] [PubMed] [Google Scholar]
- 17. Kuo Y‐F, Goodwin JS, Chen N‐W, Lwin KK, Baillargeon J, Raji MA. Diabetes mellitus care provided by nurse practitioners vs primary care physicians. J Am Geriatr Soc. 2015;63(10):1980‐1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nau D. Proportion of Days Covered (PDC) as a Preferred Method of Measuring Medication Adherence. 2015. http://ep.yimg.com/ty/cdn/epill/pdcmpr.pdf. Accessed March 4, 2015.
- 19. Martin BC, Wiley‐Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann Pharmacother. 2009;43(1):36‐44. [DOI] [PubMed] [Google Scholar]
- 20. Toy EL, Beaulieu NU, McHale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435‐441. [DOI] [PubMed] [Google Scholar]
- 21. Gielen SC, Dekker J, Francke AL, Mistiaen P, Kroezen M. The effects of nurse prescribing: a systematic review. Int J Nurs Stud. 2014;51(7):1048‐1061. [DOI] [PubMed] [Google Scholar]
- 22. McGrady ME, Hommel KA. Medication adherence and health care utilization in pediatric chronic illness: a systematic review. Pediatrics. 2013;132(4):730‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karve S, Markowitz M, Fu D‐J, et al. Assessing medication adherence and healthcare utilization and cost patterns among hospital‐discharged patients with schizoaffective disorder. Appl Health Econ Health Policy. 2014;12(3):335‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wroth TH, Pathman DE. Primary medication adherence in a rural population: the role of the patient‐physician relationship and satisfaction with care. J Am Board Fam Med. 2006;19(5):478‐486. [DOI] [PubMed] [Google Scholar]
- 25. Schoenthaler A, Chaplin WF, Allegrante JP, et al. Provider communication effects medication adherence in hypertensive African Americans. Patient Educ Couns. 2009;75(2):185‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harmon G, Lefante J, Krousel‐Wood M. Overcoming barriers: the role of providers in improving patient adherence to antihypertensive medications. Curr Opin Cardiol. 2006;21(4):310‐315. [DOI] [PubMed] [Google Scholar]
- 27. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self‐administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785‐795. [DOI] [PubMed] [Google Scholar]
- 28. Piette JD, Heisler M, Krein S, Kerr EA. The role of patient‐physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med. 2005;165(15):1749‐1755. [DOI] [PubMed] [Google Scholar]
- 29. National Quality Forum . Proportion of Days Covered (PDC): 3 Rates by Therapeutic Category. 2014. http://goo.gl/MjyDAC. Accessed November 1, 2014.
- 30. ResDac . Part B Physician Medicare Payments. 2016. https://www.resdac.org/cms-data/variables/Part-B-Physician-Medicare-Payments. Accessed June 2, 2017.
- 31. Pittman DG, Tao Z, Chen W, Stettin GD. Antihypertensive medication adherence and subsequent healthcare utilization and costs. Am J Manag Care. 2010;16(8):568‐576. [PubMed] [Google Scholar]
- 32. Roebuck MC, Liberman JN, Gemmill‐Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff Proj Hope. 2011;30(1):91‐99. [DOI] [PubMed] [Google Scholar]
- 33. Kimmel PL, Fwu C‐W, Abbott KC, Ratner J, Eggers PW. Racial disparities in poverty account for mortality differences in US Medicare beneficiaries. SSM Popul Health. 2016;2:123‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8‐27. [DOI] [PubMed] [Google Scholar]
- 35. Goldman DP, Joyce GF, Vogt WB. Part D formulary and benefit design as a risk‐steering mechanism. Am Econ Rev. 2011;101(3):382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. AANP . AANP ‐ State Practice Environment. 2016. https://www.aanp.org/legislation-regulation/state-legislation/state-practice-environment. Accessed August 30, 2016.
- 37. Pearson L. The Pearson report. Am J Nurse Pract. 2009;13(2):8‐82. [Google Scholar]
- 38. Pearson L. 24th Annual legislative update. Nurse Pract. 2012;37(1):22‐45. [DOI] [PubMed] [Google Scholar]
- 39. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo S, Fraser MW. Propen sity Score Analysis: Statistical Methods and Applications: Statistical Methods and Applications, vol. 11 Thousand Oaks, CA: Sage Publications; 2014. https://books.google.com/books?hl=en&lr=&id=5Y_MAwAAQBAJ&oi=fnd&pg=PP1&dq=Propensity+score+analysis:+Statistical+methods+and+applications+(2nd+ed.).+Thousand+Oaks,+CA:+Sage+Publications,+Inc.&ots=WVb3jGUu8A&sig=VMIVuFt4PR11LgWila1Tw-smHyw. Accessed June 9, 2016. [Google Scholar]
- 41. Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461‐494. [DOI] [PubMed] [Google Scholar]
- 42. Chattopadhyay A, Zangaro GA, White KM. Practice patterns and characteristics of nurse practitioners in the United States: results from the 2012 National Sample Survey of Nurse Practitioners. J Nurse Pract. 2015;11(2):170‐177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


