Abstract
Objectives
To evaluate the ability of claims‐based risk adjustment and incremental components of clinical data to identify 90‐day episode costs among lower extremity joint replacement (LEJR) patients according to the Centers for Medicare & Medicaid Services (CMS) Comprehensive Care for Joint Replacement (CJR) program provisions.
Data Sources
Medicare fee‐for‐service (FFS) data for qualifying CJR episodes in the United States, and FFS data linked with clinical data from CJR‐qualifying LEJR episodes performed at High Value Healthcare Collaborative (HVHC) and Mayo Clinic in 2013. HVHC and Mayo Clinic populations are subsets of the total FFS population to assess the additive value of additional pieces of clinical data in correctly assigning patients to cost groups.
Study Design
Multivariable logistic models identified high‐cost episodes.
Data Collection/Extraction Methods
Clinical data from participating health care systems merged with Medicare FFS data.
Principal Findings
Our three populations consisted of 363 621 patients in the CMS population, 4881 in the HVHC population, and 918 in the Mayo population. When modeling per CJR specifications, we observed low to moderate model performance (CMS C‐Stat = 0.714; HVHC C‐Stat = 0.628; Mayo C‐Stat = 0.587). Adding CMS‐HCC categories improved identification of patients in the top 20% of episode costs (CMS C‐Stat = 0.758, HVHC C‐Stat = 0.692, Mayo C‐Stat = 0.677). Clinical variables, particularly functional status in the population for which this was available (Mayo C‐Stat = 0.783), improved ability to identify patients within cost groups.
Conclusions
Policy makers could use these findings to improve payment adjustments for bundled LEJR procedures and in consideration of new data elements for reimbursement.
Keywords: health care expenditures, health care reform, medicare, reimbursement
1. INTRODUCTION
In the United States, the annual number of total hip arthroplasties (THAs) among patients over the age of 45 years doubled over 11 years to approximately 310 800 in 2010.1 During this same period, the rate of total knee arthroplasties (TKAs) increased 86% to 45.3 per 10 000 for men and 99% to 65.5 per 10 000 for women.2 From 2005 to 2030, the number of THAs performed in the United States is projected to increase by nearly 200% and the number of TKAs is projected to increase by over 600%.3 Lower extremity joint replacement (LEJR) is one of the most common procedures performed among Medicare beneficiaries.4
Significant variation in spending for surgical procedures exists across US hospitals.5 Variability in cost is explained by the postacute episode of care,6, 7 type of joint implant,8 geographic region,9 and type of medical center.9 Age,9, 10, 11, 12, 13 disease severity,6, 10, 14, 15, 16 socioeconomic status and social determinants of health,9, 17, 18, 19 and medical comorbidities are associated with increased LEJR costs.9, 11, 20, 21, 22, 23, 24, 25 In an attempt to reduce cost variability, the Centers for Medicare & Medicaid Services (CMS) issued the Comprehensive Care for Joint Replacement (CJR)26 rule mandating bundled payment for LEJR in 67 geographic areas. On August 15, 2017, CMS proposed to reduce the number of geographic areas required to participate in this program.27
The CJR program limited price stratifications to hip fracture, Medicare Severity‐Diagnosis Related Group (MS‐DRG), and geographic region. The final CJR rule restricted adjustment to these three factors arguing that no standard risk adjustment methodology exists for LEJR patients, commercially available risk adjustment tools were constructed using patients not included within the CJR model, and traditionally employed risk adjustment methodologies such as the CMS‐Hierarchical Condition Category (CMS‐HCC) had not been validated in LEJR populations. Previous work has studied the net difference in hospital payment under the CJR model as currently implemented vs the inclusion of risk adjustment for average CMS‐HCC score aggregated to the hospital level: reconciliation payments would be reduced by $827 (95% CI, −$1368 to −$285) per episode for each standard deviation increase in a hospital's patient complexity, and risk adjustment could increase reconciliation payments for hospitals caring for more complex patients.28
Bundled payment programs without appropriate risk adjustment could lead to adverse selection among patients requiring LEJR. To investigate the ability of CMS‐HCC and patient‐level factors to predict high‐cost 90‐day LEJR episodes (episodes potentially most affected by risk adjustment), we evaluated three populations: (a) all CMS patients with a qualifying LEJR procedure; (b) patients treated at any High Value Healthcare Collaborative (HVHC) member organizations; and (c) patients at the Mayo Clinic, Rochester, Minnesota campus. Encouraged by governmental programs such as the Electronic Health Record Incentive Program (“Meaningful Use”),29 health care organizations are collecting patient‐level information. However, variation exists in the availability of patient‐level data across providers and health systems. Our investigation assesses the incremental benefit of patient‐level clinical data in Medicare claims (FFS), across a collection of health care systems (HVHC), and within our own institution (Mayo Clinic). We evaluated the Medicare FFS‐based CMS‐HCC methodology independently in an effort to improve the ability to identify patient populations, and then included additional claims and clinically derived measures to assign patients to cost groups for CJR‐qualifying LEJR episodes. In the current analyses, the HVHC and Mayo Clinic populations are subsets of the total Medicare FFS population.
2. METHODS
2.1. Study population
This study performed secondary data analyses of Medicare FFS data for patients discharged from an acute care hospital for a CJR‐qualifying LEJR procedure in 2013 (Figure 1). Under the CJR program, eligible beneficiaries must have been discharged with a MS‐DRG code of 469 (major replacement or reattachment of lower extremity with major complications or comorbidities) or 470 (major replacement or reattachment of lower extremity without major complications or comorbidities). LEJR procedures defined under MS‐DRGs 469 and 470 include replacement, resurfacing, and reattachment of the hip, knee, ankle, and smaller joints of the lower extremities including those within the foot. Additionally, under the CJR beneficiaries must have had Medicare as their primary payer and been covered under both parts A and B FFS Medicare for the duration of their episode as well as the 12 months prior to their LEJR procedure. Medicare Part A is the entitlement portion of the Medicare program and includes the services under hospital care, skilled nursing facility, nursing home, hospice, and home health services. Medicare Part B coverage requires beneficiaries to pay a premium in order to receive coverage for services considered medically necessary and preventive, such as outpatient care, preventive care, ambulance services, and durable medical equipment. Medications covered under Medicare Part D are not included within the CJR provisions and have therefore been excluded within our analyses. Episodes under which Medicare was the primary payer were the population included within the CJR; individuals with private insurance or for whom Medicare was not the primary payer were therefore excluded from this study.
Figure 1.
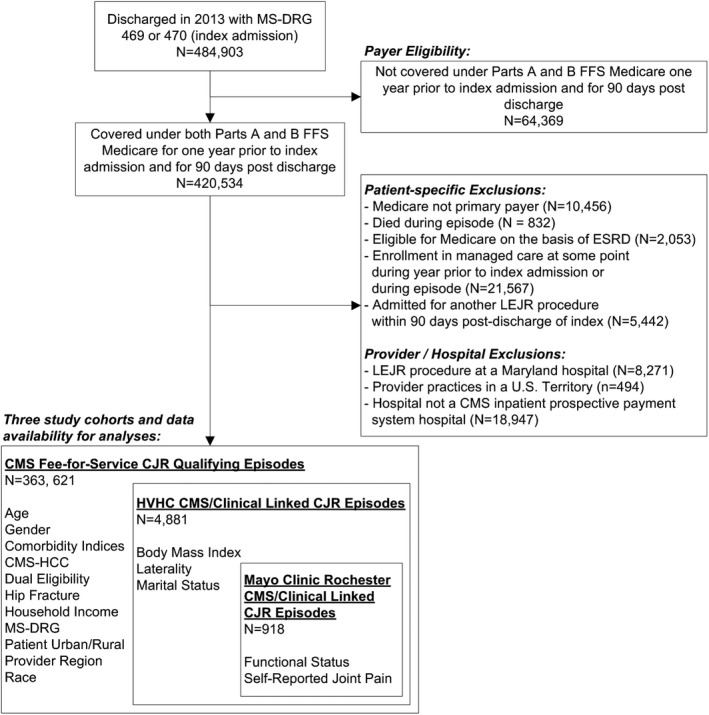
- Note: Patient and Provider/Hospital Exclusions are not mutually exclusive; A single LEJR episode may be excluded for more than one exclusion factor.
Under the CJR program, beneficiaries were excluded if they died at any point during their 90‐day episode, were eligible for Medicare on the basis of End Stage Renal Disease (ESRD), were enrolled in Medicare Managed Care at any time during or in the 12 months preceding the episode period, and were subsequently admitted to an acute care hospital during the episode period which resulted in a discharge with MS‐DRG 469 or 470 (including sequential bilateral procedures). Those who reside within a U.S. Territory or those who underwent an LEJR procedure at Maryland hospitals were also excluded due to Maryland's participation in CMS’ Maryland All‐Payer Model. These exclusions were followed based on those outlined in the CJR methodology.
We utilized all Medicare beneficiaries with a qualifying LEJR found within CMS‐claims data, and to understand the incremental value of more granular data sources for patient‐level information, we examined two populations subsets: beneficiaries with qualifying LEJR performed within one of twelve member organizations of the HVHC in which clinical and EMR data were available, and beneficiaries with a qualifying LEJR at the Mayo Clinic campus in Rochester, Minnesota. The patient‐level data elements available for each population subset are listed in Figure 1.
2.2. Patient measures
Medicare FFS data contain some patient‐level information that may be informative in correctly assigning patients to expected cost groups, which can be helpful in risk adjustment and payment stratification. Within Medicare FFS data, there are some patient‐level factors routinely collected and submitted to Medicare for qualification and billing purposes, including patient age, gender, dual eligibility status for both Medicare and Medicaid services, patient residency in an urban vs rural area, region of the country of which the LEJR procedure takes place, and patient race. Additionally, methods have been developed to use billing codes to attempt to understand patient comorbidity burden, including the Charlson comorbidity index,30 the Elixhauser comorbidity measure,31 and the CMS‐Hierarchical Condition Category (HCC) score.32 We focused on the CMS‐HCC Risk Adjustment Model, version 2132 at the request of the public commentary for the CJR final rule. This algorithm uses a combination of basic demographic characteristics and comorbidity diagnosis codes from Medicare claims to calculate a prospective risk score for each beneficiary, as well as to identify hierarchically categorized individual condition categories. CMS‐HCC condition categories were evaluated by their prevalence in the population and an assessment of the C‐statistic produced by the univariate logistic regression with the HCC condition as the predictor and the dichotomized cost group as the response (defined in detail in the next section).
Beyond the FFS/claims‐based data, health care organizations have varying degrees of patient‐level data stored and available which may help create a more comprehensive picture of patient complexity. We therefore deployed the HVHC CJR‐qualifying LEJR episodes linked with clinical data from a variety of hospital systems to help represent the data availability of a larger group of health care organizations. The HVHC is a consortium of health care delivery systems formed to accelerate process improvements and to reduce cost.33 Data available from this broader set included body mass index (BMI) to understand the impact of body fat as a proxy for complexity of patient health status and necessary recovery care, laterality of the procedure (right vs. left joint), and marital status as a proxy for social support.
To further our understanding of how patient‐reported measures could also play a role in assignment of patients to episode cost groups for CJR‐qualifying LEJR episodes, we chose to also analyze the data resources available within our own Mayo Clinic health care system. Mayo Clinic Rochester is a large multidisciplinary practice in the Midwest. The measures included questions routinely collected for clinical care within our system, which was not a standard practice among other HVHC member organizations.
2.3. Primary outcome: 90‐day episode cost
The primary outcome of interest was the total 90‐day episode cost for the LEJR procedure, and all related services rendered during the episode period as outlined under the CJR. Total 90‐day costs were calculated by aggregating Medicare FFS payments for all services billed to the beneficiary from the time of admission until 90 days postdischarge. Services deemed to be unrelated to the LEJR procedure were excluded per the stipulations outlined in the CJR federal rule: (a) unrelated readmissions identified on the basis of MS‐DRG codes; (b) Part B services identified using the principle diagnosis code on the claim line; (c) hemophilia clotting factors; (4) new technology add‐on payments for drugs, technologies, and services, as well as Outpatient Prospective Payment System (OPPS) transitional pass‐through payments for medical devices. Services that spanned outside of the defined episode period were prorated so that only services attributable to the 90‐day episode were included in the total aggregated costs. This proration was carried out per the provisions of the CJR federal rule. All Medicare prices were standardized using the method developed by The Dartmouth Institute, which standardizes the prices to adjust for sources of variation that may not be of interest to researchers, namely “variation caused by differences in utilization and variation due to Medicare's varying rates for the same medical services.”3
To understand the performance of patient‐level factors on the correct assignment of patients to cost group under a stratified payment model such as the CJR, we chose to dichotomize CJR‐qualifying LEJR episodes into two groups: the most expensive 20% of episodes, and the remaining 80%. Cut points for dichotomized 80%/20% episode costs were defined using the full CMS LEJR population, stratified by MSR‐DRG (469 vs. 470), and were then applied to the other study population subsets (HVHC and Mayo Clinic populations) to ensure consistency across populations. Therefore, each of our population subsets deployed the same cut point for analysis. This method was employed to align as closely with how federal policy may work, given the intent to reduce variability across the CMS population at large.
2.4. Statistical analysis
Descriptive statistics were calculated for all patient characteristics within the Medicare FFS CJR‐qualifying LEJR population, as well as for the two subpopulations of CJR‐qualifying LEJR procedures. Patient characteristics were presented as a prevalence estimate for the entire Medicare FFS CJR‐qualifying LEJR population to understand the magnitude of the patient factors across the entire CMS population. Additionally, proportions of patients within each study population (CMS FFS LEJR population, HVHC population, Mayo Clinic population) were presented within 90‐day episode cost groups as a method to understand the generalizability of the two population subsets (HVHC and Mayo Clinic). The distribution of the most prevalent and predictive individual conditions from the CMS‐HCC risk score was also calculated and presented for the CMS LEJR population at large, and against the dichotomized (80%/20%) episode cost groups. In order to protect the confidentiality of patient data, and for cell suppression requirements of Medicare data, we are unable to present individual Ns within each study population.
Under the CJR legislation, CMS set four target prices for each participating hospital based on MS‐DRG and patient hip fracture status upon admission. To help understand the distribution of episode costs by these CJR‐designated stratifications, we calculated the 20th percentile, median, and 80th percentile episode costs for the CMS CJR‐qualifying LEJR population as a whole, and for our two subpopulations (HVHC and Mayo Clinic).
To understand the incremental value of varying types of patient‐level information in correctly assigning CJR‐qualifying LEJR episodes to cost groups, we developed three sets of multivariable logistic models to compare predictive ability: (a) Base Model (CJR Program “as‐is”), (b) Base Model plus CMS‐HCC classifications, and (c) Base Model plus CMS‐HCC plus other factors. The base model was used to explain the predictive power of the current CJR legislation to identify high‐cost episodes. This base model included covariates currently utilized as stratification for payment under the CJR model: an indicator for whether or not the beneficiary had a hip fracture, and the location of the anchor hospitalization classified into one of the nine geographic regional census divisions, excluding the Mayo Clinic models where only one geographic regional census division is represented. This model was nested into each of the additional models, and the performance of this base model was compared to other models that included the base covariates, CMS‐HCC condition categories, age, and other claims‐based or clinical covariates with the potential to add predictive power. Models were assessed by comparing C‐statistics, sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV), as well as examining the ROC tables for each of the multivariable logistic models. The Youden Index was used to determine optimal cut points for evaluation.34 This index optimizes the cut point by maximizing the combined sensitivity and specificity measures: Youden Index = sensitivity + specificity − 1. This index was used in lieu of fixed sensitivity due to the nature of the binary predictors in our models limiting the ability to choose a fixed sensitivity.
Additionally, we estimated the same multivariate models against the continuous total 90‐day episode cost outcome for comparison. Because health care costs are not normally distributed, we estimated a generalized linear model with a gamma distribution and log link transformation. Regression estimates were converted back to costs for interpretation. To examine the incremental value of the models, we calculated R 2 estimates for generalized linear models using methods developed by Zhang.35 We report both estimated R 2 and the penalized R 2 to adjust for additional parameters in the full model. Results for these supplemental analyses can be found within Supporting information. All analysis was carried out using SAS 9.4 (Cary, NC).
3. RESULTS
Our CMS population and two population subsets consisted of 363 621 in the CMS population, 4881 in the HVHC population, and 918 in the Mayo Clinic population who underwent a qualifying LEJR procedure in 2013 (Table 1). The majority of patients were female (CMS 65.5%, HVHC 62.0%, Mayo Clinic 55.9%), between the ages of 65 and 79y (CMS 66.6%, HVHC 75.0%, Mayo Clinic 65.0%), white (CMS 91.1%, HVHC 94.3%, Mayo Clinic 97.9%), not dual eligible with Medicaid (CMS 79.9%, HVHC 92.3%, Mayo Clinic 85.6%), and resided in an urban area (CMS 72.4%, HVHC 66.4%, Mayo Clinic 50.0%). Over half of procedures were performed on the knee (CMS 58.1%, HVHC 64.2%, Mayo Clinic 58.4%), followed by on the hip (CMS 41.1%, HVHC 37.8%, Mayo Clinic 41.6%). Patients were discharged to the following locations: a skilled nursing facility (SNF), (CMS 36.4%, HVHC 34.8%, Mayo Clinic 45.2%), home with home health services (CMS 32.8%, HVHC 31.2%, Mayo Clinic 3.3%), home without additional services (CMS 17.6%, HVHC 26.6%, Mayo Clinic 48.8%), and to a postacute rehabilitation facility (CMS 11.8%, HVHC 7.2%, Mayo Clinic 2.5%).
Table 1.
Characteristics of patients undergoing lower extremity joint replacement (LEJR) for three cohorts: all qualifying episodes in the CMS population, patients with qualifying episodes at a High Value Healthcare Collaborative (HVHC) facility, patients at Mayo Clinic, Rochester, Minnesota (2013) in the bottom 80% and top 20% of episode costs
| CMS LEJR population | HVHC LEJR | Mayo LEJR | ||||
|---|---|---|---|---|---|---|
| Subpopulation | Subpopulation | |||||
| Bottom 80% | Top 20% | Bottom 80% | Top 20% | Bottom 80% | Top 20% | |
| Gender | ||||||
| Male | 84.0 | 16.0 | 90.0 | 10.0 | 90.4 | 9.6 |
| Female | 77.9 | 22.1 | 85.0 | 15.0 | 86.9 | 13.1 |
| Age | ||||||
| <65 years | 84.1 | 15.9 | 82.2 | 17.8 | 85.3 | 14.7 |
| 65‐79 years | 87.0 | 13.0 | 90.0 | 10.0 | 92.2 | 7.8 |
| 80+ years | 60.5 | 39.5 | 75.7 | 24.3 | 77.9 | 22.1 |
| Race | ||||||
| White | 80.4 | 19.6 | 87.3 | 12.7 | 88.3 | 11.7 |
| Black | 75.3 | 24.7 | 74.2 | 25.8 | 100.0 | 0.0 |
| Other/Unknown | 77.7 | 22.3 | 85.0 | 15.0 | 90.9 | 9.1 |
| Dual eligible | ||||||
| No | 81.5 | 18.5 | 88.0 | 12.0 | 89.8 | 10.2 |
| Yes | 73.9 | 26.1 | 73.4 | 26.6 | 80.3 | 19.7 |
| Urban/Rural | ||||||
| Urban | 79.1 | 20.9 | 86.4 | 13.6 | 91.8 | 8.2 |
| Rural | 82.4 | 17.6 | 87.1 | 12.9 | 87.4 | 12.6 |
| Super Rural | 82.3 | 17.7 | 88.8 | 11.2 | 81.1 | 18.9 |
| Procedure type | ||||||
| Hip | 69.2 | 30.8 | 85.1 | 14.9 | 85.9 | 14.1 |
| Knee | 87.8 | 12.2 | 87.9 | 12.1 | 90.3 | 9.7 |
| Ankle | 88.4 | 11.6 | – | – | – | – |
| Other | 54.2 | 45.8 | – | – | – | – |
| Discharge disposition | ||||||
| Home | 96.8 | 3.2 | 97.7 | 2.3 | 97.5 | 2.5 |
| Home health | 97.0 | 3.0 | 96.7 | 3.3 | 96.7 | 3.3 |
| Hospice | 70.4 | 29.6 | – | – | – | – |
| SNF | 68.9 | 31.1 | 77.3 | 22.7 | 80.7 | 19.3 |
| Rehab | 43.5 | 56.5 | 42.5 | 57.5 | 43.5 | 56.5 |
| Other | 65.5 | 34.5 | 55.6 | 44.4 | 50.0 | 50.0 |
| Marital Statusa | ||||||
| Married/Life Partner | – | – | 90.7 | 9.3 | 90.7 | 9.3 |
| Unmarried | – | – | 80.4 | 19.6 | 83.3 | 16.7 |
| BMIa | ||||||
| <30 | – | – | 87.7 | 12.3 | 90.1 | 9.9 |
| ≥30 | – | – | 87.0 | 13.0 | 87.5 | 12.5 |
| Ability to climb two flights of stairsb | ||||||
| Can't at all | – | – | – | – | 75.6 | 24.3 |
| Can with difficulty | – | – | – | – | 92.2 | 7.8 |
| Can with no difficulty | – | – | – | – | 97.7 | 2.3 |
| CMS‐HCC categories | ||||||
| Congestive heart failure | ||||||
| Present | 64.0 | 36.0 | 71.0 | 29.0 | 69.7 | 30.3 |
| Not present | 82.2 | 17.8 | 90.6 | 9.4 | 88.7 | 11.3 |
| Vascular disease | ||||||
| Present | 66.6 | 33.5 | 83.8 | 16.2 | 76.3 | 23.7 |
| Not present | 82.1 | 17.9 | 89.1 | 10.9 | 88.1 | 11.9 |
| Specified heart arrhythmias | ||||||
| Present | 69.7 | 30.3 | 80.8 | 19.2 | 78.9 | 21.1 |
| Not present | 81.9 | 18.1 | 89.9 | 10.1 | 88.3 | 11.7 |
| Dementia without complication | ||||||
| Present | 45.5 | 54.5 | 70.4 | 29.6 | 60.6 | 39.4 |
| Not present | 81.7 | 18.4 | 89.0 | 11.0 | 87.4 | 12.6 |
| COPD | ||||||
| Present | 70.2 | 29.8 | 74.7 | 25.3 | 77.1 | 22.9 |
| Not present | 81.5 | 18.6 | 89.9 | 10.1 | 87.6 | 12.3 |
BMI, body mass index; CMS, Centers for Medicare and Medicaid Services; CMS‐HCC, Centers for Medicare and Medicaid Services—Hierarchical Condition Category; COPD, chronic obstructive pulmonary disease; HVHC, high value healthcare collaborative; LEJR, lower extremity joint replacement; SNF, skilled nursing facility.
Data only available for HVHC, Mayo Clinic subpopulations.
Data only available for Mayo Clinic subpopulation.
Additional variables not found in claims had differential availability based on the subpopulation. We ascertained marital status and BMI among both the HVHC and Mayo Clinic populations, and a measure of functional ability (ability to climb two flights of stairs) among the Mayo Clinic population. The majority of the patients in our study reported to be married (HVHC 63.4%, Mayo Clinic 70.0%), and half of patients had a BMI greater than 30 (HVHC 46.0%, Mayo Clinic 50.0%). Among the Mayo Clinic population, 20.6% reported the inability to climb two flights of stairs, 37.7% reported that they could climb two flights of stairs with difficulty, and 24.0% reported that they could climb two flights of stairs with no difficulty.
3.1. Patient factors across cost categories
When patient‐level factors were modeled univariately against the 80%/20% dichotomized 90‐day episode costs, factors associated with high‐cost episodes (top 20%) included our youngest and oldest age groups (< 65 years; ≥80 years) (P < 0.0001 for CMS, HVHC, Mayo Clinic), dual eligibility status (CMS P < 0.0001; HVHC P < 0.0001; Mayo Clinic P = 0.0015), having a hip or “other” procedure (ie, complications of orthopedic device, fractures, or degeneration of the lower extremity) vs a knee or ankle procedure (CMS P < 0.0001; HVHC P = 0.0073, Mayo Clinic P = 0.0382), and residence in an urban (CMS P < 0.0001; HVHC P = NS; Mayo Clinic P = NS) or rural setting (CMS P = NS; HVHC P = NS; Mayo Clinic P = 0.0009).
Within our HVHC and Mayo Clinic subpopulations, our ability to look at different patient factors varied due to data availability. The inability to climb two flights of stairs was associated with high‐cost episodes (Mayo Clinic, P < 0.0001). Unmarried status was associated with higher cost compared to married or having a life partner (HVHC: 19.6% vs 9.3%, P < 0.0001; Mayo Clinic: 16.7% vs 9.3%, P = 0.0013). BMI (<30 and ≥30) was not associated with cost (HVHC: P = 0.495; Mayo Clinic: P = 0.2204).
The five most prevalent individual CMS‐HCC groups identified were congestive heart failure, vascular disease, specified heart arrhythmias, dementia without complication, and chronic obstructive pulmonary disease (COPD). Univariate modeling of these individual CMS‐HCC groups indicated moderate model performance (range 0.539‐0.559). When examining these variables univariately through logistic regression with total 90‐day episode cost in the top 20%, all five conditions were associated with high‐cost episodes. The only exception to this was a non‐significant association for vascular disease in the Mayo Clinic subpopulation (P = 0.0891).
3.2. 90‐day episode costs
Median total episode costs increased with the increased severity (MS‐DRG) and hip fracture status (Table 2). Median and cost distributions varied across the three populations, with the overall CMS population typically demonstrating higher costs than the HVHC and Mayo Clinic subpopulations, particularly among the MS‐DRG 470 with hip fracture group. For those patients with hip fracture grouped under MS‐DRG 469, the HVHC and Mayo Clinic sites had higher median costs.
Table 2.
Episode cost summaries for lower extremity joint replacement (LEJR) among the CMS, HVHC, and Mayo Clinic populations (2013) as grouped according to the CMS Comprehensive Care for Joint Replacement (CJR) program
| CMS LEJR population (N = 363 621) | HVHC LEJR subpopulation (N = 4881) | Mayo LEJR subpopulation (N = 918) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 20th percentile | Median | 80th percentile | 20th percentile | Median | 80th percentile | 20th percentile | Median | 80th percentile | |
| MS‐DRG 470, No HF | $17 437 | $20 331 | $28 663 | $16 181 | $19 565 | $28 114 | $14 624 | $17 288 | $24 920 |
| MS‐DRG 470, HF | $27 500 | $37 005 | $48 284 | $20 100 | $27 825 | $42 608 | $22 887 | $29 941 | $41 914 |
| MS‐DRG 469, No HF | $28 058 | $36 915 | $50 646 | $26 879 | $36 802 | $52 594 | $23 932 | $31 058 | $50 281 |
| MS‐DRG 469, HF | $39 593 | $49 710 | $63 356 | $36 181 | $57 153 | $72 098 | $35 968 | $50 504 | $65 607 |
| Overall | $17 790 | $21 818 | $33 146 | $16 276 | $19 808 | $28 807 | $14 672 | $17 881 | $26 097 |
CMS, Centers for Medicare and Medicaid Services; HF, hip fracture; HVHC, high value healthcare collaborative; MS‐DRG, Medicare severity‐diagnosis related group.
MS‐DRG 470: Episodes billed under “Major Joint Replacement or Reattachment of Lower Extremity Without Major Complication or Comorbidity (MCC).”
MS‐DRG 469: Episodes billed under “Major Joint Replacement or Reattachment of Lower Extremity With Major Complication or Comorbidity (MCC).”
3.3. Models identifying high‐cost episodes
The base model, which included hip fracture status and provider region within each clinical population, demonstrated low to moderate model performance (CMS C‐Stat = 0.714; HVHC C‐Stat = 0.628; Mayo Clinic C‐Stat = 0.587) (Table 3). The addition of HCC comorbidities increased model performance based on C‐Statistics (CMS C‐Stat = 0.758, HVHC C‐Stat = 0.692, Mayo Clinic C‐Stat = 0.677), as did the additional inclusion of categorized patient age (CMS C‐Stat = 0.780, HVHC C‐Stat = 0.721, Mayo Clinic C‐Stat = 0.721). Independent evaluation of models to determine the impact of inclusion of measures of socioeconomic status [SES] (CMS C‐Stat = 0.784, HVHC C‐Stat = 0.729, Mayo Clinic C‐Stat = 0.737), marital status (CMS C‐Stat = N/A, HVHC C‐Stat = 0.735, Mayo Clinic C‐Stat = 0.0.727), BMI (CMS C‐Stat = N/A, HVHC C‐Stat = 0.718, Mayo Clinic C‐Stat = 0.721), and patient functional status (CMS C‐Stat = N/A, HVHC C‐Stat = N/A, Mayo Clinic C‐Stat = 0.783) showed variation in impact between measure group. Patient factors listed with “Not Applicable” (N/A) indicate that these fields were not available within each given population (marital status and BMI in the CMS population; patient functional status in the CMS and HVHC populations). SES (dual eligibility) had the greatest impact on C‐Statistic, sensitivity, and PPV among the CMS population. Marital status had the greatest impact on C‐Statistic, sensitivity, and NPV but adversely impacted specificity among the HVHC population. Functional status (ability to climb two flights of stairs and BMI) had the greatest impact on C‐Statistic, sensitivity, PPV, and NPV, but adversely impacted specificity among Mayo Clinic LEJR patients.
Table 3.
Incremental modeling of 90‐day episode cost for lower extremity joint replacement (LEJR) for CMS, HVHC, and Mayo Clinic, Rochester, Minnesota populations (2013). All estimates were calculated utilizing the Youden Index for selecting optimal cut points for evaluation
| CMS LEJR population | HVHC LEJR subpopulation | Mayo LEJR subpopulation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C‐Stat | Sens | Spec | PPV | NPV | C‐Stat | Sens | Spec | PPV | NPV | C‐Stat | Sens | Spec | PPV | NPV | |
| 1. Base (CJR Program) | 0.714 | 42.4% | 93.3% | 28.9% | 85.2% | 0.628 | 53.5% | 69.5% | 20.9% | 90.9% | 0.587 | 20.8% | 96.7% | 13.2% | 90.3% |
| 2. Base + HCC | 0.758 | 59.2% | 82.4% | 49.2% | 84.2% | 0.692 | 58.4% | 70.2% | 22.8% | 91.8% | 0.677 | 40.6% | 87.6% | 27.2% | 90.3% |
| 3. Base + HCC + Age | 0.780 | 63.1% | 80.6% | 49.6% | 85.1% | 0.721 | 70.6% | 61.8% | 21.8% | 93.3% | 0.721 | 67.0% | 65.8% | 27.7% | 90.8% |
| 4. Base + HCC + SES | 0.784 | 64.8% | 78.4% | 49.4% | 85.0% | 0.729 | 62.3% | 71.1% | 24.5% | 92.6% | 0.737 | 54.7% | 83.6% | 27.5% | 91.0% |
| 5. Base + HCC + Mar | 0.735 | 70.0% | 64.6% | 22.9% | 93.4% | 0.727 | 61.3% | 73.5% | 26.7% | 91.0% | |||||
| 6 Base + HCC + BMI | 0.718 | 65.3% | 62.1% | 20.6% | 92.2% | 0.721 | 79.5% | 54.9% | 23.2% | 91.3% | |||||
| 7. Base + HCC + Fnctn | 0.783 | 76.8% | 69.2% | 18.9% | 91.0% | ||||||||||
BMI, body mass index; CJR, Comprehensive Care for Joint Replacement Program; CMS, Centers for Medicare and Medicaid Services; C‐Stat, C‐Statistic; Fnctn, functional status; HCC, Hierarchical Condition Categories (CMS Version); HVHC, High Value Healthcare Collaborative; Mar, marital status; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; SES, socioeconomic status; Spec, specificity.
1. Base (CJR Program): Hip Fracture and Provider Region (not applicable in Mayo Clinic population).
2. Base + HCC: Hip Fracture, Provider Region, and the 5 HCC Conditions.
3. Base + HCC + Age: Hip Fracture, Provider Region, the 5 HCC Conditions, and Age.
4. Base + HCC + SES: Hip Fracture, Provider Region, the 5 HCC Conditions, Age, Dual Eligibility.
5. Base + HCC + Mar Status: Hip Fracture, 5 HCC Conditions, Age, Marital Status.
6. Base + HCC + BMI: Hip Fracture, 5 HCC Conditions, Age, (BMI) Body Mass Index.
7. Base + HCC + Function: Hip Fracture, 5 HCC Conditions, Age, Ability to Climb Two Flights of Stairs.
Similar findings were observed in the continuous outcome models with increased explanatory ability (ie, higher R 2 and penalized R 2) as measured of interest were added. Additional detail regarding these results can be found in the online technical appendices.
4. DISCUSSION
We observed that younger (<65 years) and older (≥80 years) age, dual eligibility status, having a nonknee or ankle procedure, and residence in an urban setting for the entire CMS cohort, and residence in a rural setting for the Mayo Clinic cohort were associated with higher episode costs. We observed that adding CMS‐HCC categories improved our ability to identify patients who were in the top 20% of episode costs. We also observed that variables derived from administrative data and clinical sources, particularly functional status, improved our ability to identify patients within different cost groups.
Our findings add to the extant literature by demonstrating that CMS‐HCC increases the ability to identify patients who are higher cost in a population of patients undergoing LEJR. Concerns have existed about the accuracy of the CMS‐HCC for predicting patient care costs.36, 37, 38 CMS had not included CMS‐HCC risk adjustment in the CJR rule because it had not been validated in a group of patients undergoing LEJR. Our data provide support to the inclusion of CMS‐HCC risk adjustment in future bundled payment models for patients undergoing LEJR.
Our data suggest that the inclusion of clinical factors such as functional status may improve the ability to predict patients with greater episode costs. C‐statistics greater than 0.70 are considered good and clinically relevant, and C‐statistics greater than 0.80 are considered strong.39 The highest C‐statistic we observed was for the base model in addition to CMS‐HCC and functional status (defined as being ability to climb two flights of stairs) in the Mayo Clinic population (C‐statistic 0.783). The addition of functional status to the base model and CMS‐HCC increased the sensitivity for detecting patients with high‐cost episodes. In other words, if patients are able to climb two flights of stairs prior to joint surgery, they are less likely to be in the top 20% of episode costs. The CMS‐HCC model does not include adjustments for functional impairment. Previous researchers have identified that the CMS‐HCC model is likely to underpredict expenses for Medicare beneficiaries with functional status impairment, and that the level of disability accounts for a substantial portion of the difference between actual and predicted expenses.38
The inclusion of clinical variables into a risk adjusted CMS payment model would require substantial resources and infrastructure and is perhaps not justified based upon its impact in models in the LEJR population we evaluated. However, functional status may have more of impact on predicting cost in other populations with multiple medical comorbidities who may be considered for future bundled payment. Fortunately, CMS has expressed interest in the collection of patient‐reported information in the Merit‐based Incentive Payment Systems (MIPS) and Alternative Payment Models (APMs) under Medicare Access and Children's Health Insurance Program Reauthorization Act of 2015 (MACRA), and has listed patient‐reported measures as a priority focus outcome measure.40 Collection and application of measures such as patient ability to climb two flights of stairs could help to facilitate the appropriate adjustment for the potential risk that providers of care may face and safeguard patients from adverse selection.
Previous investigators have suggested that the financially dominant strategy for most hospitals under CJR would be to accept Medicare penalties and not make any investments in CJR or care management.41 This result is partially driven by the significant upfront investments in care redesign and the inability to recover these costs for low‐volume hospitals. However, for commoditized procedures like LEJR, significant cost reductions can be obtained through interventions which do not require potentially expensive care redesign efforts such as reducing implant costs or avoiding higher cost postacute care.42 Our findings provide information that could enhance the tailoring of care management pathways to reduce costs for patients more likely to incur greater costs. An unintended consequence is that our findings could be used to offer procedures to lower‐risk, lower‐cost patients. Indeed, a previous study has observed a statistically significant decrease in the average age and comorbidity of patients undergoing LEJR among academic centers during the risk‐bearing period of bundled payment for LEJR.43 The inclusion of HCC risk scores in LEJR reconciliation payments would lead to annual reductions in payments by as much as $146 360 for hospitals caring for the least medically complex patients and increases as large as $114 184 for hospitals with the most medically complex patients.28 These financial shifts in a cost‐neutral approach to reimbursement for CMS programs would serve to decrease the likelihood of adverse patient selection for higher cost patients.
Several limitations to our analysis exist. First, data availability varied across the three study cohorts used in our analysis due to the nature of data collection from Medicare claims, across differing HVHC member organizations, and at Mayo Clinic. This limited our power for detecting differences between groups and limited our ability to capture the potential effects of certain patient factors. Limited sample sizes, particularly after linking claims to administrative and clinical variables from HVHC and Mayo Clinic, also forced us to collapse certain categorical variables. Conversely, within our body of claims population, we had a very large sample size which allowed for potentially irrelevant differences to be detected.
Our study provides approaches for improving the identification of high‐cost episodes among patients undergoing LEJR. Appropriate risk adjustment may increase the likelihood that provider organizations elect to participate in bundled payment models. If bundles become part of the future health care landscape, policy makers should incorporate improved risk adjustment of payments for LEJR to minimize adverse selection of patients and limiting their access to necessary procedures to reduce pain and improve function.
CONFLICT OF INTEREST
None.
Supporting information
ACKNOWLEDGMENTS
Joint Acknowledgment/Disclosure Statement: This study was funded by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Philpot LM, Swanson KM, Inselman J, et al. Identifying high‐cost episodes in lower extremity joint replacement. Health Serv Res. 2019;54:117–127. 10.1111/1475-6773.13078
REFERENCES
- 1. Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000‐2010. NCHS Data Brief. 2015;186:1‐8. [PubMed] [Google Scholar]
- 2. Williams SN, Wolford ML, Bercovitz A. Hospitalization for total knee replacement among inpatients aged 45 and over: United States, 2000‐2010. NCHS Data Brief. 2015;210:1‐8. [PubMed] [Google Scholar]
- 3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780‐785. [DOI] [PubMed] [Google Scholar]
- 4. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991‐2010. JAMA. 2012;308(12):1227‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller DC, Gust C, Dimick JB, Birkmeyer N, Skinner J, Birkmeyer JD. Large variations in Medicare payments for surgery highlight savings potential from bundled payment programs. Health Aff (Millwood). 2011;30(11):2107‐2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plate JF, Brown ML, Wohler AD, Seyler TM, Lang JE. Patient factors and cost associated with 90‐day readmission following total hip arthroplasty. J Arthroplasty. 2016;31(1):49‐52. [DOI] [PubMed] [Google Scholar]
- 7. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991‐2008. JAMA. 2011;305(15):1560‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693‐1698. [DOI] [PubMed] [Google Scholar]
- 9. Cram P, Ravi B, Vaughan‐Sarrazin MS, Lu X, Li Y, Hawker G. What drives variation in episode‐of‐care payments for primary TKA? An analysis of Medicare administrative data. Clin Orthop Relat Res. 2015;473(11):3337‐3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87(3):570‐576. [DOI] [PubMed] [Google Scholar]
- 11. Cram P, Lu X, Li Y. Bundled payments for elective primary total knee arthroplasty: an analysis of Medicare administrative data. Geriat Orthopaed Sur Rehabilit. 2015;6(1):3‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Epps CD. Length stay, discharge disposition, and hospital charge predictors. AORN J. 79(5):975‐976. [DOI] [PubMed] [Google Scholar]
- 13. Fang M, Noiseux N, Linson E, Cram P. The effect of advancing age on total joint replacement outcomes. Geriatr Orthop Surg Rehabil. 2015;6(3):173‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adrados M, Lajam C, Hutzler L, Slover J, Bosco J. The effect of severity of illness on total joint arthroplasty costs across New York state hospitals: an analysis of 172,738 cases. J Arthroplasty. 2015;30(1):12‐14. [DOI] [PubMed] [Google Scholar]
- 15. Ashraf A, Larson AN, Maradit‐Kremers H, Kremers WK, Lewallen DG. Hospital costs of total hip arthroplasty for developmental dysplasia of the hip. Clin Orthop Relat Res. 2014;472(7):2237‐2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blanchette CM, Wang PF, Joshi AV, Kruse P, Asmussen M, Saunders W. Resource utilization and costs of blood management services associated with knee and hip surgeries in US hospitals. Adv Ther. 2006;23(1):54‐67. [DOI] [PubMed] [Google Scholar]
- 17. Cookson R, Laudicella M. Do the poor cost much more? The relationship between small area income deprivation and length of stay for elective hip replacement in the English NHS from 2001 to 2008. Soc Sci Med. 2011;72(2):173‐184. [DOI] [PubMed] [Google Scholar]
- 18. Styron JF, Koroukian SM, Klika AK, Barsoum WK. Patient vs provider characteristics impacting hospital lengths of stay after total knee or hip arthroplasty. J Arthroplasty. 2011;26(8):1418‐1426 e1411‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weaver F, Hynes D, Hopkinson W, et al. Preoperative risks and outcomes of hip and knee arthroplasty in the Veterans Health Administration. J Arthroplasty. 2003;18(6):693‐708. [DOI] [PubMed] [Google Scholar]
- 20. Bolognesi MP, Marchant MH Jr, Viens NA, Cook C, Pietrobon R, Vail TP. The impact of diabetes on perioperative patient outcomes after total hip and total knee arthroplasty in the United States. J Arthroplasty. 2008;23(6 Suppl 1):92‐98. [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez Della Valle A, Chiu YL, Ma Y, Mazumdar M, Memtsoudis SG. The metabolic syndrome in patients undergoing knee and hip arthroplasty: trends and in‐hospital outcomes in the United States. J Arthroplasty. 2012;27(10):1743‐1749 e1741. [DOI] [PubMed] [Google Scholar]
- 22. Maradit Kremers H, Visscher SL, Moriarty JP, et al. Determinants of direct medical costs in primary and revision total knee arthroplasty. Clin Orthop Relat Res. 2013;471(1):206‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Memtsoudis SG, Stundner O, Rasul R, et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesth Analg. 2014;118(2):407‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perfetti DC, Boylan MR, Naziri Q, Khanuja HS, Urban WP. Does sickle cell disease increase risk of adverse outcomes following total hip and knee arthroplasty? A nationwide database study J Arthroplasty. 2015;30(4):547‐551. [DOI] [PubMed] [Google Scholar]
- 25. Stundner O, Kirksey M, Chiu YL, et al. Demographics and perioperative outcome in patients with depression and anxiety undergoing total joint arthroplasty: a population‐based study. Psychosomatics. 2013;54(2):149‐157. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Medicare & Medicaid Services . Comprehensive Care for Joint Replacement Model. 2016; https://innovation.cms.gov/initiatives/CJR. Accessed October 14, 2016.
- 27. Centers for Medicare & Medicaid Services (CMS) . Medicare Program; Cancellation of Advancing Care Coordination Through Episode Payment and Cardiac Rehabilitation Incentive Payment Models; Changes to Comprehensive Care for Joint Replacement Payment Model (CMS‐5524‐P). https://www.federalregister.gov/documents/2017/08/17/2017-17446/medicare-program-cancellation-of-advancing-care-coordination-through-episode-payment-and-cardiac. [PubMed]
- 28. Ellimoottil C, Ryan AM, Hou H, Dupree J, Hallstrom B, Miller DC. Medicare's new bundled payment for joint replacement may penalize hospitals that treat medically complex patients. Health Aff (Millwood). 2016;35(9):1651‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Medicare & Medicaid Services . Medicare and Medicaid Programs; Electronic Health Record Incentive Program In: Services CfMM, ed. 75 FR 44313. Vol 0938‐AP78. United States of America 2010:44313‐44588. [PubMed] [Google Scholar]
- 30. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 31. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8‐27. [DOI] [PubMed] [Google Scholar]
- 32. Centers for Medicare and Medicaid Services . CMS‐Hierarchical Condition Category Model. In: Model C‐HCC, ed. 2013; https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors-Items/Risk2013.html?DLPage=1&DLEntries=10&DLSort=0&DLSortDir=descending. Accessed 12/7/2017, 2017.
- 33. Tomek IM, Sabel AL, Froimson MI, et al. A collaborative of leading health systems finds wide variations in total knee replacement delivery and takes steps to improve value. Health Aff (Millwood). 2012;31(6):1329‐1338. [DOI] [PubMed] [Google Scholar]
- 34. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32‐35. [DOI] [PubMed] [Google Scholar]
- 35. Zhang DA. Coefficient of determination for generalized linear models. Am Stat. 2017;71(4):310‐316. [Google Scholar]
- 36. Kautter J, Pope GC. CMS frailty adjustment model. Health Care Financ Rev. 2004;26(2):1‐19. [PMC free article] [PubMed] [Google Scholar]
- 37. Riley GF. Risk adjustment for health plans disproportionately enrolling frail Medicare beneficiaries. Health Care Financ Rev. 2000;21(3):135‐148. [PMC free article] [PubMed] [Google Scholar]
- 38. Noyes K, Liu H, Temkin‐Greener H. Medicare capitation model, functional status, and multiple comorbidities: model accuracy. Am J Manag Care. 2008;14(10):679‐690. [PMC free article] [PubMed] [Google Scholar]
- 39. Hosmer D, Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 40. Centers for Medicare & Medicaid Services . CMS Quality Measure Development Plan: Supporting the Transition to The Quality Payment Program 2017 Annual Report. 2016; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/2017-CMS-MDP-Annual-Report.pdf. Accessed January 29, 2018.
- 41. Maniya OZ, Mather RC 3rd, Attarian DE, et al. Modeling the potential economic impact of the Medicare comprehensive care for joint replacement episode‐based payment model. J Arthroplasty. 2017;32(11):3268‐3273 e3264. [DOI] [PubMed] [Google Scholar]
- 42. Navathe AS, Troxel AB, Liao JM, et al. Cost of joint replacement using bundled payment models. JAMA Intern Med. 2017;177(2):214‐222. [DOI] [PubMed] [Google Scholar]
- 43. Jubelt LE, Goldfeld KS, Chung WY, Blecker SB, Horwitz LI. Changes in discharge location and readmission rates under Medicare bundled payment. JAMA Intern Med. 2016;176(1):115‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


