Abstract
Perineuronal nets (PNNs) are a form of aggregate Extracellular Matrix (ECM) in the brain. Recent evidence suggests that the postnatal deposition of PNNs may play an active role in regulating neuroplasticity and, potentially, neurological disorders. Observations of high levels of PNN expression around somas, proximal dendrites, and axon initial segments of a subtype of neurons have also led to proposals that PNNs may modulate the intrinsic properties of the neurons they ensheathe. While high levels of PNNs are postnatally expressed throughout the neocortex, it is still unclear how they impact the neuronal physiology of the many classes and subtypes of neurons that exist. In this study, we demonstrate that Chondroitinase ABC digestion of PNNs from acute cortical slices from juvenile mice (P28–35) resulted in neuron-specific impacts on intrinsic physiology. Fast spiking (FS) interneurons showed decreased input resistance, resting membrane potential (RMP), reduced action potential (AP) peaks and altered spontaneous synaptic inputs. Low-Threshold Spiking interneurons showed altered rebound depolarizations and decreased frequency of spontaneous synaptic inputs. Putative excitatory neurons; regular spiking, bursting, and doublet phenotypes did not demonstrate any alterations. Our data indicate that chABC-sensitive PNNs may specifically regulate the intrinsic and synaptic physiology of inhibitory interneurons. © 2018 IBRO. Published by Elsevier Ltd. All rights reserved.
Keywords: barrel cortex, inhibitory interneurons, perineuronal nets, intrinsic physiology
INTRODUCTION
Perineuronal nets (PNNs) are an aggregate form of the brain Extracellular Matrix (ECM) consisting of proteins and proteoglycans that envelope the soma and processes of neurons. The relatively late maturation of PNNs during the second and third postnatal weeks is activity and sensory dependent (Dityatev et al., 2007; Lander et al., 1997; McRae et al., 2007; Nowicka et al., 2009) and correlates with the closing of a developmental critical window of plasticity in the visual cortex, a time when the brain is particularly susceptible to reorganization (Pizzorusso et al. 2002; Gogolla et al. 2009). Altering the expression and molecular composition of PNNs in vivo has been shown to enhance behavioral and circuit based plasticity at different levels of the central nervous system (Carulli et al., 2010; Galtrey et al., 2007; Gogolla et al., 2009; Pizzorusso et al., 2002, Favuzzi et al., 2017) and in humans diagnosed with schizophrenia, significant reductions in PNNs are detected in the neocortex (Mauney et al., 2013).
Mechanistically, the reduction of brain ECM using enzymatic digestion directly facilitates spine motility (Orlando et al., 2012) alters excitatory synaptic inputs (Bukalo et al., 2007; de Vivo et al., 2013; Saghatelyan et al., 2000) and reduces long-term and short-term synaptic plasticity in the neocortex (Bukalo et al., 2001; de Vivo et al., 2013; Frischknecht et al., 2009; Kochlamazashvili et al., 2010; Romberg et al., 2013).
While this regulation of excitatory inputs and plasticity has been widely reported, PNNs are principally enriched around Fast spiking (FS) parvalbumin + inhibitory interneurons (PV, Rossier et al., 2015), an inhibitory neuronal subtype involved in gain control, lateral inhibition, and implicated in regulating postnatal cortical plasticity (Takesian and Hensch, 2013). Recent studies have shown that PV+ interneurons are also specifically protected by PNNs from oxidative stress (Cabungcal et al., 2013; Morishita et al., 2015). However, limited PNN expression around excitatory projection neurons, somatostatin+ (SST+) interneurons as well as observations of diffuse ECM expression throughout the brain has also been observed (Alpár et al., 2006; Berretta et al., 2015; McRae et al., 2010).
Here, we demonstrate that the removal of PNNs results in neuronal subtype-specific alterations on the intrinsic physiology of FS and LTS interneurons, while sparing putative excitatory neurons.
EXPERIMENTAL PROCEDURES
Animals
Experiments were conducted with white laboratory adolescent Swiss mice of either sex (CD-1, postnatal day 28–52; n = 22) for all experiments (specified below), Charles Rivers Laboratory, Wilmington, MA) in accordance with the Institutional Animal Care and Use Committee of Queens College, CUNY and NIH guidelines for responsible use of animals in research. Both sexes were used in these experiments and were not evaluated separately.
Acute slice preparation
Adolescent CD-1 mice were placed under deep anesthesia via an intraperitoneal injection of Euthasol (Virbac ANADA, Catalog# 200–071) at 0.5–1 mL/g until unresponsive to a toe pinch. Mice were randomly chosen for chABC or control condition recordings. Animals were transcardially perfused with ice-cold sucrose artificial cerebral spinal fluid (sACSF). The brain was subsequently removed and placed in chilled (~2°C) and oxygenated (95% O2, 5% CO2) in sACSF for ~1 min with the following composition in mM: 250 Sucrose, 2.5 KCl, 3.0 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 D-Glucose. Brains were sectioned coronally at 300–350 μm using a vibratome (Leica VT 1000S). Posterior medial barrel subfield (PMBSF) was identified using anatomical references (Paxinos and Franklin, 2012). Following cutting, the brain slices were incubated at room temperature in a standard aerated beaker or a single slice incubation chamber in ACSF (in mM: NaCl 125, 2.5 KCl, 0.3 MgCl2, 2 CaCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 D-Glucose). Low Mg2+ solution was utilized in order to enhance spontaneous synaptic activity in our recordings. In experimental conditions, 10 μl of chABC (Seikagaku, Sigma Aldrich) was dissolved in 990 μl (1:100 vol./vol.) of ACSF to result in a final concentration of 0.2918 units/ml and added to the slice chamber for preincubation. chABC cleaves glycosaminoglycan side chains in chondroitin sulfate proteoglycans (Yamagata et al., 1968) and the hyaluronic acid backbone of PNNs and other ECM structures(Galtrey et al., 2007). Following chABC digestion, slices were fixed in chilled 4% paraformaldehyde (PFA) in 0.01 M phosphate buffered saline (PBS) for anatomical histochemistry and measurements. For electrophysiology, slices were incubated for a minimum of 1 h, and then placed into the recording chamber for chABC wash out and recording in a submersion chamber. Following a 3–5-day fixation at 4 °C, slices were histochemically labeled for PNNs.
Graded enzymatic digestion of PNN
Histochemistry was performed on a subset of sections to determine a baseline for PNN expression in posterior medial barrel subfield regions in the coronal plane as well as expression after a specified time in chABC. The sections were separated down the midline, with one of two hemispheres randomly assigned to either “control” incubation (ACSF only; n = 8), or digestion using chABC (n = 10). Sliced hemispheres were incubated in chABC for 60 min, then removed and immediately fixed in cold 4% PFA (in 0.01 M PBS) for 3–5 days, followed by histochemical staining. Hemisphere controls were maintained in normal aerated ACSF for the same length of time as their digestion counterparts and subsequently fixed and stained using the same procedures for comparison purposes.
Histochemistry and fluorescent immunohistochemistry
All sections were stained for PNNs using Wisteria Floribunda Agglutinin (WFA), a standard lectin that labels the N-acetylglycosamine side-chain of chondroitin sulfate proteoglycans. A subset of the slices that were recorded from were labeled with FITC-WFA (Vector Laboratories, FL-1351) to confirm the presence and absence of PNNs in control tissue and following chABC digestion. For histological analyses free floating sections were rinsed in 0.1 M PBS (3 × 10 min). Rinses were done in between all the subsequent steps unless indicated. Sections were quenched for endogenous peroxidase activity (2% H2O2, 1% MEOH in 0.1 M PBS; 1 h), blocked for 30 min using 1% BSA (Fisher Biotech, Catalog # 9048-46-8), and incubated in biotinylated Wisteria Floribunda Agglutinin (WFA; 1:500; Sigma Aldrich, Catalog #B-1355) for 2 h followed by a 1-h ABC kit reaction (Vector Laboratories, Catalog# PK-400). All steps included were washed in 0.1 M PBS with 0.5% Tween (Sigma Aldrich, P1379). 3,3′-Diaminobenzidine (DAB, Sigma Aldrich, Catalog #D4293) reaction was used as the chromogen (~10 min, 0.7 mg/ml) with H2O2 to catalyze the reaction. Mounted sections were immediately counterstained with Thionin, or dehydrated in graded alcohol baths and defatted in xylene substitute.
Identification of cell-type-specific PNN expression was conducted using standard immunohistochemistry. Briefly, mice were perfused as described above, stored in 4% PFA in 4 °C overnight in 0.01 M PBS. Brains were then embedded in OCT medium (Tissue-Tek #4583), frozen at −80 °C, and sectioned on a cryostat (Leica CM 3050S) at 25 μM. Slices were then transferred to 0.1 M PBS wells at 4 °C and stained within 72 h. Slices were washed 3 × 10 min in 0.1 M PBS, followed by - h blocking and permeabilization in 0.35% Triton-X and 1.5% Donkey serum (blocking solution). Slices were then incubated in primary antibodies for Rabbit Satb2 (Abcam, ab34735), parvalbumin (Abcam, ab11427), or Somatostatin (Santa Cruz Biotechnology, sc-7819) at 1:500 with WFA-FITC at 1:500 in blocking solution. Slices were incubated overnight at 4 °C, washed 3 × 10 min in 0.1 M PBS, then incubated in Donkey anti Rabbit Cy3 secondary antibody (Abcam, ab6949) overnight. Following appropriate washes in 0.1 M PBS, slices were mounted onto uncoated slides and coverslipped using Vectashield (VWR, H-1400). In some sections, we controlled for non-specific staining for the secondary antibody by excluding the primary. These sections yielded little nonspecific background staining. Imaging was conducted on a confocal microscope at 40× (NA 1.30, Olympus IX81, FV-1000).
PNN analysis
Slices were viewed using an Olympus (BX-51) microscope with a digital camera (Optronics Microfire). Images were taken at 10× (NA 0.25) magnifications and analyzed on a standard Windows desktop computer using Neurolucida and Neuroexplorer (MBF Biosciences Inc.). Nissl stains were conducted on a subset of WFA-labeled section and used to distinguish the cortical lamina.
PNNs were identified based on their circular hollow centers, or lattice-like mesh around cell bodies, with distinctive short radial processes. Non-specific DAB precipitate or diffuse ECM was distinguished from WFA + PNNs by darker than background staining around cell bodies with “hollow” centers and labeling around proximal processes. PNN density was calculated as PNNs counted/area (mm2) within a defined lamina contour based on Nissl counterstained references. Relative densities were defined as the ratio of chABC hemisphere density and control hemisphere density. A 2-factor mixed ANOVA was used to analyze the layer vs. experimental condition density values followed by pair-wise post hoc Tukey analyses. Two independent investigators counted PNN and an intraclass correlation coefficient was used to calculate the inter-rater reliability (Control: 0.64; chABC: 0.8). Due to variability in staining thick sections, only relative differences were used to ascertain the degree of chABC efficacy in the slice. All comparisons used an α level of 0.05 to indicate statistical significance using Statistica (Statsoft Inc) and R. All data are reported using median and interquartile range unless otherwise stated.
Electrophysiology
Whole-cell patch-clamp was conducted using pulled borosilicate pipettes (ID: 0.86 mm, OD: 1.5 mm; Sutter P-87) with 4–8 MΩ tip resistance. Glass electrodes were filled with intracellular solution of the following composition in mM: K-Gluconate 120, KCl 20, NaCl 10, Hepes 10, EGTA 0.5, Mg-ATP 2, Na-GTP 0.3 (290–310 mOsm). Slices were visualized using a Bx51WI microscope with infrared differential interference contrast optics (IR-DIC) with camera attachment (Retiga Ex).
Cells were recorded using PClamp (Version 10, Molecular Devices) under current-clamp methods as previously described at room temperature (Ramos et al., 2008). Signals were amplified with a Multiclamp 700A (Molecular Devices), digitized with a Digidata 1440A, low pass filtered at 10 kHz and sampled at 20 kHz (Molecular Devices). After achieving a stable membrane potential (Vm < −50 mV, ability to generate APs in response to a depolarizing pulse), the cells’ intrinsic properties were assessed by injecting current from −250pA to +250pA in incrementing steps of 50 pA each lasting 1 s with an inter-stimulus interval of 1000 ms, typically within 2 min of breaking into the cell. Estimated Rheobase was calculated as the minimum current injection required for first spike initiation. Following data collection, all traces were analyzed offline using Clampfit (Molecular Devices) unless otherwise specified. Active properties were analyzed using Clampfit threshold detection algorithms. Baseline and threshold markers were set 0.1 mV apart for spike properties, and at half of the maximum action potential (AP) amplitude for spike frequency. The maximum input resistance (Rin) was calculated by taking the slope of the voltage current relationship during hyperpolarizing current steps (−250 to −50 pA). Resting membrane potentials (RMP) were collected during 10–20 ms prior to onset of each current step. Latencies for spike onset and amplitude change before spike onsets measured manually for all steps eliciting spikes. Spike threshold, amplitudes and after-hyperpolarization (AHP) were measured from baseline by setting markers at the AP threshold (dV/dt = 20–25 mV/ms) and lowest Vm following an AP was used to define peak AHP for both first and last AP’s. Rebound spike latency, amplitude and slopes were measured by analyzing the change in time and Vm from the offset of the hyperpolarizing current step, and the threshold for the initiation of the rebound spike. Spiking probability was defined as the proportion of cells firing AP’s at each level of stimulation current. Interspike interval (ISI) ratio was calculated as the first ISI/last ISI during the +250pA current injection train. Junction potentials were not corrected. Sag ratio was defined as the Vm following >990 ms of hyperpolarizing current injection/minimum Vm within the first 30 ms of the hyperpolarizing current step. RMP was not adjusted in order to evaluate differences in RMP following sensory deprivation and ECM digestion. dV/dt ratio for APs were calculated and visualized on phase plots in Axograph (ver. 1.5.4).
Following the recording of intrinsic membrane and spike properties, spontaneous excitatory post synaptic potentials (sEPSP) were recorded at rest. Traces (5–15 min) were filtered at 1000 Hz (Gaussian). Unstable membrane potentials with >10 mV baseline oscillations or continual depolarization’s were discarded from the analysis. Manual baseline adjustment was done to correct for membrane oscillations (<10 mV) using the Clampex manual baseline adjust function, which applies a slope correction to adjust for linear drift. Specifically, Clampex baseline function was matched to the average baseline throughout segments with minimal oscillations (<10 mV) and straightened to optimize threshold detection. Unitary and compound sEPSPs were isolated using threshold event detection set to 0.75 mV. Events with amplitudes below 0.75 mV were excluded due to noise. Isolated sEPSP were analyzed as total frequency and also manually sorted into 1 mV amplitude bins (0.75–1 mV, 1–2 mV, 2–3 mV, 3–4 mV, >4 mV). 25 unitary sEPSPs were isolated based on visual inspection and collected in order from onset of the gap free recordings. The Shapiro–Wilks test was used to obtain a significance value for data normality, while Quantile Quantile-plots (QQ-plots) were used to visually assess normality. Levene’s test was used to test for homogeneity of variance between samples. Normality and equal variance tests were conducted in R (Version 0.99.902). Independent t-tests and Mann–Whitney Us were used as tests of significance between groups. Non-parametric effect size of differences were calculated with Cliffs δ (d), which measures how often values in one distribution are larger than values in another distribution (Macbeth et al., 2011). Cliffs d values range from [−1,1] where 0.33 ≤ d and < 0.474 are considered medium and large effect sizes respectively. K-S test was used to test distribution shifts in sEPSP recordings in control and experimental groups. An α level of 0.05 was used to determined statistical significance. Parametric data (PNN quantification) are reported at mean and ±1SEM. All other data are non-parametric and reported as median ±3rd–1st quantile (Table 1).
Table 1.
Summary of the intrinsic properties of RS-S, Doublet, and Burst. Data are displayed as median ± 3rd-1st interquartile range. p-Values represent results of Mann–Whitney U
| RS-S Control (n = 19) | RS-S chABC (n = 35) | p-Value | RS-D Control (n = 8) | RS-D chABC (n = 13) | p-Value | Burst Control (n = 6) | Burst chABC (n = 6) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| RMP (mV) | −68.9 ± 8.2 | −69.2 ± 7.85 | 0.81 | −69.8 ± 4.2 | −66.6 ± 5.9 | 0.32 | −70.0 ± 5.8 | −63.05 ± 5.8 | 0.13 |
| Spike delay (ms) | 0.087 ± 0.05 | 0.085 ± 0.1 | 0.62 | 0.007 ± 0.003 | 0.009 ± 0.16 | 0.52 | 0.006 ± 0.0019 | 0.004 ± 0.003 | 0.29 |
| Rin (MΩ) | 0.15 ± 0.06 | 0.14 ± 0.05 | 0.79 | 0.16 ± 0.08 | 0.13 ± 0.05 | 0.55 | 0.19 ± 0.04 | 0.17 ± 0.04 | 0.31 |
| Sag Ratio | 0.99 ± 0.05 | 0.98 ± 0.05 | 0.074 | 0.96 ± 0.02 | 0.96 ± 0.02 | 0.96 | 0.94 ± 0.02 | 0.91 ± 0.02 | 0.18 |
| Threshold (mV) | −39.06 ± 8.1 | −39.4 ± 75 | 0.75 | −37.9 ± 5.7 | −42.9 ± 12.4 | 0.43 | 81.78 ± 10.1 | 71.79 ± 6.8 | 0.065 |
| Half width (ms) | 1.87 ± 0.52 | 1.83 ± 0.32 | 0.29 | 1.24 ± 0.24 | 1.47 ± 0.32 | 0.08 | 1.56 ± 0.76 | 1.25 ± 0.4 | 0.23 |
| Rise slope (mV/ms) | 154.4 ± 47.9 | 163.9 ± 35.8 | 0.91 | 145.6 ± 28.5 | 165.2 ± 71.5 | 0.89 | 131.0 ± 35.0 | 106.2 ± 26.5 | 0.69 |
| Decay slope (mV/ms) | −33.0 ± 9.0 | −30.1 ± 9.5 | 0.92 | −48.34 ± 10.9 | −37.71 ± 9.42 | 0.053 | −46.14 ± 8.3 | −37.41 ± 12.9 | 0.39 |
| AP Amplitude (mV) | 93.9 ±11.6 | 94.1 ± 5.9 | 0.64 | 74.62 ± 11.59 | 89.42 ± 17.5 | 0.25 | 81.78 ± 10.1 | 71.79 ± 6.8 | 0.065 |
| AHP (mV) | −43.0 ± 8.9 | −41.8 ± 9.6 | 0.78 | −42.72 ± 4.27 | −41.04 ± 11.75 | 0.21 | −44.0 ±11.9 | −27.92 ± 17.4 | 0.11 |
| dV/dT ratio | 0.23 ± 0.04 | 0.24 ± 0.04 | 0.22 | 0.35 ± 0.02 | 0.30 ± 0.15 | 0.38 | 0.35 ± 0.03 | 0.40 ± 0.03 | 0.19 |
| Maximum AP Frequency (Hz) | 17.0 ± 1.4 | 16.1 ± 5 | 0.55 | 24.5 ± 8.5 | 24.0 ± 16.5 | 0.37 | 33.0 ± 23.8 | 26.5 ± 3.3 | 0.47 |
| Interspike intervals (ms) | 0.48 ± 0.23 | 0.45 ± 0.12 | 0.82 | 0.14 ± 0.11 | 0.19 ± 0.11 | 0.52 | 0.11 ± 0.66 | 0.16 ± 0.66 | 0.38 |
Cell-type identification
Regular spiking-single cells (RS-S) were defined as those with >1 ms AP half widths at half AP amplitude, AHP ratio >0.85, and first ISI >20 ms. Regular spiking-doublet cells (RS-D) were characterized as non-rebound spiking, non-FS, and responding with a pair of APs with <20 ms ISI at the onset of the 250 pA depolarizing current step. Burst firing cells (Bursting or Burst) were defined based on the same criteria as doublet cells, except that there were >2 initial AP responses, the first two occurring with an ISI of <8 ms. RS-S, RS-D and bursting cells were typically pyramidal shaped or round in layer 4 as observed under DIC and are presumed to be glutamatergic (McCormick et al., 1985). Fast spiking cells (FS) were classified by their short AP half widths at half amplitude (<1 ms), deep AHP (AHP ratio: AP threshold/maximum AHP < 0.85), and high spike frequency. Low-threshold spiking neurons (LTS) were defined as those firing rebound bursts of one or more APs following the offset of an injected hyperpolarizing step. L5 somatostatin (SST)+ inhibitory interneurons have been shown to exhibit LTS properties (Ma et al., 2006) whereas parvalbumin (PV)+ inhibitory interneurons exhibit FS properties (Kawaguchi et al., 1987).
RESULTS
Digestion of PNNs in the mouse somatosensory cortex
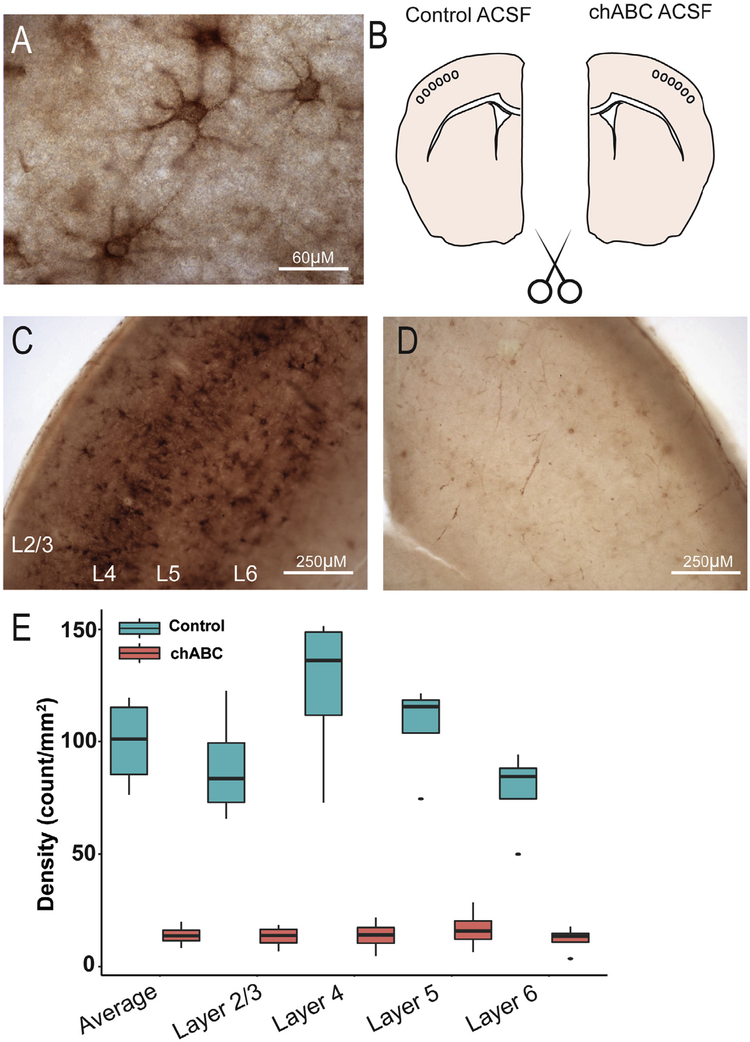
Previous studies have conducted in vivo injections of chABC (Gogolla et al., 2009; Pizzorusso et al., 2002). We sought to better control the extent of enzymatic digestion using an acute brain slice model. Quantification of PNNs in hemisected controls of barrel cortex allowed us to quantify the relative reduction in PNNs following 60 min of chABC digestion (Fig. 1). Following 60 min of chABC digestion, the average PNN density decreased by 69% relative to control at 60 min of digestion (F(1,4) = 198.82, p > 0.001, Fig. 1D,E). Post hoc comparison using Tukey HSD indicated that chABC significantly decreased the PNN density (Control: 99.45 ± 6.2; chABC: 13.91 ± 1.4; p > 0.001, Fig. 1D, E), while the effect of lamina was not significant (p < 0.05). This result indicates that the effect of chABC was uniform across all cortical layers examined.
Fig. 1.
Enzymatic digestion of PNNs in acute cortical sections. (A) Cartoon of experimental conditions used for analyzing chABC digestion of PNNs. Live sections were hemisected and one hemisphere was digested using chABC while the contralateral hemisphere was used as control. (B) Coronal section labeled with biotinylated WFA and counterstained using Nissl demonstrating dense distribution of WFA in layer 4. (C) WFA labeling of control hemisphere and (D) chABC digested hemisphere. (E) Examples of PNN labeling under high magnification. Relative decrease in PNN density (F) in chABC/Control sections. *Mann-Whitney U p < 0.05.
Intrinsic properties of cortical neurons following chABC digestion
After patching onto cells, families of depolarizing and hyperpolarizing current pulses were delivered to identify four different cellular phenotypes under control and PNN digested conditions (FS control: n = 8, FS chABC n = 9; RS-S control n = 21, RS-S chABC n = 35; RS-D control n = 6, RS-D chABC n = 12; LTS control n = 8, LTS chABC n = 8; Burst Control n = 6, Burst chABC n = 6, n = number of recorded neurons).
PNN digestion impacts FS intrinsic properties
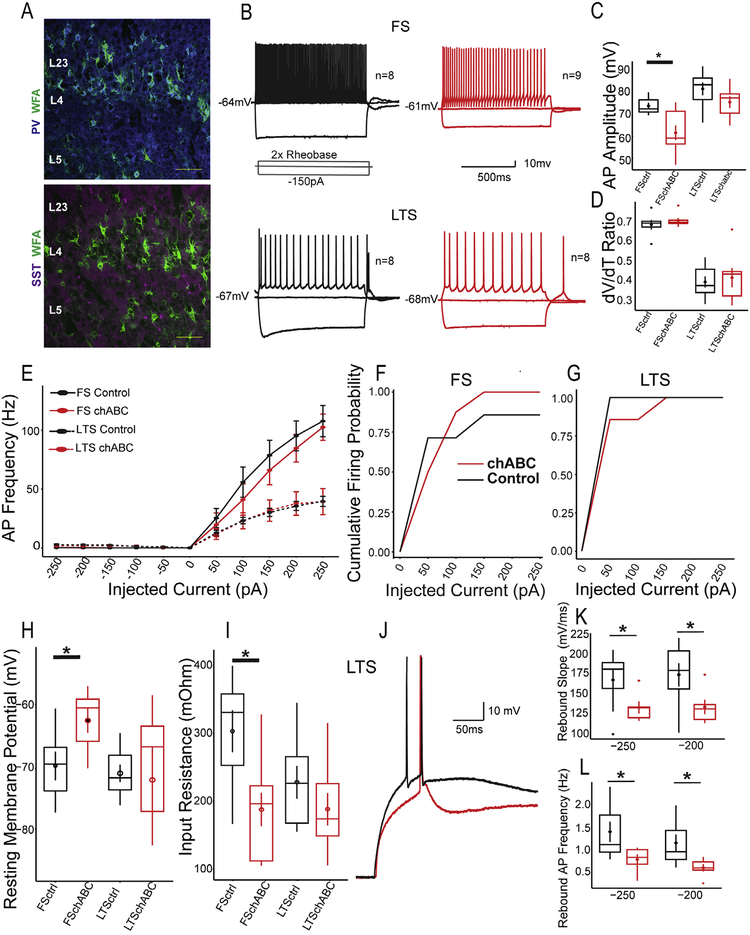
PV interneurons have been reported to express PNNs in the neocortex (McRae et al., 2007; Fig. 2A). In the barrel cortex, putative PV FS interneurons are the most abundantly ensheathed by PNNs. Therefore, we tested whether enzymatic digestion of PNNs using chABC would impact their intrinsic properties. FS interneurons exposed to PNN digesting enzyme chABC, displayed reduced AP amplitudes (Control: 71.56 ± 5.6; chABC: 58.28 ± 14.9; Mann–Whitney U p = 0.006; d = 0.61; Fig. 2B and C), but this effect did not significantly alter their dV/dT ratios (Fig. 2D). Other active properties such as maximum firing frequencies and firing probabilities were not affected (Fig. 2E–G). However, the RMP of chABC FS neurons were significantly more depolarized compared to controls (Control: −65.3 ± 5.2; chABC: −58.6 ± 5.05; Mann–Whitney U p = 0.034; d = 0.74; Fig. 2H). In addition, chABC digested FS neurons also displayed reduced Rin following chABC (Control: 330.75 ± 0.105 MΩ; chABC: 196.2 ± 0.11 MΩ Mann–Whitney U p = 0.011; d = 0.667, Fig. 2I). The reductions in Rin and AP amplitude suggest that PNNs may regulate the excitability and integration of FS interneurons in the barrel cortex (Table 2).
Fig. 2.
Impact of PNN digestion on putative inhibitory neurons. (A) Example images of colocalization of WFA-labeled PNNs with parvalbumin (top) and somatostatin (SST; bottom) inhibitory interneuron markers in the barrel cortex. Minimal colocalization of SST with WFA. Scale bar represents 200 μM (top) and 250 μM (bottom). Representative traces of neurons classified as Fast Spiking (FS) (B; top) and Low-Threshold Spiking (LTS)(B; bottom) in non-digested (black) and chABC digested sections (red). (C) Quantification of reduced FS amplitude after chABC digestion without changes (D) to dV/dT ratio. (E) Action potential (AP) frequencies were unchanged following chABC digestion. (F,G) Action potential spiking probability. (H) Depolarized resting membrane potential of FS interneurons. (I) Reduced input resistance in FS interneurons following chABC. Reduced rise slopes (K) and AP frequency (L) of rebound spike in LTS cells following chABC. *p < 0.05.
Table 2.
Summary of the intrinsic properties of FS and LTS. Data are displayed as median ± 3rd-1st interquartile range. p-Values represent results of Mann–Whitney U
| FS Control (n = 8) | FS chABC (n = 9) | p-Value | LTS Control (n = 8) | LTS chABC (n = 8) | p-Value | |
|---|---|---|---|---|---|---|
| RMP (mV) | −65.3 ± 5.2 | −58.6 ± 5.1 | 0.034 | −66.95 ± 4.2 | −63.3 ± 10.2 | 0.64 |
| Spike delay (ms) | 0.011 ± 0.001 | 0.017 ± 0.014 | 0.65 | 0.28 ± 0.14 | 0.20 ± 0.41 | 0.37 |
| Rin (MΩ) | 0.33 ± 0.11 | 0.19 ± 0.11 | 0.012 | 0.23 ± 0.98 | 0.17 ± 0.08 | 0.33 |
| Sag Ratio | 0.97 ± 0.098 | 0.94 ± 0.96 | 0.46 | 0.77 ± 0.11 | 0.91 ± 0.07 | 0.13 |
| Threshold (mV) | 71.56 ± 5.6 | 58.28 ± 14.9 | 0.48 | −43.94 ± 2.9 | −39.82 ± 5.1 | 0.10 |
| Half width (ms) | 0.64 ± 0.28 | 0.56 ± 0.16 | 0.099 | 1.12 ± 0.19 | 1.02 ± 0.87 | 0.72 |
| Rise slope (mV/ms) | 168.6 ± 33.8 | 166.7 ± 51.6 | 0.42 | 167.86 ± 35.9 | 133.30 ± 37.5 | 0.19 |
| Decay slope (mV/ms) | −132.1 ± 42.1 | −121.6 ± 29.0 | 0.89 | −53.66 ± 21.1 | −62.87 ± 44.7 | 0.71 |
| AP Amplitude (mV) | 71.56 ± 5.6 | 58.29 ± 14.9 | 0.0065 | 82.55 ± 9.8 | 76.59 ± 8.7 | 0.19 |
| AHP (mV) | −60.42 ± 3.1 | −59.81 ± 9.8 | 0.27 | −44.25 ± 6.5 | + 40.95 ± 7.3 | 0.43 |
| dV/dT ratio | 0.69 ± 0.03 | 0.69 ± 0.02 | 0.53 | 0.38 ± 0.11 | 0.43 ±0.12 | 0.98 |
| Maximum AP Frequency (Hz) | 83.5 ± 48.5 | 80.0 ± 49.0 | 0.70 | 38.5 ± 4.7 | 32.0 ± 48.5 | 0.79 |
| Interspike intervals (ms) | 0.75 ± 0.08 | 0.69 ± 0.3 | 0.60 | 0.28 ± 0.14 | 0.20 ± 0.41 | 0.65 |
The effect of chABC on LTS cell intrinsic properties
LTS neurons have been reported to express somatostatin (SST, Ma et al., 2006). LTS cells exhibited rebound spiking following the offset of a hyperpolarizing current step. We observed a small number of SST neurons colocalized with PNNs in the barrel cortex (Fig. 2A). However, most of the intrinsic properties we measured in LTS neurons were not changed (Fig. 2C–I). It has been reported that PNNs can regulate calcium currents, which mediate the rebound spiking exhibited by LTS neurons (Vigetti et al., 2008; Zhan et al., 1999). Therefore, we used the latency and voltage change between the offset of the hyperpolarizing step and onset of a rebound spike to estimate the properties of the rebound spike after PNN digestion.
All LTS cells fired rebound spikes at the offset of −250 and −200 pA in both control and chABC. In response to a −150 pA pulse only a subset of chABC LTS cells (4/8 = 50%) fired rebound spikes whereas all of the LTS control cells fired rebound spikes (8/8 = 100%). The average slope of the rebound depolarization prior to AP was significantly decreased following chABC (Control: 132.73 ± 18.6 mV/ms, chABC: 182.16 ± 36.8 mV/ms, Mann Whitney U p = 0.017; d = 0.54; Fig. 2K). chABC also reduced the number of APs generated following a rebound depolarization (Control: 2.5 ± 2.25, chABC: 1.0 ± 0.75, Mann–Whitney U p = 0.0038, d = 0.59; Fig. 2L).
Effect of chABC on putative excitatory neurons
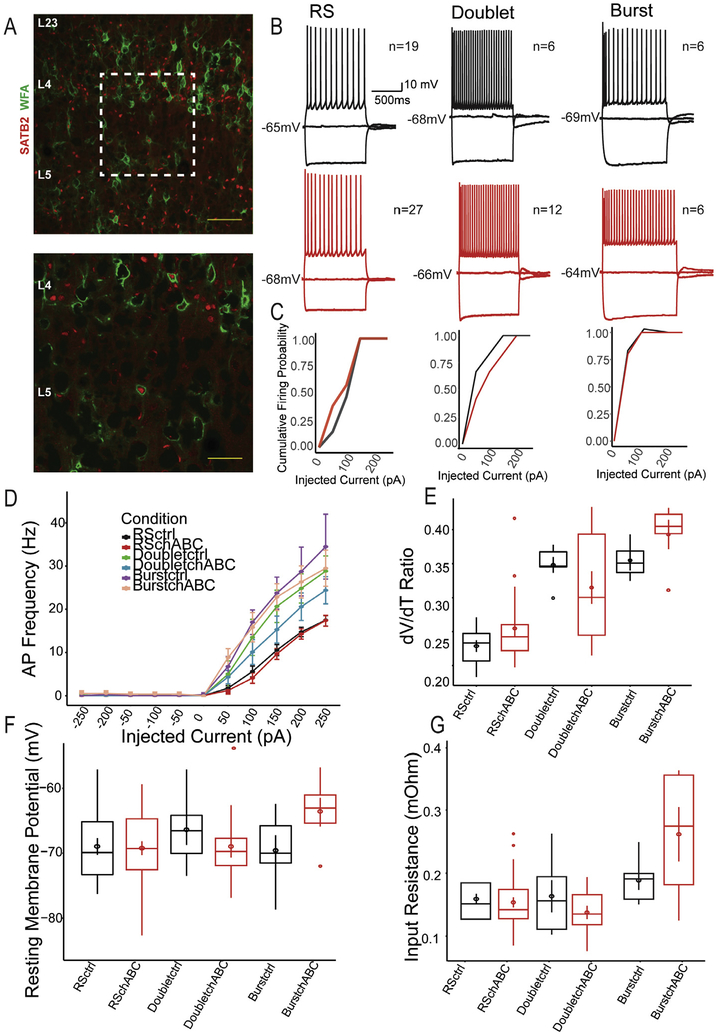
We observed PNN colocalization within a subset of pyramidal neurons using Satb2 (Fig. 3A), a chromatin remodeling protein required for callosal and subcortical projecting excitatory pyramidal neuron specification (Leone et al., 2015). These results suggest that PNNs may serve as regulators of cortical excitatory neuron physiology. We compared the intrinsic properties of putative excitatory neurons following chABC digestion of PNNs, and found that RS-S, RS-D and burst cells displayed no significant alterations to their spike frequency, spiking probabilities, dV/dt ratios, or their rheobases (Fig. 3B–G). These results suggest a lack of regulation by PNNs or an insufficient sampling rate of excitatory neurons with PNNs.
Fig. 3.
Impact of PNN digestion on putative excitatory neurons. (A) Example images of colocalization of WFA-labeled PNNs with SATB2, a marker of L5 pyramidal neurons in the barrel cortex. Scale bar represents 200 μM (top) and 100 μM (bottom). Representative traces of neurons classified as Regular Spiking (RS) Doublet and Burst neurons in control (top, black) and chABC digested sections (bottom, red). (C) Firing probabilities for RS, Doublet and Burst neurons. Putative excitatory neurons did not result in alterations to dV/dT ratio (E), resting membrane potential (F) or input resistance (G).
PNN digestion impacts spontaneous synaptic potentials
Previous reports have demonstrated that postsynaptic AMPA receptors are regulated by PNNs (Frischknecht et al., 2009). Excitatory and inhibitory inputs are located on the soma and dendrites of inhibitory interneurons, where PNNs are primarily located (Kameda et al., 2012). Therefore, we sought to test whether chABC digestion of PNNs impacted the total excitatory drive onto different neuronal classes by recording sEPSPs in current clamp.
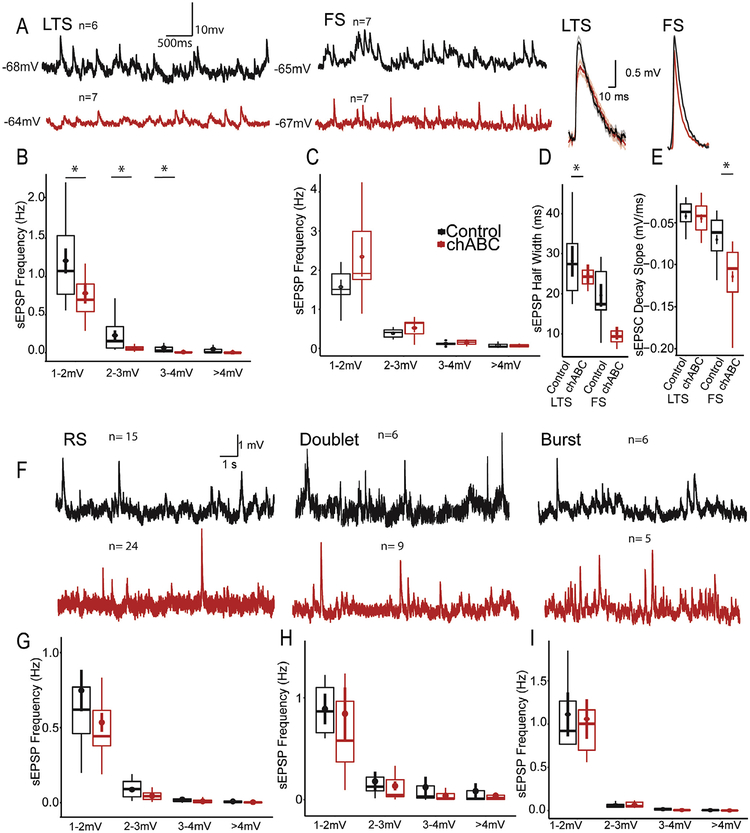
LTS interneurons showed decreased spontaneous sEPSP frequencies following chABC digestion (1–2 mV, Control: 1.07 ± 0.76 Hz; chABC: 0.69 ± 0.36 Hz, Mann Whitney p = 0.02; 2–3 mV, Control: 0.151 ± 0.28; chABC: 0.049 ± 0.04; Mann Whitney U p = 0.015; 3–4 mV, Control: 0.027 ± 0.05; chABC: 0.005 ± 0.005; Mann Whitney U p = 0.012; >4 mV, Control: 0.006 ± 0.041; chABC: 0.001 ± 0.0025; Mann Whitney U p = 0.013; Overall d = 0.59, Fig. 4A and B). The digestion of PNNs did not significantly shift the overall distribution of EPSP amplitudes (Kolmogorov–Smirnov test, p = 0.067).
Fig. 4.
PNNs regulate the spontaneous synaptic inputs of inhibitory interneurons. (A) Significant reductions in average frequency and amplitude without a shift in distribution (K-S test > 0.05) for LTS interneurons in slices digested by chABC. (B) FS interneurons do not demonstrate alterations in spontaneous inputs following chABC. (D) FS interneurons, but not LTS interneurons sEPSP inputs with faster time courses. (E) Faster spontaneous FS sEPSP inputs are driven by decreases in decay slopes(H-K). (A-C) sEPSP inputs onto RS-S, RS-D, and Burst neurons were unchanged following chABC digestion. Note that outliers have been removed to improve data visualization. *p < 0.05.
In FS cells, PNN digestion did not change sEPSP frequencies (Fig. 4A, C). However, chABC reduced sEPSP half widths onto FS neurons (Control: 17.42 ± 9.6; chABC: 9.34 ± 2.7, Mann Whitney U p = 0.048; d = 0.67, Fig. 2D) which was driven by increased decay slopes (Control: 0.061 ± 0.04; chABC: 0.10 ± 0.05, Mann–Whitney U p < 0.001; d = 0.72, Fig. 2E). Although LTS cells showed decreased EPSP frequencies, those remaining did not show changes to their temporal characteristics (Fig. 4D–E). We found no changes to the spontaneous sEPSPs for RS-S, RS-D, or bursting cells (Fig. 4F–I). These results demonstrate that spontaneous inputs onto inhibitory neurons are altered following PNN enzymatic digestion, while excitatory inputs onto putative excitatory neurons are unaffected.
DISCUSSION
PNNs have been implicated in the regulation of oxidative stress, synaptic physiology, intrinsic physiology as well as network and behavioral plasticity. The expression of PNNs occurs postnatally in mice and continues well into adulthood (Karetko-Sysa et al., 2014; Nowicka et al., 2009; Pizzorusso et al., 2002) and may serve as a developmental brake for elevated forms of neural plasticity. Our findings implicate inhibitory interneurons as effectors of PNN regulation. Consistent with our findings, previous researchers have identified a specific inhibitory alteration following chABC digestion of PNNs (Dityatev et al., 2007; Lensjo et al., 2017).
chABC digestion of PNNs impacts neuronal AP dynamics
Acute digestion of PNNs using chABC alters the intrinsic properties of both excitatory and inhibitory neurons in the barrel cortex. The effect on RS-S and RS-D AP decay slopes point to a possible role for PNNs in affecting the temporal fidelity of excitatory neurons in the sensory barrel cortex. This effect has been observed using hippocampal culture (Dityatev et al., 2007) where it was found that following chABC, interneurons displayed decreased AHPs and lowered rheobase. A role for PNNs in regulating AP gain has recently been reported for FS neurons, using white noise current steps in the brain stem auditory nucleus (Balmer, 2016). It has recently been reported that the brevican component of PNN controls the AP properties of FS interneurons in the hippocampus, as well as the expression of the Kv1.1 and Kv 3.1 subunits of voltage gated potassium channels (Favuzzi et al., 2017). Our data, while not recapitulating the specific alterations seen in the hippocampus and subcortical sensory nuclei, indicate that AP dynamics can also be regulated by PNN neocortex.
One possible role of PNNs is the regulation of local Ca2+ dynamics around the neuronal membrane following chABC (Härtig et al., 1999). Indeed, studies have shown increases in Ca2+ diffusion with removal of PNNs in brain slices (Hrabetová et al., 2009) and shifted activation curves of voltage gated calcium channels (Vigetti et al., 2008). Shifts in Ca2+ dynamics could help explained the changes to rebound depolarization rates and spiking we observed in LTS neurons that are known to be partially mediated by T-type calcium channels (Zhan et al., 1999).
Other possible mechanisms for future exploration are sodium channels that interact with complexes of neuronal cell adhesion molecules, including Ankyrin and β-spectrin that link to the ECM (For review, see Freeman et al., 2016). Voltage-gated Na+ channels also directly interact with ECM through the β-subunit (Srinivasan et al., 1998). Disrupted PNNs around the soma, or axon initial segment (Brückner et al., 2006), could potentially reduce the clustering of sodium channels and reduce the maximal AP response. In agreement with this hypothesis, others have reported decreased AP conduction velocity in a Tenascin-R knockout model (Weber et al., 1999), a molecule present in PNNs.
PNNs and inhibitory interneuron physiology
The potential significance of PNN maintenance of excitatory neuron AP dynamics, can be observed in postsynaptic neurons. SST+ interneurons exert gain control through strong disynaptic inhibition between pyramidal neurons, disinhibition through other inhibitory interneurons and receive strong glutamatergic inputs from neighboring pyramidal neurons (Urban-Ciecko and Barth, 2016 for review). In our assay, excitatory spontaneous inputs were specifically changed in LTS interneurons, which could be a result of pre-synaptic or postsynaptic modulation, by PNNs. In the present study, we did not specifically isolate the potential presynaptic or post-synaptic sources of the EPSP alterations that we observed. However, previous studies using single particle tracking, have shown that ECM digestion increases postsynaptic AMPA receptor diffusion in the hippocampus (Frischknecht et al., 2009, but see Klueva et al., 2014). In addition, super-resolution microscopy has identified PNN molecules in both pre and postsynaptic excitatory synapses onto PV somas in the hippocampus (Favuzzi et al., 2017). Pre-synaptic excitatory inputs onto PV+ interneurons are depressing, while inputs onto SOM+ interneurons are facilitating (Reyes et al., 1998). Thus, it is possible that the role of PNNs on neuronal physiology will depend on both pre-synaptic and post-synaptic neuron identity. While we did not evaluate the miniature sEPSPs, future studies could isolate the effects of pre vs. postsynaptic changes following PNN perturbation of the neocortex.
Caveats
Our purpose in chABC digestion and staining thick sections were to create an isolated model of controlled PNN digestion that could be used for patch-clamp electrophysiology. We caution against interpreting precise estimates of PNN densities in the barrel cortex using this method. However, we believe that the relative reductions between hemisected sections could serve as a reasonable estimate for PNN reduction following chABC. In addition, our measurements yielded similar density counts as has been previously reported using thinner sections (Nowicka et al., 2009).
Recent experimental evidence has arisen demonstrating that distinct populations of PV interneurons are likely to exist (Favuzzi et al., 2017; Li and Huntsman, 2014; Rossier et al., 2015). PV interneurons lacking the brevican isoform of the PNN show lower input resistances, lower AP frequency, and longer AP half widths, compared to those with brevican PNNs (Favuzzi et al., 2017). In addition, FS interneurons expressing endogenous enzymes implicated in PNN digestion are selectively expressed in PNN-ensheathed PV interneurons (Rossier et al., 2015). These neurons exhibit FS properties and were distinct from PV interneurons co-expressing SOM, which had higher input resistance, lower rheobase, lower AP frequency, and did not express PNNs. While we cannot rule out the possibility of a bias in our sampling toward PV-SOM neurons or brevican negative neurons, we did not find significant changes in AP frequency between the two groups, and AP half widths were slightly shorter in our chABC digestion group. Furthermore, interneurons co-expressing both SOM and PV are <5% of all interneurons in the barrel cortex (Lee et al., 2010). The development of genetically enabled fluorescent ECM reporters would facilitate the detection of neuronal subtype-specific regulation of excitatory and inhibitory neurons.
It is currently unknown whether glia cells are directly affected or have their functions modulated by PNNs. Anatomical evidence linking PNNs and astrocytes are lacking (Blumcke et al., 1995). In addition, it has been reported that PNNs resist degradation by microglia in a trimethyltin model of induced neurodegeneration (Schuppel et al., 2002). However, it is possible that enzymatic lysis of proteoglycan components of PNNs may activate micro-glia and have downstream consequences on synaptic physiology (Schafer et al., 2012). Further investigation is necessary to clarify this issue. Finally, it is unclear whether there are non-specific effects of chABC on neurons themselves. Similar results on visual cortex plasticity have been obtained from genetic models of PNN perturbation and chABC digestion (Carulli et al., 2010; Pizzorusso et al., 2002). Overall, there was minimal modulation of the intrinsic properties of the excitatory neurons in our population suggesting that there were minimal if any non-specific effects of chABC in our population. However, further investigation and direct comparisons must be conducted to elaborate on any non-specific effects on neuronal intrinsic physiology.
Previous studies have reported PNNs around a subset of glutamatergic neurons in the neocortex (Wegner et al., 2003). We find that PNNs ensheathe a subset of excitatory neurons in layer 5 expressing SATB2. However, we do not find significant alterations to excitatory neuron intrinsic physiology. This apparent contradiction is likely due to the sampling procedures we used to record putative excitatory neurons. Future studies should use genetically encoded reporters for both PNNs and the neuronal sub-type (Favuzzi et al., 2018) in order to fully isolate the contribution of PNNs to different subclasses of neurons.
SUMMARY
The postnatal maturation of inhibitory interneurons has received considerable attention as regulators of excitatory-inhibitory balance (E/I) in the neocortex (Fagiolini and Hensch, 2000; Fagiolini et al., 2004; Iwai et al., 2003). In our preparation, putative inhibitory interneurons are primarily regulated by PNNs and may play a key role in sensory circuit function. While most isoforms of PNNs have been reported to be around PV interneurons, the cell-type-specific expression of PNNs and brain ECM is not conclusive, and depends on the expression of a broad and heterogeneous class of molecules (Carulli et al., 2010; Deepa et al., 2006; Dityatev et al., 2010). We find that chABC does not appear to impact excitatory neurons and that WFA detected PNNs are expressed around a subset of excitatory neurons in addition to two classes of inhibitory interneurons (PV, SST). We also find that enzymatic digestion of PNNs and ECM using chABC impact the intrinsic and synaptic physiology of both excitatory and inhibitory interneurons (FS, LTS) in the barrel cortex. While future developments in genetic markers and tools will improve our understanding of the role of PNNs, our data suggest that they regulate both the intrinsic and synaptic properties of interneurons in the brain to regulate neuronal function and plasticity.
ACKNOWLEDGMENTS
We thank Dr.’s Carolyn Pytte, Raddy L. Ramos, Rick Matthews, Stephan F. Brumberg and Chia-Chien Chen for their helpful comments on the manuscript. Emma Keteku helped with early stages of the immunohistochemistry. We would also like to thank Jane Geam for insightful discussions. This work was supported by the CUNY Doctoral Student Research Grant #6, #8, and 10, the Sigma Xi Grants in Aid of Research G20130315164428 to PC and NIGMS1SC3GM122657, QC URME Award and PSC-CUNY# 67668–00 45 awards to JCB.
Abbreviations:
- AHP
after-hyperpolarization
- AP
action potential
- ECM
Extracellular Matrix
- FS
Fast Spiking
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PMBSF
posterior medial barrel subfield
- PNNs
perineuronal nets
- RMP
resting membrane potentials
- sACSF
sucrose artificial cerebral spinal fluid
- WFA
Wisteria Floribunda Agglutinin
REFERENCES
- Alpár A, Gärtner U, Härtig W, Brückner G (2006) Distribution of pyramidal cells associated with perineuronal nets in the neocortex of rat. Brain Res 1120(1):13–22. [DOI] [PubMed] [Google Scholar]
- Balmer TS (2016) Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro 3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET (2015) Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res 167(1–3):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumcke I, Egglie P, Celio MR (1995) Relationship between astrocytic processes and “Perineuronal nets” in rat neocortex. Glia 15 (2):131–140. [DOI] [PubMed] [Google Scholar]
- Brückner G, Szeöke S, Pavlica S, Grosche J, Kacza J (2006) Axon initial segment ensheathed by extracellular matrix in perineuronal nets. Neuroscience 138(2):365–375. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Schachner M, Dityatev A (2001) Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104(2):359–369. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Schachner M, Dityatev A (2007) Hippocampal metaplasticity induced by deficiency in the extracellular matrix glycoprotein tenascin-R. J Neurosci 27(22):6019–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal J-H, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, Do KQ (2013) Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci 110(22):9130–9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JCF, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW (2010) Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133(Pt 8):2331–2347. [DOI] [PubMed] [Google Scholar]
- de Vivo L, Landi S, Panniello M, Baroncelli L, Chierzi S, Mariotti L, Spolidoro T, Pizzorusso L, Ratto GM (2013) Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat Commun 4:1484. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW (2006) Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem 281(26):17,789–17,800. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Brückner G, Dityateva G, Grosche J, Kleene R, Schachner M (2007) Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol 67:570–588. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P (2010) The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11(11):735–746. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK (2000) Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404(6774):183–186. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy J-M, Löw K, Möhler H, Rudolph U, Hensch TK (2004) Specific GABAA circuits for visual cortical plasticity. Science 303(5664):1681–1683. [DOI] [PubMed] [Google Scholar]
- Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sanchez-Aguilera A, Mantoan L, Maeso P, Fernandes C, Ewers H, Rico B (2018) Activity-dependent gating of parvalbumin interneuron function by perineuronal net protein brevican. Neuron 95(3):639–655. [DOI] [PubMed] [Google Scholar]
- Freeman SA, Desmazières A, Fricker D, Lubetzki C, Sol-Foulon N (2016) Mechanisms of sodium channel clustering and its influence on axonal impulse conduction. Cell Mol Life Sci 73(4):723–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED (2009) Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 12(7):897–904. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Asher RA, Nothias F, Fawcett JW (2007) Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain 130(Pt 4):926–939. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Lüthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325 (5945):1258–1261. [DOI] [PubMed] [Google Scholar]
- Härtig W, Derouiche A, Welt K, Brauer K, Grosche J, Mäder M, Reichenbach A, Brückner G (1999) Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res 842(1):15–29. [DOI] [PubMed] [Google Scholar]
- Hrabetová S, Masri D, Tao L, Xiao F, Nicholson C (2009) Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J Physiol 587(Pt 16):4029–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK (2003) Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci 23 (17):6695–6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda H, Hioki H, Tanaka YH, Tanaka T, Sohn J, Sonomura T, Furuta T, Fujiyama F, Kaneko T (2012) Parvalbumin-producing cortical interneurons receive inhibitory inputs on proximal portions and cortical excitatory inputs on distal dendrites. Eur J Neurosci 35(6):838–854. [DOI] [PubMed] [Google Scholar]
- Karetko-Sysa M, Skangiel-Kramska J, Nowicka D (2014) Aging somatosensory cortex displays increased density of WFA-binding perineuronal nets associated with GAD-negative neurons. Neuroscience 277:734–746. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Katsumaru H, Kosaka T, Heizmann CW, Hama K (1987) Fast spiking cells in rat hippocampus (CA1 region) contain the calcium-binding protein parvalbumin. Brain Res 416:369–374. [DOI] [PubMed] [Google Scholar]
- Klueva J, Gundelfinger ED, Frischknecht RR, Heine M (2014) Intracellular Ca2+ and not the extracellular matrix determines surface dynamics of AMPA-type glutamate receptors on aspiny neurons. Philos Trans R Soc Lond 369(1654):20, 130,, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochlamazashvili G, Henneberger C, Bukalo O, Dvoretskova E, Senkov O, Lievens PM-J, Westenbroek R, Engel AK, Catterall WA, Rusakov DA, Schachner M, Dityatev A (2010) The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron 67(1):116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander C, Kind P, Maleski M, Hockfield S (1997) A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J Neurosci 17(6):1928–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B (2010) The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci 30 (50):16796–16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjo KK, Leppord ME, Dick G, Hafting T, Fyhn M (2017) Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increase gama activity. J Neurosci 37(5):1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Heavner WE, Ferenczi EA, Dobreva G, Huguengard JR, Grosschedl R, McConnell SK (2015) Satb2 regulates the diferentiation of both callosal and subcerebral projection neurons in the developing cerebral cortex. Cereb Cortex 25 (10):3406–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Huntsman MM (2014) Two functional inhibitory circuits are comprised of a heterogeneous population of fast-spiking cortical interneurons. Neuroscience 18(265):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A (2006) Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci 26(19):5069–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth G, Razumiejcyzk E, Ledsma RD (2011) Cliffs delta calculator: A non-parametric effect size program for two groups of observations. Univ Psychol:545–555. [Google Scholar]
- Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, Woo T-UW (2013) Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry 74(6):427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA (1985) Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54 (4):782–806. [DOI] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT (2007) Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci 27 (20):5405–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae PA, Baranov E, Sarode S, Brooks-Kayal AR, Porter BE (2010) Aggrecan expression, a component of the inhibitory interneuron perineuronal net, is altered following an early-life seizure. Neurobiol Dis 39(3):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Cabungcal J-H, Chen Y, Do KQ, Hensch TK (2015) Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol Psychiatry:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka D, Soulsby S, Skangiel-Kramska J, Glazewski S (2009) Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex. Eur J Neurosci 30(11):2053–2063. [DOI] [PubMed] [Google Scholar]
- Orlando C, Ster J, Gerber U, Fawcett JW, Raineteau O (2012) Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci 32(50). 18009–17, 18017a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K (2012) Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. 4th ed. Elsevier. [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science (New York, N.Y.) 298(5596):1248–1251. [DOI] [PubMed] [Google Scholar]
- Ramos RL, Tam DM, Brumberg JC (2008) Physiology and morphology of callosal projection neurons in mouse. Neuroscience 153(3):654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B (1998) Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci 1(4):279–285. [DOI] [PubMed] [Google Scholar]
- Romberg C, Yang S, Melani R, Andrews MR, Horner AE, Spillantini MG, Bussey TJ, Fawcett JW, Pizzorusso T, Saksida LM (2013) Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J Neurosci 33(16):7057–7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier J, Bernard A, Cabungcal J-H, Perrenoud Q, Savoye A, Gallopin T, Hawrylycz M, Cuenod, Do K, Urban A, Lein ES (2015) Cortical fast spiking parvalbumin interneurons enwrapped in the perineuronal net express the metallopeptidases Adamts8, Adamts15 and Neprilysin. Mol Psychiatry 20:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelyan AK, Gorissen S, Albert M, Hertlein B, Schachner M, Dityatev A (2000) The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur J Neurosci 12(9):3331–3342. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lerhman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neuronal circuits in an activity and complement-dependent manner. Neuron. 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppel K, Brauer K, Hartig W, Grosche J, Earley B, Leonard BE, Bruckner G (2002) Perineuronal nets of extracellular matrix around hippocampal interneurons resist destruction by activated microglia in trimethyltin-treated rats. Brain Res 958 (2):448–453. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Schachner M, Catterall WA (1998) Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc Natl Acad Sci 95(26):15753–15757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Hensch TK (2013) Balancing plasticity/stability across brain development. Prog Brain Res 207:3–34. [DOI] [PubMed] [Google Scholar]
- Urban-Ciecko J, Barth AL (2016) Somatostatin-expressing neurons in cortical networks. Nat Rev Neurosci 17:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigetti D, Andrini O, Clerici M, Negrini D, Passi A, Moriondo A (2008) Chondroitin sulfates act as extracellular gating modifiers on voltage-dependent ion channels. Cell Physiol Biochem 22(1–4):137–146. [DOI] [PubMed] [Google Scholar]
- Weber P, Bartsch U, Rasband MN, Czaniera R, Lang Y, Bluethmann H, Margois RU, Levinson SR, Shrager P, Montag D, Schachner M (1999) Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci 19(11):4245–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner F, Härtig W, Bringmann A, Grosche J, Wohlfarth K, Zuschratter W, Brückner G (2003) Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABA(A) receptor alpha1 subunit form a unique entity in rat cerebral cortex. Exp Neurol 184(2):705–714. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Saito H, Habuchi O, Suzuki S (1968) Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem 243(7):1523–1535. [PubMed] [Google Scholar]
- Zhan XJ, Cox CL, Rinzel J, Sherman (1999) Current clamp and modeling studies of low-threshold calcium spikes in cells of the cat’s lateral geniculate nucleus. J Neurophysiol 81(5):2360–2373. [DOI] [PubMed] [Google Scholar]