Abstract
The Xian-Ling-Gu-Bao capsule (XLGB) is a famous traditional Chinese medicine prescription (TCMP), which has proven effective in osteoporosis treatment. However, due to the lack of a dynamic XLGB profile, the in vivo pharmacokinetics of multiple bioactive components within this medicine remains unknown. In the present study, ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UPLC/Q-TOF-MS) identified a total of eighteen prototypes (using reference standards) in rat serum after oral administration of XLGB. These prototypes were subsequently evaluated to ascertain their effects on the proliferation and alkaline phosphatase activity of UMR106 cells and the adipogenesis of 3T3-L1 cells. Furthermore, a rapid and sensitive UPLC/Q-TOF-MS method was developed and validated for simultaneous quantitative analysis of 11 prototypes in rat serum. Chromatographic separation was achieved using a Waters Acquity BEH C18column (2.1 × 100 mm, 1.7μm) and linear gradient elution employing a mobile phase consisting of water and acetonitrile (both containing 0.1% formic acid). All calibration curves showed excellent linearity (r2 > 0.99) within the sampling ranges considered. The assay was accurate, precise and reproducible, as demonstrated by the obtained intra-and inter-day precisions (less than 12.3%) and accuracies (between −12.7% and 11.0%), and the matrix effects, extraction recoveries and stabilities were all satisfactory. Moreover, pharmacokinetic parameters were calculated from the plasma concentration-time data. Compared to single-compound do sing, significantly enhanced responses were obtained when several analytes were administered simultaneously, indicating possible drug-drug interactions among the complex ingredients of TCMP. This work provides an experimental baseline regarding the clinical applications and medicinal effectiveness of XLGB in the treatment of osteoporosis.
Keywords: Xian-Ling-Gu-Bao capsule, Main prototypes, Bioactivity evaluation, Pharmacokinetics, Rat serum, UPLC/Q-TOF-MS
1. Introduction
Traditional Chinese medicine prescriptions (TCMPs), which combine several single TCMs at intrinsic mass ratios, have proven to be effective in extensive clinical practices that have been used to treat chronic and complex diseases for thousands of years [1,2]. In general, the the rapeutic and pharmacological effects of TCMP have been attributed to the synergistic properties of multiple Chinese medicines and various bioactive ingredients that appear to minimize adverse effects and improving therapeutic efficacy, termed “TCMP compatibility” [3]. Given the usefulness of pharmacokinetic studies for explaining and predicting a variety of events related to the efficacies and toxicities of drugs, performing pharmacokinetic studies to evaluate the effectiveness and compatibility of TCM or TCMPs is both rational and valuable [4,5].
The Xian-Ling-Gu-Bao capsule (XLGB), a famous TCMP consisting of six commonly used herbs (Herba Epimedii (70%), Radix Dipsaci (10%), Fructus Psoraleae (5%), Rhizoma Anemarrahenae (5%), Radix Rehmanniae (5%) and Radix Salviae Miltiorrhizae (5%)), is widely used to treat osteoporosis, fractures, steoarthritis and aseptic bone necrosis [6–10]. Furthermore, the safety and efficacy of XLGB with regard to its use to treat osteoporosis have been confirmed by a randomized, multicentre, double-blind, placebo-controlled clinical trial [10]. Moreover, XLGB is the only anti-osteoporosis TCMP listed in the China National Basic Drugs Catalogue (http://www.nhfpc.gov.cn/, 2013 edition).
Until recently, studies have appeared to concentrate on the chemical constituents, pharmacology, quality control and in vivo metabolism of XLGB [11–16]. A total of 61 compounds have been isolated and identified using nuclear magnetic resonance (NMR) data obtained from bioactive fractions of XLGB [11]. Furthermore, 118 components have been identified and characterized in XLGB extracts using LC-linear ion trap/orbitrap mass spectrometry [12]. Meanwhile, no adverse effects were observed in rats after oral administration of XLGB at a dose of 1000 mg/kg, which is equivalent to 3.3 times the human dose based on conversion of body surface area [13]. Moreover, ten major compounds from Herba Epimedii and Fructus Psoraleae in XLGB were simultaneously determined using HPLC-UV and detection at 270 nm [14]. To better understand the components that demonstrate effectiveness in vivo, LC–MS has been used to identify or characterize the XLGB xenobiotics absorbed by rat blood [15,16]. However, no published information is currently available regarding the pharmacokinetics of XLGB, presenting a sizable obstacle to understanding the pharmacological mechanisms underlying the therapeutic effects of XLGB. Therefore, simultaneous quantification of multiple bioactive components deriving from the different herbal components of XLGB is indispensable.
Recently, ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UPLC/Q-TOF-MS) has been introduced as an effective analytical technique for rapidly screening and quantifying bioactive components in biological samples [17,18]. In the present study, UPLC/Q-TOF-MS was successfully used to identify 18 prototypes in rat serum after oral administration of XLGB. Furthermore, in vitro bioactivity evaluation of these 18 compounds showed that most were responsible for promoting osteoblasts formation and inhibiting fat formation to different degrees. Moreover, 11 bioactive prototypes were selected for simultaneous quantitative analysis by UPLC/Q-TOF-MS, and their concentration-time dynamic profiles were successfully used to determine the pharmacokinetics parameters involved. These results provide a basis by which the mechanisms of action and further pharmacological studies of XLGB can be determined.
2. Materials and methods
2.1. Chemicals and reagents
Xian-Ling-Gu-Bao capsules (Batch No.: 100840) were provided by Guizhou Tongjitang Pharmaceutical Co., Ltd. (Guiyang, Guizhou Province in China) and contained the following: 1.118 mg/g sweroside, 10.64 mg/g magnoflorine, 11.436 mg/g epimedin C, 0.539 mg/g psoralen, 0.602 mg/g isopsoralen, 17.549 mg/g akebia saponin D, 0.708 mg/g neobavaisoflavone, 0.309 mg/g icariside II, 0.117 mg/g psoralidin and 0.833 mg/g bavachinin. The reference standards (excepting Timosaponin B II) used for qualitative analysis were isolated from the bioactive fraction of Xian-Ling-Gu-Bao, and the detailed separation process of these chemical compounds was shown in Fig. S1 [11]. The reference standards for quantitative analysis (purity > 98%) were all purchased from Shanghai Winherb Medical Technology Co., Ltd. LC–MS-grade methanol, acetonitrile and water were purchased from Fisher Scientific (Fair Lawn, New Jersey, USA). LC–MS-grade formic acid was obtained from Sigma-Aldrich (St. Louis, USA). All other chemicals were analytical grade.
2.2. Animals
Specific pathogen free (SPF) male Sprague-Dawley rats (250 ± 20 g) were provided by Guangdong Medical Laboratory Animal Center (Guangdong, P.R. China). The rats were kept in a designated animal room at constant temperature (25 ± 2°C) and humidity (55 ± 10)% with 12 h of light/dark per day and free access to water and food. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Jinan University (Ethical Review NO. 20130301003). All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health).
2.3. Preparation of XLGB samples and standard solutions
XLGB powder was extracted twice with 60% ethanol-water for 2 h, and the resulting extracts were concentrated to 1.0 g/Ml for oral administration.
An appropriate amount of each authentic standard was dissolved in methanol to prepare each reference standard solution. These reference standard solutions were mixed together to ascertain a solution with reasonable reference concentrations. All XLGB samples and reference standard solutions were stored at 4°C until use.
2.4. Serum sample preparation
Each serum sample (500 μL) was transferred to a 10-mL polypropylene tube containing 100 μL of IS solution and 100 μL of methanol. Two millilitres of methanol-acetonitrile (2:1, V/V) was added, after which the mixture were vortex-mixed vigorously for 1 min and centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was then transferred and evaporated to dryness using N2 at 37°C. The residue was dissolved in 200 μL of methanol, and 4 μL aliquots were then injected into the UPLC/Q-TOF-MS system.
2.5. UPLC/Q-TOF-MS conditions
UPLC was performed using an ACQUITY™ UPLC system (Waters Corporation, Manchester, U.K.) with an auto-sampler at 4°C. Separation was achieved on an ACQUITY UPLC™ BEH C18 Column (1.7 μm, 3.0 × 150 mm, Waters Corporation, Manchester, U.K.) maintained at 35°C. The mobile phase consisted of water (A) and acetonitrile (B) (each containing 0.1% formic acid), and the flow rate was 0.6 mL/min. The gradient elution program utilized for qualitative analysis was as follows: 0–0.11 min, 2% B; 0.11–10.26 min, 2–36% B; 10.26–12.29 min, 36–48% B; 12.29–14.32 min, 48–80% B; 14.32–14.59 min, 80–100% B; 14.59–16.53 min, 100% B; The gradient elution program utilized for quantitative analysis was as follows: 0–0.11 min, 2 B; 0.11–2. min, 2–10% B; 2.0–3.0 min, 10% B; 3.0–3.6 min, 10–13% B; 3.6–4.2 min, 13–35% B; 4.2–5.5 min, 35–37% B; 5.5–7.5 min, 37–55% B; 7.5–8.0 in, 55–100% B; 8.0–9.3 min, 100% B.
The UPLC system was coupled to a hybrid quadrupole orthogonal time-of-flight (Q-TOF) tandem mass spectrometer (SYNAPT™ G2 HDMS, Waters, Manchester, U.K.) with electrospray ionization (ESI). The operating parameters were as follows: capillary voltage, 3 kV (ESI + ); sample cone voltage, 35 V; extraction cone voltage, 4 V; source temperature, 100°C; desolvation temperature, 300°C; cone gas flow, 50 L/h and desolvation gas flow, 800 L/h. The full scan mass range was 50–1200 Da. The method employed lock spray with leucine encephalin (m/z 556.2771 in positive ion mode and m/z 554.2615 in negative ion mode) to ensure mass accuracy. Psoralen and is opsoralen were both monitored in selective ion mode (SIM) with an m/z of 187.0395. Selective reaction monitoring (SRM) was performed as follows: the m/z 197.0814 → 127.0390 transition with a collision energy (CE) of 20 eV for sweroside, 342.1705 → 297.1130 with CE 22 eV for magnoflorine, 823.3025 → 369.1338 with CE 35 eV for epimedin C, 929.5110 → 437.3418 with CE 20 eV for akebia saponin D, 299.2011 → 231.174 with CE 20 ev for norethindrone (IS), 323.1286 → 267.066 with CE 22 eV for neobavaisoflavone, 369.1338 → 313.0710 with CE 25 eV for icariside II, 321.1126 → 137.0242 with CE 29 eV for corylin, 337.1071 → 281.0468 with CE 24 eV for psoralidin, 339.1591 → 219.1026 with CE 2 5 eV for bavachinin. All experimental data were collected in centroid mode and processed using Masslynx™ 4.1 software and a Quanlynx™ program.
2.6. Cell culture
UMR 106 cells and 3T3-L1 preadipocytes were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% foetal bovine serum in an incubator with 5% CO2 at 37°C [19].
The colourimetric MTT (tetrazolium) assay for cell proliferation is as follows. UMR106 cells were placed into a 96-well plate and maintained for 24 h, before being treated with the compounds of interest at different concentrations. After 24 h, 50 μL/well of MTT solution (1 mg/mL, Sigma, St. Louis, MO, USA) at 37°C was added. The cells were incubated for 4 h. The solution was then removed, and 200 μL of DMSO was added to each well. The plates were then read using a micro-plate reader system (Microplate Spectro, Biotek Instrument Inc., USA) with a test wavelength of 570 nm and a reference wavelength of 650 nm [19].
Alkaline phosphatase (ALP) activity was evaluated as follows. After 500 μL of 104 cells/well of UMR 106 cells was seeded into 24-well plates and incubated for 48 h. The culture medium contained compounds at different concentrations, and the osteogenic medium was a basal medium with 10 mM glycerophosphate and 50 g/mL ascorbic acid. After culturing for 2 days, the ALP activities of UMR 106 cells were determined [19].
Adipocyte differentiation and oil red O staining in 3T3-L1 cells was performed as follows. To induce adipogenesis, 3T3-L1 cells (5×104 cells/well) were plated into 6-well plates and maintained for 2 days after reaching confluence (designated as day 0). The culture medium was then exchanged with differentiation medium (DMEM containing 10% FBS, 0.5 mM IBMX, 1 μM dexamethasone, 2 μg/mL insulin, and 200 μM indomethacin) for 2 days. The cells were then incubated in adipocyte growth medium (DMEM supplemented with 10% FBS and 1 μg/mL insulin) for 2 days and maintained thereafter with 10% FBS DMEM to day 8 based on a published protocol. Tested compounds were added to the medium over the full course of differentiation. The medium was changed every other day. On day 8 the cells were stained with Oil Red O, an indicator of cell lipid content. This experiment was repeated 3 times [20].
2.7. Pharmacokinetic analysis
After fasting with free access to water for 12 h, rats were orally administered XLGB. Blood samples were then collected from the rats’ external jugular veins into tubes at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 36 and 48h after administration. The blood samples were placed at room temperature for 2 h, after which they were centrifuged at 3000 rpm for 10 min. The serum was separated and stored at −80°C until experimental use. For pharmacokinetic parameters analysis, WinNonlin 6.3 was used to calculate the analytes’ pivotal pharmacokinetic parameters.
3. Results and discussions
3.1. Selection of internal standard (IS)
Choosing an appropriate internal standard is important to obtaining satisfactory methods validation. The eleven bioactive components considered herein exhibited various types of structures. Different internal standards were optimized in this regard, including carbamazepine, daidzein, genistein, norethindrone and levonorgestrel. Norethindrone was selected as the internal standard due to its superiority with regard to analytical responses and resolutions.
3.2. Optimization of sample pre-treatments
Protein precipitation, liquid-liquid extraction (LLE) and solid phase extraction (SPE) were all tested for their suitability in sample pre-treatment. Recoveries of these analytes via LLE (ethyl acetate or extraction with other extraction reagents) were lower than those obtained via protein precipitation. Meanwhile, SPE was very labour-intensive and time-consuming. Therefore, protein precipitation was used to pre-treat serum samples. Methanol-acetonitrile was selected as the precipitation agent [21].
3.3. Identification of major component absorbed by rat serum
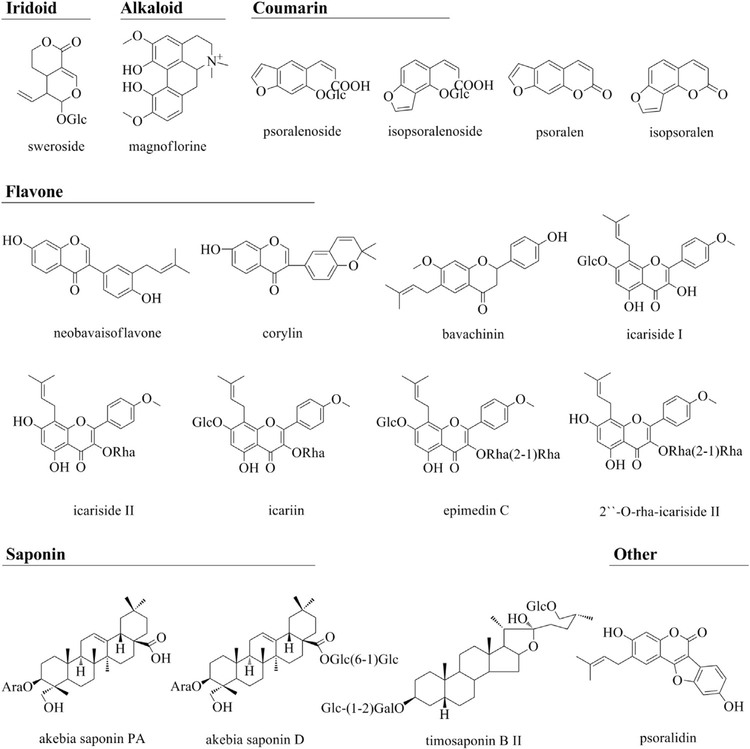
By comparing the retention times, accurate ions and fragment ions (Table S1) of XLGB-containing serum versus blank serum, 18 prototypes, including flavonoids, alkaloids, iridoid, coumarin and saponins, were identified in extract ion chromatograms (EICs) of rat serum after oral XLGB administration (Fig. S2). The structures of these prototypes were shown in Fig. 1, and two of these examples were used to demonstrate structure characterization.
Fig. 1.
Chemical structures of eighteen prototypes detected in rat serum after oral administration of Xian-ling-gu-bao extracts based on MS full scan and MS/MS scan by UPLC/Q-TOF-MS system with electrospray ionization (ESI). The rat serum were pretreated with methanol-acetonitrile (2:1, V/V) to perform protein precipitation, and then the concentrated supernatant was injected into UPLC/Q-TOF-MS system.
Peak 2 pertains to an [M+H]+ ion at m/z 342.1702 in positive ion mode, indicating an alkaloid. The ion at m/z 297.1126 in the MS/MS spectra (Fig. S3) indicates the loss of C2 H6 NH from the [M+H]+ ion. Moreover, elimination of a neutral fragment of CH3OH yielded an ion at m/z 265.0864. Subsequently, a neutral loss of CO, produced an ion at m/z 237.0912, indicating a rearrangement. Compared to the reference standard and results reported in the literature [22], peak 2 was determined to be magnoflorine.
Peak 5 has been identified unambiguously as psoralen. With regard to the MS/MS spectra (Fig. S3), the neutral loss of CO or CO2 is the predominant elimination reported in the literature [23]. The fragment ions at m/z 159.0437, 143.0495 and 131.0476 correspond to a series loss of CO, CO2, and two molecules of CO, respectively.
3.4. Evaluation of in vitro activity
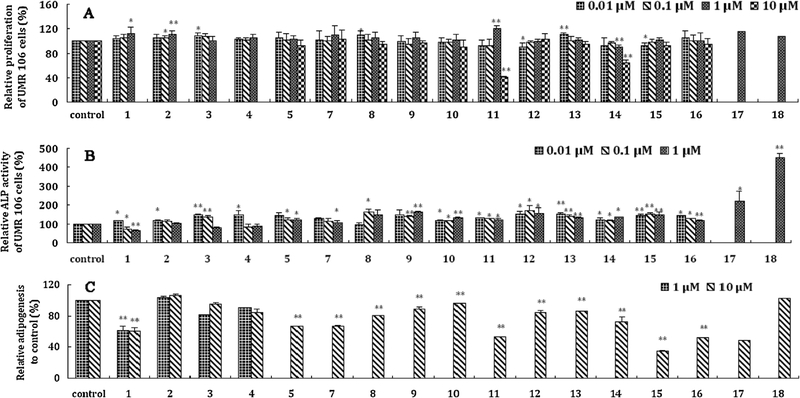
Among the 18 prototypes identified, seventeen compounds were isolated from the bioactive fraction(s) of XLGB. These 17 pure compounds were evaluated with regard to their effects on the proliferation and alkaline phosphatase (ALP) activity of UMR 106 cells and the adipogenesis of 3T3-L1 cells. All data were expressed as the means ± S.D.s and were collected from at least three independent experiments, each performed in tetraplicate. Statistical analysis was by way of one-way ANOVA with post hoc multiple comparisons. A p value less than 0.05 was regarded as significant [19,20]. The results (Fig. 2) obtained indicated that most of these compounds promoted proliferation and ALP activity and inhibited adipocyte formation to different degrees. Among the compounds evaluated, sweroside exhibited excellent bioactivities with regard to the three evaluation indexes above, while psoralidin obviously inhibited adipocyte formation.
Fig. 2.
Anti-osteoporosis related in vitro effects of the components absorbed in rat serum after oral administration of Xian-ling-gu-bao extracts. (A): Analysis of UMR 106 cells proliferation following treatment of individual compound. UMR 106 cells were treated (24 h) at increasing doses (0.01, 0.1, 1, 10 μM) of 17 individual compound. Cell proliferation was evaluated by MTT assay. (B): Alkaline phosphatase (ALP) activity was evaluated after incubated for 48 h treated with different concentration (0.01, 0.1, 1 μM) of 17 analytes. (C): Adipogenesis effect of 3T3-L1 cells after treated with each compound (1 or 10 μM). On day 8, the 3T3-L1 cells were stained with Oil Red O after tested compounds were added to the medium over the full course of differentiation. Columns, mean (n = 3, in triplicate); bars, SD. * P < 0.05, ** P < 0.01. (1: sweroside; 2: magnoflorine; 3: psoralenoside; 4: isopsoralenoside; 5: psoralen; 7: isopsoralen; 8: epimedin C; 9: icariin; 10: akebia saponin D; 11: icariside I; 12: 2”-rha-icariside II; 13: neobavaisoflavone; 14: icariside II; 15: corylin; 16: akebia saponin PA; 17: psoralidin; 18: bavachinin).
3.5. Method validation
Whether the responses of these prototypes were over the lower limit of quantitation (LLOQ), eleven components exhibiting significant anti-osteoporosis activities were selected for simultaneous quantitative analysis in rat serum via UPLC/Q-TOF-MS. The method was validated for selectivity, linearity, extraction recovery, matrix effects, precision, accuracy and stability according to the Guidance for Industry: bioanalytical method validation from the US Food Drug Administration (FDA) [24].
To ensure there were no significant endogenous interferences, selectivity was determined by comparing the chromatograms obtained for each blank serum sample (from six different rats), drug serum samples obtained 1 h after oral administration of XLGB and blank serum samples spiked with standard solutions at LLOQ concentrations. As shown in Fig. S4, no interference peaks (from endogenous entities) were detected at the retention times of the analytes and the IS.
Calibration curves were constructed by plotting analyte:IS peak area ratios (y) versus respective serum concentrations (x) using a 1/x2 weighting factor and linear least-squares regression analysis, and the slope, intercept and correlation coefficient of each curve were determined. The 1/x2 weighting factor was greater than 0.99. Linear ranges, regression equations, correlation coefficients and LLOQs were shown in Table 1.
Table 1.
Linear correlation parameters and LLOQs of each bioactive component in serum after oral administration of Xian-ling-gu-bao extracts.
| Compounds | Slope | Intercept | Concentration range (ng/mL) | r2 | LLOQ (ng/mL) |
|---|---|---|---|---|---|
| Sweroside | 2.1 × 10−4 | 7.6 × 10−4 | 20–2000 | 0.9950 | 20 |
| Magnoflorine | 5.28 × 10−3 | 1.512 × 10−2 | 1–1500 | 0.9958 | 1 |
| Epimedin C | 4.86 × 10−3 | −1.42 × 10−3 | 2–2000 | 0.9951 | 2 |
| Akebia saponin VI | 6.9 × 10−4 | −4.1 × 10−4 | 20–2000 | 0.9951 | 20 |
| Psoralen | 5.3 × 10−4 | 2.66 × 10−3 | 5–3000 | 0.9950 | 5 |
| Isopsoralen | 3.8 × 10−4 | 3.01 × 10−3 | 5–1500 | 0.9918 | 5 |
| Neobavaisoflavone | 1.661 × 10−2 | 4.0 × 10−4 | 0.5–300 | 0.9951 | 0.5 |
| Icariside II | 2.827 × 10−2 | −2.79 × 10−3 | 0.5–200 | 0.9936 | 0.5 |
| Corylin | 5.12 × 10−3 | −0.3.0 × 10−5 | 1–100 | 0.9944 | 1 |
| Psoralidin | 7.35 × 10−3 | 3.57 × 10−3 | 1–500 | 0.9953 | 2 |
| Bavachinin | 7.44 × 10−3 | 3.28 × 10−3 | 1–100 | 0.9950 | 2 |
Extraction recoveries (ER) and matrix effects (ME) were evaluated using a published experimental protocol [25]. The peak areas of 3 different concentrations of analytes within quality control (QC) samples were defined as A1. A2 referred to the peak areas pertaining to analytes within extracted control serum samples reconstituted with standard solutions at three concentrations. A3 corresponded to the responses of analytes obtained by directly injecting the corresponding pure reference standards at three QC levels. Extraction recoveries and matrix effects were calculated as follows: ER% = A1/A2 × 100%, and ME% = A2/A3 × 100%. The absolute recoveries of these analytes were all above 71.7% for the three concentration levels investigated, while the matrix effects of these analytes were found to range from 89.1 to 110.7% at different concentration levels. The results (Table S2) suggested that the recoveries obtained were consistent and reproducible and did not indicate significant enhancement or suppression of ionization for any of the analytes studied.
The accuracies and inter/intra-day precisions of the assay were evaluated by determining six replicates of QC samples (at low, middle and high concentrations) on three consecutive days and were listed in Table 2. The inter-day and intra-day precisions ranged from 0.9% to 7.9% and 3.3% to 12.3%, respectively, and the accuracies ranged from −12.7% to 11.0%. The data obtained was within acceptable limits.
Table 2.
Inter/intra-day accuracy and precision for each component in serum after oral administration of Xian-ling-gu-bao extracts.
| Compounds | Conc. (ng/mL) | Intra-day (n = 6) |
Inter-day (n = 18) |
||||
|---|---|---|---|---|---|---|---|
| Measured conc. (ng/mL) | RE% | RSD% | Measured conc. (ng/mL) | RE% | RSD% | ||
| Sweroside | 50 | 50.9 ± 5.1 | 5.1 | 10.0 | 51.3 ± 1.0 | 2.7 | 2.0 |
| 200 | 203.1 ± 25.1 | 3.8 | 12.3 | 204.1 ± 3.1 | 2.1 | 1.5 | |
| 1600 | 1617.3 ± 143.0 | −10.1 | 8.8 | 1524.2 ± 89.5 | −4.7 | 5.9 | |
| Magnoflorine | 3 | 3.0 ± 0.3 | 6.8 | 8.3 | 3.1 ± 0.1 | 4.6 | 2.9 |
| 150 | 155.1 ± 10.9 | 8.1 | 7.0 | 159.2 ± 3.7 | 6.2 | 2.3 | |
| 1200 | 1090.8 ± 35.8 | −11.0 | 3.3 | 1077.7 ± 11.6 | −10.2 | 1.1 | |
| Epimedin C | 5 | 5.1 ± 0.5 | −2.6 | 9.1 | 5.0 ± 0.1 | 0.7 | 2.9 |
| 200 | 212.1 ± 13.5 | 10.8 | 6.4 | 217.0 ± 4.7 | 8.5 | 2.2 | |
| 1600 | 1486.2 ± 55.4 | −11.6 | 3.7 | 1451.7 ± 36.0 | −9.3 | 2.5 | |
| Akebia saponin D | 50 | 50.3 ± 5.2 | 8.4 | 10.3 | 52.6 ± 2.0 | 5.2 | 3.9 |
| 200 | 215.2 ± 19.2 | 11.0 | 8.9 | 216.0 ± 5.6 | 8.0 | 2.6 | |
| 1600 | 1460.9 ± 71.5 | −11.0 | 4.9 | 1427.9 ± 31.7 | −10.8 | 2.2 | |
| Psoralen | 10 | 10.1 ± 1.0 | −6.0 | 10.0 | 9.9 ± 0.4 | −1.4 | 4.0 |
| 300 | 289.4 ± 13.5 | 5.2 | 4.6 | 304.4 ± 13.5 | 1.5 | 4.4 | |
| 2400 | 2507.9 ± 185.9 | −10.7 | 7.4 | 2343.1 ± 184.5 | −2.4 | 7.9 | |
| Isopsoralen | 10 | 10.5 ± 1.1 | 4.3 | 10.4 | 10.0 ± 0.4 | −0.2 | 4.1 |
| 150 | 145.4 ± 12.5 | −3.1 | 8.6 | 150.2 ± 4.2 | 0.1 | 2.8 | |
| 1200 | 1172.3 ± 75.2 | −9.3 | 6.4 | 1162.5 ± 69.5 | −3.1 | 6.0 | |
| Neobavaisoflavone | 1 | 1.0 ± 0.1 | 2.4 | 8.9 | 1.0 ± 0.1 | 1.6 | 1.9 |
| 30 | 31.4 ± 2.4 | 9.5 | 7.6 | 32.1 ± 0.7 | 7.0 | 2.3 | |
| 240 | 230.3 ± 10.0 | −11.9 | 4.3 | 220.7 ± 9.4 | −8.1 | 4.2 | |
| Icariside II | 1 | 1.1 ± 0.1 | 8.0 | 4.8 | 1.1 ± 0.1 | 7.8 | 0.9 |
| 20 | 20.8 ± 2.0 | 8.3 | 9.5 | 21.3 ± 0.5 | 6.5 | 2.2 | |
| 160 | 146.5 ± 5.3 | −11.2 | 3.6 | 149.7 ± 9.1 | −10.2 | 6.1 | |
| Corylin | 2.5 | 2.4 ± 0.3 | 5.6 | 11.4 | 2.5 ± 0.1 | 0.4 | 5.4 |
| 10 | 10.2 ± 1.1 | 3.2 | 10.7 | 10.2 ± 0.1 | 1.9 | 1.1 | |
| 80 | 76.0 ± 5.2 | −8.3 | 6.8 | 75.7 ± 2.1 | −5.4 | 2.8 | |
| Psoralidin | 2 | 2.1 ± 0.2 | 4.7 | 10.0 | 2.1 ± 0.1 | 2.4 | 2.5 |
| 50 | 55.3 ± 2.5 | 10.6 | 4.5 | 54.7 ± 0.5 | 9.5 | 0.9 | |
| 400 | 360.4 ± 11.9 | −12.7 | 3.3 | 354.7 ± 5.6 | −11.3 | 1.6 | |
| Bavachinin | 2 | 1.9 ± 0.2 | −9.5 | 9.1 | 1.9 ± 0.1 | −5.6 | 3.8 |
| 10 | 9.9 ± 1.0 | 8.7 | 10.2 | 10.4 ± 0.5 | 4.1 | 4.7 | |
| 80 | 73.0 ± 3.6 | −12.3 | 4.9 | 72.1 ± 1.7 | −9.8 | 2.4 | |
Conc. means concentration.
The stabilities of 11 analytes in the serum samples were assessed under different conditions and at three concentration levels, including extracted samples stored at 24 h at room temperature, those kept at −80°C for 24 h, those undergoing three freezing cycles at −80°C with thawing at 25°C, and serum samples maintained at room temperature for 8 h. The sample sets were compared via three QC replicates at each concentration investigated. Stability results (Table S3) indicated that all the analytes were stable under different storage conditions.
3.6. Pharmacokinetic analysis
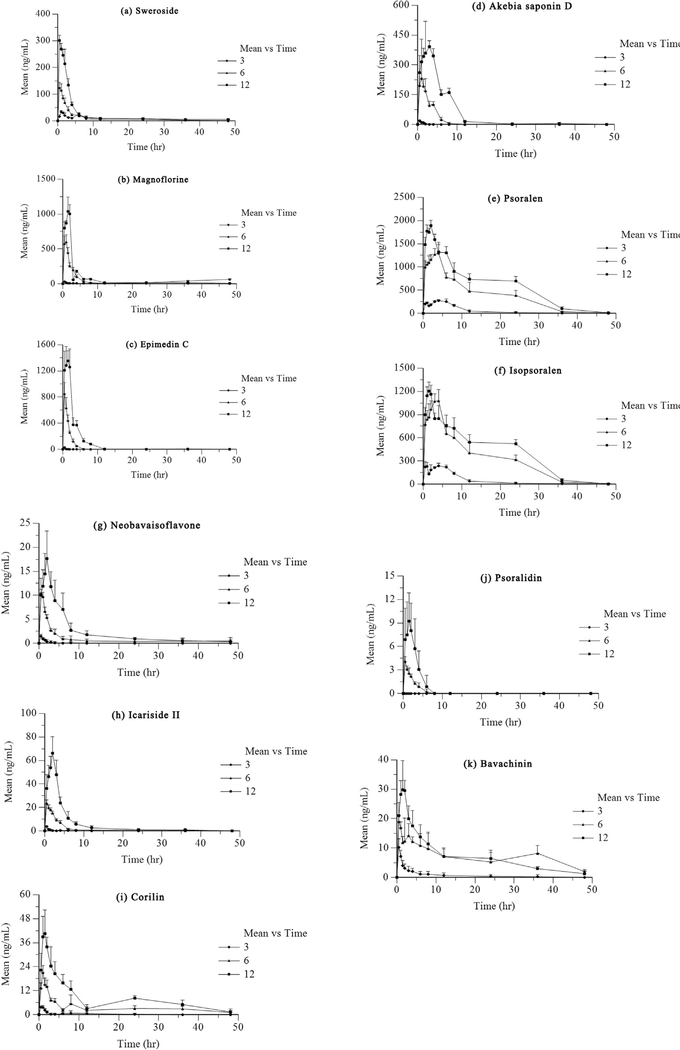
The method validated above was successfully applied in a pharmacokinetic study after oral administration of XLGB. The mean concentration-time profiles of these bioactive components shown in Fig. 3. The pharmacokinetic parameters of interest calculated using the Phoenix WinNonlin 6.3 program and are illustrated in Table 3.
Fig. 3.
Mean concentration-time profiles of these bioactive components after oral administration of Xian-ling-gu-bao extracts (n = 6) (a: sweroside; b: magnoflorine; c: epimedin C; d: akebia saponin D; e: psoralen; f: isopsoralen; g: neobavaisoflavone; h: icariside II; i: corylin; j: psoralidin; k: bavachinin).
Table 3.
Pharmacokinetic parameters of each component after oral administration of Xian-ling-gu-bao extracts.
| Dose | Compounds | Tmax (h) | Cmax (ng/mL) | t1/2 (h) | AUC0-t (ng*h/mL) | AUC0–∞ (ng*h/mL) | MRT0–t (h) | MRT0–∞ (h) |
|---|---|---|---|---|---|---|---|---|
| 12 g/kg | Sweroside | 0.50 ± 0 | 300.95 ± 19.36 | 23.67 ± 12.32 | 1146.72 ± 35.28 | 1333.04 ± 97.91 | 8.25 ± 0.83 | 19.19 ± 6.86 |
| Magnoflorine | 1.67 ± 0.26 | 1157.65 ± 101.54 | 13.40 ± 0.75 | 3325.47 ± 130.35 | 3483.38 ± 121.29 | 6.67 ± 0.32 | 9.43 ± 0.47 | |
| Epimedin C | 1.42 ± 0.38 | 1411.56 ± 200.86 | 11.13 ± 13.85 | 4397.49 ± 435.63 | 4527.69 ± 474.54 | 3.07 ± 0.63 | 5.10 ± 4.50 | |
| Akebia saponin D | 2.67 ± 0.52 | 451.92 ± 91.76 | 5.51 ± 1.33 | 2642.49 ± 233.18 | 2658.47 ± 241.31 | 5.03 ± 0.32 | 5.91 ± 0.50 | |
| Psoralen | 1.83 ± 0.41 | 1910.32 ± 117.87 | 4.20 ± 0.42 | 28308.75 ± 898.59 | 28395.45 ± 877.42 | 13.19 ± 1.14 | 13.31 ± 1.18 | |
| Isopsoralen | 1.67 ± 0.41 | 1239.77 ± 72.15 | 6.86 ± 3.44 | 19394.94 ± 1997.00 | 19853.64 ± 2015.85 | 13.31 ± 0.43 | 14.17 ± 1.43 | |
| Neobavaisoflavone | 1.83 ± 0.26 | 18.37 ± 5.40 | 16.18 ± 10.31 | 110.08 ± 30.32 | 127.48 ± 32.27 | 8.79 ± 2.43 | 17.21 ± 9.50 | |
| Icariside II | 1.83 ± 0.26 | 68.11 ± 12.99 | 9.90 ± 5.47 | 280.72 ± 40.81 | 293.14 ± 38.79 | 5.33 ± 0.84 | 7.36 ± 1.41 | |
| Corylin | 1.92 ± 0.58 | 44.22 ± 9.07 | 13.51 ± 3.81 | 386.15 ± 84.00 | 442.39 ± 107.76 | 14.73 ± 1.76 | 20.65 ± 3.78 | |
| Psoralidin | 1.33 ± 0.52 | 10.52 ± 4.11 | 1.40 ± 0.36 | 27.78 ± 13.97 | 34.27 ± 16.04 | 2.05 ± 0.45 | 2.78 ± 0.68 | |
| Bavachinin | 1.58 ± 0.38 | 36.00 ± 4.29 | 12.57 ± 3.06 | 343.30 ± 61.62 | 379.76 ± 59.32 | 13.56 ± 2.69 | 18.40 ± 3.38 | |
| 6 g/kg | Sweroside | 0.67 ± 0.26 | 135.49 ± 10.25 | 13.80 ± 7.36 | 597.74 ± 59.35 | 689.54 ± 110.18 | 8.31 ± 2.11 | 16.67 ± 7.30 |
| Magnoflorine | 0.67 ± 0.26 | 651.67 ± 64.61 | 21.78 ± 4.20 | 2597.64 ± 248.53 | 4610.73 ± 571.75 | 17.27 ± 0.63 | 44.36 ± 6.88 | |
| Epimedin C | 0.67 ± 0.26 | 854.12 ± 167.28 | 0.76 ± 0.10 | 1361.31 ± 79.46 | 1365.29 ± 80.31 | 1.45 ± 0.08 | 1.47 ± 0.08 | |
| Akebia saponin D | 1.17 ± 0.26 | 249.85 ± 64.31 | 1.37 ± 0.23 | 731.52 ± 131.54 | 756.83 ± 140.72 | 2.41 ± 0.25 | 2.63 ± 0.25 | |
| Psoralen | 2.92 ± 1.28 | 1384.02 ± 150.23 | 6.78 ± 1.00 | 18300.54 ± 510.87 | 18494.30 ± 528.74 | 11.79 ± 0.99 | 12.22 ± 0.90 | |
| Isopsoralen | 3.67 ± 0.52 | 1136.28 ± 100.43 | 6.78 ± 1.09 | 15120.11 ± 1290.85 | 15295.1307.27± | 11.72 ± 0.83 | 12.19 ± 0.75 | |
| Neobavaisoflavone | 0.58 ± 0.20 | 10.68 ± 0.59 | 11.22 ± 9.11 | 41.14 ± 18.98 | 60.56 ± 41.04 | 9.13 ± 9.19 | 19.26 ± 20.70 | |
| Icariside II | 0.67 ± 0.41 | 23.64 ± 2.84 | 9.93 ± 8.71 | 85.46 ± 15.26 | 94.10 ± 16.91 | 6.45 ± 3.98 | 10.51 ± 6.10 | |
| Corylin | 1.00 ± 0 | 21.09 ± 3.40 | 23.44 ± 10.31 | 166.50 ± 26.45 | 227.72 ± 52.75 | 16.55 ± 4.24 | 33.27 ± 11.40 | |
| Psoralidin | 0.58 ± 0.20 | 4.21 ± 0.45 | 2.09 ± 0.31 | 8.80 ± 1.38 | 11.58 ± 0.94 | 1.78 ± 0.36 | 3.13 ± 0.36 | |
| Bavachinin | 1.25 ± 1.04 | 20.41 ± 2.63 | 19.08 ± 8.31 | 346.62 ± 36.95 | 406.85 ± 56.64 | 19.50 ± 3.03 | 28.15 ± 8.89 | |
| 3 g/kg | Sweroside | 1.17 ± 0.26 | 35.58 ± 2.27 | 21.29 ± 3.33 | 313.05 ± 39.12 | 452.16 ± 50.32 | 12.42 ± 3.30 | 31.70 ± 4.64 |
| Magnoflorine | 0.50 ± 0 | 31.78 ± 4.77 | 20.00 ± 8.43 | 263.57 ± 76.19 | 319.78 ± 96.94 | 16.39 ± 4.54 | 26.17 ± 8.51 | |
| Akebia saponin D | - | - | - | - | - | - | - | |
| Epimedin C | 0.50 ± 0 | 29.07 ± 9.19 | 14.88 ± 13.40 | 21.70 ± 3.40 | 69.33 ± 37.54 | 1.41 ± 1.01 | 20.72 ± 19.70 | |
| Psoralen | 4.50 ± 1.22 | 290.47 ± 23.33 | 6.39 ± 3.73 | 2738.61 ± 681.96 | 2807.61 ± 701.19 | 7.55 ± 2.44 | 8.40 ± 2.93 | |
| Isopsoralen | 3.50 ± 2.49 | 270.33 ± 35.05 | 6.88 ± 4.89 | 2390.74 ± 460.21 | 2473.66 ± 494.00 | 7.49 ± 2.83 | 8.73 ± 3.76 | |
| Neobavaisoflavone | 0.50 ± 0 | 1.46 ± 0.38 | 6.28 ± 7.25 | 2.10 ± 0.78 | 7.24 ± 6.25 | 1.57 ± 0.81 | 10.51 ± 13.50 | |
| Icariside II | 0.50 ± 0 | 3.74 ± 0.85 | 12.23 ± 9.22 | 5.52 ± 3.08 | 17.00 ± 9.29 | 2.40 ± 1.54 | 16.51 ± 12.48 | |
| Corylin | 0.75 ± 0.27 | 4.15 ± 0.59 | 30.71 ± 20.02 | 10.62 ± 3.66 | 86.37 ± 67.67 | 5.60 ± 5.20 | 51.11 ± 29.69 | |
| Psoralidin | - | - | - | - | - | - | - | |
| Bavachinin | 0.50 ± 0 | 10.25 ± 2.84 | 11.51 ± 9.48 | 31.63 ± 11.39 | 53.60 ± 27.93 | 6.26 ± 4.26 | 18.47 ± 15.68 |
Tmax, peak time; Cmax, peak concentration; t1/2, apparent elimination half-life; AUC0–t: area under the curve calculated at the last experimental point; AUC0–∞: area under the plasma concentration-time curve from zero to infinity; MRT0–t, mean residence time at the last experimental point; MRT0–∞: mean residence time from zero to infinity.
In the present study, we different oral doses (3, 6 and 12 g/kg) to rats to investigate the pharmacokinetic parameters involved. Except for magnoflorine, psoralen, isopsoralen and bavachinin, all bioactive components exhibited dose-dependent relationships when mean AUC0-t values were plotted against the different doses employed. The pharmacokinetic profile was not available for psoralidin because the analytical signals were lower than the LLOQ after oral administration of XLGB at a dose of 3 g/kg. The concentrations of other components, such as akebia saponin D, neobavaisoflavone, icariside II and corylin, were a slightly higher than the corresponding LLOQs.
As shown in Table 3, the parameters evaluated, such as Tmax, Cmax, t1/2, AUC0-t, AUC0−∞, MRT0-t and MRT0−∞, differed from those evaluated after oral administration of a single compound. For instance, sweroside demonstrated an obvious improvement in t1/2, implying delayed absorption [26]. Compared to previously published data, magnoflorine exhibited greater Tmax and t1/2 values, indicating that magnoflorine absorption was enhanced by intragastric administration of Xian-Ling-Gu-Bao compared to administration of the compound independently [27]. Akebia saponin D showed clear improvements in Cmax and Tmax [28,29], indicating that the complex ingredients in the TCM formula induced drug-drug interactions [30] and affected pharmacokinetic behaviour via solubilisation, complexation and hydrolytic decomposition, thereby improving bioavailability [31,32]. In addition, obvious double absorption peaks appeared in previously published reports, indicating that AUC0−∞ was increased in comparison to the present study’s results. Previous research implicated icariside II as one of the major intermediate decomposition products of other flavonoids [33]. This may explain the higher absorption of Cmax observed for icariside II in the present study compared to previously published reports [34]. Similarly, the persistently high concentrations of psoralen and isopsoralen can been explained by the hydrolysis of psoralenoside and isopsoralenoside, respectively [35]. The obvious decrease in Cmax and AUC observed for bavachinin indicated that some of the ingredients present reduced the absorption and bioavailability of bavachinin [36]. Double absorption peaks were observed for several bioactive components in the present study, which may be related to enterohepatic circulation.
4. Conclusions
The method developed and validated above was successfully used for qualitative and quantitative analyses of prototypes in rat serum after oral XLGB administration. Meanwhile, evaluation of these prototypes (except Timosaponin B II) revealed different degrees of anti-osteoporosis activity. Moreover, the pharmacokinetic parameters of these bioactive components were successfully obtained using WinNolin 6.3 software. Compared with single dosing of individual compounds, significant improvements in Cmax and Tmax were observed for sweroside, magnoflorine, akebia saponin D, icariside II, psoralen and isopsoralen, indicating that the complex multiple ingredients in XLGB may induce drug-drug interactions that influence their pharmacokinetic parameters. Likewise, the obvious attenuation of the Cmax and AUC of bavachinin indicates corresponding reductions in absorption and bioavailability. These results facilitate our understanding of the material basis of the therapeutic effects of XLGB and should be valuable to further studies and therapeutic applications involving XLGB.
Supplementary Material
Acknowledgements
The work was supported by the Major Project for International Cooperation and Exchange of the National Natural Science Foundation of China (Grant No. 81220108028), the Project of National Natural Science Foundation of China (Grant No. 81073003), the National Major Scientific and Technological Special Project for Significant New Drugs Development of China (Grant No. 2011ZX09201–201-05), the Project of Natural Science Foundation of Guangdong Province (Grant No. S2013010012373) and the Science and Technology Projects of Guangdong Province (Grant No. 2011B031700068). The authors would like to thank their colleague Feng-Juan Tu, Ph.D., for supplying the reference standards.
Footnotes
Conflicts of interest
These authors declare that there are no financial or commercial conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jchromb.2016.12.026.
References
- [1].Normile D, The new face of traditional Chinese medicine, Science 299 (2003) 188–190. [DOI] [PubMed] [Google Scholar]
- [2].Jiang Y, David B, Tu P, Barbin Y, Recent analytical approaches in quality control of traditional Chinese medicines-a review, Anal. Chim. Acta 657 (2010) 9–18. [DOI] [PubMed] [Google Scholar]
- [3].Zhang A, Sun H, Qiu S, Wang X, Evidence-Based Complementary Altern. Med. 2013 (2013), 402159–402159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sun H, Dong W, Zhang A, Wang W, Wang X, Pharmacokinetics study of multiple components absorbed in rat plasma after oral administration of Stemonae radix using ultra-performance liquid-chromatography/mass spectrometry with automated MetaboLynx software analysis, J. Sep. Sci 35 (2012) 3477–3485. [DOI] [PubMed] [Google Scholar]
- [5].Wang X, Sun H, Zhang A, Jiao G, Sun W, Yuan Y, Pharmacokinetics screening for multi- components absorbed in the rat plasma after oral administration traditional Chinese medicine formula Yin- Chen- Hao- Tang by ultra performance liquid chromatography- electrospray ionization /quadrupole- time- of- flight mass spectrometry combined with pattern recognition methods, Analyst 136 (2011) 5068–5076. [DOI] [PubMed] [Google Scholar]
- [6].Wu W, Li D, Zhi X, Han M, Preventive and therapeutic effects of Xianling Gubao capsules for postmenopausal osteoposis, J. Guangzhou Univ. Tradit. Chin. Med 22 (2005) 191–193. [Google Scholar]
- [7].Wang X, Zhang F, Fu S, Shi W, Du N, Li G, Biomechanical effects of Jiangu Chongji (a TCM decoction) on femur and lumbar vertebrae of ovariectomy female rats, China J. Orthop. Trauma 12 (2001) 601–603. [Google Scholar]
- [8].Cheng T, Zhang L, Li Z, Li Q, Mu SL, Tian FM, Liu YQ, Effect of XLGB treatment in the rabbit anterior cruciate ligament transaction models of osteoarthritis, Orthopedic J. China 17 (2009) 530–533. [Google Scholar]
- [9].Tian WM, Wang WZ, Wang XG, Xu GH, Effect of Gufuhuo decoction on local apoptosis of steroid-induced necrosis of femoral head, Chin. J. Chin. Mater. Med 30 (2005) 1848–1851. [PubMed] [Google Scholar]
- [10].Zhu HM, Qin L, Garnero P, Genant HK, Zhang G, Dai KR, Yao XS, Gu GS, Hao YQ, Li ZS, Zhao YL, Li WL, Yang JW, Zhao X, Shi DP, Fuerst T, Lu Y, Li HL, Zhang XL, Li CS, Zhao JX, Wu QL, Zhao SJ, The first multicenter and randomized clinical trial of herbal Fufang for treatment of postmenopausal osteoporosis, Osteoporosis Int. 23 (2010) 1317–1327. [DOI] [PubMed] [Google Scholar]
- [11].Tu F, Study on The Anti-Osteoporosis Material Basis of Xian-Ling-Gu Bao, A Traditional Chinese Medicine Prescription, Shenyang Pharmaceutical University, 2011. [Google Scholar]
- [12].Dai Y, Tu F, Yao Z, Ding B, Xu W, Qiu X, Yao X, Rapid identification of chemical constituents in traditional Chinese medicine fufang preparation xianling gubao capsule by LC-linear ion trap/Orbitrap mass spectrometry, Am. J. Chin. Med 41 (2013) 1181–1198. [DOI] [PubMed] [Google Scholar]
- [13].Cheng YM, Liu YZ, Wang H, Li J, Ren J, Zhu L, Gong LK, A 26-week repeated dose toxicity study of Xian-ling-gu-bao in Sprague-Dawley rats, J. Ethnopharmacol 145 (2013) 85–93. [DOI] [PubMed] [Google Scholar]
- [14].Guang XY, Li HF, Yang WZ, Lin CH, Sun C, Wang BR, Guo DA, Ye M, HPLC-DAD-MS(n) analysis and HPLC quantitation of chemical constituents in Xian-Ling-Gu-Bao capsules, J. Pharm. Biomed. Anal 55 (2011) 923–933. [DOI] [PubMed] [Google Scholar]
- [15].Qin Z, Yao Z, Dai Y, Geng J, Lin S, Wu X, Yao X, HPLC-MS/MS analysis of the absorbed components in serum after intragastric administration of Xianlinggubao to rats, J. Instrum. Anal 32 (2013) 420–426. [Google Scholar]
- [16].Geng JL, Dai Y, Yao ZH, Qin ZF, Wang XL, Qin L, Yao XS, Metabolites profile of Xian-Ling-Gu-Bao capsule a traditional Chinese medicine prescription, in rats by ultra performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry analysis, J. Pharm. Biomed. Anal 96 (2014) 90–103. [DOI] [PubMed] [Google Scholar]
- [17].Zhao M, Du L, Tao J, Qian D, Guo J, Jiang S, Shang EX, Duan JA, Wu C, Ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry for rapid analysis of the metabolites of morroniside produced by human intestinal bacteria, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 976–977C (2015) 61–67. [DOI] [PubMed] [Google Scholar]
- [18].Qin ZF, Dai Y, Yao ZH, He LL, Wang QY, Geng JL, Chen HF, Yao XS, Study on chemical profiles and metabolites of Allii Macrostemonis Bulbus as well as its representative steroidal saponins in rats by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry, Food Chem. 192 (2016) 499–515. [DOI] [PubMed] [Google Scholar]
- [19].Wang XL, Zheng LZ, Zhang G, Wong MS, Yao XS, Qin L, Osteogenic effects of flavonoid aglycones from an osteoprotective fraction of Drynaria fortunei-an in vitro efficacy study, Phytomedicine 18 (2011) 868–872. [DOI] [PubMed] [Google Scholar]
- [20].Wang XL, Wang N, Zheng LZ, Xie XH, Yao D, Liu MY, Yao ZH, Dai Y, Zhang G, Yao XS, Qin L, Phytoestrogenic molecule desmethylicaritin suppressed adipogenesis via Wnt/β-catenin signaling pathway, Eur. J. Pharmacol 714 (2013) 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xiang C, Qiao X, Wang Q, Li R, Miao W, Guo D, Ye M, From single compounds to herbal extract: a strategy to systematically characterize the metabolites of licorice in rats, Drug Metab. Dispos 39 (2011) 1597–1608. [DOI] [PubMed] [Google Scholar]
- [22].Zhong X, Guo J, Wang L, Luo D, Bei W, Chen Y, Yan K, Peng J, Analysis of the constituents in rat serum after oral administration of Fufang Zhenzhu Tiaozhi capsule by UPLC-Q-TOF-MS/MS, Chromatographia 75 (2011) 111–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sun MQ, Lu JQ, Zhang HG, Fragmentation pathways of the furocoumarins in electrospray ionization mass spectrometry, Chin. J. Pharm. Anal 29 (2009) 82–85. [Google Scholar]
- [24].US FDA, Guidance for Industry: Bioanalytical Method Validation, 2001.
- [25].Matuszewski BK, Constanzer ML, Chavez-Eng CM, Matrix effect in quantitative LC/MS/MS analyses of biological fluids: a method for determination of finasteride in human plasma at picogram per milliliter concentrations, Anal. Chem 70 (1998) 882–889. [DOI] [PubMed] [Google Scholar]
- [26].Luo YD, Chen J, Cao J, Wen XD, Li P, Determination of sweroside in rat plasma and bile for oral bioavailability and hepatobiliary excretion, Chem. Pharm. Bull 57 (2009) 79–83. [DOI] [PubMed] [Google Scholar]
- [27].Tian X, Li Z, Lin Y, Chen M, Pan G, Huang C, Study on the PK profiles of magnoflorine and its potential interaction in Cortex phellodendri decoction by LC-MS/MS, Anal. Bioanal. Chem 406 (2014) 841–849. [DOI] [PubMed] [Google Scholar]
- [28].Li K, Ding L, Yang ZL, Liu EH, Qi LW, Li P, Hu YZ, Determination of asperosaponin VI in rat plasma by HPLC-ESI–MS and its application to preliminary pharmacokinetic studies, Biomed. Chromatogr 24 (2010) 550–555. [DOI] [PubMed] [Google Scholar]
- [29].Zhu H, Ding L, Shakya S, Qi X, Hu L, Yang X, Yang Z, Simultaneous determination of asperosaponin VI and its active metabolite hederagenin in rat plasma by liquid chromatography–tandem mass spectrometry with positive/negative ion-switching electrospray ionization and its application in pharmacokinetic study, J. Chromatogr. B: Anal. Technol. Biomedica. Life Sci 879 (2011) 3407–3414. [DOI] [PubMed] [Google Scholar]
- [30].Zhang X, Zhang D, Xu J, Gu J, Zhao Y, Determination of 25-OH-PPD in rat plasma by high-performance liquid chromatography–mass spectrometry and its application in rat pharmacokinetic studies, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 858 (2007) 65–70. [DOI] [PubMed] [Google Scholar]
- [31].Li Y, Duan J, Guo T, Xie W, Yan S, Li B, Zhou Y, Chen Y, In vivo pharmacokinetics comparisons of icariin emodin and psoralen from gan-kang granules and extracts of herba Epimedii, Nepal dock root, Ficus hirta yahl, J. Ethnopharmacol 124 (2009) 522–529. [DOI] [PubMed] [Google Scholar]
- [32].Zhang F, Zhan Q, Gao S, Dong X, Jiang B, Sun L, Tao X, Chen WS, Chemical profile- and pharmacokinetics-based investigation of the synergistic property of Platycodonis Radix in Traditional Chinese Medicine formula Shengxian Decoction, J. Ethnopharmacol 152 (2014) 497–507. [DOI] [PubMed] [Google Scholar]
- [33].Zhao H, Fan M, Fan L, Sun J, Guo D, Liquid chromatography-tandem mass spectrometry analysis of metabolites in rats after administration of prenylflavonoids from Epimedium, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 878 (2010) 1113–1124. [DOI] [PubMed] [Google Scholar]
- [34].Xu W, Zhang Y, Yang M, Shen Z, Zhang X, Zhang W, Li H, LC-MS/MS method for the simultaneous determination of icariin and its major metabolites in rat plasma, J. Pharm. Biomed. Anal 45 (2007) 667–672. [DOI] [PubMed] [Google Scholar]
- [35].Wang YF, Liu YN, Xiong W, Yan DM, Zhu Y, Gao XM, Xu YT, Qi AD, A UPLC-MS/MS method for in vivo and in vitro pharmacokinetic studies of psoralenoside isopsoralenoside, psoralen and isopsoralen from Psoralea corylifolia extract, J. Ethnopharmacol 151 (2014) 609–617. [DOI] [PubMed] [Google Scholar]
- [36].Liu L, Liu KN, Wen YB, Zhang HW, Lu YX, Yin Z, Development of a fully automated on-line solid phase extraction and high-performance liquid chromatography with diode array detection method for the pharmacokinetic evaluation of bavachinin: a study on absolute bioavailability and dose proportionality, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 893–894 (2012) 21–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.