Abstract
Purpose
To appraise the ability of a radiomics signature to predict clinical outcome after stereotactic body radiation therapy (SBRT) for pancreas carcinoma.
Methods
A cohort of 100 patients was included in this retrospective, single institution analysis. Radiomics texture features were extracted from computed tomography (CT) images obtained for the clinical target volume. The cohort of patients was randomly divided into two separate groups for the training (60 patients) and validation (40 patients). Cox regression models were built to predict overall survival and local control. The significant predictors at univariate analysis were included in a multivariate model. The quality of the models was appraised by means of area under the curve and concordance index.
Results
A clinical-radiomic signature associated with Overall Survival (OS) was found significant in both training and validation sets (p = 0.01 and 0.05 and concordance index 0.73 and 0.75 respectively). Similarly, a signature was found for Local Control (LC) with p = 0.007 and 0.004 and concordance index 0.69 and 0.75. In the low risk group, the median OS and LC in the validation group were 14.4 and 28.6 months while in the high-risk group were 9.0 and 17.5 months respectively.
Conclusion
A CT based radiomic signature was identified which correlate with OS and LC after SBRT and allowed to identify low and high-risk groups of patients.
Introduction
Patients affected by pancreatic adenocarcinoma have a typically un-favourable prognosis, with a 5-year overall survival (OS) rate as low as 6% [1]. Surgery, as a treatment of choice, leads to 5-year OS rates of about 20 to 25%. Nevertheless, a large fraction of the total patients, are unfit to surgery already at diagnosis (due to the stage of the disease or other concomitant exclusion criteria) [2]. For this reason, chemo-radiotherapy is a frequently adopted solution for many patients but with the drawback of some significant rate of severe toxicity (grade 3 to 4) and a still low rate of survival. The median OS is in the range of 5 to 15 months and the 2-year OS is about 30% [3]. The role of stereotactic body radiotherapy (SBRT) was investigated [4], but only few trials were published with reports about significant late toxicity rates [5–11]. Our institutional experience [12] demonstrated that SBRT with a fractionation of 45Gy in 6 sessions is an effective and safe therapeutic option for non-operable as well as for isolated local recurrences with a median OS of 13 months for the non-operable cases. 1 and 2 year OS were 59±7% and 18±9% respectively. In the same study freedom from local progression (FFLP) was 87±6% at 2 years [12]. The comparative assessment of the clinical use of SBRT in the management of pancreatic cancer demonstrated (Table 5 in that original publication), with a quite variable range of fractionation regimens, very consistent findings in terms of FFLP and median OS times ranging from 8 to 20 months, again consistent with our findings [12].
In this context, it would be advisable to introduce in the clinical practice, methods and tools suitable to predict the probability of tumor control and/or of survival enabling a better treatment personalization and to stratify patients in risk classes. The quantitative analysis of textural featuers (radiomics) of the tumor tissues from the quantification of various imaging sets might contribute to the definition of these tools. Advanced computational methodologies could enable the identification of quantitative predictive descriptors (signatures) of biological features cancer tissues [13–14].
Limited literature is available on the use of radiomics for pancreas cancer. Yue [15] stratified patients in various risk groups using pre- and post-radiotherapy positron emission tomography and computed tomography (PET, CT, PET/CT) images in a small group of 26 patients and determined the value of texture features with respect to response to treatment. The authors concluded that texture analysis of metabolic imaging is feasible and might contribute positively to the outcome prediction.
Hanania [16] investigated CT data to assess degree of malignancy of intraductal papillary mucinous neoplasms (IPMNs) for 53 patients and found that high-grade IPMNs can be significantly distinguished from lower grade disease.
Permuth [17], by means of a retrospective analysis on 38 patients with surgically-resected and pathologically-confirmed IPMNs assessed the capability of ‘radiomic’ CT features with respect to standard radiological methods to identify predict IPMN lesions.
Chen [18] assessed the response during chemoradiation therapy by means of radiomic analysis of daily CT images in a group of 20 patients. Authors found that the CT based radiomic features underwent significant changes during the course of therapy, with larger variations observed in the patients with good response to therapy.
Eilaghi [19] investigated the role of CT texture features with respect to overall survival in pancreatic ductal adenocarcinoma on 30 patients finding a signature of five features statistically associated to survival.
Our experience with hepatocellular carcinoma [20] and lung cancer [21] suggested the possibility to derive predictive models from the use of limited number of textural features computed from radiotherapy planning CT scans. The objective of the present study is to appraise the role of radiomics features computed from CT images to correlate with local control and overall survival in patients treated with SBRT for inoperable pancreatic cancer. The aim was therefore to identify, if existing, a purely radiomics based signature suitable to stratify the patients in outcome related risk groups. The inclusion of further clinical factors (like the other treatments or other known risk factors) might further strengthen the signature but was out of the scope of this first investigation.
Materials and methods
Study design
This is a retrospective investigation performed on 100 patients treated in one single institute. The retrospective study was approved by the Humanitas Cancer Center Ethics Committee by written notification. The ethical committee waived the requirement of further written informed consent for this retrospective, non clinical, study. All data were fully anonymized prior to access and use. All patients had histologically proven locally advanced pancreatic cancer and were treated with SBRT with a prescription dose of 45Gy in 6 fractions. Inclusion criteria were, as described in the original study [12]: Histologically-proven inoperable primary pancreatic adenocarcinoma, Age ≥18 years, Karnofsky Performance score of at least 70, lesions with maximum diameter not exceeding 5cm, negative lymph-node, absence of distant metastasis. Exclusion criteria were: previous abdominal RT, nodal and/or metastatic disease, gastric or duodenal obstruction, and concurrent chemotherapy. For all patients, complementarily to the textural features and the outcome data, some clinical parameters such as the age, the sex, the various chemotherapy regimens and the volume of lesions treated with SBRT were recorded.
Treatment details can be found in our original clinical report [12]. In summary, SBRT treatments were delivered by means of Volumetric Modulated Arc Therapy (VMAT) in its RapidArc with 6MV flattening filter free photon beams generated by a TrueBeam linear accelerator (Varian Medical Systems, Palo Alto, USA). Two partial arcs were used as a class solution for all the patients. Treatment planning and dose calculation was performed using the Eclipse planning system, version 11 (Varian Medical Systems, Palo Alto, USA) For all patients, the planning aim was to achieve a target coverage of V95% = 100% for the clinical target volume (where Vx% is the percentage volume covered by the x% isodose). The dose–volume constraints for the OARs were duodenum D1cm3 < 36 Gy (where Dxcm3 is the dose received by x cm3 of the structure), stomach and small bowels D3cm3 < 36Gy, kidneys V15Gy < 35%, liver, total spared volume (Vtot _ V21Gy,) > 700 cm3, and spinal cord D1cm3 < 18Gy.
All patients underwent a pretreatment contrast-free planning CT imaging and the clinical target volume contoured on these images for the radiation treatment was used as the input for the textural analysis. The segmentation was pre-processed to exclude from the contours the presence of vessels or biliary stent, calcifications of any potential artifact. The entire processing of the target volumes was performed by a team of senior radiation oncologist, radiologist and physicists and consensus volumes were used for this study.
Textural radiomics analysis
All the textural features were computed using the LifeX software tools [22]. A total of 41 features were derived from the CT images and grouped according to intensity, shape and second and higher order features [20]. In particular the following families of featuers were extracted: the gray-level co-occurrence matrix (GLCM); the neighborhood gray-level different matrix (NGLDM); the grey level run length matrix (GLRLM) and the grey level zone length matrix (GLZLM). A detailed description of all the features, can be found in [23]. From the histogram of the gray level distribution in the volume, the following features were extracted: the minimum, maximum, mean and standard deviation of the hounsfield units (HU) distribution as well as the skewness, the kurtosis, the entropy and the energy derived form this distribution [24]. The shape of the volumes was quantified by means of the spericity and the compacity. In the S1 Table from the supplementary materials to this article we detailed the list of the features computed and used for the analysis. Images were sampled to symmetrical voxels of 2 mm. A HU binning was applied resampling the images in intervals of 10 HU.
Statistical analysis
The statistical analysis of the data was performed by means of dedicated routines based on the open source R platform (version 3.3) [25]. The entire cohort of patients was randomly split in two sub-cohorts of 60 and 40 patients respectively. These two groups were used for the training and validation phases of the analysis. In the training phase, all the radiomic features, as well as the clinical predictors age and sex, were investigated for their prognostic value relatively to overall survival and local control (LC) with univariate Cox regression. Optimal separation thresholds were determined per each predictor. These were chosen in correspondance of the minimum of the p-value distribution obtained from a running threshold from the Wilcoxon test. The Pearson’s correlation among the pre-selected features was determined and the correlated features were identified and excluded from further analysis. The feature’s prognostic value was assessed by means of the concordance index [26].
Multivariate Cox regression was then performed either keeping all the significant features (model A) or including in the model only the un-correlated ones (model B) as input for the backward elimination phase. This strategy was applied to both OS and LC analysis and therefore four models were built: A-OS, B-OS (and similarly for LC). Elastic net regularization, dealing with multiple cross-related variables, was used to select the most significant covariates [20]. The elastic net regularization does automatic variable selection eliminating groups of correlated variables allowing to identify the best predictors when a set is larger than the number of cases. Calibration was evaluated with Hosmer and Lemen test [20]. The data of both training and validation sets were split into low- (below threshold) and high- risk (above threshold) groups by the median of the Cox’s prediction. The multivariate models were verified on the independent validation set.
Results
The main characteristics of the cohort of 100 patients are summarized in Table 1. The mean volume of the target was 24.59±17.6 cm3 (range: 2.8–109.5 cm3). Concerning the SBRT treatment, all patients respected the planning aims for target coverage. Table 2 summarizes the data for the training and validation sets.
Table 1. Summary of patient characteristics.
| Parameter | Number of patients (and %) |
|---|---|
| Number of patients | 100 |
| Age (years) | Mean: 70.5 Range: 41–91 |
| Sex | Males: 47 Females: 53 |
| Tumor site: | Head: 65 (65%) Body/Tail 35 (35%) |
| Stage | Locally advanced: 98 (98%) Borderline Resectable 2 (2%) Patients with detectable CA19-9 (>1.5U/ml): 72 (72%) |
| Chemotherapy before SBRT | Yes: 55 (55%) No: 45 (45%) |
| Response after chemotherapy | Partial response: 31 (56%) Stable disease: 17 (31%) Progressive disease: 7 (13%) |
Table 2. Summary of the patients’ demographics for the training and validation cohorts.
| Training | Validation | |
|---|---|---|
| Number of cases | 60 | 40 |
| Sex: | Male: 28 (47%) Female: 32 (53%) | Male: 19 (48%) Female: 21 (52%) |
| Age [years] | Median: 71.0 Range: 41–90 |
Median: 70.5 Range: 48–91 |
| Target Volume [cm3] | Mean: 23.2±14.1 Range: 2.8–92.8 |
Mean: 27.0±18.8 Range: 3.9–109.5 |
From the analysis of clinical and textural data, 9 predictors resulted significant at univariate test for OS and 4 for LC. Table 3 summarizes the relative findings while Fig 1 illustrates the OS (and LC) graphs stratified according to the nine and four, respectively, significant predictors for the training cohort.
Table 3. List of the clinical and radiomics features found to be significant at univariate analysis.
| Feature name | threshold | p-value | Concordance index |
|---|---|---|---|
| Overall Survival | |||
| Age | 76.4 | 0.002 | 0.66 |
| MeanValue | 15.1 | 0.02 | 0.66 |
| StdValue | 25.3 | 0.04 | 0.64 |
| EntropyH | 1.9 | <0.001 | 0.69 |
| EnergyH | 0.02 | <0.001 | 0.70 |
| Compacity | 3.5 | 0.05 | 0.63 |
| Homogeneity_GLCM | 0.5 | 0.004 | 0.67 |
| Contrast_GLCM | 5.5 | 0.009 | 0.65 |
| Dissimilarity_GLCM | 1.7 | 0.005 | 0.66 |
| Local Control | |||
| Volume | 27.5 | 0.02 | 0.62 |
| Compacity | 3.6 | <0.001 | 0.63 |
| SRHGE_GLRLM | 9076.0 | 0.04 | 0.65 |
| GLNU_GLRLM | 400.8 | 0.02 | 0.63 |
Fig 1. Overall survival (OS) and local control (LC) curves stratified according to the best threshold for the clinical and radiomic features found to be significant at univariate analysis.
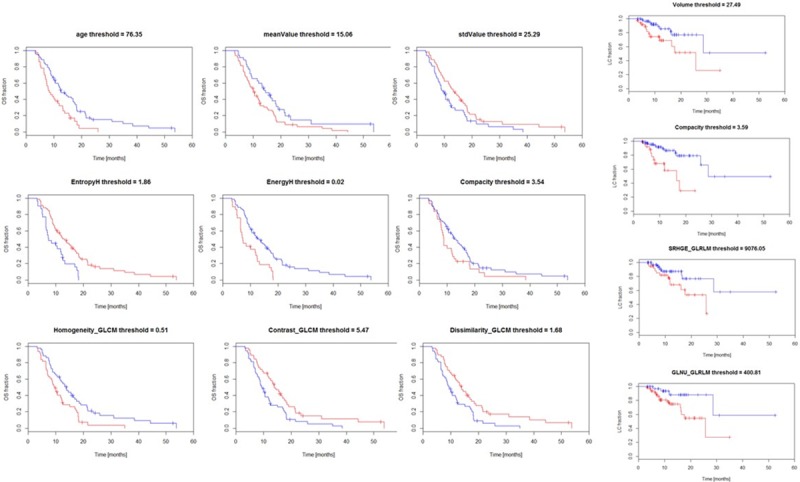
Data are shown for the training dataset. In the figures the blue lines correspond to the stratum above the threshold.
Concerning the model A-OS, of the 9 input predictors, only 4 were retained as significant(the mean value (meanValue) and the Homogeneity_GLCM as significant with p<0.01 and the standard deviation value (stdValue) as well as the Dissimilarity_GLCM with p<0.1). The area under the curve (AUC) from the receiver operating characteristic (ROC) curve built out of the model resulted 0.81 (95% confidence interval (95%CI): 0.64–0.98) for the training set and 0.73 (95%CI: 0.54–0.92) for the validation set. The concordance index for this model was 0.74±0.03 in the training and 0.72±0.03 in the validation.
In the case of the model B-OS, only two variables resulted significant (Age and Homogeneity_GLCM with p<0.01), the AUC was 0.80 (95%CI: 0.66–0.94) in the training and of 0.73 (95%CI: 0.53–0.93) in the validation. The concordance index for this model was 0.73±0.03 in the training and 0.75±0.03 in the validation.
Concerning Local Control, the model A-LC retained 2 features (Volume and grey level non-uniformity (GLNU_GLRM) with p<0.01). The AUC was 0.72 (95%CI: 0.55–0.88) for the training and 0.70 (95%CI: 0.52–0.89) for the validation. The concordance index was 0.69±0.08 for training and 0.75±0.10 for validation. The model B-LC provided similar results with 2 retained features (short run low grey level emphasis and grey level non-uniformity). The AUC was 0.65 (95%CI: 0.52–0.81) for the training and 0.61 (95%CI: 0.50–0.78) for the validation. The concordance index was 0.70±0.07 for training and 0.73±0.09 for validation.
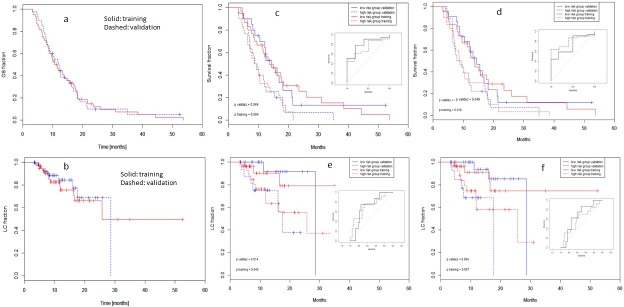
Fig 2A and Fig 2B show the OS and LC for the two cohorts in the training and validation sets. The median OS for the entire cohort was: 11.6 months (CI95%:9.6–13.9); the median LC was: 28.6 months (CI95%: 25.7–32.1). Fig 2 shows also the result of the multivariate Cox model built for Overall Survival and Local Control. OS from the Model A-OS is shown in panel (c) and the corresponding ROC curves as an internal sub-panel for the training and validation sets. In the survival curves, solid lines correspond to the low risk group of patients; blue lines to the validation (red for the training) set. In the ROC curves, solid line is for the training, dashed for the validation. Panel (d) reports the same OS data for the Cox model B-OS built using in input only the set of uncorrelated variables. The p-values from the Wilcoxon test comparison between high and low risk groups resulted 0.004 and 0.05 in the training and validation cohorts for the models A-OS and B-OS. Panel (e) reports the LC curves from Model A-LC and panel (f) from Model B-LC with the same conventions as above.
Fig 2.
a) and b): Overall survival (OS) and Local Control (LC) curves for the training (solid red line) and validation (blue dashed line) cohorts of patients without any stratification; c) and d): Overall survival (OS) curves for the multivariate models A and B respectively. The sub-panels represent the ROC curves built out of the models. e) and f): Local Control (LC) curves as for the above. In the survival curves, solid lines correspond to the low risk group of patients; blue lines to the validation (red for the training) set. In the ROC curves, solid line is for the training, dashed for the validation.
Table 4 summarizes the median times and the 95% confidence interval for OS and LC derived from the two models A and B.
Table 4. Median and 95% confidence interval for the overall survival time and for the local control time.
| Training | Validaz | |||
|---|---|---|---|---|
| Low risk | High risk | Low risk | High risk | |
| Model A-OS, Overall Survival | ||||
| Median | 13.8 | 9.2 | 14.4 | 9.0 |
| CI95% | 11.4–23.5 | 7.0–13.9 | 12.2–21.5 | 7.2–15.4 |
| Model B-OS, Overall Survival | ||||
| Median | 13.8 | 8.8 | 14.4 | 8.6 |
| CI95% | 114–23.5 | 7.0–16.8 | 12.2–21.2 | 7.0–18.0 |
| Model A-LC, Local Control | ||||
| Median | Not reached | 27.5 | 28.6 | 17.5 |
| CI95% | Not reached | 11.8-not reached | 17.5-not reached | 16.0-not reached |
| Model B-LC, Local Control | ||||
| Median | Not reached | Not reached | 28.6 | 17.5 |
| CI95% | 25.7 | 11.8-not reached | 12.5-Not reached | 7.62-not reached |
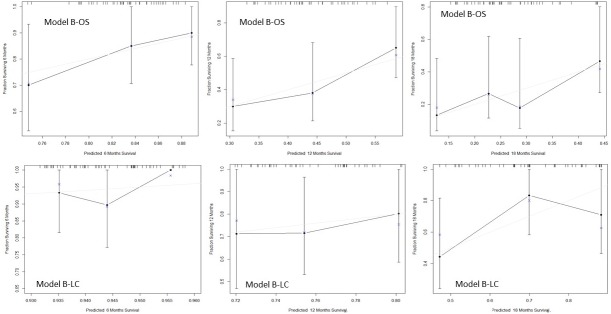
Fig 3 shows the calibration plots at 6,12 and 18 months for the models B-OS and B-LC. Considering these graphs, the best survival and local control estimates were achieved at 6 and 12 months while at 18 months the deviation of the estimated from the observed values becomes more relevant due to the paucity of events and the limited sample.
Fig 3. Calibration plots at 6,12 and 18 months for the Cox models B for overall survival and local control.
Discussion
This study aimed to appraise the correlation between some radiomic signatures and the clinical outcome in a retrospective analysis of 100 patients with pancreas cancer treated according the institutional protocols. As noticed, textural analysis has been rarely applied to pancreatic cancer and almost no efforts, so far, were put in the radiomic assessment of outcome data [17–20] and none in association to SBRT. In this study, planning CT scans were used as a basis of the analysis to derive the textural features which demonstrated the possibility to be modelled vs OS and LC. The methodology adopted in the present study is derived from similar other investigations [27–28] and aims to simplicity. The panel of features available for testing did not included higher order texture (e.g. wavelets). Our hypothesis was that a radiomics signature, if existing, should be found within the set of most robust and easy to compute classes of features. As discussed in [20], the use of conventional planning CT and the clinical target volumes (with minor pre-processing edits) is another factor of simplicity which can guarantee a straightforward implementation of the radiomic methodology in the clinical practice. Although final scope of a “predictive” tool is the classification of patients in risk groups (or any similar stratification) prior to therapy, in practice, the models has to be trained and tuned retrospectively on cohorts of patients where the outcome of treatments is known. In this perspective, our investigation covers the first elements of the entire chain, i.e. the determination of a (potentially) useful signature, in a retrospective investigation on a cohort of patients completely treated (with radiotherapy and/or chemotherapy). Further prospective studies shall be designed to validate the predictive models on un-treated cohort of patients to measure their reliability and performance. Another limiting factor in the study was the exclusion, from the predictive model, of any clinical factor which might have influenced the outcome. In practice, all patients received the same SBRT treatment but some different chemotherapy regimen. The inclusion of clinical factors might strengthen the predictive value of the predictive models but, in the present study, the aim was to identify, if existing, a purely radiomic feature. Further studies will be devoted to the refinement of the models and the elimination (or explicit inclusion) of all the valuable factors. Concerning chemotherapy, 55% of the patients received it prior to SBRT. These pre-treated patients were evaluated after the end of chemotherapy with Torax-abdominal CT scan with the evidence of partial response (56%), stable disease (31%) or local progression limited to pancreatic cancer (13%). No patient showed distant progression of disease, therefore all patients were eligible for SBRT.
The study was performed by evaluating only one random split of the cohort into training and validation sets (60–40% split). We shall acknowledge that this procedure might have some inherent selection bias due to the single evaluation. Multiple repetition of the random split could have been introduced as a mitigation factor but, if this seems to be relatively easy in a simple classification experiment, the procedure creates some methodological problem when combining multiple actuarial curves. We decided to stick on the single split which could be of course seen as a reasonable simulation of a typical prospective study. Increasing significantly the sample size would have been the other option.
Unfortunately, the consistency of the cohort of patients in the current study is limited due to the relative paucity of patients with advanced pancreatic cancer treated with SBRT. This is also acknowledged as a limit of the study. This is in particular true given the need to split the total sample into validation and training, and the further stratification in low- and high-risk groups limits the number of events per class of patients. Longer follow up and larger cohorts would be needed to improve this aspect.
Nevertheless, the data available allowed to identify radiomics signatures with two features for both OS and LC and to identify the low- and high- risk groups significantly separated at the median of the predictions. A search for an optimal threshold did not lead to significant improvements and was not considered as robust and as simple as the median. Despite we performed an independent validation on our own data, further assessment of the robustness of the thresholds should be performed by enlarging the population under investigation, possibly to data from other centers.
Conclusions
A radiomics signature made of simple clinical and textural features allowed to generate a predictive model for OS and LC in patients affected by advanced pancreatic cancer treated with SBRT. A fair discrimination power was found applying the model to training and validatin samples. Further validation studies would be advisable to confirm these findings.
Supporting information
For the explicit mathematical definitions readers are referred to ref 22 and further references therein.
(DOCX)
Data Availability
All relevant data are within the paper.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. (2013) CA Cancer J Clin 63:11–30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R. Pancreatic cancer. (2011) Lancet. 378:607–20. 10.1016/S0140-6736(10)62307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Josef E, Schipper M, Francis I, Hadley S, Ten-Haken R, Lawrence T et al. (2012) A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 84:1166–1171 10.1016/j.ijrobp.2012.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunner T, Nestle U, Grosu A, Partridge M. (2015) SBRT in pancreatic cancer: What is the therapeutic windows? Radiother. Oncol. 114:109–16 [DOI] [PubMed] [Google Scholar]
- 5.Chang D, Schellenberg D, Shen J, Kim J, Goodman K, Fisher G et al. : (2009) Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 115:665–672. 10.1002/cncr.24059 [DOI] [PubMed] [Google Scholar]
- 6.Schellenberg D, Kim J, Christman-Skieller C, Chun C, Columbo L. Ford J et al. : (2011) Single-Fraction Stereotactic Body Radiation Therapy and Sequential Gemcitabine for the Treatment of Locally Advanced Pancreatic Cancer. Int J Radiat Oncol Biol Phys 81:181–188. 10.1016/j.ijrobp.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 7.Polistina F, Costantin G, Casamassima F, Francescon P, Guglielmi R, Panizzoni G et al. : (2010) Unresectable locally advanced pancreatic cancer: A multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol 17:2092–2101. 10.1245/s10434-010-1019-y [DOI] [PubMed] [Google Scholar]
- 8.Mahadevan A, Miksad R, Goldstein M, Sullivan R, Bullock A, Buchbinder E et al. : (2011) Induction Gemcitabine And Stereotactic Body Radiotherapy For Locally advanced Nonmetastatic Pancreas Cancer. Int J Radiat Oncol Biol Phys 81:615–622. 10.1016/j.ijrobp.2010.06.044 [DOI] [PubMed] [Google Scholar]
- 9.Rwigema J, Parikh S, Heron D, Howell M, Zeh H, Moser A et al. : (2011) Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol 34:63–69. 10.1097/COC.0b013e3181d270b4 [DOI] [PubMed] [Google Scholar]
- 10.Chuong M, Springett G, Freilich J, Park C, Weber J, Mellon E et al. (2013) Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 86:516–522. 10.1016/j.ijrobp.2013.02.022 [DOI] [PubMed] [Google Scholar]
- 11.Herman J, Chang D, Goodman K, Dholakia A, Raman S, Hacker-Prietz A et al. (2015) Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 121:1128–3 10.1002/cncr.29161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comito T, Cozzi L, Clerici E, Franzese C, Tozzi A, Iftode C et al. (2017) Can stereotactic body radiation therapy be a viable and efficient therapeutic option for unresectable locally advanced pancreatic adenocarcinoma? Results of a phase 2 study. Technol. Cancer Res Treat. 16:295–301 10.1177/1533034616650778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout R, Granton P, et al. (2012) Radiomics: Extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–6 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aerts H, Velazquez E, Leijenaar R, Parmar C, Grossmann P, Carvalho S, et al. (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yue Y, Osipov A, Fraass B, Sandler H, Zhang X, Nissen N et al. (2017) Identifying prognostic intratumor heterogeneity using pre- and post-radiotherapy 18F-FDG PET images for pancreatic cancer patients. J Gastrointest Oncol. 8:127–138 10.21037/jgo.2016.12.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanania A, Bantis L, Feng Z, Wang H, Tamm E, Katz M et al. (2016) Quantitative imaging to evaluate malignant potential of IPMNs. Oncotarget. 7:85776–85784 10.18632/oncotarget.11769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Permuth J, Choi J, Balarunathan Y, Kim J, Chen D, Chen L et al. (2016) Combining radiomic features with a miRNA classifier may improve prediction of malignant pathology for pancreatic intraductal papillary mucinous neoplasms. Oncotarget. 7:85785–85797 10.18632/oncotarget.11768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Oshima K, Schott D, Wu H, Hall W, Song Y et al. (2017) Assessment of treatment response during chemoradiation therapy for pancreatic cancer based on quantitative radiomic analysis of daily CTs: an exploratory study. PlosOne 12:e0178961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eilaghi A, Baig S, Zhang Y, Zhang J, Karanicolas P, Gallinger S et al. (2017) CT texture features are associated with overall survival in pancreatic ductal adenocarcinoma–a quantitative analysis. BMC Med. Imag. 17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cozzi L, Dinapoli N, Fogliata A, Hsu W, Reggiori G, Lobefalo F et al. (2017) Radiomics based analysis to predict local control and survival in hepatocellular carcinoma patients treated with volumetric modulated arc therapy. BMC Cancer 17:829 10.1186/s12885-017-3847-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirienko M, Cozzi L, Antonovic L, Lozza L, Fogliata A, Voulaz E et al. (2018) Prediction of disease free survival by the PET/CT radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur. J. Nucl Med Mol Imag. 45:207–271 [DOI] [PubMed] [Google Scholar]
- 22.Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C et al. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 2018;78:4786–4789 10.1158/0008-5472.CAN-18-0125 [DOI] [PubMed] [Google Scholar]
- 23.Sollini M, Cozzi L, Antunovic L, Chiti A, Kirienko M. (2017) PET Radiomics in NSCLC: state of the art and a proposal for harmonization of methodology. Sci Rep. 7:358 10.1038/s41598-017-00426-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sollini M, Cozzi L, Peper G, Antunovic L, Lania A, Di Tommaso L et al. (2017) [18F]FDG-PET/Ct texture analysis in thyroid incidentalomas: preliminary results. Eur. J. Hybr. Imaging. 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- 26.Harrel F, Lee K, Mark D. (1996) Multivariable prognostic models: issues in developing models, adequacy and measuring and reducing errors. Stat Med. 15:361–387 [DOI] [PubMed] [Google Scholar]
- 27.Bogowicz M, Riesterer O, Ikenberg K, Stieb S, Moch H, Studer G et al. (2017) Computed tomography radiomics predicts HPV status and local control after definitive radiochemotherapy in head and neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 99:921–928 10.1016/j.ijrobp.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Bogowicz M, Leijneaar R, Tanadini-Lang S, Riesterer O, Pruschy M, Unkelbach J et al. (2017) Post-radiochemotherapy PET radiomics in head and neck cancer- the influence of radiomics implementation on the reproducibility of local control tumor models. Radiother. Oncol. 125:385–391 10.1016/j.radonc.2017.10.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For the explicit mathematical definitions readers are referred to ref 22 and further references therein.
(DOCX)
Data Availability Statement
All relevant data are within the paper.