Abstract
Purpose
Biologic heterogeneity is a feature of diffuse large B-cell lymphoma (DLBCL), and the existence of a subgroup with poor prognosis and phenotypic proximity to Burkitt lymphoma is well known. Conventional cytogenetics identifies some patients with rearrangements of MYC and BCL2 and/or BCL6 (double-hit lymphomas) who are increasingly treated with more intensive chemotherapy, but a more biologically coherent and clinically useful definition of this group is required.
Patients and Methods
We defined a molecular high-grade (MHG) group by applying a gene expression–based classifier to 928 patients with DLBCL from a clinical trial that investigated the addition of bortezomib to standard rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) therapy. The prognostic significance of MHG was compared with existing biomarkers. We performed targeted sequencing of 70 genes in 400 patients and explored molecular pathology using gene expression signature databases. Findings were validated in an independent data set.
Results
The MHG group comprised 83 patients (9%), with 75 in the cell-of-origin germinal center B-cell-like group. MYC rearranged and double-hit groups were strongly over-represented in MHG but comprised only one half of the total. Gene expression analysis revealed a proliferative phenotype with a relationship to centroblasts. Progression-free survival rate at 36 months after R-CHOP in the MHG group was 37% (95% CI, 24% to 55%) compared with 72% (95% CI, 68% to 77%) for others, and an analysis of treatment effects suggested a possible positive effect of bortezomib. Double-hit lymphomas lacking the MHG signature showed no evidence of worse outcome than other germinal center B-cell-like cases.
Conclusion
MHG defines a biologically coherent high-grade B-cell lymphoma group with distinct molecular features and clinical outcomes that effectively doubles the size of the poor-prognosis, double-hit group. Patients with MHG may benefit from intensified chemotherapy or novel targeted therapies.
INTRODUCTION
Aggressive B-cell non-Hodgkin lymphomas, including diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL), comprise a heterogeneous class of related malignancies for which response and survival on standard treatment vary substantially, with significantly worse outcomes in some subtypes. DLBCL incidence is high and carries a significant disease burden,1 whereas BL is a distinct and highly proliferative entity that requires substantially more intensive chemotherapy. Within DLBCL, the cell-of-origin (COO) variants germinal center B-cell-like (GCB) and activated B-cell-like (ABC) DLBCL have been defined by gene expression patterns.2 These have different underlying molecular pathology and prognosis, but internal heterogeneity in their genetic and phenotypic features indicates that further stratification is necessary for precision treatment.3
Several groups recently have considered DLBCL stratification by using integrated genetic information, providing prognostic models,4 or separating patients further into smaller subgroups on the basis of shared genetic features.5,6 Earlier work identified patients with key chromosomal rearrangements of MYC and BCL2 and/or BCL6 genes (double and triple hits) that correlated with poor response to standard therapy.7,8 MYC rearrangement (MYC-R) is a feature shared with BL, and such tumors often have some BL-like genomic features and patterns of gene expression.9,10 Gene expression profiling also has been used to distinguish DLBCL and BL,11,12 but intermediate categories of high-grade DLBCL remain, including those with double hits, those whose overall pattern of gene expression resembles that of BL, and those that strongly express both MYC and BCL213 proteins, for which the optimal group definition and treatment choices are still unclear. These MYC and BL-related groups do not feature clearly in the recent genetic classifications,5,6 but they are present in two new WHO designations as high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 translocation and high-grade B-cell lymphoma not otherwise specified.14 The difficulty in defining the optimum approach to this group is partly explained by the low frequency of groups such as double-hit lymphomas, and the absence of a clear biologic definition. With the benefit of a large clinical trial data set, we suggest here a unifying definition of a molecular high-grade (MHG) class that is based on gene expression and propose that it should form part of our evolving understanding of DLBCL.
The Randomized Evaluation of Molecular-Guided Therapy for DLBCL With Bortezomib (REMoDL-B) clinical trial15 tested standard therapy for DLBCL (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone [R-CHOP]) against its combination with the proteasome inhibitor bortezomib (RB-CHOP). The hypothesis was that bortezomib indirectly inhibits the nuclear factor kappa-light-chain-enhancer of activated B cells pathway believed to be specifically active in the ABC variant.16 We present our analysis of the trial data focused on the MHG group by showing that a biologically coherent and distinctive group with significantly poorer prognosis can be identified and validated in independent data. We suggest that this group should be targeted in the future with precision medicine approaches.
PATIENTS AND METHODS
Data Set Summary
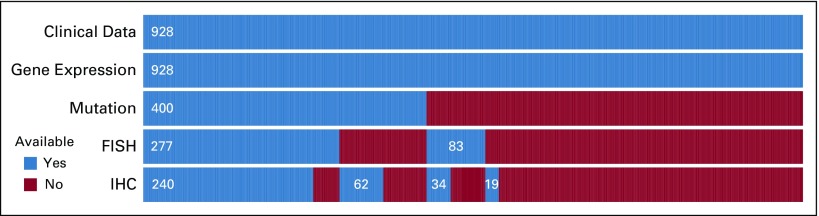
A total of 928 patients treated in the REMoDL-B trial15 (Data Supplement) were included in this retrospective study. Genome-wide gene expression data were available for all patients from formalin-fixed paraffin-embedded tissue samples. A subset of 400 patient samples was sequenced for a 70-gene panel, chosen by known relevance to DLBCL, with HaloPlexHS (Agilent Technologies, Santa Clara, CA) target enrichment and HiSeq 4000 (Illumina, San Diego, CA) sequencing, and analyzed for somatic mutations (Data Supplement). Furthermore, for the purpose of comparison with other known biomarkers, a subset of 360 patient samples was tested for MYC, BCL2, and BCL6 chromosomal rearrangements with fluorescent in situ hybridization assays, and a subset of 355 samples was tested for MYC and BCL2 protein expression with immunohistochemistry using tissue microarrays. Clinical features, treatment, progression status, and follow-up data (median, 30 months) were available for all patients. The available data are summarized in Figure 1, and full details are provided in the Data Supplement. Methodological detail in addition to that given here is provided in the Data Supplement.
FIG 1.
Data set summary. The analysis included 928 patients from the Randomized Evaluation of Molecular-Guided Therapy for Diffuse Large B-Cell Lymphoma With Bortezomib (REMoDL-B) clinical trial. All had full clinical data (diagnostic variables, treatment, treatment response, progression status, and follow-up time) and whole-genome expression profiling assayed by the DASL version 4 array (Illumina, San Diego, CA). A subset of 400 patients was analyzed for somatic mutations with a targeted 70-gene panel. In addition, 360 patients were tested for MYC, BCL2, and BCL6 rearrangements by fluorescent in situ hybridization (FISH), and 355 patients were tested for MYC and BCL2 protein expression with immunohistochemistry (IHC).
COO Classification and the MHG Subgroup
Gene expression data–based COO classification was performed in the trial with the DLBCL automatic classifier17 in real-time for random assignment to R-CHOP or RB-CHOP in the second to sixth treatment cycles. For this analysis, the COO classification was repeated with the same method to take advantage of higher-quality samples that became available for some patients after randomization and improved data normalization over the complete trial data set. The overall concordance between this retrospective COO classification (255 ABC, 543 GCB, and 130 unclassified [UNC]) and the real-time prospective classification (244 ABC, 475 GCB, and 199 UNC) from the trial randomization was 87%. The main change between prospective and retrospective COO DLBCL automatic classification was the reduction of UNC patients who were reassigned to ABC and GCB in the retrospective classification. The classification shift between GCB and ABC was low (4.5%). Full details of the prospective and retrospective classifications are provided in the Data Supplement.
Our previous work had shown that patients with DLBCL with a BL-like pattern of gene expression had poor prognosis.18 Accordingly, we applied the gene expression classifier developed in that work to REMoDL-B patients, using all those with BL and DLBCL in our local database for the normalization background, to define the MHG class. Of note, the definition of the MHG class was determined in previous work with an earlier data set and was not trained or determined in any way from the REMoDL-B trial data. Conventional diagnosis of all identified patients with MHG was checked for this study (Data Supplement) and indicated that this group had DLBCL by morphology and immunophenotype, thus excluding the possibility of contamination with patients with BL.
External Validation
Although the MHG group was defined independently of the clinical trial data, we validated it further on another independent and recently published data set4 (European Genome-Phenome Archive study accession EGAS00001002606) by using the core set of 624 patients whose gene expression profiles were examined in that study by RNA sequencing. Adaptation of our classifier to these data was straightforward because the classifier was designed and tested18 for cross-platform applicability. We note, however, that there are no diagnoses of BL in this data set, which could have a minor effect on the overall normalization, and that in this data set four classifier genes (BMP7, TCL6, SOX11, and C7orf10) had very low estimated expression levels from the RNA sequencing data. The classifier, therefore, was retrained using the original training data,18 with the gene set reduced by these four genes for application to this data set. COO classification was that provided by the authors. Analysis of mutation frequencies in this data set used the 150 identified driver genes from the original article filtered by at least 5% mutation frequency in at least one subgroup and significantly different frequency (Fisher’s exact P < .05) between any two groups of MHG, GCB, and ABC.
Statistical Analysis
All survival analyses were carried out using the survival package in R (https://cran.r-project.org), using single-factor and multivariable Cox proportional hazards regression models and likelihood ratio tests. Associations in count data related to clinical variables, chromosomal rearrangements, mutations, and so forth were analyzed with Fisher’s exact test. For continuous variables, differences between groups were tested with Mann-Whitney U or t tests as appropriate. All quoted P values are two-sided.
RESULTS
Definition and Clinical Outcome of MHG Lymphoma
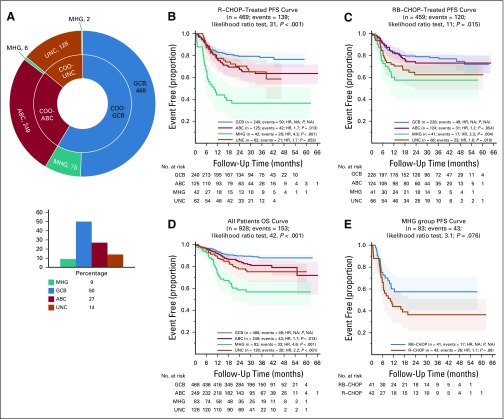
Our gene expression classifier assigned 83 REMoDL-B patients as MHG (9%; Fig 2). Seventy-five patients in the MHG group (90%) were within the original GCB group (Fig 2A) and were considered separately in the subsequent analysis, with GCB, ABC, and UNC referring (unless otherwise stated) to patients within those classes but not identified as MHG. A full analysis of associations between the COO and MHG groups and other clinical prognostic factors (Data Supplement) showed that MHG has a significantly higher International Prognostic Index19 (IPI; P = .004), tumor bulk (P = .007), and stage (P = .06). Median lactate dehydrogenase levels in patients with MHG were higher by almost 1.5-fold (P < .001), which reflects higher proliferation and cell turnover.
FIG 2.
Retrospective Randomized Evaluation of Molecular-Guided Therapy for Diffuse Large B-Cell Lymphoma With Bortezomib (REMoDL-B) trial analysis with the molecular high-grade (MHG) group. (A) Number of REMoDL-B patients with standard cell-of-origin (COO) classification (inner circle) and with MHG patients separated into a new class (outer circle). For the latter, the overall distribution between classes is shown in the histogram. (B) Progression-free survival (PFS) curves for rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)–treated patients by classification, with MHG as a separate class. (C) As in (B) but for patients treated with R-CHOP with the proteasome inhibitor bortezomib (RB-CHOP). (D) As in (B), but overall survival (OS) for all patients. (E) Progression-free survival for MHG patients separated by treatment. P values given for overall difference in survival are from the likelihood ratio test and for individual groups with reference to the best-surviving group from a Cox proportional hazards regression model. ABC, activated B-cell-like; GCB, germinal center B-cell-like; HR, hazard ratio; NA, not applicable; UNC, unclassified.
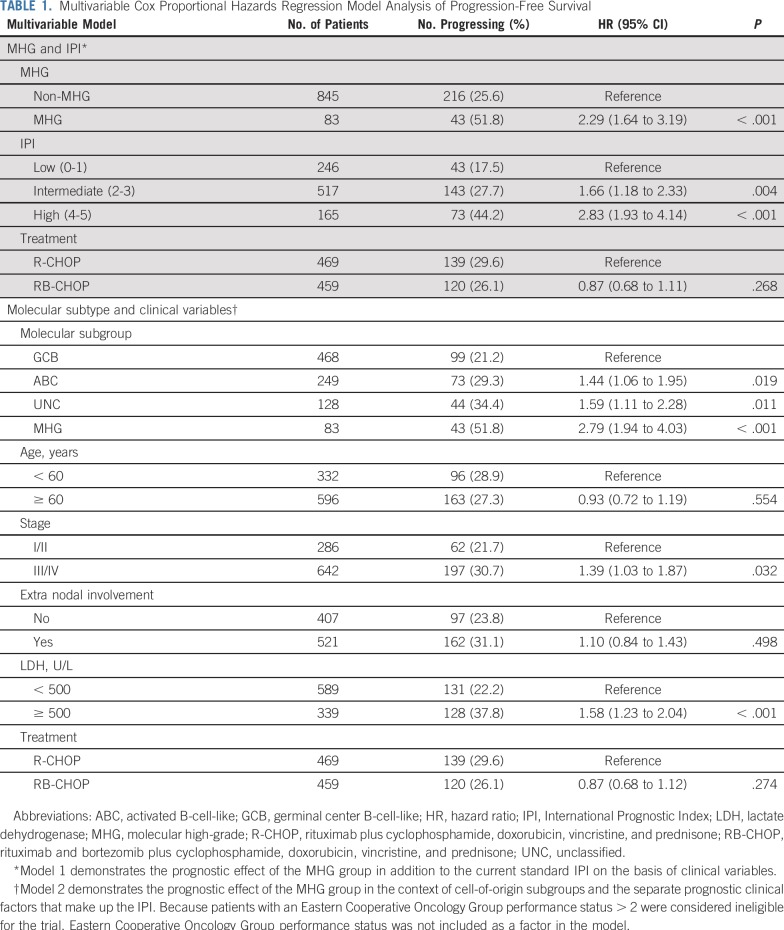
Significant differences in progression-free survival (PFS) and overall survival were observed between MHG and other COO groups. After treatment with R-CHOP, three-year PFS rate estimates were 37% for MHG, 78% for GCB, 64% for ABC, and 65% for UNC (Fig 2B-2E). Multivariable Cox proportional hazards regression models were used to assess the additional prognostic information provided by the MHG group (Table 1). The first model showed that MHG provided additional information to that from clinical variables as encapsulated in the standard IPI (P < .001), and the second showed that MHG provided additional information to relevant clinical variables from the IPI and other COO groups. In the RB-CHOP arm (Fig 2C), the results in the MHG group showed a nonsignificant trend toward improvement (3-year PFS rate, 58%), which provides possible evidence of a positive effect of bortezomib despite the small number of patients (P = .08 Fig 2E).
TABLE 1.
Multivariable Cox Proportional Hazards Regression Model Analysis of Progression-Free Survival
Molecular Characteristics of the MHG Group
To clarify the molecular characteristics of the MHG group, we augmented the trial data set with 70 patients with confirmed BL from a previous study18 that used the same platform to measure gene expression (Data Supplement) and performed differential gene expression and gene expression signature analyses. Differential expression analysis (Data Supplement) revealed that BL is characterized by a large number of upregulated genes compared with both GCB (2,483 genes) and MHG (1,784 genes). In contrast, the comparison of MHG and GCB revealed only 382 upregulated genes. Downregulated genes had a similar pattern, and together, these figures indicate that MHG is an intermediate group but closer to GCB than to BL.
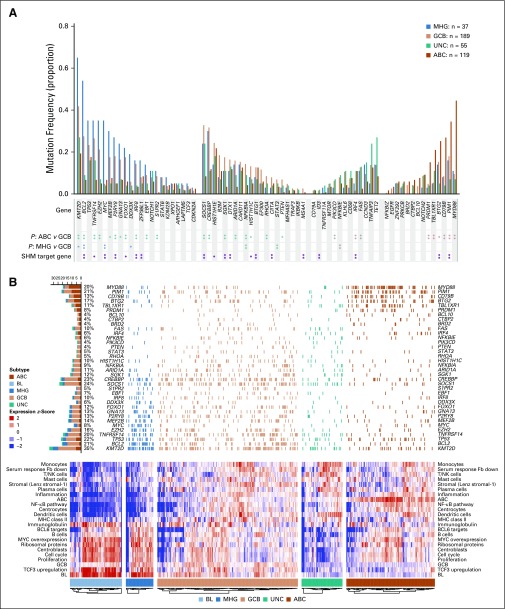
Gene signature databases were used to obtain functional insights into MHG biology (Fig 3). Figure 3B shows the results from a compact and lymphoma-enriched database20 for patients with mutation data available (an analysis of all patients revealed the same patterns). To simplify the analysis, signatures were first clustered, each cluster was named according to the function of its constituent signatures and their genes, and expression values were plotted in the heat map for a chosen representative signature for each cluster. This shows that MHG and BL share high expression of signatures that contain cell cycle genes, ribosome biogenesis, MYC overexpression, and TCF3 targets, which suggests a shared proliferative phenotype. Of note, BL and MHG showed high expression of the germinal center centroblast (dark zone) signature and lower expression of the germinal center centrocyte (light zone) signature relative to other subgroups.21 (We note that some patients with ABC showed relatively high expression of both centrocyte and centroblast signatures, which is likely due to cell cycle genes in the latter signature and may reflect proliferative ABCs that resemble plasmablasts.) Signatures that show low expression in MHG and BL include those involved with MHC class II, stromal, and immune response. Of note, our differential expression analysis shows that FOXP1, which has a number of functions, including the control of apoptotic genes, immune response signatures, and MHC class II,22,23 is upregulated in BL and MHG relative to GCB. A more comprehensive gene set enrichment analysis24 using MSigDB25 to analyze the differential expression gene lists (Data Supplement) confirmed these results.
FIG 3.
Molecular characteristics of the molecular high-grade (MHG) group. (A) Mutation frequencies for 400 Randomized Evaluation of Molecular-Guided Therapy for Diffuse Large B-Cell Lymphoma With Bortezomib (REMoDL-B) patients in MHG, germinal center B-cell-like (GCB), unclassified (UNC), and activated B-cell-like (ABC) subgroups for the 70-gene panel (statistical significance of differences at P < .05 [single asterisks]) and P < .01 [double asterisks] by Fisher’s exact test). Known (double asterisks) and predicted (single asterisks) aberrant somatic hypermutation (SHM) target genes from Schmitz et al.6 (B) Heat map of gene expression signatures (bottom) and associated mutations (top; limited to genes with mutation frequency > 5% in at least one group and significantly different [P < .05] between any two groups of MHG, GCB, and ABC). The heat map shows the mean gene expression level (red = high to blue = low) over genes in the chosen signature cluster representative and is augmented in 70 patients with Burkitt lymphoma (BL) for comparison of gene expression patterns. The left-side bar chart (top) recapitulates the incidence of the corresponding mutations and their distribution among subgroups. Fb, fibroblast; NA, not applicable; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cell; NK, natural killer cell.
Somatic mutation data (Fig 3A; Data Supplement) revealed the expected associations for the ABC and GCB groups,26,27 with the former enriched for mutations in MYD88, PIM1, CD79B, BTG2, TBL1XR1, and PRDM1 and the latter for BCL2, EZH2, KMT2D, and MEF2B. MHG had significantly higher mutation frequencies than GCB in KMT2D, BCL2, MYC, and DDX3X, whereas some frequent mutations in GCB (eg, B2M, SGK1, NFKBIA) were rare in MHG. These mutation patterns share some features (MYC, DDX3X) but not all (KMT2D, BCL2) with BL.28,29 In a similar vein, MHG did not have a high rate of mutation of TCF3 or its negative regulator ID3 typical of BL.28-30 Expression of ID3 was reduced in MHG compared with BL, whereas TCF3 was expressed at similar levels (Data Supplement), which suggests that alternative regulatory mechanisms operate for these genes in MHG.
Aberrant somatic hypermutation31 probably explains the high mutation rates of MYC and BCL2 in MHG. Of note, MYC mutations are associated with MYC-R within the MHG class (12 of 16 rearranged cases also are mutated) but not in other classes (only one of 12 rearranged cases is mutated), which suggests that MYC-Rs outside MHG are biologically different.
Comparison With Other Established Biomarkers
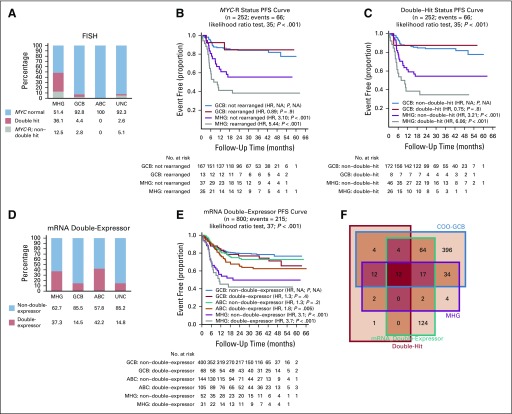
We assessed the relationship of the MHG group to biomarkers commonly used to characterize related high-risk DLBCL (Fig 4). Of the 360 patients for whom fluorescent in situ hybridization data were available, 51 (14%) had MYC-R, and 35 of these (67%) were double-hit (also with BCL2 and/or BCL6 rearrangement). Most MYC-Rs (75%) were in the MHG group, with the remainder lying in GCB and UNC (MHG enrichment by Fisher’s exact test, P < .001), but only 48.6% and 36.1% of the MHG group were MYC-R and double-hit, respectively (Fig 4A).
FIG 4.
Molecular high-grade (MHG) group in relation to MYC rearrangement (MYC-R), double-hit, and double-expressor biomarkers. (A) The proportion of MYC-R, double-hit (MYC-R as well as BCL2 and/or BCL6 rearranged), and normal in each class as a percentage of those for which fluorescent in situ hybridization (FISH) data were produced. (B) Progression-free survival (PFS) by dividing MHG and germinal center B-cell-like (GCB) classes according to MYC-R status. (C) as in (B) by dividing according to double-hit status. (D) The percentage of mRNA double-expressor and normal status in each class. (E) PFS by dividing MHG, GCB, and activated B-cell-like (ABC) classes by double-expressor (mRNA) status. (F) Venn diagram showing overlaps among GCB, MHG, double-hit, and double-expressor groups (note that here, cell of origin [COO]-GCB is the original classification that includes patients with MHG and that some outside the double-hit category have not been tested for double-hit status). P values given for overall difference in PFS are from the likelihood ratio test and for individual groups with reference to best-surviving (GCB) group from a Cox proportional hazards regression model. HR, hazard ratio; NA, not applicable; UNC, unclassified.
Both patients with MYC-R and patients with double-hit status had worse PFS than those who were MYC-normal (Data Supplement). Furthermore, irrespective of MYC-R or double-hit status, the MHG group had a lower PFS than the GCB group (Figs 4B and 4C). Of note, although patient numbers were small in the GCB group, there is no evidence of an effect of MYC-R or double-hit status on PFS, but in MHG, both confer even lower PFS. A comparative gene expression analysis (Data Supplement) showed no differentially expressed genes between MYC-R and MYC-normal within the MHG group but did show 54 differentially expressed genes between MYC-R MHG and MYC-R GCB, which supports the biologic distinctiveness of MHG.
A subset of 355 samples also was investigated for MYC and BCL2 protein expression by IHC to identify cases of double-expressors, and at the mRNA level all trial samples were assessed for combined high expression of MYC and BCL2. In contrast to double-hits, double-expressors were found in significant numbers in all groups, including ABC (Figs 4D and 4F). Although protein expression was correlated with mRNA level (Data Supplement), the immunohistochemistry-identified double-expressor group did not show significant PFS separation in this study (Data Supplement). However, patients with double-expressor status defined by mRNA level where data covered all samples had a significantly lower PFS and further separated patients within subtypes (Fig 4E; Data Supplement). Patients with MHG lymphoma nevertheless showed worse outcome regardless of double-expressor status relative to ABC and GCB subgroups (Fig 4E). Details of the distribution of all biomarkers in the COO and MHG groups are provided in the Data Supplement as is a full analysis of the prognostic effects of these biomarkers.
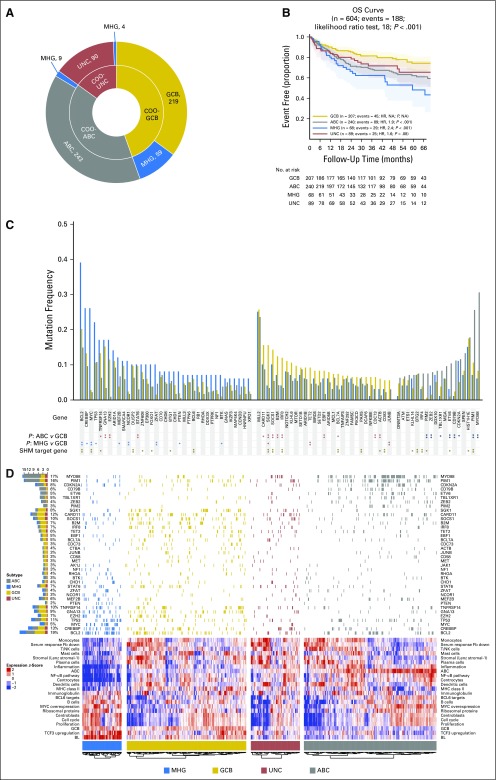
External Validation of the MHG Group
Our analysis of the external validation data set4 is shown in Fig 5. Seventy-two of patients (11.5%) were classified as MHG, and consistent with the first data set (Fig 2A), the majority (82%) of the MHG group was derived from the GCB subtype (Fig 5A). The MHG group showed similar associations with clinical variables (Data Supplement) and similar mutation spectrum in the REMoDL-B and validation data sets (Figs 5C and 5D; Data Supplement). Gene expression signature analysis in the validation data set (Fig 5D), which used our signature set from Figure 3, or the authors’ signature set used in their original article (Data Supplement), showed the same proliferation and centroblast-related biology. MHG had a higher risk in these authors’ prognostic model compared with the remaining GCBs (P < .001) and a poor outcome, with a significantly lower overall survival than the other GCBs (P < .001; Fig 5B).
FIG 5.
Validation of the molecular high-grade (MHG) group in an external data set. (A) Number of patients in each group, cell-of-origin (COO) classification (inner circle), and MHG classification of patients separated into a new group (outer circle). (B) Overall survival (OS) curves by group. (C) Mutation frequencies by group (statistical significance of differences at P < .05 [single asterisks] and P < 0.01 [double asterisks] by Fisher’s exact test). Known (double asterisks) and predicted (single asterisks) aberrant somatic hypermutation (SMH) target genes from Schmitz et al.6 (D) Gene expression signature heat map (bottom using the same signatures as Fig 3B) and mutations (top; limited to genes with mutation frequency > 5% in at least one group and significantly different [P < .05] between any two groups of MHG, germinal center B-cell-like [GCB], and activated B-cell-like [ABC]) for the patients analyzed. The heat map shows the mean gene expression level (red = high to blue = low) over genes in the chosen signature cluster representative. The left-sidebar chart (top) recapitulates the incidence of the corresponding mutations and their distribution among subgroups. BL, Burkitt lymphoma; Fb, fibroblast; HR, hazard ratio; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cell; NK, natural killer; UNC, unclassified.
DISCUSSION
We have defined the MHG group of patients with DLBCL that identifies a poor-risk subgroup primarily within the conventional GCB COO class. This encompasses most patients with double-hit lymphoma but extends the molecular identification to more than double the size of this poor-prognosis group, and significantly, this also reciprocally enriches the remaining patients with GCB DLBCL as a very good–prognosis group. Our analysis indicates that MHG is a robust and distinct group that is identifiable in independent data sets. MHG lymphoma has similarity in gene expression to both BL and GCB-DLBCL but with immunophenotype in keeping with DLBCL rather than BL and a characteristic pattern of genomic mutation. The poor prognosis for this group when treated with R-CHOP suggests that different approaches are required: either intensification of the type increasingly used for the double-hit lymphomas or, potentially, targeted agents that may preferentially affect more rapidly cycling cells. Gene expression patterns indicate that MHG has a highly proliferative phenotype and shares features with centroblasts of the germinal center dark zone in contrast to the centrocyte or light zone features of other GCBs.21
Recent analyses have suggested new taxonomies of DLBCL on the basis of genetic characteristics.5,6 Whereas Schmitz et al6 used a data set focused strongly on the ABC and UNC COO groups, where the MHG group would be under-represented, Chapuy et al5 commented on the genetic complexity of MYC and BCL2 dysregulation that is represented in more than one of their clusters. Although there are limitations to our genetic data, which are based on a small gene panel without germline control and do not include copy number and other structural variations, our data reinforce the distinction among BL, MHG, GCB, and ABC. However, the reproducibly poor outcome in the MHG group suggests that in this case, the gene expression state captures biologically and clinically important features that are not readily identified from the use of genetics alone. Indeed, our mutation data suggest that the expression state may result from a number of different genetic drivers, including MYC and BCL2 rearrangements, epigenetic effects related to mutations in KMT2D32 and EZH2, and mutations that affect other pathways. Although only a small number of MYC-Rs were identified outside the MHG group, their outcomes were similar to those of the other GCBs. Our data also indicate that MYC mutation levels of MYC-Rs differ between MHG and other groups, which suggests a different biology that could be related to different translocation partners.33
Evidence from the trial of a possible positive effect of bortezomib in the MHG group, although lacking statistical power, suggests a potential treatment option for this highly aggressive subtype. In future studies, it will be important to explore this mechanism, which seems unlikely to be mediated by the nuclear factor kappa-light-chain-enhancer of activated B cells pathway that is not believed to be active in GCB or MHG tumors.
ACKNOWLEDGMENT
We thank J. Fitzgibbon, PhD, U. Klein, PhD, E. Roman, PhD, S. Crouch, PhD, and P. Beer, PhD, for discussions and critical comments on the manuscript.
Footnotes
Presented at the Cambridge Lymphoma Biology International Symposium, Cambridge, UK, July 17-18, 2018.
Supported by Bloodwise grant number 15002: Precision Medicine for Aggressive Lymphoma. The Randomized Evaluation of Molecular-Guided Therapy for DLBCL With Bortezomib (REMoDL-B) trial was endorsed by Cancer Research UK, reference number CRUKE/10/024, and Janssen-Cillag provided funding. A.S. is partly funded by the National Institute for Health Research Oxford Biomedical Research Centre. D.R.W. acknowledges UK Medical Research Council grant MR/L01629X/1 for infrastructure support.
See accompanying editorial on page 175
See accompanying article on page 190
AUTHOR CONTRIBUTIONS
Conception and design: David R. Westhead, Reuben Tooze, Catherine Burton, Ming-Qing Du, Peter W.M. Johnson
Provision of study material or patients: Andrew Davies, Peter W.M. Johnson
Collection and assembly of data: Sharon Barrans, Francesco Cucco, Hannah Kennedy, Rahman Uddin, Lisa Worrillow, Rebecca Chalkley, Moniek van Hoppe, Sophia Ahmed, Tom Maishman, Josh Caddy, Christoph Mamot, Andrew Davies, Peter W.M. Johnson, David R. Westhead
Data analysis and interpretation: Chulin Sha, Sharon Barrans, Francesco Cucco, Thomas Cummin, Michael A. Bentley, Matthew A. Care, Hannah Kennedy, Joe S. Thompson, Rebecca Chalkley, Anna Schuh, Christoph Mamot, Reuben Tooze, Andrew Davies, Peter W.M. Johnson, David R. Westhead
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Molecular High-Grade B-Cell Lymphoma: Defining a Poor-Risk Group That Requires Different Approaches to Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Sharon Barrans
Travel, Accommodations, Expenses: HTG Molecular Diagnostics
Thomas Cummin
Research Funding: Plexxikon
Rebecca Chalkley
Research Funding: Cizzle Biotech (Inst)
Moniek van Hoppe
Employment: Leeds Teaching Hospitals NHS Trust
Travel, Accommodations, Expenses: Leeds Teaching Hospitals NHS Trust (I)
Anna Schuh
Consulting or Advisory Role: Gilead Sciences, AbbVie, Roche, Janssen Pharmaceuticals
Research Funding: Johnson & Johnson
Travel, Accommodations, Expenses: AbbVie, Gilead Sciences
Christoph Mamot
Consulting or Advisory Role: Roche, MSD, Bristol-Myers Squibb, Boehringer Ingelheim, Takeda Pharmaceuticals, Novartis, AstraZeneca
Catherine Burton
Honoraria: Takeda Pharmaceuticals, Roche
Consulting or Advisory Role: Celgene, Bristol-Myers Squibb
Speakers’ Bureau: Roche, Celgene
Travel, Accommodations, Expenses: Takeda Science Foundation
Reuben Tooze
Honoraria: Roche, Celgene
Research Funding: UCB
Travel, Accommodations, Expenses: Celgene
Andrew Davies
Honoraria: Gilead Sciences, Roche, Janssen Pharmaceuticals, Celgene
Consulting or Advisory Role: Gilead Sciences, Roche, Acerta Pharma, Kite Pharma, MorphoSys, Janssen Pharmaceuticals, Celgene
Research Funding: Roche (Inst), Bayer AG (Inst), Gilead Sciences (Inst), GlaxoSmithKline (Inst), Takeda Pharmaceuticals (Inst), Celgene (Inst), Karyopharm Therapeutics (Inst), Acerta Pharma (Inst), ADC Therapeutics (Inst)
Expert Testimony: Roche
Travel, Accommodations, Expenses: Roche, Gilead Sciences, Celgene
Ming-Qing Du
Employment: GlaxoSmithKline (I)
Peter W.M. Johnson
Honoraria: Takeda Pharmaceuticals, Bristol-Myers Squibb, Novartis, Celgene, Kite Pharma, Genmab, Incyte
Consulting or Advisory Role: Janssen Pharmaceuticals, Epizyme, Boehringer Ingelheim
Research Funding: Epizyme (Inst), Janssen Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Combined use of Fc gamma RIIb (CD32b) and CD20-specific antibodies, WO patent PCT/GB2011/051572; EU11760819.0
Travel, Accommodations, Expenses: Zenyaku Kogyo
No other potential conflicts of interest were reported
REFERENCES
- 1.Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004-2014: Sub-type analyses from the UK’s Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575–1584. doi: 10.1038/bjc.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 3.Intlekofer AM, Younes A. Precision therapy for lymphoma--current state and future directions. Nat Rev Clin Oncol. 2014;11:585–596. doi: 10.1038/nrclinonc.2014.137. [DOI] [PubMed] [Google Scholar]
- 4. Reddy A, Zhang J, Davis NS, et al: Genetic and functional drivers of diffuse large B cell lymphoma. Cell 171:481-494.e15, 2017. [DOI] [PMC free article] [PubMed]
- 5.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24:679–690. doi: 10.1038/s41591-018-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akyurek N, Uner A, Benekli M, et al. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer. 2012;118:4173–4183. doi: 10.1002/cncr.27396. [DOI] [PubMed] [Google Scholar]
- 8.Snuderl M, Kolman OK, Chen YB, et al. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol. 2010;34:327–340. doi: 10.1097/PAS.0b013e3181cd3aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Momose S, Weißbach S, Pischimarov J, et al. The diagnostic gray zone between Burkitt lymphoma and diffuse large B-cell lymphoma is also a gray zone of the mutational spectrum. Leukemia. 2015;29:1789–1791. doi: 10.1038/leu.2015.34. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017;31:37–42. doi: 10.1016/j.blre.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354:2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 12.Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 13.Staiger AM, Ziepert M, Horn H, et al. Clinical impact of the cell-of-origin classification and the MYC/BCL2 dual expresser status in diffuse large B-cell lymphoma treated within prospective clinical trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2017;35:2515–2526. doi: 10.1200/JCO.2016.70.3660. [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies AJ, Barrans S, Maishman T, et al. Differential efficacy of bortezomib in subtypes of diffuse large B‐cell lymphoma (DLBL): A prospective randomised study stratified by transcriptome profiling: REMoDL-B. Hematol Oncol. 2017;35:130–131. [Google Scholar]
- 16.Shaffer AL, III, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Care MA, Barrans S, Worrillow L, et al. A microarray platform-independent classification tool for cell of origin class allows comparative analysis of gene expression in diffuse large B-cell lymphoma. PLoS One. 2013;8:e55895. doi: 10.1371/journal.pone.0055895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sha C, Barrans S, Care MA, et al. Transferring genomics to the clinic: Distinguishing Burkitt and diffuse large B cell lymphomas. Genome Med. 2015;7:64. doi: 10.1186/s13073-015-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Non-Hodgkin’s Lymphoma Prognostic Factors Project A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer AL, Wright G, Yang L, et al. A library of gene expression signatures to illuminate normal and pathological lymphoid biology. Immunol Rev. 2006;210:67–85. doi: 10.1111/j.0105-2896.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- 21.Victora GD, Dominguez-Sola D, Holmes AB, et al. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood. 2012;120:2240–2248. doi: 10.1182/blood-2012-03-415380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown PJ, Wong KK, Felce SL, et al. FOXP1 suppresses immune response signatures and MHC class II expression in activated B-cell-like diffuse large B-cell lymphomas. Leukemia. 2016;30:605–616. doi: 10.1038/leu.2015.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gascoyne DM, Banham AH. The significance of FOXP1 in diffuse large B-cell lymphoma. Leuk Lymphoma. 2017;58:1037–1051. doi: 10.1080/10428194.2016.1228932. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberzon A, Subramanian A, Pinchback R, et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43:830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Grubor V, Love CL, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:1398–1403. doi: 10.1073/pnas.1205299110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44:1321–1325. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. doi: 10.1101/cshperspect.a014282. Schmitz R, Ceribelli M, Pittaluga S, et al: Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb Perspect Med 4:a014282, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khodabakhshi AH, Morin RD, Fejes AP, et al. Recurrent targets of aberrant somatic hypermutation in lymphoma. Oncotarget. 2012;3:1308–1319. doi: 10.18632/oncotarget.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega-Molina A, Boss IW, Canela A, et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development. Nat Med. 2015;21:1199–1208. doi: 10.1038/nm.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copie-Bergman C, Cuillière-Dartigues P, Baia M, et al. MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: A GELA/LYSA study. Blood. 2015;126:2466–2474. doi: 10.1182/blood-2015-05-647602. [DOI] [PubMed] [Google Scholar]