Abstract
Just as medicines are delivered to produce effects in the endocrine system, environmental chemicals can be similarly delivered to produce unwanted effects on the endocrine system, resulting in a staggering increase in several diseases. These effects on endocrine and other physiological systems can have significant population-level impacts and thus require public health approaches to disease control.
Keywords: Endocrine Disruptor, Environment, Development, Regulation
Contribution of the environment to disease
Over the past forty years, we have seen a 57% increase in prostate cancer, 40% in breast cancer, 85% increase in hypospadias (penile defects) and a 50% reduction in sperm count [15]. In addition, attention-deficit hyperactivity disorder has increased by 30% and autism-spectrum disorders have both doubled in the last 10 years, obesity has doubled in the past 30 years [8] and the number of U.S. adults with diabetes has more than tripled since 1980. Clearly we must look to the environment as the primary cause of such increases since the human genome has not changed over the same time period. Endocrine disruptors are likely a key factor in the surge of many diseases and disorders.
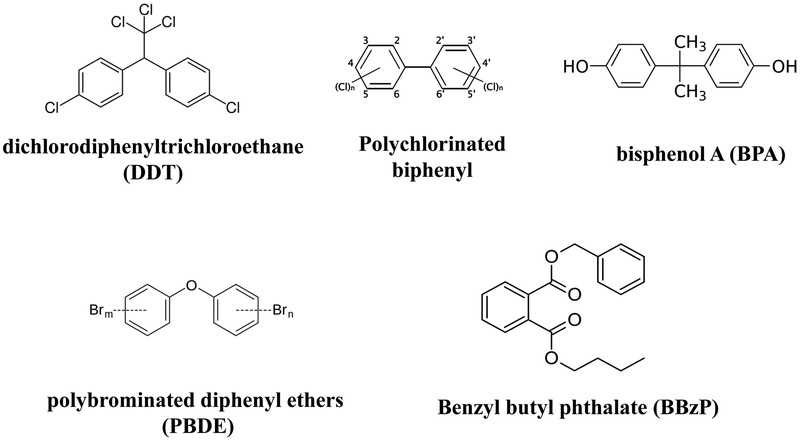
Endocrine disruptors (figure 1) are "exogenous agents that interfere with the production, release, transport, metabolism, binding, action or elimination of the natural hormones in the body responsible for the maintenance of homeostasis and the regulation of developmental processes." [1]. The case linking environmental chemicals with altered or abnormal endocrine activity is strong.
Figure 1:
Common endocrine disruptors and their chemical structure.
Endocrine disruptors are chemicals that interfere with the hormonal system in animals, including humans and with serious consequences that include cancer, birth defects, and other developmental disorders. The chemical structure of some common endocrine disruptors is shown.
Early Puberty
In 2008, a U.S. consensus panel of scientists reached general agreement that the available science showed the age of reaching puberty has decreased, specifically breast development and onset of menarche. In addition, studies suggest and experts agree that endocrine-disrupting chemicals and body fat are associated with this altered timing of puberty. [2]. A 2010 multi-site study supported this consensus and showed significant increases in the proportion of girls with early pubertal development, compared with similarly aged girls 10 to 30 years earlier [3].
Breast Cancer
High levels of the organochlorine insecticide dichlorodiphenyltrichloroethane (DDT) are associated with increased breast cancer risk, and women who were exposed to DDT before the age of 4 exhibited the highest risk [4]. High concentrations of serum polybrominated diphenyl ethers (PBDE), a common flame retardant, during adolescence, are associated with a younger age of menarche which in turn might contribute to increased risk of breast cancer [5].
Anogenital Distance
Higher exposure to phthalates results in shorter anogenital distance in boys, supporting the hypothesis that prenatal phthalate exposure at environmental levels adversely affects male reproductive development. This shortened anogenital distance is also associated with a decrease in sperm count [6].
Obesity and Diabetes
Obesity like other complex diseases is caused by a complex interaction between genetic, behavioral and environmental factors. While there is certainly an important genetic component to obesity, the recent epidemic cannot be due to genetic changes in the population and therefore must be due to changes in environmental influences. There is no doubt that over-nutrition and lack of exercise are important factors in obesity. However, the view that these two factors alone are the primary variables that explain the obesity epidemic is being challenged as far too simplistic, and other factors are now being considered as contributing to the obesity epidemic. Maternal chemical exposures have been shown to be associated with childhood obesity, and these exposures may find their way to offspring through gametes, placenta, or milk [7].
Environmental obesogens and the “developmental obesogen” hypothesis
In 2011, the National Toxicology Program sponsored a workshop to review environmental chemicals that may contribute to the diabetes and obesity epidemics. A diverse group of scientists evaluated the current literature for consistency and biological plausibility, with the ultimate goal of providing advice to the National Institute of Environmental Health Sciences for developing a research agenda on these environmental obesogens. Overall, the review of the existing literature identified linkages between several of the environmental exposures and type 2 diabetes -- nicotine likely acts as a developmental obesogen in humans, bisphenol A (BPA) affects insulin release and cellular signaling in pancreatic beta cells, there is a positive association between diabetes and certain organochlorine persistent organic pollutants (POPs), and exposure to multiple classes of pesticides may affect risk factors for diabetes and obesity.
The workshop also supported the “developmental obesogen” hypothesis, which suggests that chemical exposures may alter neural development that regulates feeding behavior later in life. When combined with a high-calorie and high-fat diet, this neuro-disregulation may contribute heavily to obesity and diabetes [8].
In addition to the endocrine system, environmental chemicals can affect other physiological systems. For example, perfluorinated compounds (PFCs) can adversely affect the immune system, resulting in reduced vaccine effectiveness, and prenatal exposure to butylbenzyl phthalate (BBzP), a chemical used in vinyl flooring and frequently found in house dust and indoor air, is a risk factor in the development of eczema in early childhood. The neurodevelopment system is also a sensitive target. Organophosphate pesticides can affect neurodevelopment, resulting in cognitive decline [9], lower IQ, and decreased attention levels in children. Other pesticides have been linked with Parkinson×s disease. Exposure to traffic-related air pollution during pregnancy and the first year of life has been associated with autism.
Environmental chemicals can disturb endocrine and other physiological systems, but new concepts in endocrine disruption have illuminated the behavior of environmental chemicals and enhanced our understanding of their relationship to disease: low-dose and non-monotonic dose response. The classical dichotomy of toxic vs. non-toxic no longer holds. The non-monotonic dose response curve shows that chemicals may have some effects at low doses and other effects at higher doses, and these can be time- and life stage-specific; this effect can be seen with the chemotherapeutic agent tamoxifen, which enhances tumor growth at low doses, but inhibits growth at high doses.[10]. This new paradigm demands that regulatory testing requirements keep pace with the science.
The human body as a delivery vehicle
The human body is not a closed system -- just as medicines are delivered orally, intranasally, and transdermally, environmental chemicals are delivered similarly, in the water we drink, the food we eat, and the air we breathe. In fact, environmental chemicals can act like bona fide medicines on endocrine pathways, and should thus be considered likewise. And just as physicians endeavor to understand and monitor the effect of medicines on endocrine pathways, we ought to achieve the same understanding and control of the effects of environmental chemicals. Physicians who disregard medicines delivered to a patient may be disciplined for inadequately obtaining a sufficiently detailed patient history; but there is no consequence for medical professionals who ignore the delivery of endocrine disrupting chemicals into the environment, other than a shocking increase in disease. Unlike medicines, which must undergo strict scrutiny by the U.S. Food & Drug Administration, where health benefits are weighed against side effects, environmental chemicals are not required to be tested for side effects and potential hazards prior to their commercial use.
Taking action
The proliferation of inadequately tested chemicals in commerce may be contributing to the skyrocketing rates of disease. The relatively new field of green chemistry attempts to design harmful effects out of synthetic chemicals. A new protocol to detect endocrine disruption in early stages of chemical design may provide a useful tool to remove hazards from future chemicals [11].
A population-based, public health approach may provide the best perspective in understanding the effect of this problem. Adverse health outcomes that may be minor on an individual level must not be disregarded; the effect size for health measures impacted by environmental chemicals, such as IQ, may be small or even clinically insignificant at an individual level, but these effects can be compounded to a staggering cost on the population level [11]. By understanding the population-level scope of the problem, preventive measures can be effectively applied.
A recent analysis by Chokshi and Farley found that environmental prevention is the most cost-effective intervention to address disease [13]. Individual interventions cannot be discounted, but when we consider population health, measures such as tobacco taxes, smoke-free-air laws, banning of trans fats from restaurants, and calorie-labeling initiatives can be effective methods of reducing disease burden on a large scale. Without action, the cost can be staggering; analyses have shown that the economic consequences of environmentally-mediated disease such as lead poisoning, prenatal mercury exposure, childhood cancers, asthma, autism, intellectual disability, and attention deficit hyperactivity disorder (ADHD) is $76.7 billion per year [14].
Animal studies, mechanistic studies, and epidemiological studies all show associations between various environmental chemicals and disease. If these associations are real, given the general population's exposure to environmental chemicals such as BPA, phthalates, mercury, lead, pesticides, and others, it only makes sense that we control these exposures to reverse the dramatic increase in chronic, non-communicable diseases that we've observed over the past 40 years. As patients do not take medications that affect their health without significant safety testing and marketing regulations, their exposure to environmental chemicals should be similarly controlled.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.U.S. Environmental Protection Agency. Endocrine Disruptors Research. 2012. Available at: http://www.epa.gov/endocrine/
- 2.Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics. 2008. February;121 Suppl 3:S167–71. [DOI] [PubMed] [Google Scholar]
- 3.Biro FM, Galvez MP, Greenspan LC, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010. September;126(3):e583–90. doi: 10.1542/peds.2009-3079. Epub 2010 Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn BA, Wolff MS, Cirillo PM and Sholtz RI 2007. DDT and breast cancer in young women: New data on the significance of age at exposure. Environ Health Perspect 115:1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen A, Chung E, DeFrance EA, et al. 2011. Serum PBDEs and age at menarche in adolescent girls: analysis of the National Health and Nutrition Examination Survey 2003-2004. Environ Res. 111 (6): 831–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendiola J, Stahlhut RW, Jørgensen N, et al. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect. 2011. July;119(7):958–63. doi: 10.1289/ehp.1103421. Epub 2011 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med. 2011. Jan-Feb;78(1):22–48. doi: 10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. 2012. Role of Environmental Chemicals in Diabetes and Obesity: A National Toxicology Program Workshop Report. Environ Health Perspect. 2012 June; 120(6): 779–789. Published online 2012 February 1. doi: 10.1289/ehp.1104597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starks SE, Gerr F, Kamel F, et al. 2011. High pesticide exposure events and central nervous system function among pesticide applicators in the Agricultural Health Study. Int Arch Occup Environ Health; doi: 10.1007/s00420-011-0694-8 [Online 7 September 2011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandenberg LN, Colborn T, Hayes TB. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine Reviews. er.2011–1050; doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schug TT, Abagyan R, Blumberg B et al. Designing endocrine disruption out of the next generation of chemicals. Green Chem. 2013. Advance Article. DOI: 10.1039/c2gc35055f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellinger DC. A Strategy for Comparing the Contributions of Environmental Chemicals and Other Risk Factors to Neurodevelopment of Children. Environ Health Perspect. 2012. April; 120(4): 501–507. Published online 2011 December 19. doi: 10.1289/ehp.1104170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chokshi DA and Farley TA. The Cost-Effectiveness of Environmental Approaches to Disease Prevention. N Engl J Med 2012; 367:295–297July 26, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Trasande L, Liu Y. Reducing the staggering costs of environmental disease in children, estimated at $76.6 billion in 2008. Health Aff (Millwood). 2011. May;30(5):863–70. doi: 10.1377/hlthaff.2010.1239. Epub 2011 May 4. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe RM, Irvine DS. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? BMJ. 2004. February 21;328(7437):447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]