Abstract
Obesity has been shown to increase risk for a number of different disorders, including cancer. In addition, obesity is also associated with immune dysfunction, which could contribute to its strong association with other comorbidities. Recently, the immune system has been found to be heavily regulated by changes in metabolism. In particular, T cells are able to respond to intrinsic metabolic regulatory mechanisms, as well as extrinsic factors such as the changes in metabolite availability. The dysfunctional metabolic environment created by obesity could therefore have a direct impact on T cell responses. In this review, we highlight recent findings in the fields of T cell biology and obesity, with a focus on mechanisms driving T cell dysfunction and potential implications for immunotherapeutic treatment of cancer.
Introduction
The incidence of obesity has risen drastically over the past few decades, and is reaching pandemic levels in the developed world [1,2]. Approximately 36% of the adult population in the U.S. is obese, and this number is expected to rise over the next decade [2]. Obesity is a major risk factor for a number of other comorbidities including diabetes, kidney disease, liver disease, cardiovascular disease, musculoskeletal disorders and cancer [3]. In addition, obesity has been associated with increased incidence of infectious disease [4]. It is not surprising, therefore, that the impact of obesity on healthcare spending is significant. Some estimates attribute up to $190 billion spent on obesity-related medical care in 2005 alone, or around 20 percent of the total annual U.S. healthcare expenditure that year [5]. Many of the comorbidities associated with obesity have been linked to dysfunction of the immune system [6].
T cells represent a major component of the immune system, orchestrating and regulating major aspects of an immune response. Recently, they have begun to be appreciated for their dynamic metabolic tuning, that can change depending on activation or availability of different metabolites (i.e. glucose, fatty acids, etc.) [7,8]. In addition, checkpoint blockade therapies aimed at reinvigorating exhausted T cell responses are showing great promise in treating cancer patients in the clinic [9]. T cell exhaustion is characterized as a progressive and hierarchal loss of effector function following chronic antigen exposure and/or inflammation, and has been characterized in cases of viral infection, autoimmunity and cancer [10]. Obesity leads to a state of chronic inflammation, which can augment the T cell pool as well as lead to accelerated thymic aging [11,12]. Therefore, because of the large impact of obesity, and the promise of checkpoint blockade therapy in treating cancer, a better understanding of the effects of obesity on T cells and T cell exhaustion is needed to better utilize these therapies on the growing population of obese patients.
Effects of obesity on the immune system
Obesity is associated with a state of chronic inflammation, characterized by a number of different changes in the immune system, including increases in serum pro-inflammatory cytokines such as IL-6 and TNFα as well as shifts in the memory to naive ratio of T cells [12,13]. Importantly, a similar phenotype has been characterized not just in mice and humans, but also canines and non-human primates [14–17]. Increases in adipose tissue lead to this inflammatory state, by recruiting different immune cell populations into the adipose tissue, notably macrophages and T cells. The inflammatory state is amplified by M1 macrophage polarization, as well as shifts to Th1/Th17 T cell populations [7,13,18]. An unknown question is the source of antigen(s) driving T cell activation in obesity, with some postulating there is an autoimmune component of obesity due to limited TCR repertoire diversity noted in T cell populations in adipose tissue [19,20].
In general, obesity leads to a state of accelerated immune senescence, similar to what is seen in aged individuals [11,21,22]. This accelerated immune aging phenotype has even been noted in obese children [23]. In line with this, obesity has been found to lead to poor vaccination responses [24,25]. A number of studies have examined the impact of obesity on immune challenge, with conflicting results. Most studies find obesity correlates with dysfunctional immunity. Notably, obesity has been found to lead to defects in memory maintenance following influenza infection, and was even found to be an independent risk factor for increased morbidity/mortality from the pandemic H1N1 infection [26–28]. However, a different study found that obesity did not impact the generation and maintenance of memory T cells following infection [29]. This highlights some of the caveats that come with studying obesity, the primary one being the variability in results due to the large number of variables inherent to obesity studies. Age is a large confounding factor in that it takes time to become obese, and we know that the immune system itself changes with time (age). Another factor is the type of diet or animal model used for obesity. Early studies on obesity relied on mutant mouse models, such as the leptin deficient ob/ob strain. More recently, diet-induced mouse models have become widely used, with both high fat diets and Western diets (i.e. NASH) being heavily reported. The age at which one starts inducing obesity can also have a profound effect on results, with one study reporting significant changes in the immune phenotype of mice started on a high fat diet at 3 weeks of age that was lost if mice were instead started on the same diet at 12 weeks of age [30]. Intriguingly, caloric restriction has been found to reverse some of the effects of the accelerated immune aging seen in obesity, with reports of delays in T cell senescence compared to ad libitum fed control mice and non-human primates [14,15,31].
T cell metabolism and obesity
Obesity induces a state of chronic metabolic dysfunction, with altered serum levels of insulin/glucose, leptin/adiponectin, among other hormones and adipokines [32–35]. Many of these same molecules that are important in regulating the metabolic state of an organism, have been shown to have direct effects in regulating immune activation [7,18]. The insulin receptor is upregulated upon T cell activation to support glucose metabolism, and insulin has been shown to polarize toward a Th2 phenotype [36,37]. Adiponectin has been shown to have both pro- and anti-inflammatory properties, as well as direct negative effects on antigen-specific T cell activation [33,34]. The levels of adiponectin have been shown to decline in obesity, thus providing another source of immune dysfunction. Leptin is a hormone involved in regulating satiety, and has effects on many aspects of immune function, primarily stimulating pro-inflammatory responses [32]. It has been shown to promote Th17 differentiation, as well as have a key role in regulating regulatory T cell proliferation [38,39]. More recently, it has been shown to support T cell activation through metabolically reprogramming the T cells [40]. Leptin levels are elevated in obese individuals and could therefore provide a mechanism behind the immune dysregulation found in obese individuals.
In addition, immune cells themselves have recently become more appreciated for the dynamic metabolic shifts that occur during development and activation [7,18]. Naïve T cells remain in a quiescent state, reliant on oxidative phosphorylation for their energy needs. Upon activation, the metabolic signature changes to support increased glycolysis to support the energy needs of the cell. Finally, memory T cell formation is accompanied by another shift to fatty-acid oxidation (FAO). Clearly T cells alter their metabolic profile and needs upon antigen recognition, but what remains unclear is how the metabolic dysfunction caused by obesity might alter the balance in T cell activation. Indeed, Mauro et al. recently showed that a high-fat diet can lead to development of inflammatory effector memory CD4+ T cells [12]. This effect was mediated by inducing metabolic stress using the saturated fatty-acid palmitate. Memory T cells have been shown to be reliant on FAO for development and survival [41]. More recently, the source of fatty acid supporting FAO in these memory T cells was shown to be both exogenously and intrinsically sourced [42,43]. Pan et al. showed that tissue resident memory T cells require exogenous lipid uptake through fatty-acid-binding proteins 4 and 5 (FABP4 and FABP5), and FAO to persist in tissue and mediate protective immunity [43]. In addition, the type of fatty-acid can also have an impact on the type of immune response, with long chain fatty-acids promoting Th1 and Th17 differentiation, and short chain fatty-acids leading to increased regulatory T cell differentiation [44]. Thus, the functional response of a T cell is linked to its metabolic state which can be directly impacted by overall metabolic states of the individual.
Effects of obesity on cancer
The significance of obesity as a risk factor for cancer incidence has become clear over the last few decades, with some epidemiological studies estimating obesity to surpass smoking as the number one causative agent of cancer [45–47]. Most cancer types have been linked to obesity, but the most significant trends concern cancers of the colon, esophagus, kidney, breast and corpus uteri. In addition, obese patients face a multitude of added complications from diagnosis, to treatment and management [48]. Multiple mechanisms have been proposed for how obesity impacts cancer progression, and it is likely not a solitary factor that drives these effects, but rather a combination of metabolic and inflammatory effects on both the tumor and the immune system [49,50]. Indeed, fasting has been linked to effects on tumor sensitization and subsequent enhancement of chemotherapy as well as direct effects on immune subsets leading to enhanced immunosurveillance [31,51,52]. Brandhorst et al. provide a comprehensive examination of the effects of periodic fasting on multiple aspects of health including metabolism, cognitive function, immune function and bone loss, in yeast, mouse and human [31]. Importantly, they found that period fasting decreased spontaneous tumor incidence in C57BL/6 mice. In addition, diet-induced obese mice were shown to have increased tumor growth and metastasis using multiple strains and tumor models [53–58]. A number of difficulties exist when it comes to treating obese cancer patients, including challenges caused by other comorbidities, difficulty with diagnosis or differences in physiology and pharmacokinetics [48]. Immunotherapies aimed at stimulating the immune system have revolutionized the field of cancer treatment [9]. Being that the immunotherapy field is relatively young, a number of toxicities independent of obesity still persist in patients undergoing treatment and limit efficacy [59,60]. However, Mirsoian et al. showed obese mice undergoing strong immunostimulatory therapy (high dose IL-2 and agonistic anti-CD40) experience rapid and lethal cytokine storm, dependent on macrophage produced TNFα [61]. Interestingly, this phenotype could be reversed with caloric restriction. Based on this and the rising incidence of obesity, more research is needed on the effects of obesity on cancer therapy.
Obesity and T cell exhaustion
Obesity’s effect on the immune system can generally be described as accelerated immune aging, and one of the hallmarks of the immune aging is thymic involution and T cell senescence [62]. Consistent with this, obesity has previously been shown to accelerate thymic aging [11]. This is in contradiction to another report that found obesity lead to increases in thymic weight and cellularity, but this did not translate to significant differences in T cell numbers between lean and obese mice [63]. Therefore, one could argue that obesity lead to defects in thymic output on a per gram basis. Nonetheless, defects in thymic function could account for many of the complications with infection and cancer in obese patients. Most studies examining the functional potential of T cells in obesity find defects in their ability to mount effective immune responses in multiple species and models [14–17,23,26,27,64–66]. On the other hand, some have found enhanced T cell function, leading to exacerbated pathology and disease [67,68]. Differences in the model and experimental design could account for these discrepancies. As mentioned before, the length of time on the diet and when the diet was started could have a profound impact on the extent of obesity’s effect on T cells [30].
Chronic stimulation has been shown to lead to an exhausted phenotype in T cells, characterized by decreased proliferation and production of effector molecules, and increased expression of inhibitory receptors [10]. It is likely the chronic inflammatory environment, combined with the added changes in immune-active metabolites (leptin, adiponectin, glucose) could lead to an exhausted phenotype amongst T cells in obese individuals. TNFα was recently shown to be a critical inducer of T cell exhaustion in chronic viral infection, and obesity leads to increased levels of TNFα [69,70]. In addition, fatty acid mediated activation of T cells adds to this cascade of potential mediators of T cell exhaustion in obesity (Figure 1) [12]. PD-1 expression was even found to be linked to the metabolic status of T cells, dependent on changes in glucose availability in vitro [71]. More recently, PD-1 was shown to augment energy metabolism and mitochondrial biogenesis of exhausted T cells by regulating expression of the key metabolic transcriptional regulator PGC-1α [72]. The authors also showed that early exhausted T cells upregulated expression of Cpt1a, a key regulator of FAO. PGC-1α levels have been previously shown to be decreased in the adipose tissue of obese individuals [73]. Therefore, the constant changes in the metabolic and activation status of T cells that occurs in obesity could lead to a terminally differentiated exhausted T cell phenotype.
Figure 1: Mechanisms driving T cell dusfunction in the setting of obesity.
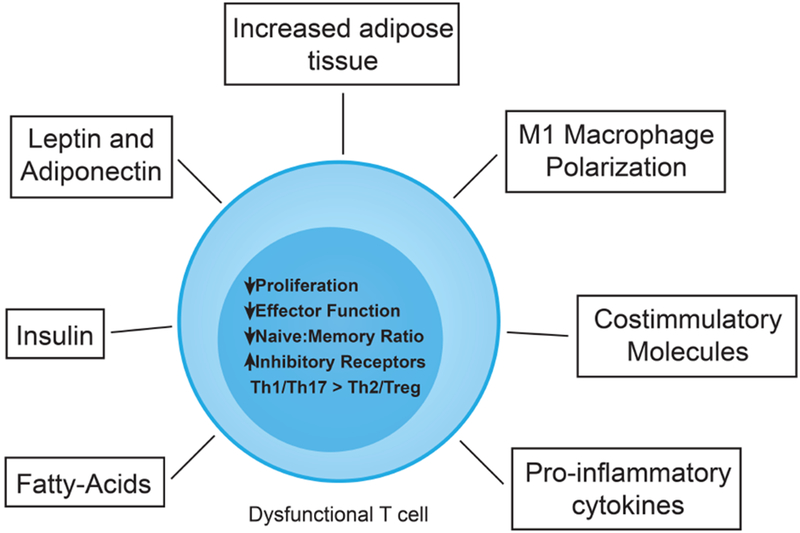
Increased adipose tissue appears to be the primary driver, producing adipokines such as leptin and adiponectin that have immune regulatory functions, and result in the recruitment of immune cells with altered levels of different metabolites such as fatty-acids, leptin or adiponectin. The local inflammatory environment drives M1 macrophage polarization as well as increased expression of co-stimulatory molecules resulting in T cell activation as well as elevated levels of proinflammatory cytokines. In addition, the altered metabolites (leptin, adiponectin and fatty-acids) can have direct effects on T cell activation. Collectively, they result in a dysfunctional T cell characterized by decreases in proliferation and effector function (i.e. cytokine production), as well as a decrease in the naive to memory T cell ratio. In addition, these changes will be accompanied by an increase in inhibitory receptor expression (i.e.PD-1) as well as imbalances of Th1 and Th17 responses over Th2 and Tregs.
The extent of T cell exhaustion or dysfunction could be dependent on the tissue examined as well, as recent work has identified a population of T cells in visceral adipose tissue displaying a senescent phenotype [74]. Shirakawa et al. show that a high fat diet caused an accumulation of CD4+ T cells with an effector memory phenotype, which expressed PD-1 and CD153 in adipose tissue. Other tissue resident T cell populations in the setting of obesity remain to be examined. Organs such as the liver, where large fat deposits are known to occur, or organs known to be adversely affected by obesity would be of interest as potential niches for exhausted T cells. The increased levels of PD-1 expression in adipose tissue, as well as the increased adiposity associated with obesity leads to the question of what effects this could have on immunotherapy treatment of cancer. Checkpoint blockade is an emerging immunotherapy aimed at reinvigorating anti-cancer T cell responses through monoclonal antibody blocking of inhibitory receptors such as PD-1 on T cells [75]. Obese patients who are found to have T cells that express high levels of PD-1 might have a better response to anti-PD-1 treatment. Conversely, due to all of the known toxicities associated with emerging immunotherapies, as well as the known issues with immunotherapy in obese mice, obese patients might be at increased risk for toxicities or complications. More research is needed on the effects of obesity on T cell exhaustion, and the implications for checkpoint blockade therapy.
Conclusion
It is clear that obesity and the metabolic dysfunctions associated with it can have a profound effect on the status of the immune system. Here, we have highlighted some of the more current findings on this front, with a focus on T cells and implications in cancer immunotherapy. A combination of mechanisms are likely responsible for the T cell dysfunction noted in obesity (Figure 1). The chronic stimulation T cells experience can lead to an exhausted-like phenotype. It is important to distinguish this from other exhaustion phenotypes as there is no known antigen driving this in obesity, and therefore it is unclear if this is exhaustion or senescence or tolerance. The impact of obesity on human health is massive, with no clear end in sight due to its continued rise. With this in mind, better pre-clinical modeling is needed to more closely represent who is being treated in the clinic, and lead to better outcomes when moving treatments into the clinic.
Highlights.
Obesity leads to a state of metabolic and immune dysfunction
T cells are highly tuned by multiple intrinsic and extrinsic metabolic mechanisms
Obesity can alter the efficacy of emerging cancer immunotherapies
Acknowledgements
We would also like to thank the other members in the Murphy lab for providing feedback and suggestions during preparation of the manuscript. This was work funded by NIH grant R01 CA095572, R01 CA195904 and R01 CA214048
The content of this publication does not necessarily reflect the view or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported in part by the Intramural Research Program of the NIH, NCI, NHLBI and Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collaboration NCDRF: Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Fryar CD, Flegal KM: Prevalence of Obesity Among Adults and Youth: United States, 2011-2014. NCHS Data Brief 2015:1–8. [PubMed] [Google Scholar]
- 3.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L: Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet 2014, 384:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milner JJ, Beck MA: The impact of obesity on the immune response to infection. Proc Nutr Soc 2012, 71:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cawley J, Meyerhoefer C: The medical care costs of obesity: an instrumental variables approach. J Health Econ 2012, 31:219–230. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin T, Ackerman SE, Shen L, Engleman E: Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest 2017, 127:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck MD, O’Sullivan D, Pearce EL: T cell metabolism drives immunity. J Exp Med 2015, 212:1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green WD, Beck MA: Obesity altered T cell metabolism and the response to infection. Curr Opin Immunol 2017, 46:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD: The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 2016, 13:273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wherry EJ, Kurachi M: Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015, 15:486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors provide a broad overview of what is known about the molecular and cellular mechanisms that drive T cell exhaustion in models of persistent infection and cancer.
- 11.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD: Obesity accelerates thymic aging. Blood 2009, 114:3803–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauro C, Smith J, Cucchi D, Coe D, Fu H, Bonacina F, Baragetti A, Cermenati G, Caruso D, Mitro N, et al. : Obesity-Induced Metabolic Stress Leads to Biased Effector Memory CD4+ T Cell Differentiation via PI3K p110delta-Akt-Mediated Signals. Cell Metab 2017, 25:593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors show that exposure of T cells to the saturate fatty acid palmitate, which is found enriched in high-fat diets, induces CD4+ T cells to acquire an inflammatory memory phenotype.
- 13.Gregor MF, Hotamisligil GS: Inflammatory mechanisms in obesity. Annu Rev Immunol 2011, 29:415–445. [DOI] [PubMed] [Google Scholar]
- 14.Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, et al. : Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A 2006, 103:19448–19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. : Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Velde H, Janssens GP, Rochus K, Duchateau L, Scharek-Tedin L, Zentek J, Nguyen P, Cox E, Buyse J, Biourge V, et al. : Proliferation capacity of T-lymphocytes is affected transiently after a long-term weight gain in Beagle dogs. Vet Immunol Immunopathol 2013, 152:237–244. [DOI] [PubMed] [Google Scholar]
- 17.Baric Rafaj R, Kules J, Marinculic A, Tvarijonaviciute A, Ceron J, Mihaljevic Z, Tumpa A, Mrljak V: Plasma markers of inflammation and hemostatic and endothelial activity in naturally overweight and obese dogs. BMC Vet Res 2017, 13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce EL, Pearce EJ: Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrante AW Jr.: The immune cells in adipose tissue. Diabetes Obes Metab 2013, 15 Suppl 3:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD: Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 2010, 185:1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bektas A, Schurman SH, Sen R, Ferrucci L: Human T cell immunosenescence and inflammation in aging. J Leukoc Biol 2017, 102:977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchlaka MN, Sckisel GD, Chen M, Mirsoian A, Zamora AE, Maverakis E, Wilkins DE, Alderson KL, Hsiao HH, Weiss JM, et al. : Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med 2013, 210:2223–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spielmann G, Johnston CA, O’Connor DP, Foreyt JP, Simpson RJ: Excess body mass is associated with T cell differentiation indicative of immune ageing in children. Clin Exp Immunol 2014, 176:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Painter SD, Ovsyannikova IG, Poland GA: The weight of obesity on the human immune response to vaccination. Vaccine 2015, 33:4422–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HL, Shim SH, Lee EY, Cho W, Park S, Jeon HJ, Ahn SY, Kim H, Nam JH: Obesity-induced chronic inflammation is associated with the reduced efficacy of influenza vaccine. Hum Vaccin Immunother 2014, 10:1181–1186.24614530 [Google Scholar]
- 26.Karlsson EA, Sheridan PA, Beck MA: Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol 2010, 184:3127–3133. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson EA, Sheridan PA, Beck MA: Diet-induced obesity in mice reduces the maintenance of influenza-specific CD8+ memory T cells. J Nutr 2010, 140:1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, Matyas BT, California Pandemic Working G: A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011, 52:301–312. [DOI] [PubMed] [Google Scholar]
- 29.Khan SH, Hemann EA, Legge KL, Norian LA, Badovinac VP: Diet-induced obesity does not impact the generation and maintenance of primary memory CD8 T cells. J Immunol 2014, 193:5873–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong L, Zhang Y, Yang L, Liu G, Ye J, Wang H: Effects of a High-Fat Diet on Adipose Tissue CD8+ T Cells in Young vs. Adult Mice. Inflammation 2017. [DOI] [PubMed] [Google Scholar]
- 31.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, et al. : A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab 2015, 22:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The authors highlight multiple benefits of a fasting diet, using a multi-system approach. Importantly, they observed rejuvenation of the hematopoietic system as well as decreased incidence of inflammatory conditions and cancer.
- 32.Naylor C, Petri WA Jr.: Leptin Regulation of Immune Responses. Trends Mol Med 2016, 22:88–98. [DOI] [PubMed] [Google Scholar]
- 33.Luo Y, Liu M: Adiponectin: a versatile player of innate immunity. J Mol Cell Biol 2016, 8:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilk S, Scheibenbogen C, Bauer S, Jenke A, Rother M, Guerreiro M, Kudernatsch R, Goerner N, Poller W, Elligsen-Merkel D, et al. : Adiponectin is a negative regulator of antigen-activated T cells. Eur J Immunol 2011, 41:2323–2332. [DOI] [PubMed] [Google Scholar]
- 35.Helderman JH: Role of insulin in the intermediary metabolism of the activated thymic-derived lymphocyte. J Clin Invest 1981, 67:1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC: Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol 2008, 84:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viardot A, Grey ST, Mackay F, Chisholm D: Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology 2007, 148:346–353. [DOI] [PubMed] [Google Scholar]
- 38.Reis BS, Lee K, Fanok MH, Mascaraque C, Amoury M, Cohn LB, Rogoz A, Dallner OS, Moraes-Vieira PM, Domingos AI, et al. : Leptin receptor signaling in T cells is required for Th17 differentiation. J Immunol 2015, 194:5253–5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G: A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007, 26:241–255. [DOI] [PubMed] [Google Scholar]
- 40.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ: Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol 2014, 192:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y: Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009, 460:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. : Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 2014, 41:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O’Malley JT, Gehad A, Teague JE, et al. : Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017, 543:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors show that tissue-resident memory T cells require exogenous lipid uptake for optimal maintenance, longevity and function, while central memory T cells do not. The fatty acid binding proteins 4 and 5 (FABP4 and FABP5) were shown to be critical mediators of exogenous FFA uptake in both mouse and humans.
- 44.Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, et al. : Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43:817–829. [DOI] [PubMed] [Google Scholar]
- 45.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working G: Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016, 375:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, Fabian CJ, Gucalp A, Hershman DL, Hudson MM, et al. : American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol 2014, 32:3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M: Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371:569–578. [DOI] [PubMed] [Google Scholar]
- 48.Tao W, Lagergren J: Clinical management of obese patients with cancer. Nat Rev Clin Oncol 2013, 10:519–533. [DOI] [PubMed] [Google Scholar]
- 49.Kanneganti TD, Dixit VD: Immunological complications of obesity. Nat Immunol 2012, 13:707–712. [DOI] [PubMed] [Google Scholar]
- 50.Khandekar MJ, Cohen P, Spiegelman BM: Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011, 11:886–895. [DOI] [PubMed] [Google Scholar]
- 51.Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, Cacciottolo M, Martin-Montalvo A, de Cabo R, Wei M, et al. : Fasting-Mimicking Diet Reduces HO-1 to Promote T Cell-Mediated Tumor Cytotoxicity. Cancer Cell 2016, 30:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, Levesque S, Castoldi F, Jacquelot N, Yamazaki T, et al. : Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 2016, 30:147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang HH, Moro A, Takakura K, Su HY, Mo A, Nakanishi M, Waldron RT, French SW, Dawson DW, Hines OJ, et al. : Incidence of pancreatic cancer is dramatically increased by a high fat, high calorie diet in KrasG12D mice. PLoS One 2017, 12:e0184455. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors show that diet-induced obese mice had significantly greater cancer incidence in KrasG12D mice compared to lean. The DIO mice showed more extensive inflammation and fibrosis, and more advanced PanIN lesions in the pancrease.
- 54.Chen J, Huang XF: High fat diet-induced obesity increases the formation of colon polyps induced by azoxymethane in mice. Ann Transl Med 2015, 3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawson DW, Hertzer K, Moro A, Donald G, Chang HH, Go VL, Pandol SJ, Lugea A, Gukovskaya AS, Li G, et al. : High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev Res (Phila) 2013, 6:1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim EJ, Choi MR, Park H, Kim M, Hong JE, Lee JY, Chun HS, Lee KW, Yoon Park JH: Dietary fat increases solid tumor growth and metastasis of 4T1 murine mammary carcinoma cells and mortality in obesity-resistant BALB/c mice. Breast Cancer Res 2011, 13:R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Neill AM, Burrington CM, Gillaspie EA, Lynch DT, Horsman MJ, Greene MW: High-fat Western diet-induced obesity contributes to increased tumor growth in mouse models of human colon cancer. Nutr Res 2016, 36:1325–1334. [DOI] [PubMed] [Google Scholar]
- 58.Yakar S, Nunez NP, Pennisi P, Brodt P, Sun H, Fallavollita L, Zhao H, Scavo L, Novosyadlyy R, Kurshan N, et al. : Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology 2006, 147:5826–5834. [DOI] [PubMed] [Google Scholar]
- 59.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et al. : Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts K, Culleton V, Lwin Z, O’Byrne K, Hughes BG: Immune checkpoint inhibitors: Navigating a new paradigm of treatment toxicities. Asia Pac J Clin Oncol 2017, 13:277–288. [DOI] [PubMed] [Google Scholar]
- 61.Mirsoian A, Bouchlaka MN, Sckisel GD, Chen M, Pai CC, Maverakis E, Spencer RG, Fishbein KW, Siddiqui S, Monjazeb AM, et al. : Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med 2014, 211:2373–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linton PJ, Dorshkind K: Age-related changes in lymphocyte development and function. Nat Immunol 2004, 5:133–139. [DOI] [PubMed] [Google Scholar]
- 63.Trottier MD, Naaz A, Li Y, Fraker PJ: Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci U S A 2012, 109:7622–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen S, Akbar SM, Miyake T, Abe M, Al-Mahtab M, Furukawa S, Bunzo M, Hiasa Y, Onji M: Diminished immune response to vaccinations in obesity: role of myeloid-derived suppressor and other myeloid cells. Obes Res Clin Pract 2015, 9:35–44. [DOI] [PubMed] [Google Scholar]
- 65.James BR, Tomanek-Chalkley A, Askeland EJ, Kucaba T, Griffith TS, Norian LA: Diet-induced obesity alters dendritic cell function in the presence and absence of tumor growth. J Immunol 2012, 189:1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pizzolla A, Oh DY, Luong S, Prickett SR, Henstridge DC, Febbraio MA, O’Hehir RE, Rolland JM, Hardy CL: High Fat Diet Inhibits Dendritic Cell and T Cell Response to Allergens but Does Not Impair Inhalational Respiratory Tolerance. PLoS One 2016, 11:e0160407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Endo Y, Asou HK, Matsugae N, Hirahara K, Shinoda K, Tumes DJ, Tokuyama H, Yokote K, Nakayama T: Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep 2015, 12:1042–1055. [DOI] [PubMed] [Google Scholar]
- 68.Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, Dosch HM: Obesity predisposes to Th17 bias. Eur J Immunol 2009, 39:2629–2635. [DOI] [PubMed] [Google Scholar]
- 69.Beyer M, Abdullah Z, Chemnitz JM, Maisel D, Sander J, Lehmann C, Thabet Y, Shinde PV, Schmidleithner L, Kohne M, et al. : Tumor-necrosis factor impairs CD4(+) T cell-mediated immunological control in chronic viral infection. Nat Immunol 2016, 17:593–603. [DOI] [PubMed] [Google Scholar]
- 70.Moon YS, Kim DH, Song DK: Serum tumor necrosis factor-alpha levels and components of the metabolic syndrome in obese adolescents. Metabolism 2004, 53:863–867. [DOI] [PubMed] [Google Scholar]
- 71.Chang CH, Curtis JD, Maggi LB Jr., Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. : Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013, 153:1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, Stelekati E, McLane LM, Paley MA, Delgoffe GM, et al. : Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity 2016, 45:358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The authors show the PD-1 signaling in early exhausted T cells leads to repressed expression of the key metabolic regulator PGd-α in CD8+ T cells during chronic LCMV infection. Importantly, this also lead to increased expression of the key fatty acid oxidation regulatory gene Cpt1a and altered metabolic profile of the exhausted T cells.
- 73.Semple RK, Crowley VC, Sewter CP, Laudes M, Christodoulides C, Considine RV, Vidal-Puig A, O’Rahilly S: Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. Int J Obes Relat Metab Disord 2004, 28:176–179. [DOI] [PubMed] [Google Scholar]
- 74.Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, et al. : Obesity accelerates T cell senescence in murine visceral adipose tissue. J Clin Invest 2016, 126:4626–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The authors found an accumulation of CD44hiCD62LloCD4+ T cells that constitutively expressed PD-1 and CD153, and displayed a senescent phenotype in the visceral adipose tissue (VAT) of diet-induced obese mice. Intriguingly, these T cells could be adoptively transferred into the VAT of lean mice and recapitulate the essential features of VAT inflammation and insulin resistance in a osteopontin dependent manner
- 75.Catakovic K, Klieser E, Neureiter D, Geisberger R: T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun Signal 2017, 15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


