Introduction
Acute kidney injury (AKI) occurs in approximately 20% of hospitalized patients, and the incidence doubles in patients admitted to the intensive care units (ICUs) (1,2). AKI carries high morbidity, resource utilization and mortality, particularly in critically ill patients in whom mortality rates could be as high as 50% (3–5). Survivors of AKI are susceptible to kidney and non-kidney related complications such as the development or progression of chronic kidney disease (CKD) (6), end-stage renal disease (ESRD) (6), and cardiovascular disease (7–9). Moreover, survivors of AKI are also at higher risk of early re-hospitalization (10) and increased risk of long-term mortality (10,11).
Frailty is a clinical syndrome characterized by a physiological age-related decline in functional reserve of several organs (12). It comprises domains such as nutritional status, energy expenditure, metabolic rate, cognitive function and sarcopenia. Frailty occurs most frequently in older adults, and similar to AKI, carries high risk of poor outcomes such as physical disability, functional decline, frequent hospitalizations and increased mortality, particularly in critically ill elderly patients admitted to the ICU (13–15).
Several clinical tools have been developed to help in the diagnosis of the frailty syndrome. The most commonly used are: (I) the physical frailty phenotype (PFP), which identifies frailty phenotypes based on the examination of changes in weight, weakness and walking speed (16); (II) the comprehensive geriatric assessment (CGA), which examines medical, psychosocial, and functional limitations of older adults by a multidisciplinary team of healthcare professionals with the objective of creating a treatment plan of long-term support and rehabilitation for frail adults (17); and (III) the clinical frailty scale (CFS), which uses pictographs to subjectively stratify older adults according to their level of vulnerability to have poor outcomes such as prolonged hospitalizations and increased mortality (14,18,19). Several studies have attempted to validate and compare these tools, but none of the tools has shown to be superior to their counterparts and therefore there is no single tool for assessment of frailty postulated as standard of care (20–22).
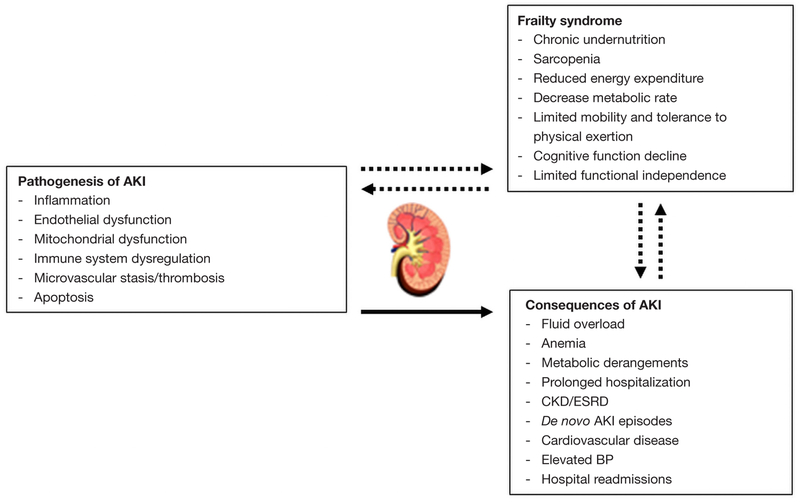
Despite frailty and AKI are commonly encountered in critically ill and elderly patients, their interplay and interaction remain unclear. Nonetheless, it is possible that they predispose to each other in a vicious circle and therefore worsen patient’s overall prognosis (Figure 1). To date, most of the frailty studies have evaluated this syndrome in patients with ESRD on prevalent HD but not in critically ill patients with or without AKI. In ESRD patients, for example, the prevalence of frailty ranged from 35% to 73%, and frailty was associated with higher mortality rates (14,23–25). In these studies, frailty was examined with heterogeneous tools such as PFP and CFS.
Figure 1.
Overview of the potential interplay between frailty and acute kidney injury. AKI, acute kidney injury; BP, blood pressure; CKD, chronic kidney disease; ESRD, end-stage renal disease.
AKI and frailty: what is the evidence?
In a recent post-hoc analysis, Abdel-Kader et al. (26) utilized data from the prospective bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors (BRAIN-ICU) cohort (14,18,19) to examine the association between AKI and subsequent frailty status in critically ill survivors. This cohort included critically ill patients in whom their frailty status was examined at baseline, 3 months, and 12 months using the CFS tool. The study included patients that were admitted to the ICU, had acute respiratory failure and/or shock (septic or cardiogenic) and daily serum creatinine (SCr) measurements during the hospital stay. Patients with ESRD, history of kidney transplant, death before discharge from the hospital, and those without follow-up visits were excluded. A total of 371 patients comprised the cohort for the primary analysis. The authors determined baseline kidney function as follows: (I) mean of all outpatient SCr measurements from 7 days to 365 days prior to the index admission or (II) the lowest SCr during the hospitalization. Further, they performed clinical adjudication of baseline SCr if the lowest SCr during the hospitalization was ≥0.5 mg/dL below the mean of all outpatient SCr values (obtained from those with available data) or if patients had a baseline SCr that resulted in an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2. Two nephrologists blinded to the study outcomes performed the clinical adjudication. The latter resulted in 21 patients with adjusted values of baseline SCr. AKI was defined and staged according to kidney disease improving global outcomes (KDIGO)-SCr criteria (27) using the difference between baseline SCr and the maximum SCr during the hospitalization.
The authors demonstrated that AKI stages 1, 2, and 3 were independently associated with higher frailty scores (CFS-based) at 3 months (stage 1: OR, 1.92; 95% CI, 1.14–3.24; stage 2: OR, 2.40; 95% CI, 1.31–4.42; stage 3: OR, 4.41; 95% CI, 2.20–8.82) and 12 months (stage 1: OR, 1.87; 95% CI, 1.11–3.14; stage 2: OR, 1.81; 95% CI, 0.94–3.48; stage 3: OR, 2.76, 95% CI, 1.34–5.66). The multivariable adjustment included confounders such as demographic characteristics, baseline SCr, baseline CSF score, and other relevant clinical parameters such as Sequential Organ Failure Assessment Score (28) and Charlson Comorbidity Score (29). In addition, the authors performed sensitivity analyses restricting the cohort to patients with measured SCr as outpatient before the index hospitalization (n=177), to patients with a baseline CFS score ≤5 (mildly frail or no frail), and to survivors at 3 or 12 months. Overall, the sensitivity analyses were concordant with the primary analysis despite some power limitations due to reduced sample size. Finally, these associations were similar when the dialysis status at discharge, the timing of peak SCr (≤48 vs. >48 hours from hospital admission) or the length of mechanical ventilation were introduced into the multivariable models.
The strengths of the study by Abdel-Kader et al. (26) are: (I) the availability of longitudinal data evaluating the post-ICU frailty syndrome utilizing the CFS tool at baseline, 3 and 12 months; (II) the comprehensive determination of baseline SCr, including clinical adjudication by two nephrologists; (III) the use of a conventional SCr-based definition of AKI (KDIGO); and (IV) the sensitivity analyses that addressed the misclassification of AKI based on the determination of baseline SCr, the effect of high frailty scores at baseline and the competing risk of death during the post-ICU observation period.
Nonetheless, there are important limitations in this study that should be noted: (I) the authors conducted an observational study that used single-center data which may not be representative of a heterogeneous ICU population; (II) a causal effect between AKI and subsequent frailty status in ICU survivors cannot be inferred from this study; (III) additional AKI data such as etiology, urine output, and/or duration of AKI were not available and may also be useful to underpin potential hypotheses in relation to the identified association between AKI and frailty; and (IV) data pertaining to post-discharge kidney function were not available, which precludes adjustment for the potential residual confounding effect of AKI-to-CKD progression in this subset of critically ill survivors.
In a distinct single-center study, Baek et al. (30) examined the effect of frailty as a predictor of AKI in hospitalized geriatric patients admitted to a South-Korean hospital. One major difference is that they measured frailty using CGA instead of the CFS tool used in the BRAIN-ICU study. Further, this study included patients who had a single CGA examination within one year before the index hospitalization; therefore, there was a heterogeneous time from CGA assessment to hospital admission in these patients. AKI was defined according to KDIGO SCr-criteria (27). The authors found that frailty was independently associated with the development of AKI (adjusted HR =3.54, 95% CI, 1.58–7.96, P=0.002). Also, they found that frailty was associated with a higher likelihood of being discharged to a nursing home facility, and with a higher risk of short- and long-term mortality during an observation period of 1 year. Despite being a single-center observational study restricted to a specific geriatric population [mean age (SD) 76.9 (5.95), 47.3% were male, and 13.7% were admitted to the ICU], this study further adds to the evolving evidence that there is a biologically plausible connection between frailty and AKI, and perhaps a predisposition to each other in some specific clinical context that remains to be determined. Certainly, more studies addressing this relationship and the putative mechanisms of these associations are direly needed because is of paramount of importance to address functional status and quality of life in survivors from critical illness and AKI as part of patient-centered research initiatives.
Conclusions
In summary, the study by Abdel-Kader et al. (26) revealed a novel and independent association between AKI and subsequent frailty status at 3 and 12 months in survivors from critical illness. This important observational study could be hypothesis generating to investigate the mechanisms that could link both of these syndromes and how they could potentially predispose to each other. It is possible that the overall frailty status of a patient correlates with renal functional reserve and therefore constitute an independent risk factor for developing AKI. In addition, some injury patterns of AKI such as inflammation and immune system dysregulation may predispose to frailty. Further, some AKI consequences such as fluid overload, anemia, cardiovascular disease, and metabolic derangements may also affect the frailty status of some susceptible critical illness survivors. Further studies should validate these findings, underpin potential mechanisms of this—possibly bidirectional—association, and importantly, develop therapeutic strategies focused on ameliorating the burden of frailty in survivors of critical illness and AKI.
Acknowledgements
Funding: JAN is supported by an Early Career Pilot Grant from the National Center for Advancing Translational Sciences, National Institute of Health, through Grant UL1TR001998.
Footnotes
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Provenance: This is an invited Editorial commissioned by the Executive Editor Dr. Zhongheng Zhang (Department of Emergency Medicine, Sir Run-Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China).
Comment on: Abdel-Kader K, Girard TD, Brummel NE, et al. Acute Kidney Injury and Subsequent Frailty Status in Survivors of Critical Illness: A Secondary Analysis. Crit Care Med 2018;46:e380–8.
References
- 1.Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:1482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szczech LA, Harmon W, Hostetter TH, et al. World Kidney Day 2009: problems and challenges in the emerging epidemic of kidney disease. J Am Soc Nephrol 2009;20:453–5. [DOI] [PubMed] [Google Scholar]
- 3.Silver SA, Long J, Zheng Y, et al. Cost of Acute Kidney Injury in Hospitalized Patients. J Hosp Med 2017;12:70–6. [DOI] [PubMed] [Google Scholar]
- 4.Rewa O, Bagshaw SM. Acute kidney injury[mdash] epidemiology, outcomes and economics. Nat Rev Nephrol 2014;10:193–207. [DOI] [PubMed] [Google Scholar]
- 5.Lafrance J-P, Miller DR. Acute Kidney Injury Associates with Increased Long-Term Mortality. Journal of the American Society of Nephrology JASN 2010;21:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grams ME, Sang Y, Coresh J, et al. Candidate Surrogate End Points for ESRD after AKI. J Am Soc Nephrol 2016;27:2851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu VC, Wu CH, Huang TM, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol 2014;25:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu VC, Wu PC, Wu CH, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gammelager H, Christiansen CF, Johansen MB, et al. Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: a population-based cohort study. Crit Care 2014;18:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li PK, Burdmann EA, Mehta RL. World Kidney Day 2013: acute kidney injury-global health alert. Am J Kidney Dis 2013;61:359–63. [DOI] [PubMed] [Google Scholar]
- 11.Silver SA, Harel Z, McArthur E, et al. 30-Day Readmissions After an Acute Kidney Injury Hospitalization. Am J Med 2017;130:163–72.e4. [DOI] [PubMed] [Google Scholar]
- 12.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen YL, Angus DC, Boumendil A, et al. The challenge of admitting the very elderly to intensive care. Ann Intensive Care 2011;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfaadhel TA, Soroka SD, Kiberd BA, et al. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol 2015;10:832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaatten H, De Lange DW, Morandi A, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/= 80 years). Intensive Care Med 2017;43:1820–8. [DOI] [PubMed] [Google Scholar]
- 16.Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016;26:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuck AE, Siu AL, Wieland GD, et al. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 1993;342:1032–6. [DOI] [PubMed] [Google Scholar]
- 18.Pandharipande PP, Girard TD, Jackson JC, et al. Long-Term Cognitive Impairment after Critical Illness. N Engl J Med 2014;370:185–6. [DOI] [PubMed] [Google Scholar]
- 19.Brummel NE, Bell SP, Girard TD, et al. Frailty and Subsequent Disability and Mortality among Patients with Critical Illness. Am J Respir Crit Care Med 2017;196:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiely DK, Cupples LA, Lipsitz LA. Validation and comparison of two frailty indexes: The MOBILIZE Boston Study. J Am Geriatr Soc 2009;57:1532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 2008;168:382–9. [DOI] [PubMed] [Google Scholar]
- 22.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009;57:492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao Y, Dalrymple L, Chertow GM, et al. Frailty, Dialysis Initiation, and Mortality in End-Stage Renal Disease. Arch Intern Med 2012;172:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a Novel Predictor of Mortality and Hospitalization in Hemodialysis Patients of All Ages. J Am Geriatr Soc 2013;61:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAdams-DeMarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol 2013;14:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel-Kader K, Girard TD, Brummel NE, et al. Acute Kidney Injury and Subsequent Frailty Status in Survivors of Critical Illness: A Secondary Analysis. Crit Care Med 2018;46:e380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury Available online: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI%20Guideline.pdf
- 28.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707–10. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 30.Baek SH, Lee SW, Kim SW, et al. Frailty as a Predictor of Acute Kidney Injury in Hospitalized Elderly Patients: A Single Center, Retrospective Cohort Study. PLoS ONE 2016;11:e0156444. [DOI] [PMC free article] [PubMed] [Google Scholar]