Abstract
Depression in adults is associated with deficits in a number of cognitive domains, however it remains less clear how early in development theses deficits can be detected in early onset depression. There are several different hypotheses about the links between cognitive function and depression. For example, it has been argued that executive function deficits contribute to emotion regulation difficulties, which in turn increase risk for depression. Further, it has been suggested that some cognitive deficits, such as episodic memory, may reflect hippocampal abnormalities linked to both depression and episodic memory. We examined these questions in adolescents participating in a longitudinal study of preschool onset depression. We measured cognitive function at adolescence using the NIH toolbox (vocabulary, processing speed, executive function, working memory and episodic memory), and examined relationships of cognitive deficits to depression, emotion regulation, life stress and adversity, as well as hippocampal volume trajectories over three imaging assessments starting at school age. Depression related deficits in episodic memory were found. Youths with either current and past depression showed episodic memory deficits even after controlling for other psychopathology and family income. Depression severity, emotion dysregulation and life stress/adversity all predicted episodic memory impairment, as did smaller intercepts and slopes of hippocampal growth over time. Modest relationships of depression to hippocampal volume and strong relationships between emotion regulation and both episodic memory and hippocampal volume were found. These data are consistent with prior work in adults linking depression, episodic memory, emotion regulation, life stress/adversity and hippocampal volume in adults and suggest similar relations are evident as early as adolescence when memory systems are under development.
Keywords: Depression, episodic memory, hippocampus, emotion regulation
General Scientific Summary
This study suggests that problems with episodic memory are apparent even among adolescents with a history of depression, suggesting that they emerge relatively early in the course of depression. Further, this work also shows that episodic memory impairments are related to stress/adversity, emotion regulation and hippocampal volume, consistent with a developmental model of cumulative stress contributions to cognitive impairment associated with depression.
A growing body of research has begun to examine whether depression is associated with deficits in aspects of cognitive function such as executive function, working memory, inhibitory control, episodic memory and processing speed (Bora, Harrison, Yucel, & Pantelis, 2013; Gotlib & Joormann, 2010). Collectively this work confirms that individuals with clinical depression can experience impairments in a range of cognitive domains, that such cognitive deficits may be an unmet treatment need that persist beyond remission of active depression (Medicine, 2015), and further, that such cognitive deficits may play a key role in risk for depression onset and recurrence. In particular, there is a body of research pointing to impairments in both executive function and episodic memory among individuals with a history of Major Depressive Disorder (MDD). As discussed below, deficits in executive dysfunction are hypothesized to be a risk factor for depression by virtue of their contribution to difficulties with regulating control over negative thoughts and rumination (Hankin, Young, Gallop, & Garber, 2018; Snyder & Hankin, 2016). Deficits in episodic memory are thought to potentially reflect a history of life stress, altered emotion regulation, and stress reactivity that may negatively impact hippocampal structure/function and the cognitive functions this structure supports, including episodic memory. Much of the work on cognitive function in MDD has been in adults. At least some of the hypotheses about the role of cognitive function in risk for depression suggest that such deficits might be apparent earlier in adolescence when these systems are developing (Ghetti & Bunge, 2012; Lee, Ekstrom, & Ghetti, 2014; Sowell, Delis, Stiles, & Jernigan, 2001), especially among those with early signs and symptoms of depression. Thus, the goal of the current work was to examine cognitive function among adolescents with both current and past depression and to examine factors earlier in development that might contribute to this hypothesized relationship. To do so, we used data from a prospective longitudinal study that started at preschool age and extended into late adolescence with multiple imaging waves to examine whether childhood depression is associated with impairments in episodic memory and/or executive function, and whether these are related to impairments in emotion regulation and or hippocampal volume growth trajectories.
Patterns of Cognitive Impairment in Major Depressive Disorder
There are now a number of meta-analyses that have summarized the existing literature on cognitive impairment in adults with depression. For example, one meta-analysis found that MDD was reliably associated with impairments on tasks thought to measure various aspects of executive function, and that these impairments could not be accounted for simply by slower processing (Snyder, 2013). Several meta-analysis surveying a broader literature have concluded that individuals with MDD experience deficits in memory and attention domains as well in executive function (Ahern & Semkovska, 2017; Bora, et al., 2013; Rock, Roiser, Riedel, & Blackwell, 2014). Thus, together, these meta-analyses suggest that individuals with MDD experience deficits in a number of different cognitive domains. Interestingly, work has also examined deficits in future episodic simulation in MDD, which is thought to depend on many of the same psychological and neural processes as episodic recall of past memories (Mullally & Maguire, 2014; Schacter, 2012; Schacter, Benoit, De Brigard, & Szpunar, 2015; Szpunar, 2010). A further study found that individuals with MDD showed deficits in generating episodic, but not non-episodic, details about future events (King, Macdougall, Ferris, Herdman, & McKinnon, 2011). Two meta-analyses focused on younger populations also found evidence for broad cognitive impairment in child and adolescent depression, including significant impairments in episodic memory and executive function (Goodall et al., 2018; Wagner, Muller, Helmreich, Huss, & Tadic, 2015).
Comparisons against other patient groups suggests that deficits in at least some aspects of episodic memory may be particularly characteristic of MDD (Goodall, et al., 2018). For example, research has found that individuals with either MDD or schizophrenia showed impairments on a range of cognitive tests, but that individuals with MDD were more impaired than those with schizophrenia on delayed recall of information (Gooren, Schlattmann, & Neu, 2013). Other work found that inpatients with either MDD or schizophrenia showed impairments in a number of cognitive domains, including episodic memory, but that individuals with schizophrenia showed more improvement in episodic memory with clinical improvement than did individuals with MDD (Neu, Gooren, Niebuhr, & Schlattmann, 2017). Bourke and colleagues found that individuals with MDD were more impaired than those with social anxiety disorder on both verbal learning and memory and spatial working memory (Bourke et al., 2012).
Models of the Role of Cognitive Impairment in Risk for Depression
The literature reviewed above suggests that individuals with depression experience deficits in a number of different cognitive domains, with potentially particularly strong evidence for executive function and episodic memory. Further, there is some evidence that such deficits are already evident in depressed children and adolescents. As such, key questions are what mechanisms might link depression and cognitive impairment and whether such impairments are present early in the course of depression. Answers to these questions are key to inform earlier preventive interventions targeting cognitive development in depression risk trajectories.
Reduced Cognitive Reserve as a General Risk Factor of Psychopathology
One hypothesis is the possibility that lower “cognitive reserve,” as indexed by lower IQ assessed prior to illness onset, is a general risk factor for developing psychopathology of many forms, including psychosis, depression and anxiety (Koenen et al., 2009). Given that IQ is correlated with cognitive function across many domains, at least some aspects of the broad cognitive dysfunction associated with MDD may reflect this risk factor that is present prior to illness onset and which remains even when acute episodes remit. As shown in Table 1, this hypothesis would suggest that cognitive impairments should be relatively broad even among young individuals with depression (e.g., adolescents) and that they should be present even among those with a history of depression who are out of episode. Further, this hypothesis might also predict that such deficits should be worse among adolescents with more severe depression if a greater magnitude of reduced “cognitive reserve” is associated with stronger risk for depression. However, this hypotheses suggests that such cognitive impairments should not be specific to depression, but will be associated with most forms of psychopathology, including anxiety and externalizing disorders.
Table 1:
Predictions from models linking cognitive function to depression
| Model | Predictions |
|---|---|
| IQ and Cognitive Reserve | 1) Impairments across all cognitive domains 2) Impairments present in adolescents with either current or past depression 3) Greater impairments in adolescents with more severe or more recurrent depression 4) Present in individuals with other forms of psychopathology, such as anxiety and externalizing disorders |
| Executive Function and Emotion Regulation | 1) Executive function impairments present in adolescents with either current or past depression 2) Executive function impairments with emotion regulation impairments 3) Not present in individuals with externalizing disorders (might be present in anxiety) |
| Life Stress/Adversity, Emotion Regulation, Hippocampus and Episodic Memory | 1)Episodic memory impairments present in adolescents with either current or past depression 2)Episodic memory impairments associated with reduced hippocampal volumes 3)Episodic memory impairments associated with emotion regulation impairments 4)Episodic memory impairments memory associated with life stress and adversity 5)Not present in individuals with externalizing disorders (might be present in anxiety) |
Executive Function and Emotion Regulation as Risk Factors for Depression
It is also possible that at least some aspects of cognitive impairment associated with MDD reflect processes associated with either risk factors specifically for MDD or the consequences of experiencing MDD. For example, another hypothesis is that deficits in executive function contribute to impairments in emotion regulation (e.g. rumination, negative preoccupation) a process that increases risk for depression. Hankin et al found evidence for prospective associations between deficits in executive function, “dependent” stress (stress that could be created by the individual’s behavior) and subsequent rumination, which in turn predicted increases in depression (Snyder & Hankin, 2016). Relatedly, Joormann and colleagues have argued that difficulties updating the contents of working memory and inhibiting the processing of negative information contribute to disturbances in emotion regulation, including rumination, which in turn contribute to risk for depression (Foland-Ross & Gotlib, 2012; Joormann & Quinn, 2014; Kircanski, Joormann, & Gotlib, 2012; Yoon, LeMoult, & Joormann, 2014; Zetsche, D’Avanzato, & Joormann, 2012). These hypotheses both suggest that deficits in executive function are a risk factor for depression and should be (see Table 1): 1) present even among adolescents; 2) present in both depressed individuals and those with a history of depression; 3) associated with difficulties in emotion regulation; and 4) more specifically associated with depression than other forms of psychopathology, such as externalizing disorders.
Life Stress/Adversity, Hippocampus, and Episodic Memory in Relationship to Depression
As another example, a number of researchers have argued that MDD is associated with chronic stress and adversity as well as alterations in stress reactivity and emotion regulation (Andersen & Teicher, 2008; MacQueen & Frodl, 2011; Tafet & Nemeroff, 2016), providing a theoretical framework for understanding the consistent evidence for a link between MDD and reduced hippocampal volume (Schmaal et al., 2016). Given the large literature linking hippocampal function and structure to episodic memory (Cohen et al., 1999; Eichenbaum et al., 2016; Squire & Dede, 2015; Squire & Knowlton, 1995), this framework also provides context for understanding a link between episodic memory impairments and MDD. It has been suggested that early life stress and adversity contribute to early hippocampal dysfunction (Anacker, O’Donnell, & Meaney, 2014; Lajud & Torner, 2015) and associated deficits in episodic memory and emotion regulation, with the latter a risk factor for the development of depression (van Bodegom, Homberg, & Henckens, 2017). This hypothesis is consistent with the evidence for reduced hippocampal volume even among first-episode depression patients (Cole, Costafreda, McGuffin, & Fu, 2011). Further, it has also been argued that hippocampal volume loss in depression may also reflect a type of neuro-toxicity associated with a cumulative history of stress and adversity, disrupted emotion regulation, stress reactivity and excessive HPA mediated glucocorticoid release (Sheline, 1996, 2011). This latter argument is consistent with evidence suggesting that hippocampal volume reductions are even more apparent among individuals with longer illness duration or more than one episode of MDD (McKinnon, Yucel, Nazarov, & MacQueen, 2009; Sheline, Sanghavi, Mintun, & Gado, 1999; Sheline, Wang, Gado, Csernansky, & Vannier, 1996). These hypotheses predict that episodic memory deficits should be (see Table 1): 1) present early in life in individuals at risk for depression; 2) associated with life stress/adversity and emotion regulation difficulties; 3) present even among individuals with a history of depression who are not currently depressed; and 4) associated with depression, but not some other forms of psychopathology (e.g., externalizing disorders).
Somewhat surprisingly, despite the clear evidence for both hippocampal volume reductions and episodic memory impairments in MDD, there has been little research examining the links between the two. A recent exception to this was work by Schweizer and colleagues in a general population sample (Schweizer, Kievit, Emery, Cam, & Henson, 2018). They found that depression was linked to both subjective and objective memory deficits, and both these deficits were in turn linked to smaller hippocampal volume. However, the correlations of objective memory deficits with depression and hippocampal volume did not survive correction for anxiety and general cognitive function, though it is not clear whether controlling for anxiety (often comorbid with depression) and general cognition (associated with episodic memory) controls for the variance of interest. There was, however, no direct relationship between depression and hippocampal volume in this community sample even without any covariates. Further, there has been no data to our knowledge about this link in adolescent depression when both memory ad hippocampal systems are under active development (Ghetti & Bunge, 2012; Lee, et al., 2014; Sowell, et al., 2001). Thus, there is a clear need for more research in child and adolescent samples with current or past clinical depression examining potential links between hippocampal volume and episodic memory.
Adolescence may be a particularly important time for examining the relationship between depression and cognitive function, as it is a time when many cognitive functions are undergoing their final evolution, with such procesess key for many of the development tasks that predominate in this stage of development. For example, adolescence is a time when executive functions are in the final stages of maturing, and are critical for the enhanced demands on self-regulation and emotion regulation that are needed with the shift to prioritizing peer interactions and the move to independence from the family unit (Casey, 2015; Guyer, Silk, & Nelson, 2016; Modecki, Zimmer-Gembeck, & Guerra, 2017). Similarly, adolescence may be a time where disruptions in emotion regulation and the experience of prior life stress may start to manifest in episodic memory deficits, potentially via hippocampal dysfunction, though it is possible that such relationships could also be present even earlier in life.
The goal of the current study was to generate further evidence about each of the theories described above linking cognitive function with depression, using data from a longitudinal study of youth with early childhood depression. We hypothesized that youth with either current depression or past depression would show impaired executive function and episodic memory, but that they might also show impairments in other cognitive domains as well. We hypothesized that the severity of such cognitive impairments would be greater in children with more severe depression (both current and past history). We also examined whether episodic memory deficits were related to hippocampal volume in this sample, and whether hippocampal volume mediated any association between depression and episodic memory. Finally, because of the literature above suggesting a putative link among depression, hippocampal volume, stress reactivity and life stress, we also examined associations to emotion regulation and life stress/adversity. As described above, it has been hypothesized that a key reason for a link between MDD and hippocampal volume, and by extension episodic memory, is a shared association with life stress and disrupted emotion regulation (MacQueen & Frodl, 2011). If so, we would expect that both life stress/adversity and emotion dysregulation may also be associated with both episodic memory and hippocampal volume reduction.
Methods
Participants
Participants were a sub-sample of youth enrolled in an ongoing, longitudinal study focused on examining the trajectory of preschool-onset depression and brain development (see Figure S1 for a flowchart of the study). At baseline, 306 children aged 3.0-5.11 years and their primary caregivers were recruited from the St. Louis area, using a checklist to oversample preschoolers with elevated symptoms of depression (J. Luby, Heffelfinger, Koenig-McNaught, Brown, & Spitznagel, 2004) and then followed longitudinally. At school age (ages 6.11 to 12.11), healthy children and those with a history of depression from this sample were invited for participation in scanning, along with recruitment of an additional 42 healthy children (N = 210 completed the first wave of scanning). These children have had between 1 and 9 assessment waves and between 1 and 4 scan waves (Figure S1). Given the goals of the study, we focused our analysis on adolescents (N = 172) from the most recently completed assessment/scan wave (T9/MRI 4). Of these, 164 completed the NIH Toolbox Cognitive Assessment (see below). Exclusion criteria included (i) head injury with loss of consciousness >5 min; (ii) neurological illness; (iii) diagnosis of an Autistic Spectrum Disorder; (iv) treatment for lead poisoning; or (v) contraindications for MRI scanning (added starting at first scan wave). All study methods were reviewed and approved by the Institutional Review Board at the Washington University School of Medicine (IRB #201502094; PDS-III Imaging). Written informed consent and assent was obtained from all study participants.
Clinical and Emotion Regulation Assessment
Diagnostic Interviews:
Trained staff from the Early Emotional Development Program conducted up to nine in-person assessment sessions with participants and their primary caregivers over the course of the study (Figure S1). The children were between the ages of 3.0-5.11 at the time of their first interview (T1) and between the ages of 13.3-19.4 at the most recent assessment wave (T9). The first three interviews (T1-T3) used the Preschool-Age Psychiatric Assessment (PAPA) (Egger, 2009; Egger et al., 2006) as a diagnostic assessment. The PAPA is designed for diagnostic use with children ages 2.0-6.0 years (but has been used up to age 8.0), has acceptable reliability (Egger, et al., 2006), and consists of a series of developmentally appropriate questions answered by the primary caregiver which cover the DSM-IV criteria for all Axis I disorders, including MDD, ADHD, and anxiety disorders. The two-week duration requirement for MDD was omitted prior to age 8 based on prior data suggesting it is not a clinically sensitive cut-off in the preschool age group (Gaffrey, Belden, & Luby, 2011; J. L. Luby et al., 2002). The next five interviews (T4-T8) used the Childhood and Adolescent Psychiatric Assessment (CAPA) that allows for assessment of participants up to age 18. The CAPA probes for symptoms of a variety of psychiatric disorders as above but, unlike the PAPA, it makes use of reports from the child or adolescent in addition to the primary caregiver (Angold & Costello, 2000). The most recently completed interview (T9) used the KIDDIE Schedule for Schizophrenia and Affective Disorders (KSADS) (Kaufman et al., 2013; Kaufman et al., 1997), which also makes use of both primary caregiver and adolescent/young adult self-report. All diagnostic interviews were audiotaped and reviewed for reliability using the methods previously reported (J. L. Luby, Belden, Pautsch, Si, & Spitznagel, 2009). Caregiver and youth reports were combined using the methods described by Bird (Bird, Gould, & Staghezza, 1992).
We computed both categorical and dimensional childhood psychopathology scores for each child, with one version for just the most recent assessment wave (T9 – Current Depression) and one version that spanned from preschool through the assessment wave prior to the one that included the NIH Toolbox measures (i.e., T1-T8 – Cumulative Depression, see Figure S1). For the categorical scores, depression scores were coded as a 1 if the youth met criteria for either Major Depression, Depression Disorder NOS or Dysthymia based on either caregiver report or youth report and 0 if they did not. Anxiety scores were coded as a “1” if the youth met criteria for either social anxiety disorder, generalized anxiety disorder, specific phobia or post-traumatic stress disorder, and “0” if they did not. Externalizing scores were coded as “1 “if the youth met criteria for either opposition defiant disorder, conduct disorder or attention-deficit hyperactivity disorder.
We created analogous dimensional scores by summing the core depression symptoms, the core anxiety symptoms or the core externalizing symptoms as endorsed by either the caregiver or the youth for each assessment wave. For the versions that spanned from preschool through T8, we averaged across waves. Youth in the current analyses had data from a median of 8 assessment waves (range 2 to 9), with 94.5% of the youth having 4+ assessment waves, and 77% of the youth having 7+ assessment waves.
Emotion Regulation:
In order to assess the youths’ emotional regulation skills, the caregivers completed the Emotion Regulation Checklist (ERC) (Shields & Cicchetti, 1997) at each of the last four assessment waves (T6-T9). The ERC targets affective lability, intensity, valence, and flexibility and includes both positively and negatively weighted items rated on a 4-point Likert scale. It has two subscales: emotional regulation (higher scores indicate more positive/effective emotion regulation) and negative lability (higher scores indicate more emotional lability and less effective emotion regulation). In addition, the youth completed the child-report version of the Cognitive Emotion Regulation Questionnaire (CERQ-K) (Garnefski, Rieffe, Jellesma, Meerum Terwogt, & Kraaij, 2007) (Shields & Cicchetti, 1997) at each of the last four assessment waves (T6-T9). The CERQ-K uses items with a 5 point Likert scale to measure children’s tendencies to engage in a variety of emotion regulation strategies typically thought of as either associated with positive or effective emotion regulation (Acceptance, Positive Refocusing, Refocus on Planning, Positive Reappraisal, Putting it into Perspective) or negative or less effective emotion regulation (Self Blame, Rumination, Catastrophizing, Other-Blame). We created two composite scores, one for current emotion dysregulation (T9) and one for emotion dysregulation across the previous three assessment waves (T6-T8, as these measures were not available at earlier timepoints) to assess the degree to which current and historical emotion regulation related to cognitive function and hippocampal volume. For each composite score, we z-scored the individual items for parent and youth, reverse scored items so that a higher score indicated greater emotion dysregulation, and averaged them.
Life Stress and Adversity
We created a life stress/adversity score for each child, aggregating the occurrence of indicators of life stress/adversity from T1 to T9. Children received a point for each of the following, which were reported as part of the PAPA/CAPA/KSADs interviews described above: 1) single parent household; 2) parental arrest; 3) experienced foster care; 4) physical abuse; 5) sexual abuse; 6) poor coverage of financial needs; and 7) income-to-needs below the poverty line.
Cognitive Assessment
Youth completed a subset of the NIH Toolbox cognitive measures at their most recent assessment wave (T9) (see http://www.nihtoolbox.org for full description) (Bleck, Nowinski, Gershon, & Koroshetz, 2013; Gershon, Wagster, et al., 2013; Hodes, Insel, Landis, & Research, 2013). Using state-of-the-science methods, the battery was developed to be comprehensive, amenable for use in longitudinal studies, have relatively brief administration times, and to be psychometrically sound. Normative data was generated with a nationally representative sample of close to 5,000 participants. The Toolbox measures are either computer-administered and scored using algorithms embedded in the software, or tester-administered with the results input through a standard interface. We administered tasks from the following five domains, using the age-corrected scores as the dependent measures.
Toolbox Picture Sequence Memory Test (TPSMT; Episodic memory domain):
Participants are presented with a series of pictures depicting activities or events that could occur in a particular setting (i.e., working on a farm) (Bauer et al., 2013; Dikmen et al., 2014). After being shown the pictures in order, the pictures appear in scrambled order on the screen and they have to try to arrange them in correct order on the screen. They are given multiple trials with the same set of pictures. The TPSMT has moderate test-retest reliability and reasonable convergent validity (Bauer, et al., 2013; Dikmen, et al., 2014).
Toolbox List Sorting Working Memory Test (TLSWMT; Working memory domain):
This is a variant of the letter-number sequencing test (Gold, Carpenter, Randolph, Goldberg, & Weinberger, 1997) that uses pictures rather than words or letters (Tulsky et al., 2014; Tulsky et al., 2013). Participants are presented with a series of pictures of animals or foods of different sizes, accompanied by the name presented auditorily by an iPad, and asked to repeat the items back in order from smallest to largest. The TLSWMT starts with a single category (i.e., animals). Participants are presented with a 2-item list, and if they get it correct, the next trial increases to 3 items, and so on until a maximum of 7 is reached. Participants have two opportunities (different trials) to provide a correct answer at each list length, and continue on to the next length if they get at least one of the trials correct. Participants then progress to a next phase where the trials interleave two different categories (i.e., animals and food). The participant is asked to first organize and repeat back the items for one category (i.e., animals) and then the other. The TLSWMT has good test-retest reliability and reasonable convergent validity (Tulsky, et al., 2013).
Toolbox Flanker Task (TFT; Selective Attention):
This is a variant of the Eriksen Flanker task (Eriksen & Eriksen, 1974) that was adapted from the Attention Network Task (Fan, McCandliss, Sommer, Raz, & Posner, 2002; Rueda et al., 2004). There are four flanking arrows (2 on the outer left and 2 on the outer right) that are all facing the same way, either left or right. The middle arrow is then either facing the same way (congruent trial) or a different way (incongruent trial). Participants push a button to indicate whether the middle arrow is facing left or right. Scoring is based both on speed and accuracy. The TFT has excellent test-retest reliability and moderate convergent validity (Zelazo et al., 2014; Zelazo et al., 2013).
Toolbox Pattern Comparison Processing Speed Test (TPCPST; Processing Speed domain):
This task (Carlozzi, Beaumont, Tulsky, & Gershon, 2015; Carlozzi et al., 2014; Carlozzi, Tulsky, Kail, & Beaumont, 2013) was modeled on the Pattern Comparison Task developed by Salthouse (Salthouse, Babcock, & Shaw, 1991). Participants are shown two pictures and asked to determine whether the pictures are the same or not. The score is based on how many items they are able to complete correctly in a specific amount of time. The TPCPST has good test-retest reliability and moderate convergent validity (Carlozzi, et al., 2014; Carlozzi, et al., 2013).
Toolbox Picture Vocabulary Task (TPVT; Verbal IQ):
This is a variant on the Peabody Picture Vocabulary Test (PPTV) (Gershon et al., 2014; Gershon, Slotkin, et al., 2013). Participants hear audio files of words and are shown four pictures in a square, one of which depicts the concept, idea or object referenced by the auditorily presented words. The participant is asked to touch the picture that matches the word. The TPVT has good test-retest reliability and strong convergent validity (Gershon, et al., 2014; Gershon, Slotkin, et al., 2013; Mungas et al., 2014).
Imaging Acquisition
The focus of imaging data in the current manuscript is on how the trajectory of hippocampal volume development relates to episodic memory function in children with and without a history of preschool onset depression. As such, we focus on the hippocampal data from scans 1 to 3 which have already been processed through the Freesurfer Longitudinal pipeline (see below). Structural images were also acquired at Scan 4, co-incident with the time at which the cognitive data were acquired. However, Scan 4 switched to using a 3.0 Siemen’s Tesla Prisma whole-body scanner with a 32-channel head coil using Human Connectome Project style acquisitions (Glasser et al., 2016). While brain structure assessed at the same time as cognitive function is of interest, we are still in the process of determining the best way to integrate the first 3 scan waves of Trio scanner data with the newer Prisma data. Thus, the current analyses focus scan waves 1 through 3, which were performed using a 3.0 Tesla Siemen’s Tim Trio whole-body scanner with a 12-channel head coil. Quality assurance measures included having subjects practice in an MRI simulator, evaluating head motion during structural scans, and recollection of data if necessary. Structural data were obtained using two 3D T1-weighted scans (TR 2,300 ms, TE 3.16 ms, TI 1,200 ms, flip angle 8°, 160 slices, 256 × 256 matrix, field of view 256 mm, 1.0 mm3 voxels, 6:18 min per scan) in the sagittal plane using a magnetization-prepared rapid gradient echo (MPRAGE) sequence. Two resting-state fMRI (rsfMRI) scans were obtained during the same session with T2*-weighted gradient-echo echo-planar sequence; neither of these modalities is of focus here.
Structural Imaging Processing
Hippocampal volumes were generated using the same Longitudinal Freesurfer processing stream as in Luby et al. (2016)(J. L. Luby et al., 2016). Specifically, for each scan session, the two MPRAGE scans were assessed visually, and the best in terms of quality and contrast selected by blind raters. Processing of structural data was accomplished using the FreeSurfer Longitudinal pipeline v5.3 rhttp://surfer.nmr.mgh.harvard.edu1 (Reuter, Rosas, & Fischl, 2010) with visual inspection of the white and pial surfaces for errors and regeneration with manual intervention to correct for errors when necessary by an experienced rater blinded to diagnostic category. Processing steps included skull stripping, atlas registration, spherical surface registration, and parcellation. Importantly, the longitudinal ‘stream’ includes initialization from an unbiased within-subject template (created across the longitudinal scans), which reduces the bias that would otherwise be present in selecting a single scan as “baseline”. Using an unbiased longitudinal template significantly increases reliability and statistical power (Reuter, Schmansky, Rosas, & Fischl, 2012). For approximately 10% of sessions, poor scan quality (in both MPRAGEs) required excluding those sessions from the longitudinal analysis (n = 29, 22, and 18 at the three waves, respectively). In those cases, FreeSurfer’s longitudinal stream was run using the remaining available sessions for that participant. Volume of the left and right hippocampus in the subject’s ‘native space’ were obtained FreeSurfer’s “aseg.stats” report. We did not have a priori hypotheses about left or right hippocampus, and thus we averaged the two together, though the patterns were the same for left or right hippocampus. To generate individual intercepts and slopes of hippocampal volume for each youth, we used a growth curve model that included both random intercept and random slope components (with an unstructured covariance matrix between the two). Time was coded as wave number (1, 2, 3). The model included age at scan 1 (centered at mean=10.3 years), quadratic age at scan 1, and gender (1=male, 0=female) as covariates. This was the same type of model used in our prior work to examine the relationship between maternal support and hippocampal developmental trajectories (J. L. Luby, et al., 2016). Slopes and intercepts of hippocampal volume were available for 160 of the 164 children with NIH Toolbox Data. As a comparison “control” region, we examined the caudate using analogous measures given some previous research suggesting a role for caudate in links among stress/adversity and depression (Frodl et al., 2017).
Statistical Analysis
We started by using the categorical depression scores to ask whether children with either a current depression diagnosis or who ever had a depression diagnosis differed in cognitive function. To do so, we used Multivariate Analysis of Variance (MANOVA) with the five cognitive variables as the dependent measures and both age at cognitive testing and gender as covariates. Significant omnibus tests were followed up by post-hoc comparisons using false discovery rate. We examined the anxiety and externalizing scores as a comparison to address questions of specificity to depression. We then examined relationships to dimensional depression severity and recurrence scores using partial correlations controlling for age, gender, and family income. We next examined the relationships of the cognitive scales that were associated with depression to emotion regulation and life stress/adversity, again using partial correlations, though in the case of stress/adversity, we did not control for family income since that was part of the stress/adversity variable. Lastly, we examined the relationship of the hippocampal slopes and intercepts to episodic memory, depression, emotion regulation and stress/adversity, again using partial correlations. Of note, all analyses reported below held when we controlled for whether or not the child was currently taking any psychotropic medications, or had a history of taking psychotropic medications.
Results
Participant Characteristics
Of the 164 children with complete NIH Toolbox Data, there were 85 youth with no current or past history of depressive disorder (N-MDD), 58 youth with past, but not current, depressive disorders (P-MDD) and 21 youth with a current depressive disorder (C-MDD). As shown in Table 1, these three groups did not differ in age at T9 assessment, the percentage of females, or the percentage of African-American youth. However, N-MDD had a higher family income than both C-MDD and P-MDD. C-MDD were more likely to have a current anxiety disorder compared to the other groups, but both C-MDD and P-MDD were more likely to have a lifetime history of anxiety disorder than N-MDD. C-MDD were more likely to have a current externalizing disorder than P-MDD, who were in turn more likely than N-MDD. Both C-MDD and P-MDD were more likely to have a lifetime history of externalizing disorders compared to N-MDD. Of the children who were recruited into the scanning wave of the study (N = 210), those who did and did not complete the cognitive battery at T9 did not differ on any of the following characteristics at the first scan wave (MRI 1): age, family income, dimensional depression, anxiety or externalizing scores, or lifetime history of depression, anxiety or externalizing disorders (all ps > .19).
What Types of Cognitive Impairment Are Associated with Depression?
We started by using a MANOVA to examine whether youth with current or past depressive disorders differed from those without in any of the five domains of cognitive functions. The omnibus MANOVA was significant (Roy’s Largest Root F(5,156) = 3.85, p = .003, partial η2 = .11; Pillai’s Trace F(10,312) = 2.23, p = .016, partial η2 = .067). As shown in Figure 1, follow-up univariate (ANOVA) tests using the 3 groups (N-MDD, P-MDD, and C-MDD) indicated that the groups differed on the episodic memory domain (Picture Sequencing test, F(2,159) = 6.86, p = .001, partial η2 = .079, passes FDR correction), but not on any of the other four cognitive tests (all ps > .32). As can be seen in Figure 1, both the C-MDD and P-MDD groups had significantly worse performance than the N-MDD group (ps < .005), but did not differ from each other (p = .32). As noted above, the depressive disorder groups differed in family income, and family income can be related to cognitive function. Thus, we repeated this analysis with family income as a covariate, with the same results (Roy’s Largest Root F(5,156) = 3.648, p=.004, partial η2 = .107; Pillai’s Trace F(10,312) = 2.16, p = .02, partial η2 = .066).
Figure 1: Depressive Disorder Group Differences on the NIH Toolbox.
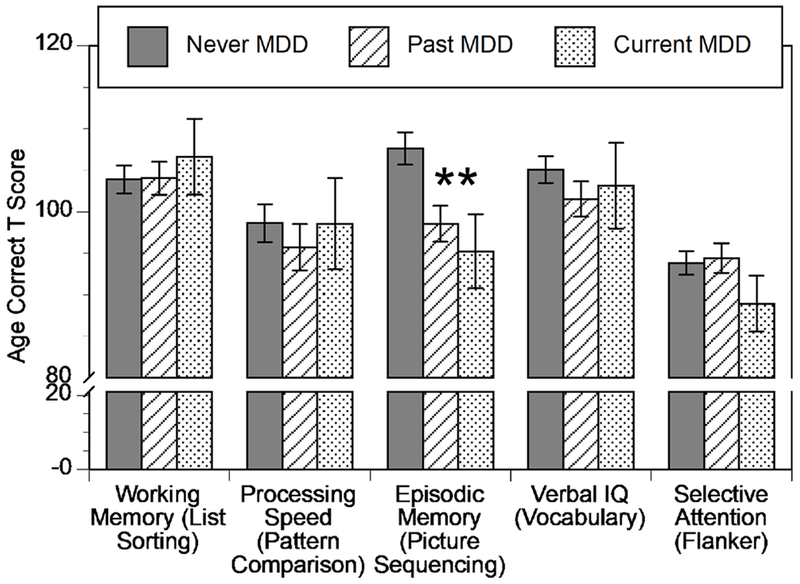
Graph of mean and standard error for each of the five NIH Toolbox measures as a function of Depressive Disorder Group. Asterisks indicate significant differences among depressive disorder groups.
How Specific are Cognitive Impairments To Depression?
The cognitive reserve theory (Table 1) would suggest that cognitive impairments should not be specific to depression, while the emotion regulation and episodic memory deficit theories would suggest more specificity to depression. Thus, we computed the same analysis for children with a current anxiety disorder (N = 51), a past but not current anxiety disorder (N = 47) or no history of anxiety disorder (N = 66). The omnibus MANOVA was not significant (Roy’s Largest Root F(5,156) = 1.56, p= .18, partial η2 = .048; Pillai’s Trace F(10,312) = 1.16, p= .32, partial η2 = .036). We also computed the same analysis for children with a current externalizing disorder (N = 22), a past but not current externalizing disorder (N = 53) or no history of externalizing disorder (N = 89). This omnibus MANOVA was significant (Roy’s Largest Root F(5,156) = 6.578, p < .001, partial η2 = .174; Pillai’s Trace F(10,312) = 3.07, p = .001, partial η2 = .09). As shown in Figure S2, follow-up univariate tests indicated that the externalizing groups differed on both the episodic memory (Picture Sequencing, F(2,159) = 8.37, p < .001, partial η2 = .095, passes FDR correction) and vocabulary domains (Picture Vocabulary, F(2,159) = 10.56, p < .001, partial η2 = .117, passes FDR correction), but not on any of the other three cognitive tests (all ps > .13). The pattern for externalizing disorders was different than for depressive disorders (Figure S2). For both episodic memory and vocabulary, the youth with current externalizing disorders differed from both the youth with only past externalizing and those with no history of externalizing (all ps < .001), who did not differ from each other (ps > .84).
As described above, both current depressive and externalizing disorders were related to episodic memory performance, though only youth with past depressive disorders, and not those with past externalizing, also showed impaired episodic memory. Nonetheless, as shown in Table 1, rates of current externalizing disorders were higher among youth with both current and past depressive disorders, raising the question of whether there is a relationship of depressive disorders with episodic memory independent of a relationship with current externalizing disorder. We addressed this by computing a univariate ANOVA with picture sequencing as the dependent variable and both depressive disorder (none, past, current) and externalizing disorder (none, past, current) as factors. This analysis indicated significant main effects of both depressive disorder (F(2,153) = 3.22, p = .043, partial η2 = .04) and externalizing disorder group (F(2,153) = 4.32, p = .015, partial η2 = .053). Further follow-up analyses showed that youth with both current and past depressive disorders had worse episodic memory than those with no current or past MDD (ps <.05), while youth with current externalizing had worse episodic memory than those with past but not current externalizing or no externalizing disorders (ps <.05).
Relationships of Episodic Memory to Depression Severity and Recurrence
Both the cognitive reserve and episodic memory hypotheses predict that greater episodic memory impairments should be associated with more severe and/or recurrent depression. Thus, we examined the relationships of episodic memory to dimensional symptom counts on the KSADs at T9 (current depression), the average dimensional symptom counts across the course of the study from T1 to T8 (cumulative depression), and the number of waves on which an adolescent had received a diagnosis of depression, using regressions that controlled for T9 age, gender and family income. As shown in Table 2, all three measures of depression severity and recurrence were associated with reduced episodic memory, with all partial correlations passing FDR correction. Importantly, cumulative depression symptoms remained a significant predictor even when anxiety and externalizing symptom counts from T1 to T8 were included in model (r = −.18, p =.037, CI+− .65 to −.02).
Table 2:
Participant Characteristics
| No Current or Past History of Depressive Disorder (N-MDD) N = 85 | Past History of Depressive Disorder, but not Current (P- MDD) N = 58 | Current Depressive Disorder (C- MDD) N = 21 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Statistic | p-value | Group Difference | |
| Age at T9 Assessment (years) | 15.6 | 1.23 | 16.0 | 1.04 | 16.05 | 1.02 | F = 2.24 | .11 | NA |
| % Female | 53% | 43% | 62% | X2 = 2.56 | .28 | NA | |||
| % African American | 34% | 40% | 38% | X2 = 8.0 | .09 | ||||
| Family Income at T9 Assessment | 3.34 | 1.00 | 2.93 | 1.17 | 2.76 | 1.12 | F = 3.67 | .028 | N>P; N>C |
| % Maternal History of Depressive Disorder | 41% | 68% | 90% | F = 4.82 | .009 | N>P; N>C | |||
| % Current Anxiety Disorder | 21% | 33% | 67% | F = 8.93 | <.001 | C>P; C>N | |||
| % Lifetime Anxiety Disorder | 39% | 78% | 95% | F = 21.17 | <.001 | C>N; P>N | |||
| % Current Externalizing Disorder | 5% | 16% | 43% | F = 12.11 | <.001 | C > P > N | |||
| % Lifetime Externalizing Disorder | 22% | 67% | 81% | F = 25.99 | <.001 | C>N; P>N | |||
Relationships of Episodic Memory to Emotion Dysregulation.
The hypotheses outlined in Table 1 suggest that worse episodic memory should be associated with greater emotion dysregulation. As shown in Table 3 and Figure 3, partial correlations controlling for age, gender and family income provided evidence for these predicted relationships, with both current emotion dysregulation and historical emotion dysregulation showing significant relationships to worse episodic memory function. Of note, contrary to the predictions made by the executive dysfunction model, we did not see any relationships between emotion regulation and executive function (ps >.5). Not surprisingly, partial correlations controlling for age, gender and family income also showed that current emotion dysregulation was associated with current depression severity (r = .51 , p <.001, CI+− .69 to .43) as were the measures of emotion dysregulation and depression from T6-T8 (r = .53 , p <.001, CI+− .72 to .46).
Table 3:
Partial correlations between measures of depression with episodic memory and hippocampal intercepts slopes, controlling for age and gender.
| Episodic Memory | Hippocampal Intercept | Hippocampal Slope | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r | + 95% CI | − 95% CI | r | + 95% CI | − 95% CI | r | + 95% CI | − 95% CI | |
| Depression Severity and Recurrence Measures | |||||||||
| T9 Depressive Disorder Symptom Count | −.16*# | −.29 | −.03 | −.003 | −.13 | .13 | −.01 | −.14 | .12 |
| T1-T8 Depressive Disorder Symptom Count | −.23***# | −.36 | −.10 | −.04 | −.17 | .09 | −.04 | −.17 | .09 |
| Number of Waves with Diagnosis of Depression | −.17*# | −.30 | −.04 | −.02 | −.15 | .11 | −.01 | −.14 | .12 |
| Emotion Regulation Measures | |||||||||
| T9 Emotion Regulation Composite | −.26***# | −.40 | −.14 | −.19**# | −.32 | −.06 | −.17*# | −.30 | −.04 |
| T6-T8 Emotion Regulation Composite | −.22***# | −.35 | −.09 | −.14*# | −.27 | −.01 | −.19**# | −.32 | −.06 |
| Life Stress/Adversity | |||||||||
| Cumulative Stress and Adversity (T1 to T9) | −.20**# | −.33 | −.07 | −.30***# | −.44 | −.18 | −.28***# | −.42 | −.16 |
p < .05, one-tail;
p < .01, one-tail;
p < .005, one-tail
Passes FDR correction
Figure 3: Episodic Memory and Hippocampal Volume.
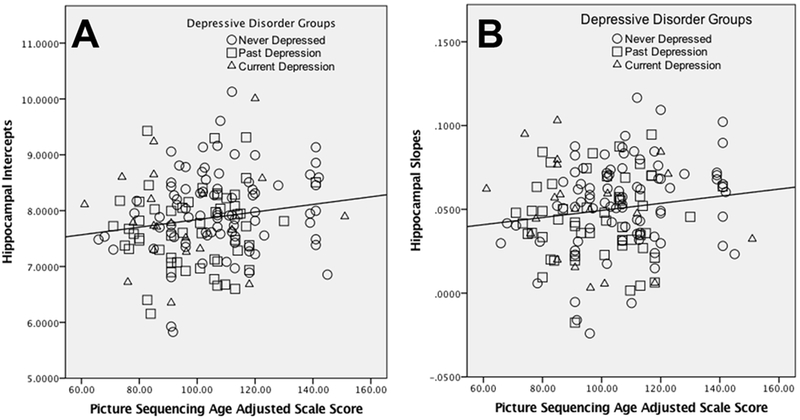
Scatterplots illustrating the relationships between hippocampal intercepts (A) and slopes (B) from the multi-level model across MRI 1-MRI 3 and episodic memory.
Relationships of Episodic Memory to Stressful Life Events and Adversity
The hypotheses outlined in Table 1 suggest that episodic memory should be associated with stressful life events and adversity. As shown in Table 3, in partial correlations controlling for age and gender (but not family income life), greater life stress/adversity was associated with worse episodic memory. Again, not surprisingly, partial correlations controlling for age and gender also showed that life stress/adversity was associated with both current depression severity (r = .26 , p <.001, CI+− .40 to .14) and depression severity from T6-T8 (r = .32 , p <.001, CI+− .46 to .20).
Hippocampal Volume
As outlined in Table 1, the life stress/adversity model suggests that the link between depression and episodic memory reflects disruption of the hippocampus. To determine whether our data are consistent with this hypothesis, we first examined the association between episodic memory and either hippocampal volume intercepts or slopes (trajectories across scans). As shown in Figure 3, larger hippocampal intercepts (i.e., mean volume, r = .17, p = .017, CI+− .30 to .04) and slopes (i.e., increase over time, r = .16, p = .025, CI+− .29 to .03) were related to better episodic memory. These results remained the same if family income was also included as a covariate. The relationship between hippocampal intercepts and episodic memory remained significant if also controlling for total gray matter volume (r = .15, p = .033, CI+− .28 to .02), with a trend for hippocampal slopes (r = .11, p = .094, CI+− .24 to −.02). We also asked whether hippocampal intercepts and slopes were independently related to episodic memory. However, neither was significantly related to episodic memory when controlling for other, suggestion that they were accounting for shared variance in episodic memory.
Further, to examine specificity, we looked at relationship to caudate, and did not find significant relationships between episodic memory and either caudate intercepts (r = .14, p = .089, CI+− .29 to −.01) or caudate slopes (r = −.08, p = .35, CI+− .23 to .07). The magnitude of the relationship of hippocampal versus caudate intercepts to episodic memory did not differ significantly, but hippocampal slopes were significantly more correlated with episodic memory than were caudate slopes (Z = 2.30, p = .01), suggest some evidence for specificity.
We next asked whether our categorical or dimensional measures of depression were related to either the hippocampal intercept or slope measures. We started by using a MANOVA to examine whether youth with current or past depressive disorders differed from those without in either hippocampal intercepts or slope. The omnibus MANOVA was trend level significant (Roy’s Largest Root F(2,162) = 3.20, p=.043, partial η2 = .38; Pillai’s Trace F(4,324) = 1.60, p = .175, partial η2 = .019). Follow-up univariate tests indicated that the three depressive disorder groups differed on intercepts (F(2,162) = 3.18, p =.044, partial η2 = .038) but not slopes, (F(2,162) = 2.30, p =.103, partial η2 = .028). Both youth with current (M = 7.87, SD = .86) and past MDD (M = 7.68, SD = .70) had lower hippocampal intercepts than those youth with no history of depression (M = 8.00, SD = .72), but only the comparison of past depression (P-MDD) to never depressed (N-MDD) was significant (p = .013), potentially due to the smaller sample size of the current depression group. However, as shown in Table 2, none of the dimensional measures of depression were significantly related to either hippocampal intercepts or slopes.
Emotion Regulation, Life Stress/Adversity, and Hippocampal Volume
The life stress/adversity model also predicts that there should be a correlation between emotion regulation and life stress/adversity and either or both hippocampal intercepts or slopes. As shown in Table 3 and Figure S3, partial correlations controlling for age, gender and family income show that that greater current emotion dysregulation was associated with smaller hippocampal intercepts and slopes, as was greater historical emotion dysregulation. These results all passed FDR correction and remained significant if the corresponding gray matter measures were included as covariates (e.g., intercept or slope). Further, partial correlations controlling for age and gender showed that life stress/adversity was related to smaller intercepts and slopes (Table 3). We asked whether hippocampal intercepts and slopes were independently related to emotion regulation or stress/adversity. For current emotion regulation, neither was significantly related when controlling for other, suggestion that they were accounting for shared variance in current emotion regulation. However, even when controlling for hippocampal intercepts, slopes remained significantly associated with both historical emotion regulation (r = .13, p = .048, CI+− .26 to .01) and stress/adversity (r = .17, p = .015, CI+− .30 to .04), though the opposite was not true for intercepts.
Figure 4 provides a schematic illustration of the interrelationships hypothesized as part of the life-stress/adversity model, along with a summary of the significant relationships outlined above that are consistent with this model. Consistent with this model, we found evidence that worse episodic memory was associated with both smaller hippocampal volume/slopes and greater depression severity, worse emotion dysregulation and greater life stress/adversity. Further, we found evidence that worse emotion dysregulation and greater life stress/adversity were associated with smaller hippocampal volume/slopes, though only modest evidence for a relationship to depression. This model also implies that hippocampal volume should mediate the relationship between either or both stress/adversity and emotion dysregulation with episodic memory. Thus we tested mediation models using the PROCESS 3.0 macro. The direct effect of life-stress/adversity to episodic memory was no longer significant with either hippocampal intercepts (t = −1.54, p = .13) or slopes (t = −1.64, p = .10) in the model, but there was not a significant indirect mediation effect for either intercepts (Boot Strap CI −.55 -- .02) or slopes (Boot Strap CI −.52 -- .04). For both current and historical emotion dysregulation, the direct effects remained significant for mediation models with either hippocampal slopes or intercepts (all ps <.05), and all of the indirect mediation confidence intervals included 0.
Figure 4: Schematic Model of the Potential Associations Among Life Stress/Adversity, Emotion Dysregulation, Depression, Hippocampal Volume and Episodic Memory.
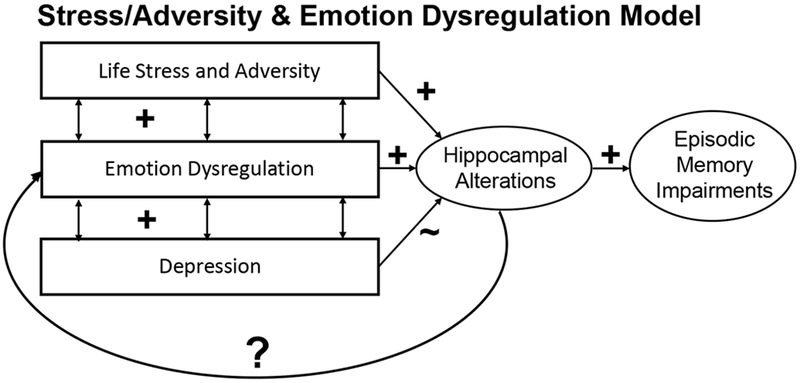
Arrows represent hypothesized associations. “+” signs indicate relationships found in the current data that are consistent with hypothesized associations. “~” indicates modest evidence. “?” indicates open questions in need of further research.
Discussion
The goal of the current study was to examine cognitive function among adolescents with current or past MDD in a longitudinal study of youth with early signs and symptoms of depression. Our goal was to test the predictions associated with several different models of the potential links between depression and different patterns of cognitive function, as outlined in Table 1. We found that both youth with current and past MDD showed impairment in episodic memory, even when controlling for internalizing and externalizing disorders and family income. Notably, we did not find MDD related deficits in other cognitive domains, suggesting that episodic memory is a specific cognitive impairment in adolescent depression. Youth with current externalizing disorders showed evidence of impairments in both vocabulary and episodic memory. Both current and cumulative depression severity, as well as number of prior depression episodes, were associated with greater episodic memory impairment. Further, both life-stress/adversity and emotion dysregulation were also associated with greater episodic memory impairment. In addition, smaller hippocampal intercepts and shallower slopes of hippocampal growth over time were also associated with greater episodic memory impairment. There were only modest relationships of depression to hippocampal volume, but robust relationships of both emotion dysregulation and life-stress/adversity to both episodic memory and hippocampal volume. These findings begin to elucidate a developmental mechanistic model of specific cognitive impairments in adolescent depression as discussed in more detail below.
We found that youth with current or past depression showed episodic memory impairments. This finding is consistent with a large body of literature in adults with depression that also shows episodic memory impairment in both individuals with current MDD and those with a prior history of MDD (Ahern & Semkovska, 2017; Bora, et al., 2013; Rock, et al., 2014; Snyder, 2013). However, we were somewhat surprised to not find evidence for impairments in other cognitive domains as well. As described in the introduction, the literature on adults with MDD suggests impairments in episodic memory, but also impairments in executive function, working memory, and processing speed. Further, the “cognitive reserve” model would have predicted broader cognitive deficits, and the emotion regulation model would have predicted more evidence for executive function deficits. While the previous literature might suggest that episodic memory may show some evidence of greater impairment in MDD as compared to some other disorder (Bourke, et al., 2012; Gooren, et al., 2013; Neu, et al., 2017), we would have expected somewhat more evidence for impairments in other cognitive domains. There are several possible reasons for this more selective cognitive impairment in the current study. First, one possibility is that episodic memory deficits are an early emerging aspect of cognitive impairment in adolescent depression given the developmental salience of this cognitive domain during this period. Along this line, it is possible that evidence for other cognitive impairments will emerge as our youth grow older. However, this explanation runs counter to the other studies in children and adolescents that have shown broader cognitive impairment (Wagner, et al., 2015) and is not consistent with suggestions that executive dysfunction is a risk factor for depression (Snyder & Hankin, 2016). Another possible explanation is that many previous studies in children and adolescents have not necessarily controlled for confounding factors such as family income and the presence of other comorbid symptoms (anxiety) and disruptive disorders, which can also be associated with cognitive impairment. However, even if we compare children with MDD to children free of any psychiatric disorder and do not control for SES, we still see deficits only in episodic memory. Another possibility is that our measure of executive function (Flanker task from the NIH Toolbox) was not optimal, and that other measures of executive function would provide greater evidence for impairment. Work with additional samples and other measures of executive function will be needed to clarify the level of impairment in other cognitive domains associated with early depression.
In contrast, consistent with the life stress/adversity model and the adult MDD literature, we found that episodic memory deficits were present in youth with a current diagnosis of MDD, as well as in those with only past and not current MDD. This provides further evidence that such episodic memory deficits continue into remission (Bora, et al., 2013; Rock, et al., 2014; Snyder, 2013). We also found that greater severity of both current and past depression, as well as the number of prior depressive episodes, was related to worse episodic memory impairment. This finding is again consistent with the adult literature (Fossati et al., 2004; McDermott & Ebmeier, 2009; Talarowska, Zajaczkowska, & Galecki, 2015) and the predictions of the life stress/adversity model, as well as speculations about the neurotoxicity of depressive experiences (Sheline, 1996, 2011). Importantly, this finding in an adolescent population highlights the need to examine whether early intervention among children with early onset depression might help stave off more severe impairment in episodic memory and suggests that early interventions should also target this cognitive domain. However, as we did not have assessments of episodic memory from when the children first entered the study in their preschool years, we cannot tell whether the greater episodic memory impairment in children with a greater cumulative history of depression severity reflects increases in episodic memory deficits over time or whether those children who go on to have a more severe depression course start out with more impaired episodic memory. Regardless, findings have implications for the clinical treatment of adolescent depression.
As noted in the introduction, it is has been hypothesized that one of the reasons that there could be a relationship between depression and episodic memory is because chronic and/or early life stress is a risk factor for MDD (Pagliaccio & Barch, 2016; Stroud, Davila, & Moyer, 2008) as well as emotion dysregulation (VanTieghem & Tottenham, 2017) and reduced hippocampal volume (Kim, Pellman, & Kim, 2015; Pagliaccio & Barch, 2016). As such, we examined whether life stress/adversity, emotion dysregulation, and/or hippocampal volume were associated with episodic memory in youth. Consistent with this model, we found that both life stress/adversity and emotion dysregulation were associated with worse episodic memory, as well as with greater depression, providing further evidence for the developmental validity of this risk trajectory established in adult populations.
We also found evidence that hippocampal volume showed some association with episodic memory. Both lower intercepts (i.e., predicted ‘baseline’ volume at the first scan wave) and smaller slopes (shallower increase in hippocampal volume across the three scan waves) were associated with worse episodic memory, though they accounted for overlapping variance. Although this relationship was predicted a priori and remained significant even when controlling for family income and gray matter volume (for intercepts, with a trend for slopes), the magnitude of these relationships was small (r ≤ 0.17). Further, we also found only a very modest relationship of hippocampal volume to depression, with a trend level reduction in hippocampal intercepts and no relationship of hippocampal volume (intercepts or slopes) to the dimensional measures of depression. This pattern of findings is generally consistent with the work of Schweizer et al. (Schweizer, et al., 2018), who found an association of hippocampal volume and episodic memory in community dwelling adults, but no relationship of hippocampal volume to depression. Further, we also directly tested the hypothesis that hippocampal volume might be mediating the associations of life stress/adversity and emotion dysregulation to episodic memory, but did not find evidence for significant mediation.
We also found evidence that both life-stress and emotion dysregulation were related to smaller hippocampal volumes with slopes, findings that are generally consistent with our prior work in this sample linking poverty and early stress to reduced hippocampal volume (J. Luby et al., 2013; J. L. Luby et al., 2012). Further, we found that reduced slopes were more strongly related than reduced intercepts to both life stress/adversity and emotion dysregulation, suggesting the possible importance of cumulative and ongoing stress and dysregulation. However, we did not find evidence that hippocampal volume mediated the relationships between either life stress or emotion dysregulation and episodic memory. It is possible that we will see a stronger relationship of depression to hippocampal volume as our participants grow older, or more support for mediation, given the evidence described in the introduction linking duration of illness and recurrence to hippocampal volume in depression (McKinnon, et al., 2009; Sheline, et al., 1999; Sheline, et al., 1996). Thus, the while the current data suggest links between episodic memory and both depression and a constellation of constructs associated with stress, emotion dysregulation and hippocampal volume, they do not provide strong support consistent with the hypothesis that the link between depression and episodic memory, or between life stress or emotion dysregulation, is mediated through hippocampal structure.
There are a number of limitations to the current study. First, some of the children were currently taking medications or had taken medications in the past, and it is possible that this could impact cognitive function. However, the results reported held when controlling for either current use of psychotropic medication or history of psychotropic medication use. Second, our assessment of cognitive function was limited to five domains and it is possible that there are other cognitive domains that we did not assess which would have been impaired in these children, such as inhibition, other aspects of executive function, or visual-spatial processing. Third, we did not have detailed assessments of cognitive function from when the children were in preschool, or throughout the course of the study, and thus we cannot tell from our findings how much the cognitive deficits we assessed resulted from the experience of having depression, versus emerged concurrently with depression, versus having been present prior to the onset of depression. Future longitudinal studies that start cognitive assessment at an early age will be necessary to identify the time course of cognitive impairment in relation to depression in children.
In summary, the current data add to the literature on cognitive function in depression by showing that both adolescents with current and those with past depression show impairments in episodic memory function. This is an important finding beyond the replication in younger samples, as memory is a salient developmental phenomenon in adolescence and therefore represents a key new early intervention target. Further, the magnitude of the episodic memory impairment was associated with depression severity, emotion dysregulation and life stress/adversity, consistent with models in the literature of these relationships. In addition, we found an association of hippocampal volume with episodic memory and depression, though the magnitudes of these relationships were modest. We also saw robust relationships among emotion dysregulation, life stress/adversity, hippocampal volume and episodic memory, though we did not find evidence that hippocampal volume was the mediator of stress/dysregulation relationships to episodic memory. Nonetheless, this later set of relationships suggests that it will be important to examine the potential role of emotion regulation in the links between hippocampal volume and episodic memory, with an eye toward possible early intervention and prevention pathways starting in childhood to target adolescent and adult depression.
Supplementary Material
Figure 2: Episodic Memory and Emotion Regulation.
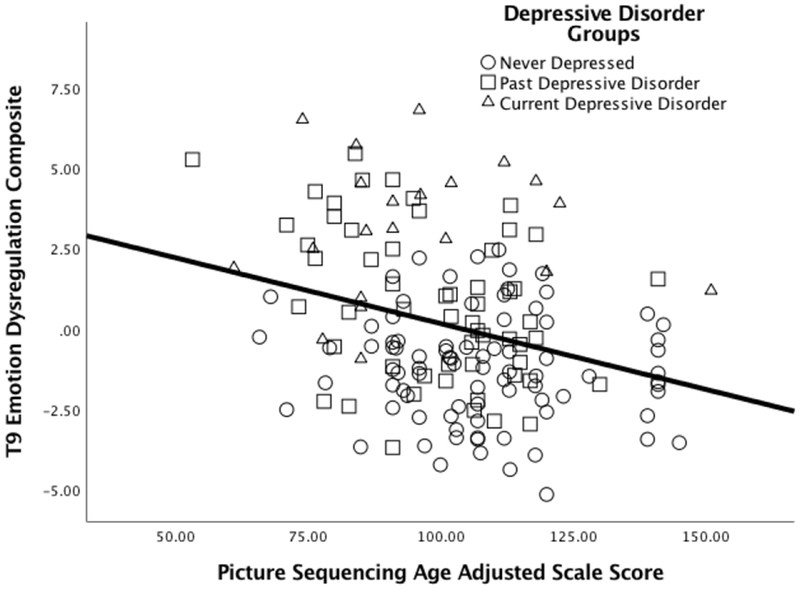
Scatterplot illustrating the relationships between the current (T9) emotion dysregulation composite and episodic memory.
Acknowledgments:
This study was supported by grants 2R01 MH064769-06 and R01 MH098454.
Contributor Information
Deanna M. Barch, Departments of Psychological & Brain Sciences, Psychiatry and Radiology at Washington University in St. Louis.
Michael P. Harms, Department of Psychiatry at Washington University in St. Louis
Rebecca Tillman, Department of Psychiatry at Washington University in St. Louis.
Elizabeth Hawkey, Department of Psychiatry at Washington University in St. Louis.
Joan L. Luby, Department of Psychiatry at Washington University in St. Louis.
References
- Ahern E, & Semkovska M (2017). Cognitive functioning in the first-episode of major depressive disorder: A systematic review and meta-analysis. Neuropsychology, 31(1), 52–72. doi: 10.1037/neu0000319 [DOI] [PubMed] [Google Scholar]
- Anacker C, O’DonneN KJ, & Meaney MJ (2014). Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues Clin Neurosci, 16(3), 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, & Teicher MH (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci, 31(4), 183–191. doi: 10.1016/j.tins.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Angold A, & Costello EJ (2000). The Child and Adolescent Psychiatric Assessment (CAPA). J Am Acad Child Adolesc Psychiatry, 39(1), 39–48. doi: S0890-8567(09)66099-8 [pii] 10.1097/00004583-200001000-00015 [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Dikmen SS, Heaton RK, Mungas D, Slotkin J, & Beaumont JL (2013). III. NIH Toolbox Cognition Battery (CB): measuring episodic memory. Monogr Soc Res Child Dev, 78(4), 34–48. doi: 10.1111/mono.12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird HR, Gould MS, & Staghezza B (1992). Aggregating data from multiple informants in child psychiatry epidemiological research. [Research Support, U.S. Gov’t, P.H.S.]. Journal of the American Academy of Child and Adolescent Psychiatry, 31(1), 78–85. doi: 10.1097/00004583-199201000-00012 [DOI] [PubMed] [Google Scholar]
- Bleck TP, Nowinski CJ, Gershon R, & Koroshetz WJ (2013). What is the NIH toolbox, and what will it mean to neurology? Neurology, 80(10), 874–875. doi: 10.1212/WNL.0b013e3182872ea0 [DOI] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Yucel M, & Pantelis C (2013). Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med, 43(10), 2017–2026. doi: 10.1017/S0033291712002085 [DOI] [PubMed] [Google Scholar]
- Bourke C, Porter RJ, Carter JD, McIntosh VV, Jordan J, Bell C, Joyce PR (2012). Comparison of neuropsychological functioning and emotional processing in major depression and social anxiety disorder subjects, and matched healthy controls. Aust N Z J Psychiatry, 46(10), 972–981. doi: 10.1177/0004867412451502 [DOI] [PubMed] [Google Scholar]
- Carlozzi NE, Beaumont JL, Tulsky DS, & Gershon RC (2015). The NIH Toolbox Pattern Comparison Processing Speed Test: Normative Data. Arch Clin Neuropsychol, 30(5), 359–368. doi: 10.1093/arclin/acv031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Tulsky DS, Chiaravalloti ND, Beaumont JL, Weintraub S, Conway K, & Gershon RC (2014). NIH Toolbox Cognitive Battery (NIHTB-CB): the NIHTB Pattern Comparison Processing Speed Test. J Int Neuropsychol Soc, 20(6), 630–641. doi: 10.1017/S1355617714000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Tulsky DS, Kail RV, & Beaumont JL (2013). VI. NIH Toolbox Cognition Battery (CB): measuring processing speed. Monogr Soc Res Child Dev, 78(4), 88–102. doi: 10.1111/mono.12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol, 66, 295–319. doi: 10.1146/annurev-psych-010814-015156 [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, & Nash C (1999). Hippocampal system and declarative (relational) memory: Summarizing from functional neuroimaging studies. Hippocampus, 9, 83–98. [DOI] [PubMed] [Google Scholar]
- Cole J, Costafreda SG, McGuffin P, & Fu CH (2011). Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J Affect Disord, 134(1–3), 483–487. doi: 10.1016/j.jad.2011.05.057 [DOI] [PubMed] [Google Scholar]
- Dikmen SS, Bauer PJ, Weintraub S, Mungas D, Slotkin J, Beaumont JL, Heaton RK (2014). Measuring episodic memory across the lifespan: NIH Toolbox Picture Sequence Memory Test. J Int Neuropsychol Soc, 20(6), 611–619. doi: 10.1017/S1355617714000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL (2009). Psychiatric assessment of young children. Child Adolesc Psychiatr Clin N Am, 18(3), 559–580. doi: S1056-4993(09)00018-2 [pii] 10.1016/j.chc.2009.02.004 [DOI] [PubMed] [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, & Angold A (2006). Test-Retest Reliability of the Preschool Age Psychiatric Assessment (PAPA). J Am Acad Child Adolesc Psychiatry, 45(5), 538–549. doi: 10.1097/01.chi.0000205705.71194.b8 S0890-8567(09)61201-6 [pii] [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Amaral DG, Buffalo EA, Buzsaki G, Cohen N, Davachi L, Witter M (2016). Hippocampus at 25. Hippocampus. doi: 10.1002/hipo.22616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, & Eriksen CW (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, & Posner MI (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–347. [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, & Gotlib IH (2012). Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Frontiers in psychology, 3, 489. doi: 10.3389/fpsyg.2012.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Harvey PO, Le Bastard G, Ergis AM, Jouvent R, & Allilaire JF (2004). Verbal memory performance of patients with a first depressive episode and patients with unipolar and bipolar recurrent depression. J Psychiatr Res, 38(2), 137–144. [DOI] [PubMed] [Google Scholar]
- Frodl T, Janowitz D, Schmaal L, Tozzi L, Dobrowolny H, Stein DJ, Grabe HJ (2017). Childhood adversity impacts on brain subcortical structures relevant to depression. J Psychiatr Res, 86, 58–65. doi: 10.1016/j.jpsychires.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Belden AC, & Luby JL (2011). The 2-week duration criterion and severity and course of early childhood depression: Implications for nosology. Journal of Affective Disorders. doi: 10.1016/j.jad.2011.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnefski N, Rieffe C, Jellesma F, Meerum Terwogt M, & Kraaij V (2007). Cognitive emotion regulation strategies and emotional problems in 9-11-year-old children: The development of an instrument. European Child & Adolescent Psychiatry, 16(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Gershon RC, Cook KF, Mungas D, Manly JJ, Slotkin J, Beaumont JL, & Weintraub S (2014). Language measures of the NIH Toolbox Cognition Battery. J Int Neuropsychol Soc, 20(6), 642–651. doi: 10.1017/S1355617714000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Slotkin J, Manly JJ, Blitz DL, Beaumont JL, Schnipke D, Weintraub S (2013). IV. NIH Toolbox Cognition Battery (CB): measuring language (vocabulary comprehension and reading decoding). Monogr Soc Res Child Dev, 78(4), 49–69. doi: 10.1111/mono.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, & Nowinski CJ (2013). NIH toolbox for assessment of neurological and behavioral function. Neurology, 80(11 Suppl 3), S2–6. doi: 10.1212/WNL.0b013e3182872e5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, & Bunge SA (2012). Neural changes underlying the development of episodic memory during middle childhood. Dev Cogn Neurosci, 2(4), 381–395. doi: 10.1016/j.dcn.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, Andersson JL, Auerbach EJ, Behrens TE, … Van Essen DC (2016). The Human Connectome Project’s neuroimaging approach. Nat Neurosci, 19(9), 1175–1187. doi: 10.1038/nn.4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, & Weinberger DR (1997). Auditory working memory and Wisconsin card sorting test performance in patients with schizophrenia. Archives of General Psychiatry, 54(2), 159–165. [DOI] [PubMed] [Google Scholar]
- Goodall J, Fisher C, Hetrick S, Phillips L, Parrish EM, & Allott K (2018). Neurocognitive Functioning in Depressed Young People: A Systematic Review and Meta-Analysis. Neuropsychoi Rev, 28(2), 216–231. doi: 10.1007/s11065-018-9373-9 [DOI] [PubMed] [Google Scholar]
- Gooren T, Schlattmann P, & Neu P (2013). A comparison of cognitive functioning in acute schizophrenia and depression. Acta Neuropsychiatr, 25(6), 334–341. doi: 10.1017/neu.2013.21 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, & Joormann J (2010). Cognition and depression: current status and future directions. Annu Rev Clin Psychol, 6, 285–312. doi: 10.1146/annurev.clinpsy.121208.131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Silk JS, & Nelson EE (2016). The neurobiology of the emotional adolescent: From the inside out. Neurosci Biobehav Rev, 70, 74–85. doi: 10.1016/j.neubiorev.2016.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Young JF, Gallop R, & Garber J (2018). Cognitive and Interpersonal Vulnerabilities to Adolescent Depression: Classification of Risk Profiles for a Personalized Prevention Approach. J Abnorm Child Psychol. doi: 10.1007/s10802-018-0401-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes RJ, Insel TR, Landis SC, & Research N. I. H. B. f. N. (2013). The NIH toolbox: setting a standard for biomedical research. Neurology, 80(11 Suppl 3), S1. doi: 10.1212/WNL.0b013e3182872e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, & Quinn ME (2014). Cognitive processes and emotion regulation in depression. Depress Anxiety, 31(4), 308–315. doi: 10.1002/da.22264 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, & Ryan N (2013). KSADS-PL. Yale University; New Haven, CT. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry, 36(7), 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Pellman B, & Kim JJ (2015). Stress effects on the hippocampus: a critical review. Learn Mem, 22(9), 411–416. doi: 10.1101/lm.037291.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MJ, Macdougall AG, Ferris S, Herdman KA, & McKinnon MC (2011). Episodic simulation of future events is impaired in patients with major depressive disorder. Psychiatry Res, 187(3), 465–467. doi: 10.1016/j.psychres.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Kircanski K, Joormann J, & Gotlib IH (2012). Cognitive Aspects of Depression. Wiley Interdiscip Rev Cogn Sci, 3(3), 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, … Caspi A (2009). Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry, 166(1), 50–57. doi: 10.1176/appi.ajp.2008.08030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajud N, & Torner L (2015). Early life stress and hippocampal neurogenesis in the neonate: sexual dimorphism, long term consequences and possible mediators. Front Mol Neurosci, 8, 3. doi: 10.3389/fnmol.2015.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Ekstrom AD, & Ghetti S (2014). Volume of hippocampal subfields and episodic memory in childhood and adolescence. Neuroimage, 94, 162–171. doi: 10.1016/j.neuroimage.2014.03.019 [DOI] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Barch D (2013). The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr, 167(12), 1135–1142. doi: 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Heffelfinger A, Koenig-McNaught AL, Brown K, & Spitznagel E (2004). The preschool feelings checklist: A brief and sensitive screening measure for depression in young children. Journal of the American Academy of Child and Adolescent Psychiatry, 43(6), 708–717. doi: 10.1097/01.chi.0000121066.29744.08 [DOI] [PubMed] [Google Scholar]
- Luby JL, Barch DM, Belden A, Gaffrey MS, Tillman R, Babb C, Botteron K (2012). Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci U S A, 109(8), 2854–2859. doi: 10.1073/pnas.1118003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden AC, Jackson JJ, Lessov-Schlaggar CN, Harms MP, Tillman R, Barch DM (2016). Early Childhood Depression and Alterations in the Trajectory of Gray Matter Maturation in Middle Childhood and Early Adolescence. JAMA Psychiatry, 73(1), 31–38. doi: 10.1001/jamapsychiatry.2015.2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden AC, Pautsch J, Si X, & Spitznagel E (2009). The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disord, 112(1-3), 111–119. doi: S0165-0327(08)00148-1 [pii] 10.1016/j.jad.2008.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger AK, Mrakotsky C, Hessler MJ, Brown KM, & Hildebrand T(2002). Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Adolesc Psychiatry, 41(8), 928–937. doi: S0890-8567(09)61073-X [pii] 10.1097/00004583-200208000-00011 [DOI] [PubMed] [Google Scholar]
- MacQueen G, & Frodl T (2011). The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry, 16(3), 252–264. doi: 10.1038/mp.2010.80 [DOI] [PubMed] [Google Scholar]
- McDermott LM, & Ebmeier KP (2009). A meta-analysis of depression severity and cognitive function. J Affect Disord, 119(1-3), 1–8. doi: 10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, & MacQueen GM (2009). A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci, 34(1), 41–54. [PMC free article] [PubMed] [Google Scholar]
- Medicine, N. A. o. (2015). Enabling discovery, development and translation of treatments for cognitive dysfunction in depression In Bain L & Stroud C (Eds.), Forum on Neuroscience and Nervous System Disorders. Washington, DC: The National Academies of Sciences, Engineering and Medicine. [PubMed] [Google Scholar]
- Modecki KL, Zimmer-Gembeck MJ, & Guerra N (2017). Emotion Regulation, Coping, and Decision Making: Three Linked Skills for Preventing Externalizing Problems in Adolescence. Child Dev, 88(2), 417–426. doi: 10.1111/cdev.12734 [DOI] [PubMed] [Google Scholar]
- Mullally SL, & Maguire EA (2014). Memory, Imagination, and Predicting the Future: A Common Brain Mechanism? Neuroscientist, 20(3), 220–234. doi: 10.1177/1073858413495091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Heaton R, Tulsky D, Zelazo PD, Slotkin J, Blitz D, Gershon R (2014). Factor structure, convergent validity, and discriminant validity of the NIH Toolbox Cognitive Health Battery (NIHTB-CHB) in adults. J Int Neuropsychol Soc, 20(6), 579–587. doi: 10.1017/S1355617714000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu P, Gooren T, Niebuhr U, & Schlattmann P (2017). Cognitive impairment in schizophrenia and depression: A comparison of stability and course. Appl Neuropsychol Adult, 1–14. doi: 10.1080/23279095.2017.1392962 [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, & Barch DM (2016). Early life adversity and risk for depression: Alterations in cortisol and brain structure and function as mediating mechanisms In Frodl T (Ed.), Systems neuroscience in depression (pp. 29–77). New York: Academic Press. [Google Scholar]
- Reuter M, Rosas HD, & Fischl B (2010). Highly accurate inverse consistent registration: a robust approach. Neuroimage, 53(4), 1181–1196. doi: 10.1016/j.neuroimage.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, & Fischl B (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage, 61(4), 1402–1418. doi: 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, & Blackwell AD (2014). Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med, 44(10), 2029–2040. doi: 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, & Posner MI (2004). Development of attentional networks in childhood. Neuropsychologia, 42(8), 1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012 [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL, & Shaw RJ (1991). Effects of adult age on structural and operational capacities in working memory. Psychol Aging, 6(1), 118–127. [DOI] [PubMed] [Google Scholar]
- Schacter DL (2012). Constructive memory: past and future. Dialogues Clin Neurosci, 14(1), 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Benoit RG, De Brigard F, & Szpunar KK (2015). Episodic future thinking and episodic counterfactual thinking: intersections between memory and decisions. Neurobiol Learn Mem, 117, 14–21. doi: 10.1016/j.nlm.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, Hibar DP (2016). Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry, 21(6), 806–812. doi: 10.1038/mp.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer S, Kievit RA, Emery T, Cam CAN, & Henson RN (2018). Symptoms of depression in a large healthy population cohort are related to subjective memory complaints and memory performance in negative contexts. Psychol Med, 48(1), 104–114. doi: 10.1017/S0033291717001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI (1996). Hippocampal atrophy in major depression: a result of depression-induced neurotoxicity? Mol Psychiatry, 1(4), 298–299. [PubMed] [Google Scholar]
- Sheline YI (2011). Depression and the hippocampus: cause or effect? Biol Psychiatry, 70(4), 308–309. doi: 10.1016/j.biopsych.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, & Gado MH (1999). Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci, 19(12), 5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, & Vannier MW (1996). Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A, 93(9), 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A, & Cicchetti D (1997). Emotion regulation among school-age children: the development and validation of a new criterion Q-sort scale. Dev Psychol, 33(6), 906–916. [DOI] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull, 139(1), 81–132. doi: 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, & Hankin BL (2016). Spiraling out of control: Stress generation and subsequent rumination mediate the link between poorer cognitive control and internalizing psychopathology. Clin Psychol Sci, 4(6), 1047–1064. doi: 10.1177/2167702616633157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, & Jernigan TL (2001). Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc, 7(3), 312–322. [DOI] [PubMed] [Google Scholar]
- Squire LR, & Dede AJ (2015). Conscious and unconscious memory systems. Cold Spring Harb Perspect Biol, 7(3), a021667. doi: 10.1101/cshperspect.a021667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, & Knowlton BJ (1995). Memory, hippocampus, and brain systems In Gazzaniga M (Ed.), The Cognitive Neurosciences (pp. 825–837). Cambridge, MA: MIT Press. [Google Scholar]
- Stroud CB, Davila J, & Moyer A (2008). The relationship between stress and depression in first onsets versus recurrences: a meta-analytic review. J Abnorm Psychol, 117(1), 206–213. doi: 10.1037/0021-843X.117.1.206 [DOI] [PubMed] [Google Scholar]
- Szpunar KK (2010). Episodic Future Thought: An Emerging Concept. Perspect Psychol Sci, 5(2), 142–162. doi: 10.1177/1745691610362350 [DOI] [PubMed] [Google Scholar]
- Tafet GE, & Nemeroff CB (2016). The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J Neuropsychiatry Clin Neurosci, 28(2), 77–88. doi: 10.1176/appi.neuropsych.15030053 [DOI] [PubMed] [Google Scholar]
- Talarowska M, Zajaczkowska M, & Galecki P (2015). Cognitive functions in first-episode depression and recurrent depressive disorder. Psychiatr Danub, 27(1), 38–43. [PubMed] [Google Scholar]
- Tulsky DS, Carlozzi N, Chiaravalloti ND, Beaumont JL, Kisala PA, Mungas D, … Gershon R (2014). NIH Toolbox Cognition Battery (NIHTB-CB): list sorting test to measure working memory. J Int Neuropsychol Soc, 20(6), 599–610. doi: 10.1017/S135561771400040X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky DS, Carlozzi NE, Chevalier N, Espy KA, Beaumont JL, & Mungas D (2013). V. NIH Toolbox Cognition Battery (CB): measuring working memory. Monogr Soc Res Child Dev, 78(4), 70–87. doi: 10.1111/mono.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bodegom M, Homberg JR, & Henckens M (2017). Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front Cell Neurosci, 11, 87. doi: 10.3389/fncel.2017.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem MR, & Tottenham N (2017). Neurobiological Programming of Early Life Stress: Functional Development of Amygdala-Prefrontal Circuitry and Vulnerability for Stress-Related Psychopathology. Curr Top Behav Neurosci. doi: 10.1007/7854_2016_42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Muller C, Helmreich I, Huss M, & Tadic A (2015). A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. Eur Child Adolesc Psychiatry, 24(1), 5–19. doi: 10.1007/s00787-014-0559-2 [DOI] [PubMed] [Google Scholar]
- Yoon KL, LeMoult J, & Joormann J (2014). Updating emotional content in working memory: a depression-specific deficit? J Behav Ther Exp Psychiatry, 45(3), 368–374. doi: 10.1016/j.jbtep.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, Conway KP, … Weintraub S (2014). NIH Toolbox Cognition Battery (CB): validation of executive function measures in adults. J Int Neuropsychol Soc, 20(6), 620–629. doi: 10.1017/S1355617714000472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, & Weintraub S (2013). II. NIH Toolbox Cognition Battery (CB): measuring executive function and attention. Monogr Soc Res Child Dev, 78(4), 16–33. doi: 10.1111/mono.12032 [DOI] [PubMed] [Google Scholar]
- Zetsche U, D’Avanzato C, & Joormann J (2012). Depression and rumination: relation to components of inhibition. Cogn Emot, 26(4), 758–767. doi: 10.1080/02699931.2011.613919 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


