Abstract
The intestinal epithelium is a rapidly renewing cellular compartment. This constant regeneration is a hallmark of intestinal homeostasis and requires a tightly regulated balance between intestinal stem cell (ISC) proliferation and differentiation. Since intestinal epithelial cells directly contact pathogenic environmental factors that continuously challenge their integrity, ISCs must also actively divide to facilitate regeneration and repair. Understanding niche adaptations that maintain ISC activity during homeostatic renewal and injury-induced intestinal regeneration is therefore a major and ongoing focus for stem cell biology. Here, we review recent concepts and propose an active interconversion of the ISC niche between homeostasis and injury-adaptive states that is superimposed upon an equally dynamic equilibrium between active and reserve ISC populations.
Keywords: Stem cell niche, intestinal homeostasis, injury, interconversion, regeneration
The Intestinal Stem Cell Niche
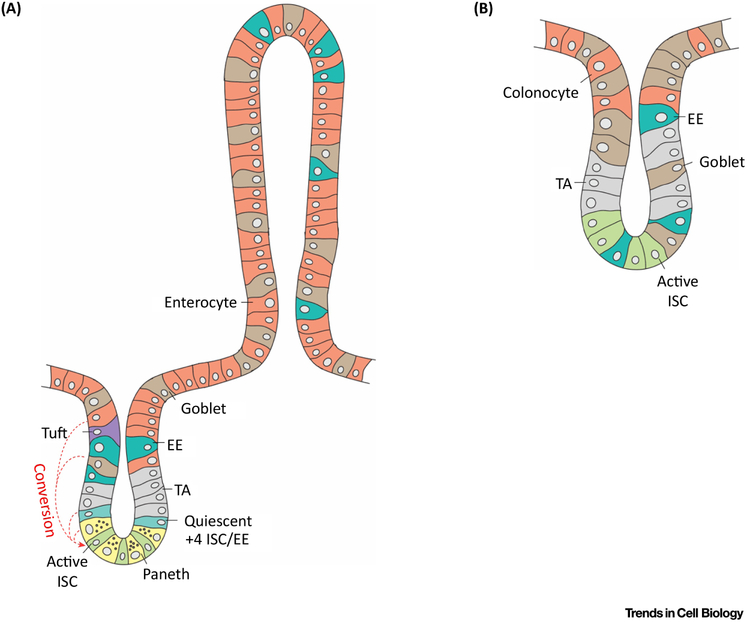
The epithelium of the small intestine is composed of a monolayer of different cell types that form serial arrays of luminal projections (villi) and cup-shaped invaginations (crypts). The homeostatic constant regeneration of the intestinal epithelium is driven by active Lgr5+ stem cells (active intestinal stem cells [ISCs]) at the crypt bases, which give rise to all the different epithelial cell types [1]. Progressing from the crypts towards the villus tips are the transit amplifying (TA) cells that differentiate into either secretory lineages, including enteroendocrine, tuft or goblet cells; or alternatively adopt absorptive cell fates, yielding enterocytes. TA cells can also migrate downwards back to the crypts as they differentiate into Paneth cells, the closest neighbors of the active ISCs. Also driven by the constant division of Lgr5+ stem cells, the epithelium of the colon has no villus projections, as it is organized in glands containing basally located crypts, but many cell types found in small intestinal villi are also found in the upper part of the colonic glands, including enterocyte-like colonocytes and Paneth-like cells (Figure 1).
Figure 1: Cell types of the small intestine and colon.
Numerous well-defined subtypes of epithelial cells can be found in the crypt/villus axis of the small intestine (A) and in the glands of the colon (B). Actively dividing intestinal stem cells (ISCs) are shown in green and TA (transit amplifying) cells are represented in gray. Absorptive enterocytes of the small intestine and colonocytes of the colon share common functions (red). Secretory cells comprise EE (Entereoendocrine) (blue); Goblet (brown); Tuft (purple); quiescent +4 ISCs, which possess enteroendocrine markers (light blue); and Paneth cells (yellow). The red arrows point at the ability of enterocytes, quiescent +4 enteroendocrine cells or Paneth cells to convert to active ISCs.
The two intestinal epithelial lineages, absorptive and secretory, define the two main functions of the gut apparatus: secretory cells secrete hormones and provide an important barrier against food-borne microorganisms, toxins and antigens, mainly through the secretion of mucus and anti-microbial peptides. In contrast, the absorptive cells conduct uptake of dietary nutrients, as they localize mainly at the tips of the villi in the small intestine or at the top of the colonic crypts, thus constituting the majority of luminal cells across the intestinal surface area.
To understand how this homeostasis is maintained it is therefore necessary to understand the different juxtracrine niche factors that maintain ISC activity and the identity of the underlying non-epithelial and/or epithelial cells that elaborate these signals, thus constituting the cellular niche. Such niche factors include canonical signals such as Wnt, R-spondin, Notch and BMP, but also inflammatory and dietary influences. Indeed, upon injury, the ISC niche adapts beyond its homeostatic state to interpret pathogenic stimuli and translate them into regeneration of the epithelium, which is mediated by either surviving Lgr5+ ISCs or other mature cell types like enterocytes, enteroendrocrine or Paneth cells, which can convert back to Lgr5+ ISCs to aid epithelial regeneration [2]. It has now become necessary to understand the cellular stem cell plasticity of reserve ISCs as well as the biology of Lgr5+ ISCs upon distinct adaptations of the niche during epithelial damage and subsequent repair. Such understanding will result in important new insights on the mechanisms underlying intestinal injury and repair and thus in potential new therapeutics for human intestinal disorders. (Figure 2).
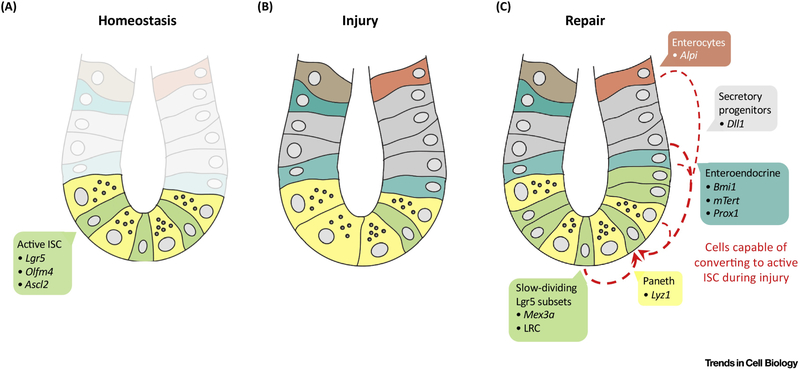
Figure 2: ISC dynamics during homeostasis, injury and repair.
(A) During intestinal homeostasis, active intestinal stem cells (ISCs) (green) marked by expression of Lgr5, Olfm4 and Ascl2 drive epithelial renewal at the bottom of the crypts neighbored by Paneth cells (yellow). (B) Intestinal injury leads to depletion of actively dividing ISCs, (C) which somehow induces enterocytes (Dll1+, Alpi+), +4/Enteroendocrine (Bmi1+, mTert+ or Prox1+) or Paneth cells (Liz1+) to convert to active ISCs (represented by the red arrows) for epithelial repair.
Homeostatic and injury-inducible ISC populations
The identification of molecular markers and location of stem cells has been transformative for the study of intestinal stem cell biology. Although the location and activity of ISCs had been long debated, recent studies using lineage tracing in animal models together with profiling transcriptomes at a single cell level have provided unique opportunities to comprehensively study the physiology and molecular mechanisms of ISCs [3, 4]. In 2007, Lgr5 was first identified as a specific marker for ISCs residing between Paneth cells at the bottom of the crypt [1]. Lgr5+ ISCs are long-term multipotent ISCs that rapidly generate all intestinal epithelial cell types of the small intestine and colon within lineage “stripes” extending from crypt to villus tip and are thus responsible for most ongoing homeostatic epithelial turnover [1]. Single Lgr5+ cells isolated from the intestine are sufficient to form intestinal enteroids, which is a 3-dimensional culture system that recapitulates all epithelial cell types and has self-renewing capacity. The intestinal enteroids also develop an architecture resembling in vivo crypt/villus units, further confirming Lgr5 as a specific ISC marker [5]. Mechanistically, Lgr5, which encodes for a G-protein-coupled receptor (GPCR)-like glycoprotein hormone receptor, was originally reported to be a canonical Wnt/β-catenin signaling-responsive gene [6]. In agreement with its critical role in Wnt/β-catenin signaling, Lgr5 binds to its ligands, the R-spondin1-4 proteins, to amplify canonical Wnt/β-catenin signaling, and crucially catalyze stem cell self-renewal [4, 7-10]. Notably, novel artificial surrogate soluble Wnt agonists defined non-equivalent functions of Wnt and R-spondin proteins where Wnts prime adult Lgr5+ ISCs for the subsequent action of R-spondins which actively catalyze stem cell division [4, 11]. Taken together, these results render Lgr5, as well as co-expressed genes such as Olfm4 and Ascl2 [10, 12] as attractive markers for active ISCs. Additional markers that allow enrichment for Lgr5+ ISCs include CD24lo, CD166+, and GPR78−[13-17].
In addition to the active Lgr5+ cells during homeostasis, the intestine harbors distinct reserve ISC populations, which seem to play an essential role upon epithelial injury by their ability to convert back to Lgr5+ ISCs to assist repair. Label-retaining cells (LRCs) were localized to approximately four cell positions from the crypt base (“+4” cells) that could serve as reservoirs of non-mutated DNA [18-20]. In vivo lineage tracing and transcriptome analyses suggested that Bmi1+ cells localized around the +4 position possess ISC properties but are functionally distinct from Lgr5+ ISCs with Wnt-insensitivity and radiation resistance/inducibility of the former versus Wnt-responsiveness and radiation-sensitivity of the latter [21, 22]. Remarkably, Lgr5+ ISC depletion does not perturb epithelial homeostasis, as Bmi1+ cells rapidly revert to Lgr5+ ISC to sustain intestinal homeostasis [23] and these de novo-generated Lgr5+ ISC are essential for post-radiation epithelial repair [24]. These results provided evidence for injury-induced plasticity in ISC regeneration. Several distinct lineage-committed populations can also acquire stem cell activity following injury. The Lgr5+ ISC pool also exhibits heterogeneity that correlates with an injury-inducible function, including slowly-cycling Lgr5+ ISC subsets such as (1) LRCs exhibiting some degree of Paneth and enteroendocrine gene expression that give rise to clonal traces uniquely after injury [25] or (2) express the RNA-binding protein Mex3a and contribute to regeneration after chemotherapy and radiation injury [26]. Other reserve ISCs are Dll1+ secretory progenitors which generate short-lived lineage traces during homeostasis but long-lived traces after irradiation injury [27]. Further, following Lgr5+ ISC ablation, Alpi+ enterocyte precursors generate lineage traces including replacement of Lgr5+ ISCs [28]; and recent evidence also shows the reserve ISC capability of Paneth cells upon depletion of Lgr5+ ISCs [29, 30]. Another example is the Bmi1-GFP knock-in allele that marks cells that resemble mTert+ ISCs, strongly express enteroendocrine markers, undergo clonogenic organoid formation and can revert to an Lgr5+-like open chromatin configuration upon injury [17, 31], substantiating earlier studies suggesting a stem cell potential of enteroendocrine cells [32-35]. The Drosophila homeobox protein Prospero is required for ISC generation of enteroendocrine cells [36]. The Prospero vertebrate homologue Prox1 is expressed in murine intestinal enteroendocrine and Tuft cells and exhibits overlap with Bmi1 expression as well as injury-inducible stem cell activity [17]. These convergent findings indicate that the intestine possesses substantial plasticity in which numerous lineage-committed cell types function as reserve injury-inducible stem cells, possibly reflecting acquired properties of the intestinal niche during damage-induced regeneration versus homeostatic renewal (Figure 2).
Interconversion of homeostatic and adaptive ISC niches
A stem cell niche can be defined as the microenvironment necessary to maintain stem cell self-renewal and proliferation. The ISC niche contains cells that provide a local source of signals that nourish stem cells to support tissue homeostasis, maintaining a crucial balance between sufficient turnover to form an effective epithelial barrier versus neoplastic overgrowth. These constituent niche cells may include both non-epithelial stromal cells as well as the epithelium itself. This spans significant intestinal diversity, where non-epithelial cells in close proximity to ISC include subepithelial mesenchymal elements such as myofibroblasts, smooth muscle and endothelium, but also hematopoietic cells (lymphocytes, macrophages) and neurons, while epithelial cells can also potently regulate ISCs. In total, these niche cell types elaborate diverse paracrine signals, including Wnt, R-spondin, Notch, BMP and Hedgehog that dictate the balance between stem cell self-renewal and differentiation, with further potential for bidirectional communication between stem cell and niche [37] (Figure 3).
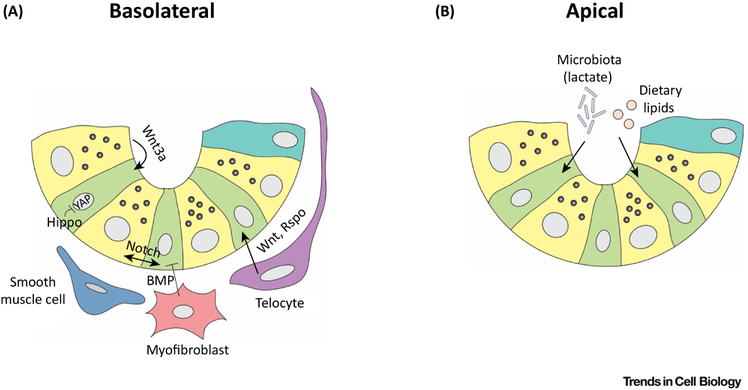
Figure 3: Canonical pathways present in the ISC niche.
Different niche factors impact the activity of active intestinal stem cells (ISCs) during homeostasis. (A) From the basolateral side, the integrity and function of ISCs is maintained directly or indirectly by “homeostatic niche” factors such as Wnts, R-spondins (Rspo), BMP and Hedgehog, secreted by stromal populations such as telocytes, myofibroblasts and smooth muscle cells; and Notch and redundant Wnt signals secreted by epithelial Paneth cells. The Hippo pathway via YAP may transduce mechanosensory signals. (B) On the apical side, dietary lipids impact directly the activity of ISCs and commensal microbiota contribute to the “homeostatic niche” by producing beneficial signals such as lactate.
In contrast to homeostasis, the intestine must also mount an active response to injury. This injury response is absolutely critical to maintain epithelial integrity after diverse insults such as radiation, chemotherapy, bacterial or viral pathogens, or chronic and/or autoimmune inflammatory states. One final common outcome of intestinal damage is loss of Lgr5+ ISCs, which is well tolerated since multiple reserve ISC populations discussed above that are activated by injury, exhibit injury-inducible lineage tracing and/or regenerate Lgr5+ ISCs [17, 22, 23, 27, 38, 39]. Potentially, these diverse reserve ISC types could funnel into the post-damage regeneration of Lgr5+ ISCs. This replacement of lost Lgr5+ ISCs via reserve ISCs, may be particularly important as a final common route for intestinal epithelial repair since Lgr5+ ISCs are radiosensitive and yet Lgr5+ ISCs are required for post-radiation epithelial recovery [24]. The mechanisms behind this so-called “injury-recovery” are poorly understood and remain a major area of inquiry in the ISC biology field. In particular, it is necessary to understand the mobilization of reserve ISCs in the context of ISC niche adaptations during epithelial damage and repair.
Canonical niche pathways
Wnt ligands
The canonical Wnt/β-catenin signaling pathway is a major driver of ISC proliferation. Wnt ligands, encoded by a family of 19 related genes, are obligately palmitoylated by the endoplasmic reticulum enzyme Porcupine (Porcn), which enables both Wnt secretion and binding to Frizzled receptors. Simultaneous Wnt binding to Frizzled and to LRP5/6 co-receptors inhibits Axin- and APC-dependent ubiquitination of β-catenin, allowing its nuclear translocation, association with LEF/TCF transcription factors and consequent trans-activation of Wnt target genes [40-42]. Genetic and pharmacologic evidence indicates a pivotal role of canonical Wnt signaling during intestinal homeostasis and Lgr5+ ISC proliferation with crypt/villus/Lgr5+ ISC loss observed upon diverse manipulations including knockout (KO) of Tcf4, an essential gene in Wnt signaling and maintenance of stem cells [43], deletions of distinct mediators of Wnt biosynthesis and secretion like Wntless (WIs) or Porcn [44, 45], overexpression of the canonical Wnt signaling inhibitor Dickkopf-1 (DKK1) [46, 47] or small molecule PORCN inhibitors [44]. Further, Wnt proteins are essential for intestinal organoid culture. Notably, in vivo Wnt ligand inhibition depletes Lgr5+ ISC via their premature lineage commitment [4], mirroring the use of Wnt withdrawal to induce in vitro differentiation of intestinal enteroids [48]. The source of Wnt in the niche has been intensively investigated. Paneth cells augment in vitro organoid formation from Lgr5+ ISC [49] and Wnt3a is produced by Paneth cells and exhibits short-range action within the crypt in vivo [50]. Further, small intestinal enteroids grown in submerged Matrigel produce their own Wnts, do not require exogenous Wnt supplementation but are appropriately growth-inhibited by Porcn antagonists [49].
On the other hand, numerous lines of evidence reveal non-epithelial stromal cells as an essential Wnt-expressing ISC niche. The absence of Paneth cells in mice does not alter ISC maintenance, proliferation or intestinal homeostasis [51, 52]. Moreover, Wnt3a or Porcn deletion in the mouse intestinal epithelium does not affect intestinal proliferation, differentiation or post-injury regeneration, indicating that intestinal epithelial Wnts, and specifically the Paneth cell-produced Wnt3a, are dispensable for intestinal homeostasis or injury recovery in vivo [44, 45, 53].
Multiple Wnts including Wnt2b, Wnt4 and Wnt5a are highly expressed in intestinal stroma [53]. A main source of intestinal Wnt is a sub-population of Foxl1+ mesenchymal stromal cells that extend long processes around the crypt and are classified as telocytes. Genetic ablation of Foxl1+ cells induced loss of Wnt2b, Wnt4 and Wnt5a expression in the crypt/villus axis and a severe disruption of the intestinal epithelium, with crypt loss and villus shortening. Ablation of Foxl1-expressing cells induces loss of Lgr5+ ISC, but not Paneth cells [54]. Importantly, conditional genetic deletion of Porcn in mouse Foxl1+ cells prevents localized Wnt signaling in intestinal crypts, which leads to loss of proliferation of stem cells and impaired epithelial renewal, indicating that telocytes are a long-sought essential Wnt source for the small intestine [55]. Moreover, Gli1+ or αSMA+ sub-epithelial stromal cells express high levels of Wnt2b, which is sufficient to rescue intestinal epithelial homeostasis when injected in mice upon lack of global Wnt secretion, further indicating an essential role of stromal cells in the ISC niche [56]. Indeed, a recent study establishes Gli1+ sub-epithelial cells as essential contributors to the integrity of the colonic epithelium since conditional KO of Wls specifically in Gli1+ cells prevents Lgr5+ ISC self-renewal in the colon and leads to destruction of the epithelium twenty-one days after KO induction [57].
Potential methods to model the mesenchymal/stromal ISC niche in vitro include Air-Liquid Interface (ALI) intestinal organoids that contain both epithelium and stromal cells. Murine ALI intestinal organoids can grow without the addition of any niche factors but are inhibited by extracellular Wnt antagonists such as DKK1 and Fzd8-Fc, suggesting functional endogenous Wnt production that is sufficient to sustain ISCs [58]. Similarly, iPSC-derived human intestinal organoids (hIOs) contain both epithelium and stroma [59, 60].
R-spondins
The R-spondins constitute another family of essential ISC niche factors. R-spondins (RSPO1–RSPO4) are secreted glycoproteins with Furin domains that do not have intrinsic Wnt signaling activity but strongly potentiate the ability of Wnt ligands to activate β-catenin-dependent transcription and canonical Wnt signaling [61]. The R-spondins are ligands for two classes of receptors – the leucine-rich repeat seven-pass transmembrane proteins Lgr4/5/6, and the transmembrane E3 ligases RNF43 and ZNRF3. These E3 ligases preferentially catalyze the ubiquitination, endocytosis and degradation of the Wnt receptors Frizzled and LRP5/6, thus damping Wnt signaling. However, Rspo binding to RNF43 and ZNRF3 inhibits this process, resulting in Frizzled and LRP5/6 accumulation and amplification of Wnt signaling [8-10, 62, 63].
Notably, R-spondins potently regulate Lgr5+ ISCs, which also express high levels of RNF43 and ZNRF3. In vivo R-spondin overexpression strongly induces expansion of Lgr5+ ISCs [4, 22, 58, 64]. Genetic deletion of both Rnf43 and Znrf3 results in crypt hyper-proliferation and intestinal overgrowth [65] while combined deletion of Lgr4 and Lgr5 induces rapid crypt and villus loss [7]. Rspo2 and Rspo3 may be particularly relevant within the ISC niche, as combined treatment with anti-Rspo2 and anti-Rspo3 neutralizing monoclonal antibodies induces loss of Lgr5+ ISC and impairs post-irradiation repair [66]. Notably, R-spondins and Wnts exert distinct, but cooperative, regulation of Lgr5+ ISCs. Taking advantage of gain-of-function approaches using a novel non-lipidated Wnt analogue, Wnt ligands alone are unable to induce Lgr5+ ISC self-renewal, but instead they induce the expression of Lgr5, RNF43 and ZNRF3 receptors on stem cells. Thus, Wnt ligands prime the Lgr5+ ISC, rendering them “competent” to engage with R-spondins, which in contrast to Wnts are fully sufficient to drive stem cell expansion [4].
The cellular sources of R-spondins within the ISC niche are presently unclear. R-spondins are expressed in Foxl1+ and other mesenchymal cells, raising the possibility that this niche cell type may be an essential source of both RSPOs and Wnts [54, 67, 68]. In agreement with this possibility, a recent report shows that sub-epithelial Pdgfrα+ myofibroblasts can support the growth of small intestinal enteroids without exogenous addition of R-spondins in the medium, but not upon deletion of RSPO3 in the same cells. Interestingly, in vivo deletion from embryogenesis of RSPO3 in Pdgfrα+ cells did not affect the intestinal epithelium during homeostasis, but affected epithelial repair upon sodium sulfate-induced colitis [69]. Similar to Foxl1+ and Pdgfrα+, pericryptal CD34+ Gp38+ αSMA− stromal cells are in close proximity with Lgr5+ ISCs and can support the growth of intestinal enteroids in the absence of added Wnt or R-spondin [45, 67]. These CD34+ Gp38+ αSMA− also express Foxl1, indicating potential relatedness.
Notch
Notch signaling plays a major role in the ISC niche by maintaining the undifferentiated status of ISCs through “lateral inhibition”. The binding between Notch ligands (Notch1-4) and Notch receptors (Jag1-2, Dll1-4) between adjacent cells establishes the foundation of Notch activation through cell-cell contact [70]. Notch pathway activation induces conformational changes of the Notch receptors, triggering a series of proteolytic cleavages to generate the Notch intracellular domain (ICD) that translocates to the nucleus, associates with the DNA-binding transcription factor CSL (RBP-Jκ in mouse) and activates target gene transcription [71].
As opposed to Wnt signals, which may be primarily elaborated from a mesenchymal/stromal niche, Notch signaling requires cell-cell contact and thus it likely operates on ISCs via adjacent epithelial cells or even stromal populations that may directly contact ISCs. Indeed, both mesenchymal and epithelial cells, including Lgr5+ ISCs, express transcripts for Notch receptors and ligands (reviewed in [72]). Notch signaling is active in Lgr5+ ISCs and directly regulates several of their specific markers, such as Olfm4 [12, 73]. Notch receptors Notch1 and Notch2 are highly expressed in the intestinal crypts, including Lgr5+ ISCs [74-76]. However, the Notch ligands Dll1 and Dll4 are mostly expressed in Atoh1+ intestinal secretory lineages, such as Paneth cells in the small intestine, and c-Kit+ or Reg4+ crypt base goblet cells in the colon [77, 78]. These Notch ligand-expressing epithelial secretory cells are located adjacent to Lgr5+ ISCs and function as important ISC niche cellular populations by directly interacting with ISCs, thus representing an epithelial niche.
Several lines of evidence have shown that disruption of Notch activity in the intestine results in Lgr5+ ISC loss and conversion of proliferating transit-amplifying cells to secretory cells. In Lgr5+ ISCs, both Notch1 and Notch2 maintain stem cell identity. Notch1 or Notch2 single deletion in the intestinal epithelium does not change ISC activity. However, simultaneous Notch1/Notch2 deletion recapitulates the global Notch inhibition phenotype of Lgr5+ ISC loss and secretory hyperplasia, suggesting Notch1 and Notch2 are functionally redundant in ISCs [79-81]. The transcription factor Atoh1 critically strengthens Notch-mediated lateral inhibition between ISCs and adjacent secretory cells. Atoh1 expression is required to generate all intestinal secretory cell lineages as intestinal epithelial Atoh1 KO engenders pan-secretory lineage loss without affecting absorptive cell differentiation [82, 83]. A recent genome-wide analysis of Atoh1 transcriptional targets identified Notch ligands Dll1 and Dll4 as direct Atoh1 targets, suggesting positive feedback in these cellular populations within the ISC niche to further reinforce Notch-mediated lateral inhibition [84]. Consistent with this concept, combined deletion of Dll1 and Dll4 in intestinal epithelium promotes ISC differentiation to secretory lineages, implicating them as primary Notch ligands maintaining ISC homeostasis [85].
Hedgehog (Hh)
Hedgehog ligands are secreted morphogens that regulate intestinal homeostasis. Intestinal epithelial cells express Hedgehog ligands, such as Sonic Hedgehog in crypts and Indian Hedgehog in villi. In a cascade of negative regulatory events, Hedgehog ligands stimulate cells by engaging with the Patched (Ptc1) receptor, leading to de-repression of Smoothened and the subsequent nuclear translocation of Gli transcription factors [86-88]. Interestingly, late embryonic genetic deletion of these two different Hedgehog ligands, Indian Hedgehog (Ihh) or Sonic Hedgehog (Shh), leads to distinct intestinal outcomes: Shh abrogation induces duodenal obstruction and abnormal intestinal innervation, whereas Ihh deletion reduces crypt proliferation and differentiation [89]. Expression of Shh and Ihh is restricted to the epithelium, while Gli transcription factors are exclusively expressed in the mesenchyme strongly indicating that Hedgehog ligands are produced and secreted by the intestinal epithelium with paracrine action on the mesenchyme [90]. Some evidence indicates that in response to Hedgehog signals sent by the epithelium, stromal cells may secrete niche factors, such as Wnts that either promote intestinal proliferation or induce differentiation. Gli1+ stromal cells secrete Wnt2b to sustain ISC integrity, which further suggests a role of Hh signaling in priming sub-epithelial stromal cells to maintain intestinal homeostasis [56]. Hedgehog autocrine signaling also occurs in Paneth cells and ISCs [91-93]. Thus, the Hedgehog ligands are paracrine and autocrine ISC factors.
Bone Morphogenetic Protein (BMP)
Wnt/β-catenin and BMP (Bone Morphogenetic Protein) signaling are opposing forces in the intestinal crypt/villi axis with contrasting gradients of activity: contrary to Wnt/β-catenin signaling, BMP signaling is low in the crypts and higher towards the top of the villi. This inhibits proliferation of the Lgr5+ cells via Smad-mediated repression of genes important for stem cell renewal, including Lgr5, thus promoting differentiation [94-96]. To counteract the inhibitory effects of BMP signaling, BMP antagonists like Noggin, Gremlin-1 and Gremlin-2 are enriched in the crypts, allowing the self-renewal of the Lgr5+ ISC. The BMP antagonists facilitating stem cell self-renewal are secreted by intestinal sub-epithelial myofibroblasts and smooth muscle cells adjacent to the crypt base [97].
Hippo
The Hippo pathway is an evolutionarily conserved signaling module first described in Drosophila. In vertebrates, mechanosensory stresses lead to activation of the core Hippo pathway in which the serine/threonine kinases MST1/2 (orthologs of Drosophila Hippo), SAV1, LATS1/2 orthologs) and MOBKL1A/1B participate in a kinase cascade that culminates in the phosphorylation of the transcriptional co-activators YAP and TAZ. The phosphorylation of YAP and TAZ leads to their exclusion from the nucleus and inability to potentiate gene expression. In contrast, in the absence of Hippo pathway signaling, the dephosphorylated forms of YAP and TAZ are nuclear and cooperate with the TEAD transcription factors to activate diverse target genes including pro-proliferative loci [98]. The Hippo pathway has been investigated in ISC biology, typically through genetic manipulation of YAP1. Overexpression of a constitutively active form of YAP1 strongly inhibits intestinal proliferation with loss of Olfm4+ ISCs while intestinal epithelial-specific YAP1 deletion is well tolerated during homeostasis but produces massive intestinal overgrowth and Lgr5+ ISC expansion upon radiation injury and/or R-spondin treatment [99]. Potential functional redundancy between YAP and TAZ has also been explored with results differing on the methods employed [100, 101]. It has been additionally proposed that YAP1 maintains the ISC pool during early intestinal regeneration, whereas late hyperproliferation is independent of YAP and TAZ [102]. The ability of mechanosensory stresses to regulate the Hippo pathway may allow changes in niche and crypt architecture to crucially govern ISC biology during regeneration.
Other canonical niche pathways
Many other pathways and cell types likely contribute functionally to the ISC niche. The intestine contains multiple stromal cell populations with distinct morphologies and functions. Mesenchymal populations including FoxL1+, CD34+ Gp38+ αSMA−, or Gli1+ may be highly interrelated and are clearly significant with regards to crypt homeostasis, as discussed above. Additional populations including fibroblasts and myofibroblasts are major lamina propria cell types, with fibroblasts providing structural support through the synthesis of extracellular matrix and myofibroblasts having both fibroblast and smooth muscle characteristics contributing to contracting ability [103]. Besides Foxl1-expressing mesenchymal cells, myofibroblasts also enclose the crypts and are thus candidate sources of ISC niche factors. Notably, Porcn deletion within MYH11+ cells, which include all muscle layers of the lamina propria and myofibroblasts, does not affect crypt homeostasis although these cells could elaborate redundant factors for ISC maintenance [45]. Besides expressing BMP antagonists [97], myofibroblasts secrete growth factors including SCF, FGF, HGF, IGF, KGF, NGF and PDGF whose impact on ISCs is less understood [104]. Since fibroblasts and myofibroblasts are heterogeneous, different sub-populations may secrete distinct niche factors. The smooth muscle cells are in close proximity with myofibroblasts, forming the muscularis mucosa, which separates the lamina propria from the submucosa. Their constant contraction and relaxation repels luminal contents from the crypts and may serve as primary or redundant sources of Wnt and BMP ligands for ISCs [97].
Emerging niche pathways
Cellular niche components mediating repair
The cellular complexity of the ISC niche provides a diversity of biosensors that can stimulate an ISC response to injury. Conceivably, different intestinal stem/progenitor cells could be induced by different cellular niches. Distinct homeostatic cellular niches may accordingly exhibit characteristic adaptations to injury that in turn induce the regenerative response of diverse ISC populations. The importance during homeostasis of mesenchymal populations such as Foxl1+, CD34+ Gp38+ αSMA− or Gli1+ may directly translate into equal significance during repair through modulation of canonical pathways such as Wnt or R-spondin. Gli1+ sub-epithelial mesenchymal cells constitute an essential Wnt source in the colon but in the small intestine Gli1+ cells are essential Wnt sources during injury repair and dispensable during homeostasis [57]. This could indicate that Gli1 possibly marks a sub-population of Foxl1+/PDGFRα+ telocytes in the small intestine, playing an essential role only upon damage. Indeed, both Foxl1+ and Gli1+ cells co-express Sfrp1 with similar morphology and tissue localization [55, 57]. Furthermore, the intestinal lamina propria also contains a plethora of additional cell types including myofibroblasts, smooth muscle cells, endothelial cells, neurons, glia, and hematopoietic cells. All these cell populations interact with the epithelium and many are implicated in post-injury intestinal recovery.
The muscular layer of the intestine ensures coordinated bowel movements to achieve suitable mixing and motion of contents during digestion, absorption and excretion. These movements are driven by the enteric nervous system (ENS), comprised of neurons and glial cells. Enteric neurons within the niche stimulate intestinal epithelial growth and repair upon injury. Glucagon-like peptide 2 (GLP-2) is secreted by the intestinal epithelium and received by enteric neurons which reciprocally signal back to the epithelium, promoting its repair via as-yet unidentified factor(s) [105]. The other cell type composing the ENS, glial cells, may elaborate ISC regulatory niche factors, via their projections in close proximity to crypts. Although genetically-induced loss of enteric glia does not perturb intestinal epithelial homeostasis [106], multiple lines of evidence point to a possible role of the enteric glial cells in injury recovery, by the release of putative niche factors such as GDNF (Glial-Derived Neurotrophic Factor), TGF-β1, 15dPGJ2 or GSNO. GDNF prevents colonic epithelial apoptosis upon damage through activation of MAPK and PI3K/AKT [107, 108]. GDNF may also exert an anti-apoptotic role on glia by an autocrine mechanism [109]. Some glial factors may act to restrain proliferation. Enteric glia secrete TGF-β1 upon injury to inhibit ISC proliferation [110, 111] and also release 15dPGJ2, a prostaglandin ligand for the intracellular PPARγ receptor [112] that promotes intestinal differentiation [113]. Further, the glial cell-secreted niche factor s-nitrosoglutathione (GSNO), besides roles in intestinal permeability, inhibits inflammation through NF-κB-dependent signaling and inhibiting expression of pro-inflammatory cytokines such as TNF-α, which in theory could aid recovery of the epithelium by attenuating inflammatory damage [114-116]. This agrees with studies indicating the ENS reduces over-proliferation of the gut microbiota and inflammation [117]. Notably, ablation of enteric glia worsens mucosal damage in colitis and intestinal recovery, while enteric glial cells enhance epithelial cell proliferation via EGF in vitro [118]. This and other studies also particularly implicate the GFAP-expressing glial sub-population during intestinal inflammation [118-121]. Lastly, glial cells form synapses with epithelial enteroendocrine cells [122] (Figure 4).
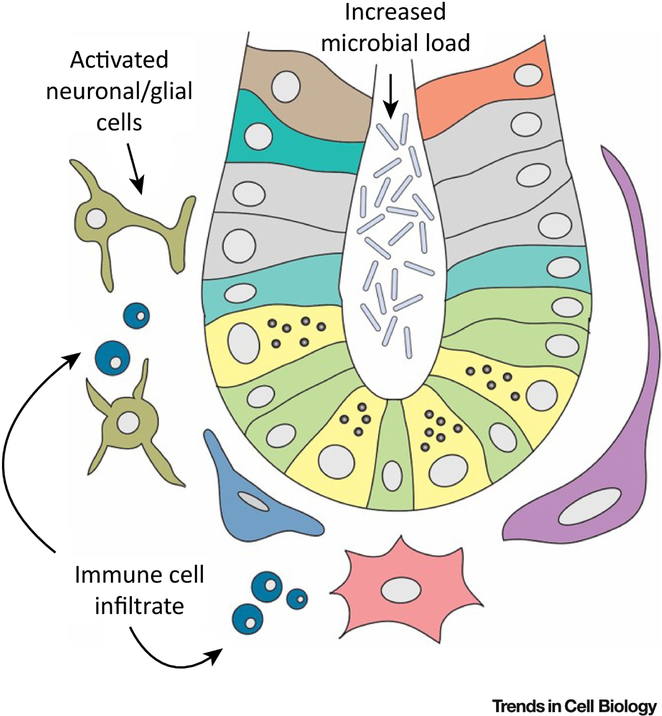
Figure 4: Niche cell changes during injury and repair.
Injury to the intestinal epithelium is often accompanied by changes in the microbiota. To aid repair upon injury and return to homeostasis, “injury niche” factors are also provided by cell populations that are dispensable during homeostasis, such as neurons, glial and immune cells.
Immune cells also contribute to intestinal epithelial protection after injury by secreting niche factors. Co-cultures of small intestine enteroids with IL-22 or IL-22-producing innate lymphoid cells (ILCs) augments enteroid growth in a Paneth cell-independent manner by inducing STAT3 phosphorylation within Lgr5+ ISC. Further, in vivo IL-22 treatment after tissue damage in a mouse graft-versus-host disease (GVHD) model aids recovery of Lgr5+ ISCs, increases epithelial regeneration and reduces mortality in mice [123]. Other immune cells may also maintain the ISC niche upon injury. For instance, the macrophage-secreted factor CSF1 supports Paneth cell maintenance, which may redundantly maintain Lgr5+ ISCs [124]. Interestingly, macrophage-specific Porcn deletion reveals that macrophages are an important source of Wnts upon radiation damage to enhance survival of ISCs and promote epithelial repair – indeed, Porcn-null mice are rescued from radiation lethality when treated with bone marrow-derived macrophage condition medium from control but not from Porcn-null mice [125]. Elaborating the role of immune cells within the ISC niche during inflammation-associated injuries such as Crohn’s disease, ulcerative colitis, or gastroenteritis should continue to be a fertile area of inquiry (Figure 4).
Endothelial cells constitute the inner lining of blood and lymph vessels and pervade the ISC niche through capillaries and lacteals. The endothelium was initially identified as an injury-regulated niche component since epithelial loss upon irradiation damage is partially dependent on endothelial apoptosis. Blocking apoptosis of endothelial cells with fibroblast growth factor (FGF) prevents radiation-induced intestinal epithelial damage, indicating that endothelial apoptosis could be a key signal that compromises the integrity and survival of the epithelium and Lgr5+ cells, possibly by the release of intracellular factors upon apoptosis [126]. While these endothelial effects could be direct or indirect, the identification of those factors and their influence on Lgr5+ ISCs are important questions.
Extrinsic ISC niche factors during homeostasis, infection and injury response
Extrinsic factors also have a demonstrated capacity to influence ISC niche components, during homeostasis and perhaps to a more pronounced extent during injury. These include microbiota and diet, which interface with the ISC niche to regulate stem cells and intestinal regeneration (Figure 3).
1. Commensal bacteria and gut pathogens
Over the last decade, it has become increasingly apparent that the intestinal interactions with microorganisms and viruses are not restricted to the immune system but extend to ISC self-renewal and differentiation. Many studies using germ-free and/or antibiotic-treated mice have demonstrated the influence of microbiota on the intestinal epithelium including regional phenotypes caused by the lack of bacteria– decreased villus height and crypt depth in the jejunum and ileum but increased villus height and decreased crypt depth in the duodenum. Moreover, germ-free animals revealed increased colonic crypt depth and epithelial proliferation after exposure to commensal bacteria. These intestinal regional differences in microbiota-lacking versus colonized animal models have been recently discussed [127]. Gavage of neonatal mice with the human-derived probiotic Lactobacillus reuteri increases enterocyte migration, proliferation and crypt height. This altered intestinal microbiota composition suggests that microbial diversity can directly influence intestinal epithelial homeostasis [128]. Another oral probiotic, Lactobacillus rhamnosus, protects the murine small intestinal epithelium from radiation injury, via action of MyD88, TLR-2 and COX-2. Contrary to Lactobacillus reuteri, Lactobacillus rhamnosus did not significantly alter microbiotal diversity, indicating that protective effects of the former were not due to a change in the microbiota composition [129].
Butyrate, a short-chain fatty acid produced by intestinal microflora, represents a clear example of microbiotal regulation of intestinal homeostasis. This organic acid produced by intestinal microbial fermentation of dietary carbohydrates is mostly present in the colon. Butyrate within the crypt bases (in case of injury or given to crypt-less animals, such as zebrafish), strongly inhibits expansion of colonic Lgr5+ ISCs via Foxo3. However, colonocytes at the upper part of the colonic glands, use butyrate preferentially as their energy source, titrating it away from the basally-located ISCs. Butyrate consumption by the colonocytes not only maintains stem cell integrity but probably also boosts overall colon fitness [130]. Administration of butyrate to young piglets improves overall jejunal and ileal epithelial health, leading to increases in crypt depth, villus length and mucosal thickness [131]. In agreement with those findings, short-chain fatty acids, including butyrate, promote growth and budding of mouse intestinal enteroids [132]. Since most microbiota reside in the colon, with high concentrations of butyrate-producing bacteria, effects of butyrate on small intestine ISCs remain unstudied.
The microbiota may directly regulate intestinal regeneration via expression of the Nod2 receptor, since the Nod2 agonist, muramyl-dipeptide (MDP), a peptidoglycan motif universal to all bacteria, promotes mouse crypt-derived organoid formation. Moreover, following doxorubicin-induced intestinal damage, MDP improves intestinal epithelial survival and regeneration in a Nod2 dependent manner [133]. ISCs from germ-free mice have very distinct microRNA profiles versus germ-free mice later colonized with murine “normal” microbiota with mIR-375 inhibiting ISC expansion in enteroids [134]. The microbiota can also impact oncogenically transformed Lgr5+ ISCs as in colorectal cancer [135, 136] which can be promoted by inflammatory pathways [137] triggered by bacteria [138, 139]. Accordingly, intestinal dysbiosis/microbial imbalance or increased expression of TLRs are linked to colorectal cancer development [140, 141]. The microbiota can contribute directly to the development of colorectal tumors – via calcineurin/NFAT dependent survival and proliferation of cancer stem cells [142].
On the other hand, pathogenic agents, like Salmonella or Heligmosomoides polygyrus also impact the fate of the stem cell lineage in the intestinal epithelium during infection. A recent study showed that Salmonella infections cause a population increase of Paneth cells and enterocytes, and a significant decrease in Lgr5+ ISCs, whilst H. polygyrus infections lead to an increase in the abundance of tuft and goblet cells, but do not change the number of Lgr5+ ISCs [143]. Gut pathogens are thus emerging niche agents capable of influencing the ISC status, as it has been shown that bacterial pathogens such as Salmonella or Shigella can enter the crypts during infection [144, 145].
The enteric pathogen rotavirus (RV) specifically infects and damages differentiated cells at villus tips, leaving intact crypt ISC populations. Interestingly, upon oral RV exposure in mice, villus RV infection stimulates Lgr5+ ISCs, crypt expansion and hyperproliferation. This effect was not observed with epithelial-specific WIs KO, suggesting a specific role for epithelial-derived Wnts; Bmi1+ quiescent ISCs populations were unaffected. These findings indicate a preferential role of the active Lgr5+ ISCs over other potential ISC populations during intestinal epithelial restitution [146], consistent with findings in radiation models [24].
2. Diet
Another extrinsic adaptive ISC niche factor is represented by diet. Paneth cells regulate ISC viability and function in response to caloric restriction through mTORC1 [147]. In addition, a recent publication showed that 24 h fasting in mice promotes ISC function by activating a fatty acid oxidation (FAO) program [148]. Intriguingly, the opposite treatment - a high-fat calorie intake in mice also promotes ISC renewal and represses differentiation [149]. Interestingly, both fasting and high-fat calorie intake act via an ISC peroxisome proliferator-activated receptor delta (PPARδ)-mediated FAO program that leads to increased circulating free fatty acids that contribute to ISC expansion. Indeed, palmitic acid or oleic acid promote intestinal organoid growth, highlighting how diet modulates ISC function and presenting dietary lipids as novel niche factors [148, 150].
Concluding Remarks and Future Directions
The intestine displays a remarkable homeostatic capacity, balancing the need for robust absorptive and barrier functions against the risks of excessive epithelial turnover and neoplastic overgrowth. Similarly, the intestinal epithelium must also repair itself in response to diverse pathologic injuries. The ISC niche is central to this ability to meet the dual challenges of homeostasis and injury repair (Figure 5). Indeed, far from a static entity, it is increasingly clear that the ISC niche can nimbly adapt beyond its homeostatic extent to address vastly different insults, spanning infectious, inflammatory and treatment-related conditions. Thus, one important future direction in the intestinal stem cell field is to identify cellular and humoral components that distinguish the homeostatic versus the injury-adapted niche, or more specifically, to pinpoint the exact niche cells that elaborate the paracrine signals that regulate ISCs during homeostasis and those that regulate ISCs during repair (see Outstanding Questions). Certainly, the adaptive and homeostatic niches could simply interconvert, via the appropriate injury-inducible modulation of canonical pathways operative during homeostasis such as Wnt, R-spondin, Notch, BMP and Hedgehog, and cell types such as Foxl1+ mesenchymal cells. In such a reductionist model, these same cell populations and pathways operative during homeostasis also mediate intestinal repair. However, the diversity of intestinal pathogenic conditions may necessitate the incorporation of additional biosensor niche cell types and repair mechanisms during injury into the adaptive ISC niche (Figure 5). Such an understanding would allow further insight into how the homeostatic and injury-adapted ISC niches differentially regulate the equilibrium between active and quiescent/reserve ISCs, and which exact factors guide the cellular conversion from reserve to active ISCs. Multiple different pathways, including inflammation, the ENS, microbiota and diet, may be of particular significance during injury and thus in activating different quiescent/reserve ISCs. Lastly, it will be absolutely necessary to reconcile the emerging distinctions between the homeostatic and adaptive ISC niche against an equally emerging biology of interconversion between active versus injury-inducible ISCs. This task may be compounded by the extensive plasticity displayed by numerous cell types that are now appreciated to function as reserve ISCs, as well as the possibility that Lgr5+ ISC generation may be a final common pathway of repair. Despite these manifold challenges, the resultant understanding should yield significant insights into basic biology, disease mechanisms and therapeutic approaches for intestinal disorders.
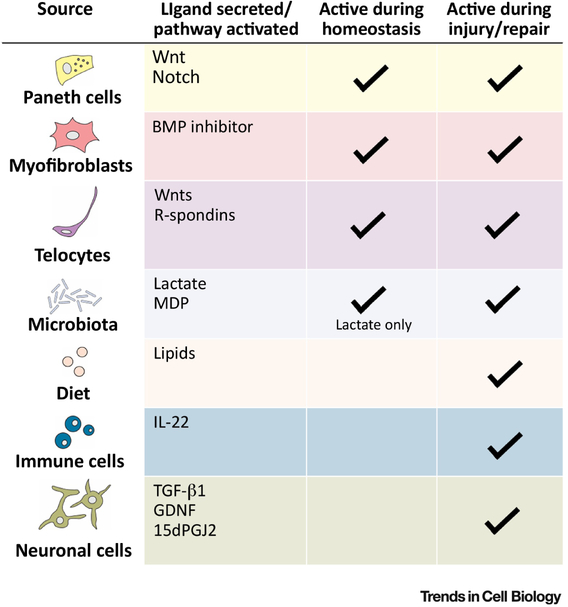
Figure 5: Interconversion of ISC niches during homeostasis and repair.
Summary table depicting the differences between the homeostatic niche and the injury niche – different cell types come into play upon injury and provide different niche factors that help to repair the epithelial damage and the return to homeostasis.
GDNF, glial-derived neurotrophic factor; MDP, muramyl-dipeptide.
Outstanding questions.
-
1)
What cellular and humoral components distinguish the homeostatic versus injury-adapted ISC niches?
-
2)
Which intestinal niche populations elaborate the paracrine signals that regulate ISCs during homeostasis and repair?
-
3)
What is the correspondence between the homeostatic and injury-adapted ISC niches, versus active and quiescent/reserve ISCs?
-
4)
How do the homeostatic and injury-adapted ISC niches differentially regulate the dynamic equilibrium between active and quiescent/reserve ISCs?
-
5)
Which factors guide the cellular reversion from quiescent to active ISCs?
Highlights.
The homeostatic niche maintains the activity of Lgr5+ intestinal stem cells and the relative quiescence of reserve ISCs.
Upon injury, the ISC niche undergoes substantial adaptation to effect epithelial repair, potentially by activating reserve ISC populations.
The intestinal stroma, epithelium and paracrine signals may all underlie niche adaptations to injury.
Microbes, viruses, diet and inflammation are external factors that impact the integrity of ISCs thus affecting intestinal epithelial health in homeostasis and influencing recovery upon injury.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barker N et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449 (7165), 1003–7. [DOI] [PubMed] [Google Scholar]
- 2.Beumer J and Clevers H (2016) Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 143 (20), 3639–3649. [DOI] [PubMed] [Google Scholar]
- 3.Barker N and Clevers H (2010) Lineage tracing in the intestinal epithelium. Curr Protoc Stem Cell Biol Chapter 5, Unit5A 4. [DOI] [PubMed] [Google Scholar]
- 4.Yan KS et al. (2017) Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature 545 (7653), 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato T et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459 (7244), 262–5. [DOI] [PubMed] [Google Scholar]
- 6.van de Wetering M et al. (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111 (2), 241–50. [DOI] [PubMed] [Google Scholar]
- 7.de Lau W et al. (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476 (7360), 293–7. [DOI] [PubMed] [Google Scholar]
- 8.Carmon KS et al. (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 108 (28), 11452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glinka A et al. (2011) LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep 12 (10), 1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuijers J et al. (2015) Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 16 (2), 158–70. [DOI] [PubMed] [Google Scholar]
- 11.Janda CY et al. (2017) Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature 545 (7653), 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Flier LG et al. (2009) OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 137 (1), 15–7. [DOI] [PubMed] [Google Scholar]
- 13.Dalerba P et al. (2007) Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A 104 (24), 10158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin TG et al. (2010) Characterization of the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology 139 (6), 2072–2082 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Furstenberg RJ et al. (2011) Sorting mouse jejunal epithelial cells with CD24 yields a population with characteristics of intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 300 (3), G409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F et al. (2013) Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 145 (2), 383–95 e1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan KS et al. (2017) Intestinal Enteroendocrine Lineage Cells Possess Homeostatic and Injury-Inducible Stem Cell Activity. Cell Stem Cell 21 (1), 78–90 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potten CS et al. (1974) Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet 7 (3), 271–83. [DOI] [PubMed] [Google Scholar]
- 19.Potten CS (1975) Kinetics and possible regulation of crypt cell populations under normal and stress conditions. Bull Cancer 62 (4), 419–30. [PubMed] [Google Scholar]
- 20.Potten CS (1977) Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 269 (5628), 518–21. [DOI] [PubMed] [Google Scholar]
- 21.Sangiorgi E and Capecchi MR (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40 (7), 915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan KS et al. (2012) The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A 109 (2), 466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian H et al. (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478 (7368), 255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalfe C et al. (2014) Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14 (2), 149–59. [DOI] [PubMed] [Google Scholar]
- 25.Buczacki SJ et al. (2013) Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495 (7439), 65–9. [DOI] [PubMed] [Google Scholar]
- 26.Barriga FM et al. (2017) Mex3a Marks a Slowly Dividing Subpopulation of Lgr5+ Intestinal Stem Cells. Cell Stem Cell 20 (6), 801–816 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Es JH et al. (2012) Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 14 (10), 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tetteh PW et al. (2016) Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell 18 (2), 203–13. [DOI] [PubMed] [Google Scholar]
- 29.Roth S et al. (2012) Paneth cells in intestinal homeostasis and tissue injury. PLoS One 7 (6), e38965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu S et al. (2018) Paneth Cell Multipotency Induced by Notch Activation following Injury. Cell Stem Cell 23 (1), 46–59 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadhav U et al. (2017) Dynamic Reorganization of Chromatin Accessibility Signatures during Dedifferentiation of Secretory Precursors into Lgr5+ Intestinal Stem Cells. Cell Stem Cell 21 (1), 65–77 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schonhoff SE et al. (2004) Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol 270 (2), 443–54. [DOI] [PubMed] [Google Scholar]
- 33.Gross S et al. (2015) Nkx2.2 is expressed in a subset of enteroendocrine cells with expanded lineage potential. Am J Physiol Gastrointest Liver Physiol 309 (12), G975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sei Y et al. (2011) A stem cell marker-expressing subset of enteroendocrine cells resides at the crypt base in the small intestine. Am J Physiol Gastrointest Liver Physiol 300 (2), G345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Landeghem L et al. (2012) Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 302 (10), G1111–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng X and Hou SX (2015) Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development 142 (4), 644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sailaja BS et al. (2016) The regulatory niche of intestinal stem cells. J Physiol 594 (17), 4827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montgomery RK et al. (2011) Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A 108 (1), 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda N et al. (2011) Interconversion between intestinal stem cell populations in distinct niches. Science 334 (6061), 1420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willert K et al. (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423 (6938), 448–52. [DOI] [PubMed] [Google Scholar]
- 41.Janda CY et al. (2012) Structural basis of Wnt recognition by Frizzled. Science 337 (6090), 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clevers H et al. (2014) Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346 (6205), 1248012. [DOI] [PubMed] [Google Scholar]
- 43.van Es JH et al. (2012) A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol 32 (10), 1918–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabiri Z et al. (2014) Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141 (11), 2206–15. [DOI] [PubMed] [Google Scholar]
- 45.San Roman AK et al. (2014) Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Reports 2 (2), 127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhnert F et al. (2004) Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A 101 (1), 266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinto D et al. (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17 (14), 1709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato T et al. (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141 (5), 1762–72. [DOI] [PubMed] [Google Scholar]
- 49.Sato T et al. (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469 (7330), 415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farin HF et al. (2016) Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 530 (7590), 340–3. [DOI] [PubMed] [Google Scholar]
- 51.Durand A et al. (2012) Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci U S A 109 (23), 8965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim TH et al. (2012) Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci U S A 109 (10), 3932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farin HF et al. (2012) Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143 (6), 1518–1529 e7. [DOI] [PubMed] [Google Scholar]
- 54.Aoki R et al. (2016) Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol 2 (2), 175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoshkes-Carmel M et al. (2018) Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valenta T et al. (2016) Wnt Ligands Secreted by Subepithelial Mesenchymal Cells Are Essential for the Survival of Intestinal Stem Cells and Gut Homeostasis. Cell Rep 15 (5), 911–918. [DOI] [PubMed] [Google Scholar]
- 57.Degirmenci B et al. (2018) GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 558 (7710), 449–453. [DOI] [PubMed] [Google Scholar]
- 58.Ootani A et al. (2009) Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15 (6), 701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCracken KW et al. (2011) Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc 6 (12), 1920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munera JO et al. (2017) Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell Stem Cell 21 (1), 51–64.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Lau WB et al. (2012) The R-spondin protein family. Genome Biol 13 (3), 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zebisch M et al. (2013) Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat Commun 4, 2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie Y et al. (2013) Interaction with both ZNRF3 and LGR4 is required for the signalling activity of R-spondin. EMBO Rep 14 (12), 1120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim KA et al. (2005) Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309 (5738), 1256–9. [DOI] [PubMed] [Google Scholar]
- 65.Koo BK et al. (2012) Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488 (7413), 665–9. [DOI] [PubMed] [Google Scholar]
- 66.Storm EE et al. (2016) Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nature 529 (7584), 97–100. [DOI] [PubMed] [Google Scholar]
- 67.Stzepourginski I et al. (2017) CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A 114 (4), E506–E513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang E et al. (2016) R-Spondins Are Expressed by the Intestinal Stroma and are Differentially Regulated during Citrobacter rodentium- and DSS-Induced Colitis in Mice. PLoS One 11 (4), e0152859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greicius G et al. (2018) PDGFRalpha(+) pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc Natl Acad Sci U S A 115 (14), E3173–E3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kopan R and Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137 (2), 216–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kovall RA et al. (2017) The Canonical Notch Signaling Pathway: Structural and Biochemical Insights into Shape, Sugar, and Force. Dev Cell 41 (3), 228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demitrack ES and Samuelson LC (2016) Notch regulation of gastrointestinal stem cells. J Physiol 594 (17), 4791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.VanDussen KL et al. (2012) Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139 (3), 488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schroder N and Gossler A (2002) Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns 2 (3-4), 247–50. [DOI] [PubMed] [Google Scholar]
- 75.Sander GR and Powell BC (2004) Expression of notch receptors and ligands in the adult gut. J Histochem Cytochem 52 (4), 509–16. [DOI] [PubMed] [Google Scholar]
- 76.Fre S et al. (2011) Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS One 6 (10), e25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rothenberg ME et al. (2012) Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology 142 (5), 1195–1205 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sasaki N et al. (2016) Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci U S A 113 (37), E5399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riccio O et al. (2008) Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep 9 (4), 377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carulli AJ et al. (2015) Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol 402 (1), 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y et al. (2010) Therapeutic antibody targeting of individual Notch receptors. Nature 464 (7291), 1052–7. [DOI] [PubMed] [Google Scholar]
- 82.Yang Q et al. (2001) Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294 (5549), 2155–8. [DOI] [PubMed] [Google Scholar]
- 83.Shroyer NF et al. (2007) Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 132 (7), 2478–88. [DOI] [PubMed] [Google Scholar]
- 84.Lo YH et al. (2017) Transcriptional Regulation by ATOH1 and its Target SPDEF in the Intestine. Cell Mol Gastroenterol Hepatol 3 (1), 51–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pellegrinet L et al. (2011) Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140 (4), 1230–1240 e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mao J et al. (2010) Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development 137 (10), 1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kolterud A et al. (2009) Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology 137 (2), 618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang H et al. (2013) Specific requirement of Gli transcription factors in Hedgehog-mediated intestinal development. J Biol Chem 288 (24), 17589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramalho-Santos M et al. (2000) Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127 (12), 2763–72. [DOI] [PubMed] [Google Scholar]
- 90.Madison BB et al. (2005) Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 132 (2), 279–89. [DOI] [PubMed] [Google Scholar]
- 91.Varnat F et al. (2006) PPARbeta/delta regulates paneth cell differentiation via controlling the hedgehog signaling pathway. Gastroenterology 131 (2), 538–53. [DOI] [PubMed] [Google Scholar]
- 92.Regan JL et al. (2017) Non-Canonical Hedgehog Signaling Is a Positive Regulator of the WNT Pathway and Is Required for the Survival of Colon Cancer Stem Cells. Cell Rep 21 (10), 2813–2828. [DOI] [PubMed] [Google Scholar]
- 93.Varnat F et al. (2009) Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med 1 (6-7), 338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haramis AP et al. (2004) De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303 (5664), 1684–6. [DOI] [PubMed] [Google Scholar]
- 95.He XC et al. (2004) BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet 36 (10), 1117–21. [DOI] [PubMed] [Google Scholar]
- 96.Qi Z et al. (2017) BMP restricts stemness of intestinal Lgr5+ stem cells by directly suppressing their signature genes. Nat Commun 8, 13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kosinski C et al. (2007) Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A 104 (39), 15418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mo JS et al. (2014) The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 15 (6), 642–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barry ER et al. (2013) Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493 (7430), 106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Azzolin L et al. (2014) YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158 (1), 157–70. [DOI] [PubMed] [Google Scholar]
- 101.Imajo M et al. (2015) Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol 17 (1), 7–19. [DOI] [PubMed] [Google Scholar]
- 102.Gregorieff A et al. (2015) Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature 526 (7575), 715–8. [DOI] [PubMed] [Google Scholar]
- 103.Roulis M and Flavell RA (2016) Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 92 (3), 116–131. [DOI] [PubMed] [Google Scholar]
- 104.Baum J and Duffy HS (2011) Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol 57 (4), 376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bjerknes M and Cheng H (2001) Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci U S A 98 (22), 12497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rao M et al. (2017) Enteric Glia Regulate Gastrointestinal Motility but Are Not Required for Maintenance of the Epithelium in Mice. Gastroenterology 153 (4), 1068–1081 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steinkamp M et al. (2003) Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology 124 (7), 1748–57. [DOI] [PubMed] [Google Scholar]
- 108.Zhang DK et al. (2010) Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J Pathol 222 (2), 213–22. [DOI] [PubMed] [Google Scholar]
- 109.Steinkamp M et al. (2012) GDNF protects enteric glia from apoptosis: evidence for an autocrine loop. BMC Gastroenterol 12, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miyoshi H et al. (2012) Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science 338 (6103), 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Munoz NM et al. (2008) TGF-beta has paradoxical and context dependent effects on proliferation and anoikis in human colorectal cancer cell lines. Growth Factors 26 (5), 254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bull AW et al. (2003) Activation of PPAR gamma in colon tumor cell lines by oxidized metabolites of linoleic acid, endogenous ligands for PPAR gamma. Carcinogenesis 24 (11), 1717–22. [DOI] [PubMed] [Google Scholar]
- 113.Bach-Ngohou K et al. (2010) Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12,14-prostaglandin J2. J Physiol 588 (Pt 14), 2533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Savidge TC et al. (2007) Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132 (4), 1344–58. [DOI] [PubMed] [Google Scholar]
- 115.Costantini TW et al. (2010) Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am J Physiol Gastrointest Liver Physiol 299 (6), G1308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reynaert NL et al. (2004) Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A 101 (24), 8945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rolig AS et al. (2017) The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol 15 (2), e2000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Van Landeghem L et al. (2011) Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol 300 (6), G976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosenbaum C et al. (2016) Activation of Myenteric Glia during Acute Inflammation In Vitro and In Vivo. PLoS One 11 (3), e0151335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cornet A et al. (2001) Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci U S A 98 (23), 13306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.von Boyen GB et al. (2004) Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 53 (2), 222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bohorquez DV et al. (2015) Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 125 (2), 782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lindemans CA et al. (2015) Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528 (7583), 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Akcora D et al. (2013) The CSF-1 receptor fashions the intestinal stem cell niche. Stem Cell Res 10 (2), 203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saha S et al. (2016) Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat Commun 7, 13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paris F et al. (2001) Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293 (5528), 293–7. [DOI] [PubMed] [Google Scholar]
- 127.Peck BCE et al. (2017) Gut Microbial Influences on the Mammalian Intestinal Stem Cell Niche. Stem Cells Int 2017, 5604727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Preidis GA et al. (2012) Probiotics stimulate enterocyte migration and microbial diversity in the neonatal mouse intestine. FASEB J 26 (5), 1960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ciorba MA et al. (2012) Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 61 (6), 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaiko GE et al. (2016) The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 165 (7), 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kotunia A et al. (2004) Effect of sodium butyrate on the small intestine development in neonatal piglets fed [correction of feed] by artificial sow. J Physiol Pharmacol 55 Suppl 2, 59–68. [PubMed] [Google Scholar]
- 132.Park JH et al. (2016) Promotion of Intestinal Epithelial Cell Turnover by Commensal Bacteria: Role of Short-Chain Fatty Acids. PLoS One 11 (5), e0156334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nigro G et al. (2014) The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe 15 (6), 792–8. [DOI] [PubMed] [Google Scholar]
- 134.Peck BC et al. (2017) Functional Transcriptomics in Diverse Intestinal Epithelial Cell Types Reveals Robust MicroRNA Sensitivity in Intestinal Stem Cells to Microbial Status. J Biol Chem 292 (7), 2586–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Sousa e Melo F. et al. (2017) A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature 543 (7647), 676–680. [DOI] [PubMed] [Google Scholar]
- 136.Shimokawa M et al. (2017) Visualization and targeting of LGR5(+) human colon cancer stem cells. Nature 545 (7653), 187–192. [DOI] [PubMed] [Google Scholar]
- 137.Terzic J et al. (2010) Inflammation and colon cancer. Gastroenterology 138 (6), 2101–2114 e5. [DOI] [PubMed] [Google Scholar]
- 138.Grivennikov SI et al. (2012) Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491 (7423), 254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kostic AD et al. (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22 (2), 292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Castellarin M et al. (2012) Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 22 (2), 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marchesi JR et al. (2011) Towards the human colorectal cancer microbiome. PLoS One 6 (5), e20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peuker K et al. (2016) Epithelial calcineurin controls microbiota-dependent intestinal tumor development. Nat Med 22 (5), 506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haber AL et al. (2017) A single-cell survey of the small intestinal epithelium. Nature 551 (7680), 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Santos AJM et al. (2016) Clustered Intracellular Salmonella enterica Serovar Typhimurium Blocks Host Cell Cytokinesis. Infect Immun 84 (7), 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Iwai H et al. (2007) A bacterial effector targets Mad2L2, an APC inhibitor, to modulate host cell cycling. Cell 130 (4), 611–23. [DOI] [PubMed] [Google Scholar]
- 146.Zou WY et al. (2018) Epithelial WNT Ligands Are Essential Drivers of Intestinal Stem Cell Activation. Cell Rep 22 (4), 1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yilmaz OH et al. (2012) mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 486 (7404), 490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mihaylova MM et al. (2018) Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell 22 (5), 769–778 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mah AT et al. (2014) Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology 155 (9), 3302–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Beyaz S et al. (2016) High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531 (7592), 53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]