Abstract
Cell polarity identifies the asymmetry of a cell. Various types of cells, including odontoblasts and epithelial cells, polarize to fulfil their destined functions. Odontoblast polarization is a prerequisite and fundamental step for tooth development and tubular dentin formation. Current knowledge of odontoblast polarization, however, is very limited, which greatly impedes the development of novel approaches for regenerative endodontics. Compared to odontoblasts, epithelial cell polarization has been extensively studied over the last several decades. The knowledge obtained from epithelia polarization has been found applicable to other cell types, which is particularly useful considering the remarkable similarities of the morphological and compositional features between polarized odontoblasts and epithelia. In this review, we first discuss the characteristics, the key regulatory factors, and the process of epithelial polarity. Next, we compare the known facts of odontoblast polarization with epithelial cells. Lastly, we clarify knowledge gaps in odontoblast polarization and propose the directions for future research to fill the gaps, leading to the advancement of regenerative endodontics.
Keywords: cell polarity, epithelial cell, odontoblast, polarization
1. Introduction
In a broad sense, cell polarity can be defined by either the structural or the functional asymmetry of a cell. Basically, all types of mammalian cells experience polarization process during their lifecycles either transiently or permanently. Cells undergo transient polarization process when they respond to exterior stimuli. However, some cells undergo permanent polarization process to achieve destined cellular fates, including epithelia, neurons, and odontoblasts.
Odontoblasts are a layer of dental cells that orderly align along the dental pulp. Odontoblast polarization marks the morphological change from a symmetrical mesenchymal cell to an asymmetrical odontoblast, with a columnar cell body aligned in a single layer and a cytoplasmic process extending from the cell body to the predentin. During primary odontogenesis, the polarized arrangement and morphological change of odontoblasts is a fundamental step for tubular dentin formation. It ensures the formation of tubular dentin and subsequently enables the existence of dentinal fluid and neural fibers within the dentinal tubules. In dentin regeneration, a similar transition of odontoblasts is eagerly desired, since the reconstruction of the tubular microstructure is now a major challenge in developing novel dentin regeneration strategies. Using current strategies, the regenerated dentin is morphologically more similar to bone, rather than dentin since the organized tubular structure is usually missing. This “osteodentin” explicitly impairs the biological properties of regenerated dentin, including its sensation transduction and mechanical properties. Therefore, understanding the mechanism of odontoblast polarization is pivotal for functional dentin regeneration. However, apart from the descriptions of cell morphology, there are few publications on odontoblast polarity.
The polarization process of epithelial cells has been extensively studied and major characteristics have been described in detail. First, their plasma membranes are divided into apical, lateral, and basal domains that exhibit different features; for example, the different distribution of membrane lipids between apical and basal membranes and the existence of intracellular junctions between adjacent cells. Within the cells, the asymmetric cytoskeleton distribution and the repositioning of organelles mark the intracellular polarity of a cell, together with the dispersion of epithelial polarity protein complexes. Each of these components is controlled by multiple factors, including Rho GTPases that are the predominant ones. All these components form a delicate molecular network, and contribute to the establishment of epithelial cell polarity. Moreover, these critical factors, especially the regulatory molecules, are also applicable in other types of cell polarity, such as neurons or migrating cells.
Multiple similarities have been found between epithelial cells and odontoblasts, including the features of cell morphology, development, function, and composition (McGuire et al., 2014). Therefore, in this paper we use epithelial cell polarity as a template to analyze the “knowns” and “unknowns” of odontoblast polarity. After introducing the features of epithelial cell polarity, regulatory factor Rho GTPases and the polarization process, we summarize the current understanding of odontoblast polarization and compare the differences between the two cell types. We hope that this review will shine a light on the “unknowns” of odontoblast polarization for further dental studies.
2. Characterizations of epithelial cell polarization
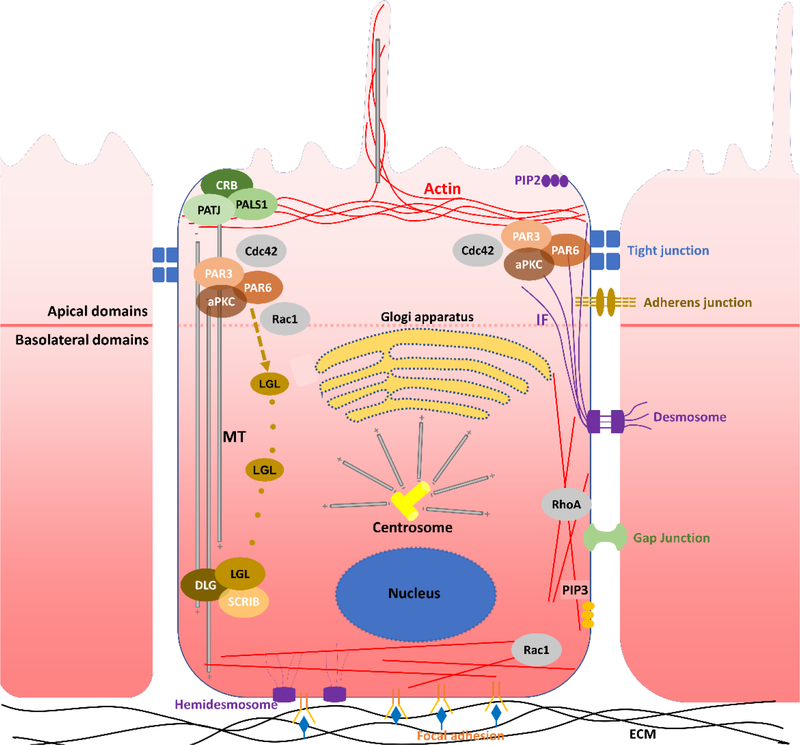
Epithelial cells exhibit apicobasal polarity defined by three plasma membrane domains, including apical, lateral, and basal domains (Rodriguez-Boulan and Macara, 2014). The apical domains face the lumen and enable material exchange. The lateral domains connect adjacent cells via specialized intercellular junctional structures. The basal domains adhere to the underlying basement membrane or extracellular matrix (ECM). The basal and lateral domains have similar components and are thereby named as basolateral domains (Román‐Fernández and Bryant, 2016). The characteristic components in epithelial polarization include the formation of intercellular junctions, the asymmetric cytoskeleton distribution, the reposition of organelles, and the distinct distribution of polarity complexes and membrane lipids. Each of these components is precisely regulated by multiple signaling pathways, among which the Rho GTPases are the predominant ones throughout the whole process (Mack and Georgiou, 2014). Moreover, these components themselves also act as intermediate scaffolds in constructing a polarized epithelial cell. A precise coordination of all components is required to guarantee an accurate polarization process (Figure 1).
Figure 1.
Illustration of epithelial cell polarity elements. Several distinct features, apart from the apparent difference in cell morphology, could be seen, including polarity complexes, intracellular junctions, reorganized cytoskeletons, repositioned organelles and regulating molecules. 1. Polarity complexes include apical PAR6/PAR3/aPKC complex, apical CRB/PALS1/PATJ complex, and basolateral SCRIB/LGL/DLG complex. 2. Intracellular junctions related to epithelial polarity include adherens junctions and tight junctions. Tight junctions mark the boundary of apical surface domains and basolateral surface domains. 3. Actin filaments and microtubules are reorganized. Actin majorly form the cortical belt encircling the most apical end of the lateral membrane domain and support apical junctions. Microtubules are majorly noncentrosomal and polarized with their plus ends stabilized at the basal cell cortex and minus ends anchored to cell-cell junctions or at the apical pole. Besides, a part of microtubules also orientates apically from the centrosome. Moreover, microtubules also form the primary cilium, where MTs originate from the centrosome toward the tip of the cilium. 4. Specific organelles are repositioned, including the nucleus, the centrosome and Golgi apparatus. The nucleus is located near the basal pole of a cell, and the centrosome is localized near the apical surface above the nucleus, and Golgi apparatus accompanies the movement of centrosome and remains adjacent to the centrosome. 5. Regulatory factors mainly include Rho GTPase members Rac1, Cdc42 and RhoA. The three molecules coordinate in regulating cytoskeleton reorganization to generate epithelial cell polarity. Furthermore, they can also directly interact with polarity complexes and even colocalize with specific organelles and regulate their activities. Other features, like the distribution of lipids PIP3 and PIP2 are also shown.
2.1. Intercellular junctions
There are several types of junctions around a polarized epithelial cell. Tight junctions (TJ), adherens junctions (AJ), desmosomes, and gap junctions are located at the intercellular space from apical to basal regions sequentially, while hemidesmosomes and focal adhesions are located between basal cell domains and underlying ECM. Despite the strict structural demarcation among the junctions, they are closely related during junction formation and epithelial polarity development (Giepmans and van IJzendoorn, 2009).
TJs are the most featured intercellular junctions during epithelial polarization. They function as a “gate” to occlude intercellular gaps for selective para-cellular permeability, and as a “fence” to mechanically segregate protein or lipid dispersion within the lipid bilayer, thereby defining the boundary of apical and basolateral domains (Zihni et al., 2016). TJs are composed of transmembrane structural proteins that constitute diffusion barriers and cytosolic adaptor proteins that connect the surface membrane to the cytoskeleton network. The structural proteins include tetraspans of the claudin family and MARVEL-domain proteins like occludin (Van Itallie and Anderson, 2013). Cytosolic adaptor plaque is a network of proteins containing complicated protein-protein interacting motifs, including zonula occludens (ZOs), cingulin, and JACOP. In short, occludins maintain the stability and barrier function of a TJ, claudins regulate the permeability (Günzel and Alan, 2013), and ZOs function in TJ assembly. ZO-1, the most important regulator of TJ formation, functions in various ways, including assembling occludins and claudins as scaffolding proteins, binding to and regulating actin cortex components, and promoting cadherin-mediated intercellular adhesion via regulating the spatial organization of tension (Balda and Matter, 2016). Moreover, ZOs can also be imported into the nucleus and regulate the expression of genes related to epithelial cell growth and differentiation (Caplan et al., 2008). ZOs are also components of AJs in non-epithelial cells and partners of gap junctions (van Zeijl et al., 2007). It was surmised that ZO proteins directly interacted with AJ proteins during AJ assembly and subsequently recruited TJ-related proteins to form TJs (Fanning and Anderson, 2009).
2.2. Polarity complexes
Three groups of polarity complexes are involved in epithelial polarity, including PAR6/PAR3/aPKC complex, CRB/PALS1/PATJ complex, and SCRIB/LGL/DLG complex (Assémat et al., 2008; Cereijido et al., 2008; Ebnet, 2015). The CRB and PAR complexes determine the apical domain of epithelial cells and exclude the SCRIB complex from the apical domain, while the SCRIB complex expels CRB and PAR complexes at the basolateral domains. This mutual exclusion between the polarity complexes results in the establishment and maintenance of apicobasal polarity (Ebnet, 2015).
In polarized epithelia, the PAR and CRB complexes locate primarily close to the TJs (Cereijido et al., 2008). The PAR complex is the major protein complex that dominates the polarization process (Chen and Zhang, 2013; Ebnet, 2015). PAR3 is recruited to the initial sites of cell-cell contact, and serves as a scaffold protein to recruit follow-up proteins to form apical junctions, including PAR6-aPKC. PAR6 functions as a conjugator that brings TJ-related proteins together (Chen and Zhang, 2013). aPKC plays a crucial role in the establishment of cell polarity in various biological contexts. In mammalian cells, aPKC is recruited to the initial spot-like AJs via a direct interaction with PAR-3, JAM-1, and nectin-1/3 upon cell-cell contact (Suzuki and Ohno, 2006), assists in promoting the transition of primordial spot-like AJs into continuous belt-like AJs via myosin II modulation (Kishikawa et al., 2008), and finally accumulates in TJs as polarization progresses. Apart from its role in junctional development, aPKC is fundamental in regulating cell polarity owing to its kinase role in phosphorylating various proteins. A detailed discussion of aPKC targets has been summarized recently (Hong, 2018).
The CRB complex associates with TJs through the interactions of PATJ with ZO-3, claudin1, and JAMA (Michel et al., 2005), and contributes to the apical junction stabilization, apical membrane differentiation, and cilium formation in epithelial cells (Bulgakova and Knust, 2009). Over-expression of CRB3 induces TJ formation in non-polarized epithelial cells (Fogg et al., 2005). PALS1 acts as a linker of CRB3 and PATJ (Assémat et al., 2008).
The SCRIB complex locates at the basolateral pole of a polarized epithelial cell. Suppression of SCRIB (Qin et al., 2005) or DLG (Iizuka-Kogo et al., 2007) expression causes a delay in TJ assembly. After the detachment from the PAR6/aPKC dimer, LGL moves towards the basolateral domain where DLG and SCRIB localize, therefore creating a direct connection between the apical PAR complex and the basolateral SCRIB complex.
2.3. Cytoskeleton distribution
Cytoskeleton network includes three components: actin filaments, microtubules, and intermediate filaments. In polarized epithelia, actin filaments form a belt-like cortex that encircles the most apical domains of the lateral membrane to support the apical junction complex, and form stress fibers at the basal domains to support focal adhesions (Miyoshi and Takai, 2008). Microtubule rearrangement is also dramatic — from a radial centrosomal array, to a highly asymmetric distribution with distinct orientations. The majority of microtubules are not centrosome-orientated and instead apicobasal polarized. Their plus ends are rooted at the basal cell cortex and their minus ends are anchored to the intercellular junctions or to the apical domains. The release and rearrangement of microtubules may associate with γ-tubulin ring complex (Oakley et al., 2015), E-cadherin mediated AJs formation (Li and Gundersen, 2008), or PAR3-regulated release of microtubule nucleators (Feldman and Priess, 2012). Another two minor populations include microtubules orientating from the centrosome to the apical membranes, which are related to apical protein trafficking, and microtubules forming the primary cilium, where microtubules originate from the centrosome toward the tip of the cilium (Sugioka and Sawa, 2012). A thick layer of intermediate filaments lie within the terminal web and below at the rootlets of the cellular processes. A few isolated intermediate filament bundles extend along the apical half of the lateral membrane, where they bind to desmosomes (Oriolo et al., 2007). Moreover, a faint but distinct intermediate filament network has also been observed at the basal pole where it attaches to hemidesmosomes or focal adhesions (Franke et al., 1979; Oriolo et al., 2007).
2.4. Organelle repositioning
Translocations of several organelles are observed responding to polarization stimuli, which include nucleus, centrosomes, and Golgi apparatus. In non-polarized cells, the centrosomes reside in the central area close to the nucleus, and the Golgi is usually adjacent to the centrosome. In a polarized epithelial cell, the nucleus resides at the basal half of the cell body, the centrosome at the apical surface above the nucleus (Feldman and Priess, 2012), and the Golgi apparatus accompanies the movement of centrosome and remains adjacent to the centrosome. The relative centrosome-to-nucleus localization corresponds to the main polarization axis (Elric and Etienne-Manneville, 2014), and the relative Golgi-to-nucleus defines an axis of secretion relevant to the proper orientation of membrane trafficking (Roux et al., 2009).
2.5. Membrane lipids
The polarized distribution of lipids is equivalently significant as proteins. PIP3 stably disperses at the basolateral domains, but is expelled from the apical domain. In contrast, PIP2 localizes exclusively at the apical domains (Martin-Belmonte et al., 2007). The phosphorylation of PIP2 generates PIP3 via PI3K, and dephosphorylation of PIP3 generates PIP2 via PTEN. The minute a cell begins to develop the apicobasal polarity, PTEN is recruited to the apical domains, making this process critical in membrane polarity establishment (Devergne et al., 2014).
3. Rho GTPases as the regulatory factors of polarization
The most extensively characterized Rho GTPases related to epithelial polarity include RhoA, Rac1, and Cdc42. As small GTPases, Rho proteins bind to GTP to form an active status, and bind to GDP to form an inactive status. The switch between the two statuses is regulated by various types of GTPase-binding proteins, including guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). GEFs promote the activation of the GTPases. GAPs can accelerate the hydrolyzation of GTP to activate GTPases. GDIs inhibit the dissociation of the guanine nucleotide and prevent the activation of GTPases. Rho GTPases also modulate each other’s activity via the complex crosstalk among them (Iden and Collard, 2008; Wu and Lew, 2013). As the major regulatory molecules in epithelial polarization, Rho GTPases not only regulate cytoskeleton organization and remodeling for transporting purposes, but also interact directly with polarity complexes (Mack and Georgiou, 2014; Ngok et al., 2014) and junction proteins to establish epithelial polarity.
3.1. Rho GTPases and cytoskeletal components
The downstream molecules of Rho GTPases targeting actin activities have been described in detail (Hall, 1998; Sit and Manser, 2011; Spiering and Hodgson, 2011). The RhoA effector ROCK directly phosphorylates actin regulators such as myosin phosphatase and LIM domain kinase (LIMK), which regulate actin contractility and depolymerization, respectively. Another RhoA effector is the formins that regulate unbranched actin filaments nucleation and elongation. WASP and WAVE are effectors of Cdc42 and Rac1 respectively, and they promote actin branching to augment the amount of free barbed ends to accelerate actin polymerization (Spiering and Hodgson, 2011). Actin dynamics also regulate Rho GTPase activity. This feedback loop ensures a precise regulation of actin activities (Li and Gundersen, 2008). Meanwhile, Rho GTPases also regulate microtubule dynamics (Müsch, 2004; Watanabe et al., 2005; Wojnacki et al., 2014). RhoA regulates microtubule stability through a mDia-APC-EB1 pathway (Quinones et al., 2011), and integrin-FAK mediated cell adhesion facilitates this process [46]. Moreover, ROCK phosphorylates MAP-2 and Tau, two microtubule-associated proteins critical in microtubule dynamics (Watanabe et al., 2005). Rac1 and Cdc42 regulate microtubule polarization by stabilizing these microtubules, providing the docking sites for microtubule plus ends and regulating the location of CLASPs. Microtubules also regulate Rho GTPase. Microtubule outgrowth activates Rac1. Microtubule depolymerization activates RhoA at focal adhesions and promotes the formation of contractile actin bundles via GEF-H1 (Birkenfeld et al., 2008; Kaverina et al., 2002).
3.2. Rho GTPases and polarity complexes
Rho GTPases not only play roles in cytoskeleton dynamics, but also interact directly with polarity complexes (Mack and Georgiou, 2014; Ngok et al., 2014). Rac1 activity is high at AJs and low at TJs, which is probably owing to the PAR3-mediated inhibition of Tiam1-Rac1activity, a process required for TJ biogenesis (Chen and Macara, 2005; Mack and Georgiou, 2014). Moreover, Rac1 is lower in apical membranes compared to lateral membranes, because Rac1 GAP chimaerin reduces Rac1 activity apically (Yagi et al., 2012). RhoA activity at apical membranes is also limited, that it is inactivated by Rac1 via recruiting p190RhoGAP to cell junctions to help define the apical domains (Ngok et al., 2014). Additionally, PAR6 targets RhoA degradation at TJs, which limits the activity of local RhoA. Cdc42 is vital in the apical localization of PAR complex and the establishment of epithelial polarity. An earlier theory suggested that Cdc42 promoted apical differentiation through binding to PAR6 and caused its conformational change, which enhanced its affinity for aPKC substrates. Cdc42 also increased the phosphorylation activity of aPKC and subsequent PAR3 activation, resulting in the correct positioning of the apical-basolateral border. However, other evidence showed the interruption of Cdc42-PAR6 interaction did not impair aPKC activity. Therefore, the function of Cdc42-PAR6 in aPKC stimulation remains unclear. Moreover, a network that involves Cdc42, Tuba, annexin2 and PAR complex guides Cdc42-dependent exocytosis and targeted apical transportation (Bryant et al., 2010), resulting in apical localization of CRB and exclusion of apical Baz/PAR3 to define the apicobasal border (Schlüter et al., 2009).
3.3. Rho GTPases and junction proteins
Rho GTPases also directly interact with junction adaptor proteins and transfer junction signals to the cell interior (Zihni et al., 2014a). Rac1 is recruited and activated by nascent cell-cell contacts, and it stimulates lamellipodia formation which initiates AJ formation. As AJs mature, the intercellular accumulation of E-cadherin widely diminishes Rac1 activity (Zihni et al., 2014a). Rac1 moving in the lamellipodia promotes Arp2/3-mediated actin polymerization, and drives the expansion of cell-cell adhesion (Ehrlich et al., 2002), together with the concurrent activation of RhoA/ROCK/myosin pathway (Yamada and Nelson, 2007).
RhoA promotes both the organization and the maintenance of tight junctions (Quiros and Nusrat, 2014; Zihni et al., 2016). First, E-cadherin-based puncta clustering is the initiator of apical junction formation and it’s dependent on RhoA/mDia-dependent actin polymerization, as well as Rac1-WAVE-Arp2/3-dependent and Cdc42-NWASP-Arp2/3-dependent pathways. Second, the direct interactions between actomyosin and TJ plaque proteins stabilize the junctions and provide force against the disruption of intercellular junctions. Third, Rho GEFs p114RhoGEF (Terry et al., 2011) and ARHGEF11 (Itoh et al., 2012) are recruited by adaptor proteins JACOP or ZO-1 to mediate the formation of junctions. Last, RhoA regulates contractile activity via associating with ROCK/cingulin/myosin complex or ATJ/Lulu2 complex, which causes the expansion and linearization of initial AJ-like junctions. The formation of the circumferential actomyosin belt and the specialized morphology of a polarized epithelial cell are also regulated by RhoA (Nakajima and Tanoue, 2011; Terry et al., 2011). However, there is also evidence suggesting that RhoA negatively regulates TJ activity. GEF-H1, the microtubule-related RhoA GEF, was inhibited by association with cingulin, and can promote TJ disassembly (Aijaz et al., 2005; Samarin et al., 2007).
Cdc42 is also a critical factor in tight junction assembly. The activated Cdc42 binds to PAR6 and the Cdc42-PAR6 complex activates aPKC, which results in PAR3 phosphorylation and PAR3-PAR6-aPKC complex dissociation. PAR3 remains at the tight junction area while the PAR6-aPKC complex migrates to the apical membrane. This process drives the accumulation of apical signaling proteins and the construction of TJs, during which a Cdc42 GEF, Dbl3 plays a critical role. Dbl3 is not indispensable in tight junction formation, instead it activates the PAR3-PAR6-aPKC complex that promotes apical PAR3 expulsion and apical membrane differentiation, thereafter regulating the size of apical domains and the position of TJs. Dbl3 localizes along the apical membrane and is enriched apical to tight junctions similar to other apical proteins (Zihni et al., 2014b). Other Cdc42 GEFs like Etc2 and Tuba are also involved in apical polarization. Previously Etc2 was proposed to regulate Cdc42 activity and associate with the PAR3-PAR6-aPKC complex in the initial establishment of epithelial polarity (Liu et al., 2006), but a later study showed that it specifically regulated RhoA signaling at AJs (Ratheesh et al., 2012). Tuba is recruited to TJs by ZO-1 and tricellulin, and it modulates the apical actin network by binding to N-WASP (Otani et al., 2006). Furthermore, a Rac1/Rich1 (a Cdc42 GAP) complex is also recruited and associated with the CRB complex and PAR3 to sustain the stability of tight junction by precisely controlling the local Cdc42 activity (Wells et al., 2006).
3.4. Rho GTPases and organelles
Rho GTPases regulate organelle positioning through cytoskeleton dynamics and direct interaction. Microtubules are critical forces in regulating nuclear position, especially the centrosomal microtubules. Their plus ends are anchored to cortical proteins via +TIPS including dynein, dynactin, CLASPs, IQGAP1 and APC, while the minus ends are directly linked to the nuclear envelope via liners of nucleoskeleton and cytoskeleton (LINC) to apply forces to modulate nuclear positioning (Razafsky and Hodzic, 2009). Actin cytoskeleton contributes to nuclear positioning via increasing microfilament stability, regulating actomyosin contraction and self-reorganizing at specific sites to restrain movement of the nucleus. (Huelsmann and Brown, 2014; Starr and Han, 2003). Actin filaments regulated by Cdc42 are directly linked to the nucleus by SUN2 to position the nucleus. Myosin II-mediated actomyosin contraction may power and accelerate this process (Gomes et al., 2005). Intermediate filaments also participate and exhibit a critical role in this actin-dominated nucleus movement (Dupin et al., 2009; Dupin et al., 2011).
The centrosome movement precedes and might initiate nuclear translocation (Dupin and Etienne-Manneville, 2011). The location of the centrosome is regulated by PAR proteins and contributes to epithelial polarity, mainly by regulating the apical accumulation of microtubule nucleators and the formation of an apicobasal microtubule network (Feldman and Priess, 2012). Centrosome positioning requires an active force generated by both microtubule and actin(Piel et al., 2000). Proteins mediating cytoskeleton-centriole interaction include actin, dynein and TBCCD1, a centrosome-associated protein (Gonçalves et al., 2010), and ninein, a microtubule-anchoring protein (Matsumoto et al., 2008).
The reassembly, location, and trans-Golgi transport of the Golgi requires both microtubule and actin cytoskeleton (Sütterlin and Colanzi, 2010). For instance, Cdc42 regulates the position of the Golgi via microtubules, which together with MAPs, determine the location and organization of the Golgi ribbon around the centrosome during Golgi remodeling and allow a directed transportation to specific membrane domains. Apart from centrosomal microtubules, a subset of microtubules can be nucleated at the Golgi independent of the centrosome. Their functions include assisting post-mitotic assembly of Golgi complex, post-Golgi trafficking, and establishing cell architecture (Sanders and Kaverina, 2015; Zhu and Kaverina, 2013). Although the exact mechanism remains unclear, the formation of Golgi-derived microtubules was found associated with γ-tubulin (Efimov et al., 2007; Ori-McKenney et al., 2012), microtubule-stabilizing protein CLASPs (Efimov et al., 2007), AKAP proteins, and microtubule motor complex dynein/dynactin (Rivero et al., 2009). The actin cytoskeletons have multiple effects on the Golgi. They facilitate post-Golgi transport by providing actin-based motors(Vicente-Manzanares et al., 2007), prevent the Golgi from spontaneous swelling by providing mechanical stability, maintain the Golgi position by providing structural support through the dynamic instability of actin filaments via Arp2/3 and cofilin, and assist in Golgi reconstruction (Gurel et al., 2014). For example, there are 3 actin-relevant proteins linked to Golgi: FMNL1g and INF2 assist in Golgi compaction and mDia1 promotes Golgi fragmentation.
Apart from the cytoskeleton dynamics, Rho GTPases also have direct roles in organelles (Farhan and Hsu, 2016; Long and Simpson, 2017). Both Cdc42 and RhoA are localized at the Golgi apparatus. Cdc42 localizes at the Golgi apparatus and the endoplasmic reticulum (ER), and regulates cell polarity by controlling the secretory and endocytic transport (Farhan and Hsu, 2016). Apart from accumulating Cdc42 as a reservoir to supplement the membranous Cdc42, Golgi-located Cdc42 also promotes the polarized transportation via regulating cargo sorting and carrier formation within the Golgi cisternae (Long and Simpson, 2017). Moreover, RhoA also influences the morphology and function of the Golgi (Long and Simpson, 2017). Constitutive overexpression of RhoA disrupted Golgi ribbon integrity and increased Rab6-positive carriers issued from the Golgi (Zilberman et al., 2011), which may associate with a RhoA GAP, DLC3 (Braun et al., 2015).
4. The polarization process of epithelial cells
During the epithelial polarization process, a cascade activation of Rho GTPases, polarity complexes, and junction proteins dominate the formation of intercellular junctions and demarcated plasma membrane domains. The cytoskeletal components function as the transportation carriers in delivering the proteins and organelles. An intricate interaction network among all these elements ensures an accurate polarization process.
4.1. Epithelial cell polarization induced by cell-cell contact
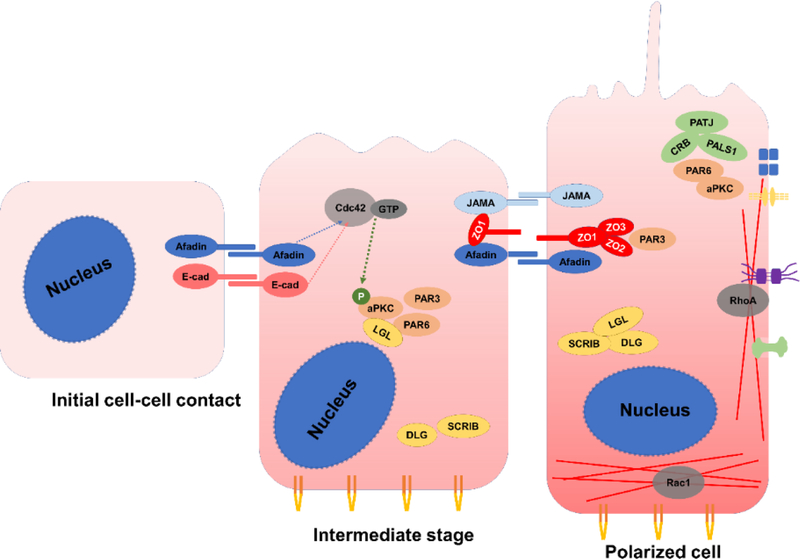
Normally, the initiation of polarization begins with cell-cell contacts between adjacent cells (Assémat et al., 2008; Etienne-Manneville, 2004; Goehring, 2014; Mack and Georgiou, 2014; Martin-Belmonte and Perez-Moreno, 2012; Quiros and Nusrat, 2014; Rodriguez-Boulan and Macara, 2014). Cell-cell contact initiated by adhesion proteins nectin and E-cadherin induced Cdc42 activation. Nectin recruits JAMA through afadin with the help of ZO-1, then JAMA recruits PAR3 to initial tight junctions, establishing the apicobasal border. PAR3 serves as a scaffold protein to recruit relevant proteins for junction assembly like PAR6 and aPKC. Ezrin recruits GEF Dbl3 and GEF Dbl3 triggers the activation of Cdc42 and then the phosphorylation of aPKC, which subsequently phosphorylates LGL. Phosphorylated LGL detaches from the PAR6/aPKC dimer and moves towards the lateral membrane where DLG/SCRIB resides. aPKC phosphorylates PAR3 to induce the assembly of activated PAR complex at the apical junctions. After being stabilized at tight junctions and phosphorylated by aPKC, PAR3 thereby detaches from the aPKC/PAR6 complex. The functional kinase domain of aPKC is freed up and able to phosphorylate other proteins. The PAR6-aPKC complex moves towards the differentiating apical domains while PAR3 stays at tight junctions. This process drives the accumulation of apical signaling proteins, such as CRB3, and the construction of tight junction. CRB3 interacts with PAR6 either directly or through PALS1 to induce the differentiation and maturation of the apical junctions (Figure 2).
Figure 2.
Polarization process of epithelial cells induced by cell-cell contact. From initial mesenchymal cell to the polarized epithelial cell, a cascade activation of Rho GTPases, polarity complexes and junction proteins is seen. The cytoskeletal components function as the transportation carriers in delivering those proteins and organelles repositioning. An intricate interaction network among all these elements ensures an accurate polarization process. Details of this figure can be found within the text.
4.2. Epithelial cell polarization independent of cell-cell contact
A single LKB1-activated epithelial cell can be fully polarized without the induction from cell–cell contact. Moreover, sustained AMPK activation restored a majority of the polarity defects exhibited in LKB1-null epithelial cells, indicating the necessity of AMPK in inducing epithelial polarization. LKB1/AMPK pathway mediates in both tight junction formation and plasma membrane domains differentiation and its effect on epithelial polarity may involve the regulation of actin redistribution via its motor protein myosin II (Martin-Belmonte and Perez-Moreno, 2012).
4.3. Epithelial cell polarization induced by cell-ECM interaction
The interaction between cell and extracellular matrix also regulates epithelial polarity. β1 integrins are critical mediators in forming focal adhesion between an epithelial cell and the surrounding ECM. Genetic deletion approaches have verified the importance of β1 integrins in polarity establishment of an epithelial cell. After the attachment to the ECM, β1 integrin is activated and followed by Rac1 activation for accurate basement membrane assembly, or ILK activation for correct intracellular polarity orientation. Integrins control the alignment of noncentrosomal microtubules along the apicobasal axis and their dynamic stability via molecular links between integrin-mediated focal adhesion proteins and the microtubule plus-end-binding protein EB1. The links may involve ILK-LL5α/β-CLASP-EB1 pathway or ILK-IQGAP1-CLIP170-EB1 pathway (Lee and Streuli, 2014). Moreover, integrins may impact centrosomal microtubules since their deletion impair Golgi positioning and the Golgi is closely related to the centrosome both spatially and functionally (de Forges et al., 2012; Rios, 2014; Sütterlin and Colanzi, 2010). Therefore, integrin adhesions tether microtubule with their plus-ends, while microtubules deliver adhesion-relevant proteins to ensure correct focal adhesion formation. These two mechanisms interact with each other and form a positive feedback loop to assist in the establishment of the basolateral polarity.
Apart from the “outside-in” and “inside-out” integrin pathways, focal adhesions also regulate cell polarization in a negative way and a series of complicated signaling pathways have been found to participate in this process (Burute and Thery, 2012; Manninen, 2015; Miyoshi and Takai, 2008; Yan et al., 2009; Zihni et al., 2016). The intercellular adhesion and the cell-matrix adhesion negatively affect each other by a process of local negative feedback. For example, cell-matrix adhesion activates the Src/FAK pathway, which leads to the activation of β-catenin and its detachment from the cadherin complex, and the disruption of cell-cell adhesion. Meanwhile, cell-cell adhesion disruption activates the NCAM/FAK pathway and promotes cell-matrix adhesion assembly (Lehembre et al., 2008). Moreover, actin-binding proteins such as zyxin, vinculin and talin, shuffle between cell-cell adhesion and cell-matrix adhesion and participate in the balance between the two attachment complexes. Other regulatory factors related to Rho GTPase also contribute to the two adhesion networks (Miyoshi and Takai, 2008; Papusheva and Heisenberg, 2010). For instance, JAMA-mediated intercellular adhesion signals via two RAS-related proteins, RAP1 and RAP2. RAP2 enhances AJ stabilization while RAP1 promotes cell-matrix adhesion by influencing the expression and recycling of β1 integrins.
5. Comparison of polarization between epithelial cells and odontoblasts
Odontoblasts are another type of polarized cells (Figure 3) which possess a number of distinct features when compared to epithelial cells. In contrast to epithelial cells, much less is known about odontoblast polarization. Therefore, we compare both the similarities and differences between odontoblast and epithelial cells to underline the knowledge gap in odontoblast polarization.
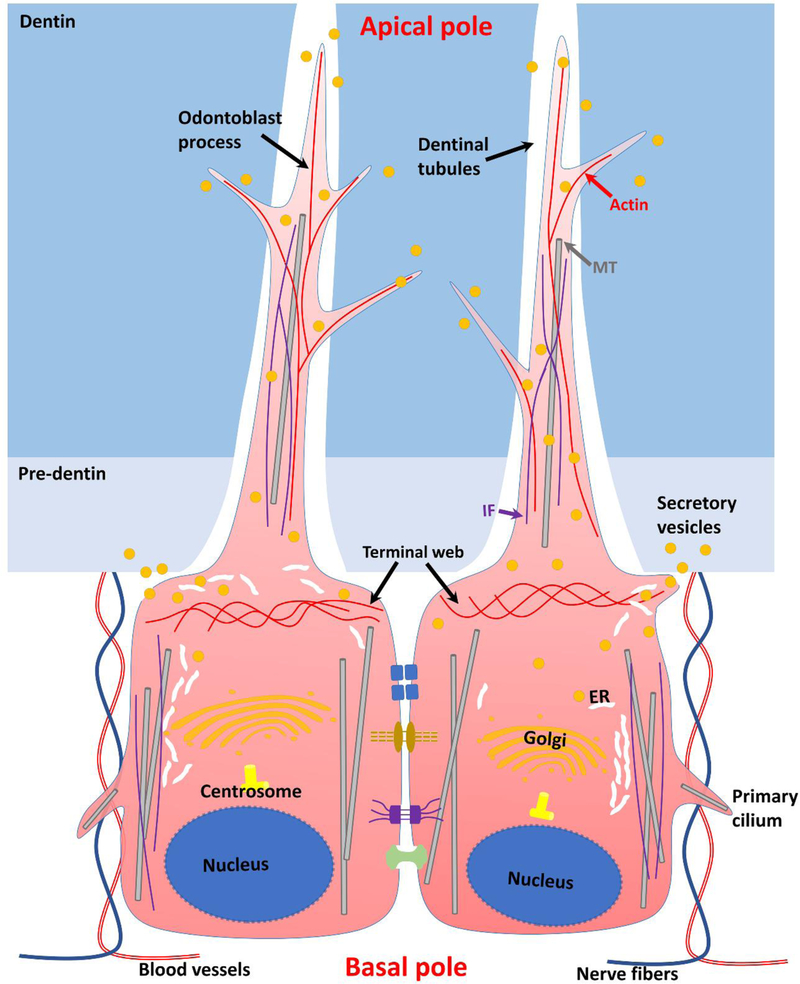
Figure 3.
Illustration of polarized odontoblasts. Unique features of odontoblasts are shown, including the polarized cell morphology, cellular processes (including odotoblast process and primary cilium), organelles distribution (including the nucleus, Golgi apparatus, ER, centrosome and secretory vesicles), cytoskeleton arrangement, and cell-cell junctions.
5.1. Cell morphology
Epithelial cells can be divided into squamous, cuboidal and columnar epithelial cells based on cell morphology. In contrast, the morphology of odontoblasts is more consistent. Generally, odontoblasts exhibit a large columnar cell body aligning at the pulp periphery and a long process inside the dentinal tubule.
5.2. Cellular process
Epithelial cellular processes are seen at the apical pole of epithelial cells and can be divided into primary cilia (immotile cilia), motile cilia, and microvilli. Primary cilia are short processes that exist on all mammalian cells and only one primary cilia can be seen within one epithelial cell. The main function of primary cilia is sensory receptors. Contrary to the “9+0 axoneme” microtubule structure of primary cilia, motile cilia contain “9+2 axoneme” and usually exist in respiratory tract to help remove mucus and dirt. Microvilli only enclose microfilaments and cytoplasm and usually exist in the small intestine to enhance absorption efficiency. In terms of odontoblast, only one long process is seen in an odontoblast and multiple branches can be observed from the trunk of the process. The cytoskeletal constitutes of odontoblast process include actin filaments, microtubules and intermediate filaments. Actin filaments and microtubules concentrated on the apical pole of odontoblasts with the onset of tooth development (Diekwisch, 1989). Vimentin, a type III intermediate filament protein, exhibits a change in the distribution during odontoblast polarization, that it was uniformly localized within the cytoplasm of pre-odontoblasts while it accumulated in the apical pole of polarized odontoblasts (LESOT et al., 1982). Apart from cytoskeletons, the odontoblast process also contains vesicles related to exocytosis and endocytosis (Linde and Goldberg, 1993), which differs from epithelial processes. The function of odontoblast processes mainly involve collagen secretion and mineralization during dentin formation (Holland, 1985). Primary cilia have also been detected in the supranuclear region at the basal pole of odontoblasts (Magloire et al., 2004; Thivichon-Prince et al., 2009) and function in both microenvironment sensation and dentin modelling via Shh and Wnt signaling pathways (Couve et al., 2013; Magloire et al., 2009).
5.3. Apical-basolateral polarity
The apical pole of epithelial cells lines on the outside (skin) or inside cavities and lumina of bodies, while the basal pole opposites the apical pole and faces connective tissues. The apical pole of odontoblasts is usually defined as the pole of odontoblast process which faces the predentin/dentin, while the basal pole faces the dental pulp (Ruch et al., 1995), although an opposite polarity has been announced based on an inverted expression of virus-carrying proteins and an inverted distribution pattern of aquaporin 4/5, a pair of epithelial polarity proteins (Tjäderhane et al., 2013).
5.4. The basement membrane
Epithelial cells interact with underlying connective tissues via the basement membrane, a network formed by fibrous proteins and glycosaminoglycans. Reconstruction of ECM occurs during epithelial polarization, which is mainly mediated by integrin via an “outside-in” and “inside-out” pathway (Lee and Streuli, 2014). Instead of basement membrane, the interaction between odontoblasts and inner dental pulp fibroblasts is intermediated by a sub-odontoblastic cell layer called Hohl’s cells (Tziafas, 2003), which may differentiate into functional odontoblasts in reparative dentinogenesis. Moreover, a basement membrane-like tissue initially formed between pre-ameloblasts and pre-odontoblasts during primary odontogenesis, while it was reconstructed and finally degraded with the onset of predentin mineralization (Kjoelby et al., 2002).
5.5. Blood vessels and nerve fibers
Epithelial layers contain no blood vessels or nerve fibers, therefore nourishment is acquired via diffusion from underlying connective tissues through the basement membrane. On the contrary, both capillaries (James, 1955) and neural fibers (Heymann, 2002; Magloire et al., 2009; Thivichon-Prince et al., 2009) are found within the odontoblast layer, in which the capillaries provide nutrition support for odontoblasts and the neural fibers sense and respond to stimuli.
5.6. Cell-cell junctions
Cell-cell junctions of epithelial cells have been described in detail above. All four intercellular junctions including AJs (Heymann et al., 2002), TJs, gap junctions (Arana-Chavez and Katchburian, 1997; Sasaki and Garant, 1996), and desmosomes (Callé, 1985; Iguchi et al., 1984) have been found between adjacent odontoblasts. Although TJs are demonstrated as a critical factor in inducing epithelial polarization, their structure (macular or continuous) and function (barrier only or also a polarization inducer) in odontoblasts remain controversial (Arana‐Chavez and Katchburian, 1998; Bishop, 1985; Hoshino et al., 2008; João and Arana‐ Chavez, 2004; Xu et al., 2016). Moreover, as the key factor in AJs, the expression of cadherins in odontoblasts is also worth noticing (Heymann, 2002). E-cadherin expression is absent in differentiating odontoblasts and weak in mature odontoblasts, while N-cadherin can be found in differentiated odontoblasts and Hohl’s cells. Both proteins are down-regulated in adult teeth, but N-cadherin can be re-expressed in the dental pulp of carious and injured teeth (Heymann et al., 2002). However, contradictory results were also reported from in vitro studies that E-cadherin was up-regulated by Nfic with the differentiation of odontoblast while N-cadherin was down-regulated (Lee et al., 2014).
5.7. Polarity complexes
The dynamic expression and function of polarity complexes in epithelial cells have been described throughly above. In odontoblasts, however, relevant studies are scarce and fail to provide a theoretical support in explaining odontoblast polarization. Up until now, none of the PAR complexes have been described in odontoblasts, although PAR3 has been detected at the proximal TJs in ameloblasts and is proposed to mediate in the formation and maintenance of the proximal TJs (Inai et al., 2008). Similarly, no reports discussing the expression of CRB/PALS1/PATJ and SCRIB/LGL/DLG complexes in odontoblasts have been found.
5.8. Cytoskeleton distribution
In odontoblasts, the distribution of actin filaments, microtubules, and intermediate filaments varies with that in epithelial cells. Actin filaments form a terminal web apically where the process originates from the cell body, and meanwhile stretch into the odontoblast process and its branches (Magloire et al., 2009). Microtubules align parallel with the long axis of the odontoblast cell body and form the odontoblast process in the core and the primary cilia (Diekwisch, 1989). Intermediate filaments similarly align parallel along the axis of the cell body and form the odontoblast process in the core (Magloire et al., 2009).
5.9. Organelles
In odontoblasts, the positions of organelles also change with the maturation of odontoblasts (Ruch et al., 1995). During odontoblast polarization, the nucleus moves to the basal pole, the Golgi apparatus moves to the supranulear region, and the centrosome locates between these two organelles [102]. ER is distributed at two regions within the cytosol, that a well-developed and flattened ER is found laterally and parallel to the long axis of the cell, and a secretory vesicle-rich RER-filled area where the RER is abruptly interrupted is simutaneously found proximal to the process (Ruch et al., 1995).
5.10. Membrane lipids
In contrast to epithelial cells, the distribution of PIP3 and PIP2 have not been reported in odontoblasts. While changes in odontoblast plasma membrane components, including increased adenylate cyclase activity, and redistribution of concanavalin binding sites have been correlated with odontoblast terminal differentiation (Ruch et al., 1995).
5.11. Regulatory factors
The distribution and function of GTPases members in epithelial cells have been discussed above. As to odontoblasts, RhoA (Biz et al., 2010; Xu et al., 2016) and Cdc 42 (Biz et al., 2010) has been reported to express uniformly in odontoblast before and during cytodifferentiation stages, while Rac1 was found to express uniformly in odontoblast at initiation stages and disappeared at cytodifferentiation stages (Biz et al., 2010). However, the exact roles of these factors in odontoblast polarization remain unclear.
5.12. Polarization signals
It was widely accepted that epithelio-mesenchymal interactions are necessary for odontogenesis (Ruch et al., 1995). There are 3 major components involved in the interactions, including the inner dental epithelium, the dental basement membrane and the growth factors. It was initially reported that dental epithelium was indispensable to initiate odontoblast polarization, while later non-dental epithelium was also found to possess such functions. For non-dental epithelium, the dental mesenchymal tissues would first promote the transformation of those non-dental epithelium into an inner dental epithelium, which then induced odontoblast differentiation. Second, the epithelio-mesenchymal basement membrane was believed necessary that its removal impedes the odontoblast polarization process. Moreover, when co-cultured with epithelium, the removed basement membrane would be reconstructed before the initiation of dental mesenchyme differentiation (Karcher-Djuricic et al., 1978). The epithelio-mesenchymal basement membrane is dynamic and its components change accompanying odontoblast polarization (Ruch et al., 1995), including the disappearance of collagen type III, the modification and turnover of glycosaminoglycans, and the restricted distribution of fibronectin at the apical pole of polarized odontoblasts which surrounded dividing pre-odontoblasts (LESOT et al., 1982). The basement membrane is gradually degraded with the onset of predentin mineralization (Kjoelby et al., 2002). Third, various growth factors from epithelium are involved in odontoblast polarization, including TGF-β superfamily (Heikinheimo et al., 1993), EGFs (Heikinheimo et al., 2002), NGFs (Byers et al., 1990), IGFs (Joseph et al., 1993) and CPNE7(Oh et al., 2015; Seo et al., 2017). Moreover, related signaling pathways involving Runx2 (Li et al., 2011a; Miyazaki et al., 2008), Wnt (Lin et al., 2011), Notum (Vogel et al., 2016), Dlx3 (Choi et al., 2010), DSPP (Gluhak-Heinrich et al., 2010) and DMP1 (Ye et al., 2004) as transcriptional factors are found critical in correct odontoblast polarization and tubular dentin formation. It’s worth noting that although the intervention of these epithelium-related growth factors or transcriptional factors have been confirmed by immunolocalization and/or in situ hybridization techniques, their exact roles in regulating cytoskeleton distribution and organelle reposition during odontoblast polarization remain unclear.
The recent development of biological engineering approaches have succeeded in inducing odontoblast polarization in vitro independent of epithelium signals. For example, by culturing dental pulp cell pellet on microfilters, odontoblast-like cells, identified by their polarized morphology and long cell processes, could be observed adjacent to the filter pores (Li et al., 2011b). Moreover, dental stem cells cultured on an artificial microtubular scaffold could polarize, differentiate and even form organized tubular dentin (Ma et al., 2017). Furthermore, single odontoblast also managed to polarize in vitro on a similar microtubular platform, further refuting the necessity of epithelial signals in inducing odontoblast polarization (Ma et al., 2018).
6. Conclusion
Cell polarity represents the asymmetric status of a cell, either in morphology or in function. Odontoblast polarization is a critical step in both the primary tubular dentin formation and dentinal tissue regeneration. Currently, there are limited data on odontoblast polarization, and the gap of knowledge in odontoblast polarization impedes the development of novel strategies for regenerative endodontics. Considering the similarities between epithelial cells and odontoblasts, we use epithelial cell polarity as a template to narrate the known and unknown facts in odontoblast polarity in this review. By summarizing the characteristic components and the network during epithelial cell polarization, we extract critical factors in epithelial polarization that might also be involved in odontoblast polarization and compare these factors between these two cell types. It is clear that despite the similarities in morphology, many characteristics of epithelial polarity at the molecular level are missing in odontoblasts, such as the distributionof inositol lipids, the existence of polarity complexes, and the effect of small GTPases. Therefore, by comparing the polarity between epithelial cells and odontoblasts, we underline the gap in odontoblast polarization and propose an orientation for future odontoblast studies, which will guide the development of new strategies in regenerative dentistry.
Acknowledgments
Funding: This work was supported by NIH/NIDCR R01DE024979 (X.L.) and China Scholarship Council (B.C.).
Abbreviation
- AJ
Adherens junction
- AKAP
A kinase (PRKA) anchor protein
- AMPK
AMP-activated protein kinase
- APC
Adenomatous polyposis coli
- aPKC
Atypical protein kinase C
- Arp2/3
Actin-related protein2/3
- Cdc42
Cell division control protein 42 homolog
- CLASP
Cytoplasmic linker associated protein
- CLIP-170
Cytoplasmic linker protein CLIP-170
- DLC3
Deleted in liver cancer 3
- DLG1
Discs large 1
- DLX-3
Distal-Less Homeobox 3
- EB1
Also known as MAPRE1, Microtubule-associated protein RP/EB family member 1
- ECM
Extracellular matrix
- ER
Endoplasmic reticulum
- Etc2
Enhancer of triptychon and caprice 2
- FAK
Focal adhesion kinase
- GAPs
GTPases activating proteins
- GDIs
Guanine nucleotide dissociation inhibitors
- GEFs
Guanine nucleotide exchange factors
- IF
Intermediate filament
- IQGAP1
IQ Motif containing GTPase activating protein
- JACOP
Junction-associated-coiled-coil protein
- JAMA
Junctional adhesion molecule A
- LGL
Lethal giant larvae
- LIMK
LIM domain kinase
- LINC
Linker of nucleoskeleton and cytoskeleton
- LKB1
Liver Kinase B1
- MAP
Microtubule-associated protein
- MARK
Microtubule affinity regulating kinase
- mDia1
Diaphanous homolog 1
- NCAM
Neural cell adhesion molecule
- N-WASP
Neural Wiskott-Aldrich syndrome protein
- PALS1
Protein associated with Lin seven 1
- PAR
Protease-activated receptor
- PATJ
PALS1-associated tight junction protein
- PI3K
Phosphoinositide 3-kinase
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PIP3
Phosphatidylinositol 3,4,5-trisphosphate
- PTEN
Phosphatase and tensin homolog
- Rac1
Ras-related C3 botulinum toxin substrate 1
- RAP
Ras-related protein
- RhoA
Ras homolog gene family, member A
- Rich1
Rho GAP interacting with CIP4 homologues
- ROCK
Rho-associated, coiled-coil containing protein kinase 1
- Runx2
Runt-related transcription factor 2
- SUN
Sad1p, UNC-84
- TBCCD1
Tubulin-binding cofactor C (TBCC) domain containing 1
- TDO
Tricho-dento-osseous syndrome
- Tiam1
T-cell lymphoma invasion and metastasis-inducing protein 1
- TIPS
Microtubule plus-end tracking proteins
- TJ
Tight junction
- Tuba
Also known as DNMBP, dynamin binding protein
- WASP
Wiskott–Aldrich syndrome protein
- WAVE
Wasp-family verprolin-homologous protein
- ZO
Zonula occluden
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations: The authors declare no competing financial interest.
Availability of data and materials: Not applicable.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
References:
- Aijaz S, D’Atri F, Citi S, Balda MS, and Matter K, 2005. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell 8, 777–786. [DOI] [PubMed] [Google Scholar]
- Arana-Chavez V, and Katchburian E, 1997. Development of tight junctions between odontoblasts in early dentinogenesis as revealed by freeze-fracture. Anat. Rec 248, 332–338. [DOI] [PubMed] [Google Scholar]
- Arana‐Chavez VE, and Katchburian E, 1998. Freeze‐fracture studies of the distal plasma membrane of rat odontoblasts during their differentiation and polarisation. Eur. J. Oral Sci 106, 132–136. [DOI] [PubMed] [Google Scholar]
- Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, and Massey-Harroche D, 2008. Polarity complex proteins. BBA. Biomembranes 1778, 614–630. [DOI] [PubMed] [Google Scholar]
- Balda MS, and Matter K, 2016. Tight junctions as regulators of tissue remodelling. Curr. Opin. Cell Biol 42, 94–101. [DOI] [PubMed] [Google Scholar]
- Birkenfeld J, Nalbant P, Yoon S-H, and Bokoch GM, 2008. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol 18, 210–219. [DOI] [PubMed] [Google Scholar]
- Bishop M, 1985. Evidence for tight junctions between odontoblasts in the rat incisor. Cell Tissue Res 239, 137–140. [DOI] [PubMed] [Google Scholar]
- Biz MT, Marques MR, Crema VO, Moriscot AS, and Dos Santos MF, 2010. GTPases RhoA and Rac1 are important for amelogenin and DSPP expression during differentiation of ameloblasts and odontoblasts. Cell Tissue Res 340, 459–470. [DOI] [PubMed] [Google Scholar]
- Braun AC, Hendrick J, Eisler SA, Schmid S, Hausser A, and Olayioye MA, 2015. The Rho-specific GAP protein DLC3 coordinates endocytic membrane trafficking. J. Cell Sci 128, 1386–1399. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, and Mostov KE, 2010. A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol 12, 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakova NA, and Knust E, 2009. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J. Cell Sci 122, 2587–2596. [DOI] [PubMed] [Google Scholar]
- Burute M, and Thery M, 2012. Spatial segregation between cell–cell and cell–matrix adhesions. Curr. Opin. Cell Biol 24, 628–636. [DOI] [PubMed] [Google Scholar]
- Byers M, Schatteman G, and Bothwell M, 1990. Multiple functions for NGF receptor in developing, aging and injured rat teeth are suggested by epithelial, mesenchymal and neural immunoreactivity. Development 109, 461–471. [DOI] [PubMed] [Google Scholar]
- Callé A, 1985. Intercellular junctions between human odontoblasts. Cells Tissues Organs 122, 138–144. [Google Scholar]
- Caplan MJ, Seo-Mayer P, and Zhang L, 2008. Molecular cell biology and physiology of solute transport: Epithelial junctions and polarity: complexes and kinases. Curr. Opin. Nephrol. Hypertens 17, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, and Larre I, 2008. Tight junction and polarity interaction in the transporting epithelial phenotype. BBA. Biomembranes 1778, 770–793. [DOI] [PubMed] [Google Scholar]
- Chen J, and Zhang M, 2013. The Par3/Par6/aPKC complex and epithelial cell polarity. Exp. Cell Res 319, 1357–1364. [DOI] [PubMed] [Google Scholar]
- Chen X, and Macara IG, 2005. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat. Cell Biol 7, 262–269. [DOI] [PubMed] [Google Scholar]
- Choi S, Song I, Feng J, Gao T, Haruyama N, Gautam P, Robey P, and Hart TC, 2010. Mutant DLX 3 disrupts odontoblast polarization and dentin formation. Dev. Biol 344, 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve E, Osorio R, and Schmachtenberg O, 2013. The amazing odontoblast: activity, autophagy, and aging. J. Dent. Res 92, 765–772. [DOI] [PubMed] [Google Scholar]
- de Forges H, Bouissou A, and Perez F, 2012. Interplay between microtubule dynamics and intracellular organization. Int. J. Biochem. Cell Biol 44, 266–274. [DOI] [PubMed] [Google Scholar]
- Devergne O, Tsung K, Barcelo G, and Schüpbach T, 2014. Polarized deposition of basement membrane proteins depends on Phosphatidylinositol synthase and the levels of Phosphatidylinositol 4, 5-bisphosphate. Proc. Natl. Acad. Sci 111, 7689–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekwisch T, 1989. Localization of microfilaments and microtubules during dental development in the rat. Acta Histochem. Suppl 37, 209–212. [PubMed] [Google Scholar]
- Dupin I, Camand E, and Etienne-Manneville S, 2009. Classical cadherins control nucleus and centrosome position and cell polarity. J. Cell Biol 185, 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin I, and Etienne-Manneville S, 2011. Nuclear positioning: mechanisms and functions. Int. J. Biochem. Cell Biol 43, 1698–1707. [DOI] [PubMed] [Google Scholar]
- Dupin I, Sakamoto Y, and Etienne-Manneville S, 2011. Cytoplasmic intermediate filaments mediate actin-driven positioning of the nucleus. J. Cell Sci 124, 865–872. [DOI] [PubMed] [Google Scholar]
- Ebnet K 2015. Cell Polarity 1: Biological Role and Basic Mechanisms Springer, Switzerland. [Google Scholar]
- Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, and McLeod IX, 2007. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12, 917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich JS, Hansen MD, and Nelson WJ, 2002. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell 3, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elric J, and Etienne-Manneville S, 2014. Centrosome positioning in polarized cells: common themes and variations. Exp. Cell Res 328, 240–248. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, 2004. Cdc42-the centre of polarity. J. Cell Sci 117, 1291–1300. [DOI] [PubMed] [Google Scholar]
- Fanning AS, and Anderson JM, 2009. Zonula occludens‐1 and‐2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci 1165, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhan H, and Hsu VW, 2016. Cdc42 and cellular polarity: emerging roles at the Golgi. Trends Cell Biol 26, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, and Priess JR, 2012. A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization. Curr. Biol 22, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg VC, Liu C-J, and Margolis B, 2005. Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J. Cell Sci 118, 2859–2869. [DOI] [PubMed] [Google Scholar]
- Franke W, Appelhans B, Schmid E, Freudenstein C, Osborn M, and Weber K, 1979. The organization of cytokeratin filaments in the intestinal epithelium. Eur. J. Cell Biol 19, 255–268. [PubMed] [Google Scholar]
- Giepmans BN, and van IJzendoorn SC, 2009. Epithelial cell–cell junctions and plasma membrane domains. BBA. Biomembranes 1788, 820–831. [DOI] [PubMed] [Google Scholar]
- Gluhak-Heinrich J, Guo D, Yang W, Harris M, Lichtler A, Kream B, Zhang J, Feng J, Smith L, and Dechow P, 2010. New roles and mechanism of action of BMP4 in postnatal tooth cytodifferentiation. Bone 46, 1533–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring NW, 2014. PAR polarity: from complexity to design principles. Exp. Cell Res 328, 258–266. [DOI] [PubMed] [Google Scholar]
- Gomes ER, Jani S, and Gundersen GG, 2005. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451–463. [DOI] [PubMed] [Google Scholar]
- Gonçalves J, Nolasco S, Nascimento R, Fanarraga ML, Zabala JC, and Soares H, 2010. TBCCD1, a new centrosomal protein, is required for centrosome and Golgi apparatus positioning. EMBO Rep 11, 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzel D, and Alan S, 2013. Claudins and the modulation of tight junction permeability. Physiol. Rev 93, 525–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel PS, Hatch AL, and Higgs HN, 2014. Connecting the cytoskeleton to the endoplasmic reticulum and Golgi. Curr. Biol 24, R660–R672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, 1998. Rho GTPases and the actin cytoskeleton. Science 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Heikinheimo K, Happonen R, Miettinen PJ, and Ritvos O, 1993. Transforming growth factor beta 2 in epithelial differentiation of developing teeth and odontogenic tumors. J. Clin. Invest 91, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo K, Voutilainen R, Happonen R, and Miettinen P, 2002. EGF receptor and its ligands, EGF and TGF-alpha, in developing and neoplastic human odontogenic tissues. Int. J. Dev. Biol 37, 387–396. [PubMed] [Google Scholar]
- Heymann R 2002. Cell adhesion molecules during odontogenesis and tooth-related diseases. Institutionen för cell-och molekylärbiologi (CMB)/Department of Cell and Molecular Biology, Solna. [Google Scholar]
- Heymann R, About I, Lendahl U, Franquin J-C, Öbrink B, and Mitsiadis TA, 2002. E-and N-cadherin distribution in developing and functional human teeth under normal and pathological conditions. Am. J. Pathol 160, 2123–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland G, 1985. The odontoblast process: form and function. J. Dent. Res 64, 499–514. [DOI] [PubMed] [Google Scholar]
- Hong Y, 2018. aPKC: the Kinase that Phosphorylates Cell Polarity. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Hashimoto S, Muramatsu T, Matsuki M, Ogiuchi H, and Shimono M, 2008. Claudin rather than occludin is essential for differentiation in rat incisor odontoblasts. Oral Dis 14, 606–612. [DOI] [PubMed] [Google Scholar]
- Huelsmann S, and Brown NH, 2014. Nuclear positioning by actin cables and perinuclear actin: Special and general? Nucleus 5, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iden S, and Collard JG, 2008. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol 9, 846–859. [DOI] [PubMed] [Google Scholar]
- Iguchi Y, Yamamura T, Ichikawa T, Hashimoto S, Horiuchi T, and Shimono M, 1984. Intercellular junctions in odontoblasts of the rat incisor studied with freeze-fracture. Arch. Oral Biol 29, 487–497. [DOI] [PubMed] [Google Scholar]
- Iizuka-Kogo A, Ishidao T, Akiyama T, and Senda T, 2007. Abnormal development of urogenital organs in Dlgh1-deficient mice. Development 134, 1799–1807. [DOI] [PubMed] [Google Scholar]
- Inai T, Sengoku A, Hirose E, Iida H, and Shibata Y, 2008. Differential expression of the tight junction proteins, claudin‐1, claudin‐4, occludin, ZO‐1, and PAR3, in the ameloblasts of rat upper incisors. Anat. Rec. A Discov. Mol. Cell Evol. Biol 291, 577–585. [DOI] [PubMed] [Google Scholar]
- Itoh M, Tsukita S, Yamazaki Y, and Sugimoto H, 2012. Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc. Natl. Acad. Sci 109, 9905–9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WW, 1955. The blood capillary system of the odontoblast layer of the dental pulp. J. Anat 89, 547–549. [PMC free article] [PubMed] [Google Scholar]
- João S, and Arana‐Chavez VE, 2004. Tight junctions in differentiating ameloblasts and odontoblasts differentially express ZO‐1, occludin, and claudin‐1 in early odontogenesis of rat molars. Anat. Rec. A Discov. Mol. Cell Evol. Biol 277, 338–343. [DOI] [PubMed] [Google Scholar]
- Joseph B, Savage N, Young W, Gupta G, Breier B, and Waters M, 1993. Expression and regulation of insulin- like growth factor-I in the rat incisor. Growth Factors 8, 267–275. [DOI] [PubMed] [Google Scholar]
- Karcher-Djuricic V, Osman M, Meyer J, Staubli A, and Ruch J, 1978. Basement membrane reconstitution and cytodifferentiation of odontoblasts in isochronal and heterochronal reassociations of enamel organs and pulps. J Biol. Buccale 6, 257–265. [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, and Small JV, 2002. Regulation of substrate adhesion dynamics during cell motility. Int. J. Biochem. Cell Biol 34, 746–761. [DOI] [PubMed] [Google Scholar]
- Kishikawa M, Suzuki A, and Ohno S, 2008. aPKC enables development of zonula adherens by antagonizing centripetal contraction of the circumferential actomyosin cables. J. Cell Sci 121, 2481–2492. [DOI] [PubMed] [Google Scholar]
- Kjoelby M, Thesleff I, Sahlberg C, Fejerskov O, and Josephsen K, 2002. Degradation of the dental basement membrane during mouse tooth development in vitro. Int. J. Dev. Biol 38, 455–462. [PubMed] [Google Scholar]
- Lee H-K, Lee D-S, Park S-J, Cho K-H, Bae H-S, and Park J-C, 2014. Nuclear factor IC (NFIC) regulates dentin sialophosphoprotein (DSPP) and E-cadherin via control of Kruppel-like factor 4 (KLF4) during dentinogenesis. J. Biol. Chem 289, 28225–28236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, and Streuli CH, 2014. Integrins and epithelial cell polarity. J. Cell Sci 127, 3217–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehembre F, Yilmaz M, Wicki A, Schomber T, Strittmatter K, Ziegler D, Kren A, Went P, Derksen PW, and Berns A, 2008. NCAM‐induced focal adhesion assembly: a functional switch upon loss of E‐cadherin. EMBO J 27, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESOT H, MEYER JM, RUCH JV, WEBER K, and OSBORN M, 1982. Immunofluorescent localization of vimentin, prekeratin and actin during odontoblast and ameloblast differentiation. Differentiation 21, 133–137. [DOI] [PubMed] [Google Scholar]
- Li R, and Gundersen GG, 2008. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat. Rev. Mol. Cell Biol 9, 860–873. [DOI] [PubMed] [Google Scholar]
- Li S, Kong H, Yao N, Yu Q, Wang P, Lin Y, Wang J, Kuang R, Zhao X, and Xu J, 2011a. The role of runt-related transcription factor 2 (Runx2) in the late stage of odontoblast differentiation and dentin formation. Biochem. Biophys. Res. Commun 410, 698–704. [DOI] [PubMed] [Google Scholar]
- Li Y, Lü X, Sun X, Bai S, Li S, and Shi J, 2011b. Odontoblast-like cell differentiation and dentin formation induced with TGF-β1. Arch. Oral Biol 56, 1221–1229. [DOI] [PubMed] [Google Scholar]
- Lin M, Li L, Liu C, Liu H, He F, Yan F, Zhang Y, and Chen Y, 2011. Wnt5a regulates growth, patterning, and odontoblast differentiation of developing mouse tooth. Dev. Dyn 240, 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde A, and Goldberg M, 1993. Dentinogenesis. Crit. Rev. Oral Biol. Med 4, 679–728. [DOI] [PubMed] [Google Scholar]
- Liu XF, Ohno S, and Miki T, 2006. Nucleotide exchange factor ECT2 regulates epithelial cell polarity. Cell. Signal 18, 1604–1615. [DOI] [PubMed] [Google Scholar]
- Long M, and Simpson JC, 2017. Rho GTPases operating at the Golgi complex: Implications for membrane traffic and cancer biology. Tissue Cell 49, 163–169. [DOI] [PubMed] [Google Scholar]
- Ma C, Qu T, Chang B, Jing Y, Feng JQ, and Liu X, 2017. 3D Maskless Micropatterning for Regeneration of Highly Organized Tubular Tissues. Adv. Healthcare Mater 7, 1700738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Chang B, Jing Y, Kim H, and Liu X, 2018. Bio-inspired micropatterned platforms recapitulate 3D physiological morphologies of bone and dentinal cells. Adv. Sci 2018, 1801037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack NA, and Georgiou M, 2014. The interdependence of the Rho GTPases and apicobasal cell polarity. Small GTPases 5, e973768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloire H, Couble ML, Romeas A, and Bleicher F, 2004. Odontoblast primary cilia: facts and hypotheses. Cell Biol. Int 28, 93–99. [DOI] [PubMed] [Google Scholar]
- Magloire H, Couble ML, Thivichon‐Prince B, Maurin JC, and Bleicher F, 2009. Odontoblast: a mechano‐ sensory cell. J. Exp. Zool. (Mol Dev Evol) 312, 416–424. [DOI] [PubMed] [Google Scholar]
- Manninen A, 2015. Epithelial polarity–generating and integrating signals from the ECM with integrins. Exp. Cell Res 334, 337–349. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, and Mostov K, 2007. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, and Perez-Moreno M, 2012. Epithelial cell polarity, stem cells and cancer. Nat. Rev. Cancer 12, 23–38. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Schiller P, Dieterich LC, Bahram F, Iribe Y, Hellman U, Wikner C, Chan G, Claesson-Welsh L, and Dimberg A, 2008. Ninein is expressed in the cytoplasm of angiogenic tip-cells and regulates tubular morphogenesis of endothelial cells. Arterioscler. Thromb. Vasc. Biol 28, 2123–2130. [DOI] [PubMed] [Google Scholar]
- McGuire JD, Walker MP, Dusevich V, Wang Y, and Gorski JP, 2014. Enamel organic matrix: potential structural role in enamel and relationship to residual basement membrane constituents at the dentin enamel junction. Connect. Tissue Res 55, 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel D, Arsanto J-P, Massey-Harroche D, Béclin C, Wijnholds J, and Le Bivic A, 2005. PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J. Cell Sci 118, 4049–4057. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kanatani N, Rokutanda S, Yoshida C, Toyosawa S, Nakamura R, Takada S, and Komori T, 2008. Inhibition of the terminal differentiation of odontoblasts and their transdifferentiation into osteoblasts in Runx2 transgenic mice. Arch. Histol. Cytol 71, 131–146. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, and Takai Y, 2008. Structural and functional associations of apical junctions with cytoskeleton. BBA. Biomembranes 1778, 670–691. [DOI] [PubMed] [Google Scholar]
- Müsch A, 2004. Microtubule organization and function in epithelial cells. Traffic 5, 1–9. [DOI] [PubMed] [Google Scholar]
- Nakajima H, and Tanoue T, 2011. Lulu2 regulates the circumferential actomyosin tensile system in epithelial cells through p114RhoGEF. J. Cell Biol 195, 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngok SP, Lin W-H, and Anastasiadis PZ, 2014. Establishment of epithelial polarity–GEF who’s minding the GAP? J. Cell Sci 127, 3205–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BR, Paolillo V, and Zheng Y, 2015. γ-Tubulin complexes in microtubule nucleation and beyond. Mol. Biol. Cell 26, 2957–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H-J, Choung H-W, Lee H-K, Park S-J, Lee J-H, Lee D-S, Seo B-M, and Park J-C, 2015. CPNE7, a preameloblast-derived factor, regulates odontoblastic differentiation of mesenchymal stem cells. Biomaterials 37, 208–217. [DOI] [PubMed] [Google Scholar]
- Ori-McKenney KM, Jan LY, and Jan Y-N, 2012. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76, 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriolo AS, Wald FA, Ramsauer VP, and Salas PJ, 2007. Intermediate filaments: a role in epithelial polarity. Exp. Cell Res 313, 2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T, Ichii T, Aono S, and Takeichi M, 2006. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J. Cell Biol 175, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papusheva E, and Heisenberg CP, 2010. Spatial organization of adhesion: force‐dependent regulation and function in tissue morphogenesis. EMBO J 29, 2753–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel M, Meyer P, Khodjakov A, Rieder CL, and Bornens M, 2000. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol 149, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, and Macara IG, 2005. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol 171, 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones GB, Danowski BA, Devaraj A, Singh V, and Ligon LA, 2011. The posttranslational modification of tubulin undergoes a switch from detyrosination to acetylation as epithelial cells become polarized. Mol. Biol. Cell 22, 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros M, and Nusrat A, 2014. RhoGTPases, actomyosin signaling and regulation of the epithelial Apical Junctional Complex. Semin. Cell Dev. Biol 36, 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, and Stehbens SJ, 2012. Centralspindlin and [alpha]-catenin regulate Rho signalling at the epithelial zonula adherens. Nat. Cell Biol 14, 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky D, and Hodzic D, 2009. Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J. Cell Biol 186, 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios RM, 2014. The centrosome–Golgi apparatus nexus. Phil. Trans. R. Soc. B 369, 20130462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero S, Cardenas J, Bornens M, and Rios RM, 2009. Microtubule nucleation at the cis‐side of the Golgi apparatus requires AKAP450 and GM130. EMBO J 28, 1016–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, and Macara IG, 2014. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol 15, 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román‐Fernández A, and Bryant DM, 2016. Complex polarity: building multicellular tissues through apical membrane traffic. Traffic 17, 1244–1261. [DOI] [PubMed] [Google Scholar]
- Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, and Burke B, 2009. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc. Natl. Acad. Sci 106, 2194–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch J, Lesot H, and Begue-Kirn C, 1995. Odontoblast differentiation. Int. J. Dev. Biol 39, 51–68. [PubMed] [Google Scholar]
- Samarin SN, Ivanov AI, Flatau G, Parkos CA, and Nusrat A, 2007. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol. Biol. Cell 18, 3429–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AA, and Kaverina I, 2015. Nucleation and dynamics of Golgi-derived microtubules. Front. Neurosci 9, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, and Garant PR, 1996. Structure and organization of odontoblasts. Anat. Rec 245, 235–249. [DOI] [PubMed] [Google Scholar]
- Schlüter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu C-J, and Margolis B, 2009. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol. Biol. Cell 20, 4652–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y-M, Park S-J, Lee H-K, and Park J-C, 2017. Copine-7 binds to the cell surface receptor, nucleolin, and regulates ciliogenesis and Dspp expression during odontoblast differentiation. Sci. Rep 7, 11283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit S-T, and Manser E, 2011. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci 124, 679–683. [DOI] [PubMed] [Google Scholar]
- Spiering D, and Hodgson L, 2011. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh. Migr 5, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, and Han M, 2003. ANChors away: an actin based mechanism of nuclear positioning. J. Cell Sci 116, 211–216. [DOI] [PubMed] [Google Scholar]
- Sugioka K, and Sawa H, 2012. Formation and functions of asymmetric microtubule organization in polarized cells. Curr. Opin. Cell Biol 24, 517–525. [DOI] [PubMed] [Google Scholar]
- Sütterlin C, and Colanzi A, 2010. The Golgi and the centrosome: building a functional partnership. J. Cell Biol 188, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, and Ohno S, 2006. The PAR-aPKC system: lessons in polarity. J. Cell Sci 119, 979–987. [DOI] [PubMed] [Google Scholar]
- Terry SJ, Zihni C, Elbediwy A, Vitiello E, San IVLC, Balda MS, and Matter K, 2011. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat. Cell Biol 13, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivichon-Prince B, Couble M, Giamarchi A, Delmas P, Franco B, Romio L, Struys T, Lambrichts I, Ressnikoff D, and Magloire H, 2009. Primary cilia of odontoblasts: possible role in molar morphogenesis. J. Dent. Res 88, 910–915. [DOI] [PubMed] [Google Scholar]
- Tjäderhane L, Koivumäki S, Pääkkönen V, Ilvesaro J, Soini Y, Salo T, Metsikkö K, and Tuukkanen J, 2013. Polarity of mature human odontoblasts. J. Dent. Res 92, 1011–1016. [DOI] [PubMed] [Google Scholar]
- Tziafas D, 2003. Basic mechanisms of cytodifferentiation and dentinogenesis during dental pulp repair. Int. J. Dev. Biol 39, 281–290. [PubMed] [Google Scholar]
- Van Itallie CM, and Anderson JM, 2013. Claudin interactions in and out of the tight junction. Tissue Barriers 1, e25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeijl L, Ponsioen B, Giepmans BN, Ariaens A, Postma FR, Várnai P, Balla T, Divecha N, Jalink K, and Moolenaar WH, 2007. Regulation of connexin43 gap junctional communication by phosphatidylinositol 4, 5-bisphosphate. J. Cell Biol 177, 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, and Horwitz AF, 2007. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol 176, 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P, Read R, Hansen G, Powell D, Kantaputra P, Zambrowicz B, and Brommage R, 2016. Dentin Dysplasia in Notum Knockout Mice. Vet. Pathol 53, 853–862. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Noritake J, and Kaibuchi K, 2005. Regulation of microtubules in cell migration. Trends Cell Biol 15, 76–83. [DOI] [PubMed] [Google Scholar]
- Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, Kulkarni S, Gish G, Virag C, and Lim C, 2006. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell 125, 535–548. [DOI] [PubMed] [Google Scholar]
- Wojnacki J, Quassollo G, Marzolo M-P, and Cáceres A, 2014. Rho GTPases at the crossroad of signaling networks in mammals: impact of Rho-GTPases on microtubule organization and dynamics. Small GTPases 5, e28430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-F, and Lew DJ, 2013. Beyond symmetry-breaking: competition and negative feedback in GTPase regulation. Trends Cell Biol 23, 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Shao M, Pan H, Wang H, Cheng L, Yang H, and Hu T, 2016. Novel role of zonula occludens‐1: A tight junction protein closely associated with the odontoblast differentiation of human dental pulp cells. Cell Biol. Int 40, 787–795. [DOI] [PubMed] [Google Scholar]
- Yagi S, Matsuda M, and Kiyokawa E, 2012. Chimaerin suppresses Rac1 activation at the apical membrane to maintain the cyst structure. PLoS ONE 7, e52258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, and Nelson WJ, 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J. Cell Biol 178, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Lee WM, and Cheng CY 2009. Cross-talk between tight and anchoring junctions—lesson from the testis. Adv. Exp. Med. Biol 636, 234–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, and Feng JQ, 2004. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization and expanded cavities of pulp and root canal during postnatal tooth development. J. Biol. Chem 279, 19141–19148. [DOI] [PubMed] [Google Scholar]
- Zhu X, and Kaverina I, 2013. Golgi as an MTOC: making microtubules for its own good. Histochem. Cell Biol 140, 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihni C, Balda MS, and Matter K, 2014a. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J. Cell Sci 127, 3401–3413. [DOI] [PubMed] [Google Scholar]
- Zihni C, Mills C, Matter K, and Balda MS, 2016. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol 17, 564–580. [DOI] [PubMed] [Google Scholar]
- Zihni C, Munro PM, Elbediwy A, Keep NH, Terry SJ, Harris J, Balda MS, and Matter K, 2014b. Dbl3 drives Cdc42 signaling at the apical margin to regulate junction position and apical differentiation. J Cell. Biol 204, 111–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman Y, Alieva NO, Miserey-Lenkei S, Lichtenstein A, Kam Z, Sabanay H, and Bershadsky A, 2011. Involvement of the Rho–mDia1 pathway in the regulation of Golgi complex architecture and dynamics. Mol. Biol. Cell 22, 2900–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]