Abstract
Current literature on the relationship between dispositional fear (or threat sensitivity) and amygdala gray matter volume (GMV) is heterogeneous, with findings including positive, negative, and null correlations. A clearer understanding of this relationship would help to determine the potential utility of amygdala volume as a biomarker of anxious/depressive (internalizing) disorders and contribute to understanding of neural mechanisms for variations in fearfulness. The study reported here used voxel-based morphometry to quantify amygdala GMV scores from structural neuroimaging data in a sample of 44 monozygotic twins (i.e., 22 pairs). Dispositional threat sensitivity (THT) was quantified using a biobehavioral cross-domain score that combined neurophysiological indicators with a psychological scale measure. Analyses revealed expected high concordance for amygdala GMV between co-twins. With respect to the major question of the study, a negative correlation was found between biobehavioral THT scores and amygdala volume - with individuals higher in THT showing smaller amygdala GMV scores. More modest associations of amygdala GMV with symptoms of social phobia, and fear disorder symptomology more broadly, were mediated by THT. These results provide insight into prior mixed findings and support the combined use of biological and behavioral measures to quantify characteristics relevant to mental health problems.
Keywords: threat sensitivity, fear, amygdala, voxel-based morphometry, twins
1. Introduction
The amygdala plays a central role in defensive processing and reactivity, including activation of fear responses (Davis, 1992; Phillips & LeDoux, 1992). Aberrant processing of threat cues has been reported in relation to anxious-depressive (internalizing) disorders (e.g., Shankman et al., 2013; Yancey et al., 2015), and functional neuroimaging studies have reported enhanced amygdala activation in patients with such disorders and persons high in anxious or fearful traits (Beesdo et al., 2009; Indovina et al., 2011; Rauch et al., 2003). Considerable work has also tested for relations of amygdala structure (gray matter volume) with internalizing problems and affiliated traits, but findings here have been more mixed (e.g., Rauch et al., 2003; van der Plas et al., 2010). Further research is needed examining how variations in volume of the amygdala relate to fearful-anxious traits and internalizing disorders. The current study addressed this need in a novel way - by quantifying threat sensitivity biobehaviorally (i.e., using both physiological and psychological indicators) in a sample of identical twins, and examining its relationship with amygdala volume, both within the sample as whole and across twin-pair members.
1.1. Amygdala, threat sensitivity, and fearfulness
The amygdala is a key limbic structure involved in the processing of emotional events (Phillips & LeDoux, 1992; Phelps, 2006). In healthy controls, amygdala activation relates to negative emotional reactivity in different threat-cuing contexts (Hariri et al., 2002; Morris et al., 1996; Phillips & LeDoux, 1992). Self-reported threat sensitivity has been shown to predict metabolic processes in the amygdala during aversive cuing (Cools et al., 2005), and conversely, threat sensitivity quantified as amygdala reactivity to fearful faces predicts behavioral attentiveness to aversive cues (Ohrmann et al., 2007). Dysfunction in the amygdala has also been implicated in psychiatric conditions including fear and anxiety disorders (Kim et al., 2011; Rauch et al., 2003).
Other human studies have used structural neuroimaging to test for relations between amygdala gray matter volume and anxious-depressive conditions. Findings from these studies have been mixed and inconsistent. For example, studies of adults with specific anxiety disorders have reported negative associations in some cases (i.e., reduced amygdala volume relative to healthy controls; Hayano et al., 2009; Irle et al., 2010; Park et al., 2015; Rogers et al., 2009; Fisler et al., 2013), positive associations in others (De Bellis et al., 2000; Machado-de-Sousa et al., 2014; Tian et al., 2016), and null associations in yet others (Syal et al., 2012; Duval et al., 2015). Findings for children or adolescents have likewise been mixed (e.g., Morey et al., 2016; van der Plas et al., 2010; Juranek et al., 2006). The same is true for studies that have tested for associations between amygdala volume and major depression (De Bellis et al., 2000; Qin et al., 2014; Van der Plas et al., 2010; Milham et al., 2005; Merz et al., 2017; see also reviews by Bora et al., 2012; Hamilton et al., 2008).
A specific complicating factor in interpreting the mixed findings from studies of this type is the fairly consistent evidence that has been reported for a positive association between early adverse experience and amygdala volume (for a review, see Tottenham & Sheridan, 2010). The effects of early adversity on brain development may operate to obscure the effects of constitutional factors (e.g., heritable dispositions) and thereby contribute to the inconsistent findings of research on amygdala volume and internalizing psychopathology.
1.2. Animal research on amygdala volume and threat sensitivity
In contrast with the highly mixed findings from human studies, research with animal (rodent) subjects has reported consistent evidence for reduced amygdala volume in subjects bred to exhibit high fearfulness or stress reactivity in behavioral procedures such as aversive conditioning (e.g., Pedraza et al., 2014; Yang et al., 2008). The implication is that small amygdala volume may be related to constitutionally-based elevations in amygdala reactivity to stressors or threats. Consistent with this possibility, evidence has been reported for an inverse relationship between amygdala volume and amygdala reactivity to threat cues - both in humans (e.g., Gianaros et al., 2008; Kalmar et al., 2009; see also Zhao et al., 2013) and in animals (Pedraza et al., 2014).
A notable feature of animal studies on this topic is that they have examined amygdala volume in relation to physiological or behavioral reactivity to laboratory stressors. The implication is that amygdala morphology may relate more to fearfulness when this attribute is assessed biobehaviorally -in terms of responsiveness to aversive cues (i.e., threat sensitivity). Consistent with this, Hartley et al. (2011) reported a negative association between amygdala volume and electrodermal reactivity to cues signaling shock in an aversive conditioning procedure. Relatedly, Gianaros et al. (2008) reported lower amygdala gray matter volume (along with enhanced functional amygdala reactivity) in individuals who exhibited strong as opposed to weak cardiac reactivity (as measured via blood pressure) to a laboratory stressor. These findings suggest that quantifying fearfulness through use of physiological along with psychological indicators of threat reactivity may be important in studies examining how amygdala volume relates to dispositional fear, and in turn to internalizing psychopathology.
1.3. Biobehavioral assessment of threat sensitivity in humans
Biobehavioral traits - dispositions that relate directly to both behavior patterns and neural systems (Patrick et al., 2012) - have proven useful for integrating individual difference variables from different domains of assessment (e.g., Patrick et al., 2013; Venables et al., 2018; Yancey et al., 2016). One trait construct of this kind is threat sensitivity (THT), a brain-based characteristic that affects how people respond behaviorally and physiologically in aversive-cuing contexts. Yancey et al. (2016) established a method for quantifying THT in biobehavioral terms, through combined use of trait-scale (i.e., questionnaire) and physiological response measures of fearfulness. Scores on THT defined in this way are appreciably heritable and relate to symptoms of internalizing disorders -in particular, disorders involving excessive fear reactivity (Venables et al., 2017).
1.4. Current study
The study reported here used structural magnetic resonance imaging (MRI) data from a sample of identical (monozygotic; MZ) twins to examine relations between amygdala gray matter volume (GMV) and dispositional threat sensitivity (THT) quantified as described by Yancey et al. (2016) - i.e., using multiple neurophysiological indicators of aversive reactivity together with a psychological-scale measure of general fearfulness. Our primary hypothesis, based on animal and human studies as described above, was that amygdala GMV would be correlated with biobehavioral THT (Hypothesis 1a) in a negative direction (i.e., higher THT would be associated with smaller amygdala volume). More tentatively, we predicted that amygdala GMV would show a negative association with anxious-depressive (internalizing) symptomatology - primarily as a function of its relationship with biobehavioral THT (Hypothesis 1b). In order to test this secondary hypothesis, internalizing psychopathology was assessed using clinical interviews.
Based on prior reports of high twin-heritability for amygdala volume (e.g., Munn et al., 2007; van der Schot et al., 2009) and moderate-level heritability for biobehavioral THT (Venables et al., 2017), we expected that each of these variables would show appreciable concordance across twin-pair members (Hypothesis 2a). Further, we tested for the possibility of a genetic basis to the predicted association between biobehavioral THT and amygdala volume by examining whether, across members of MZ twin pairs, one twin’s THT score would significantly predict the co-twin’s amygdala GMV score (Hypothesis 2b).
2. Materials and Methods
2.1. Sample:
Participants were 22 pairs of adult MZ twins (n = 44 individuals; 24 female; M age = 27.7 years [SD = 4.9; range = 22 to 37]). These participants comprised a subset of a larger twin sample (n = 508) tested in a laboratory protocol involving collection of questionnaire, diagnostic, and physiological data (Yancey et al., 2016), and this lab-test sample in turn comprised a subset of an even larger twin sample (N = 2,511) administered a battery of questionnaires assessing fear/fearlessness (Kramer et al., 2012). The twin sample as a whole (N = 2,511) from which participants were selected for lab testing (n = 508), and follow-up MRI testing in a smaller portion of cases (n = 44), was recruited specifically for purposes of a grant project focusing on fear/fearlessness and threat sensitivity (see Patrick et al., 2012). Participants for this grant project were recruited from the Minnesota Twin Registry, a birth-record based listing of all twins born in the state of Minnesota during two spans of time (i.e., 1935–1955 and 1971–1989; see Iacono et al., 1999; Lykken et al., 1990), and had not taken part in any previous twin research studies. Further details regarding the overall project sample, and subsets of participants participating in lab testing and follow-up MRI testing, are provided in the part 1 of the online article Supplement, titled “Participant Recruitment and Pre-Selection.”
For each MZ twin pair in the current MRI test sample, one member (denoted ‘twin A’) was pre-selected to possess higher or lower levels of biobehavioral threat sensitivity (THT), as described in the next subsection, so as to ensure representation of ‘twin A’ participants with a broad range of scores on this dimension; no pre-selection was done for ‘twin B’ participants. Neither twin group was pre-selected with regard to disorder symptoms as assessed by clinical interview. However, given the documented relations of fear disorders and depression with dispositional THT (see, e.g., Mineka et al., 1998), twins in the MRI sample were expected to show elevated rates of these disorders.
No study participant reported any general physical or neurological condition that would exclude them from providing viable MRI data. The study was approved by the Institutional Review Board of the University of Minnesota and all participants provided written informed consent prior to commencement of testing.
2.2. Measures:
2.2.1. Biobehavioral threat sensitivity assessment
As described by Yancey et al. (2016), individual differences in threat sensitivity (THT) were quantified for 454 of the 508 lab-test participants in the above-noted grant project - i.e., those for whom requisite questionnaire-scale and physiological-response indicators of THT were available. The scale indicator of THT was a 55-item Trait Fear inventory (TF-55; Nelson et al., 2016) constructed to index the general fear/fearlessness dimension underlying various existing questionnaire measures of this domain (Kramer et al., 2012); scores on this scale correlate over 0.9 with scores on this fear/fearlessness dimension (Nelson et al., 2016). The physiological indicators of THT consisted of the following response measures from an affective picture-viewing task: (a) aversive startle potentiation, quantified as enhancement of electromyographic (EMG) eyeblink response to noise probes occurring during aversive compared to neutral pictures; (b) differential corrugator (‘frown’) EMG reactivity to aversive pictures versus neutral; and (c) midlatency heart rate (HR) acceleration to aversive pictures. A detailed description of procedures for recording and quantification of these physiological indicator variables is provided in Yancey et al. (2016).
Variations in biobehavioral THT were quantified in terms of regression-estimated scores on the common factor emerging from a factor analysis of the foregoing indicator variables in the test sample (N = 454) from which the current MRI-test sample was drawn (for details, see Yancey et al., 2016); loadings of these four indicators on the common factor in the larger sample, which determined their weightings in the computation of THT scores, were as follows: TF-55 = 0.48, startle potentiation = 0.26, corrugator EMG differentiation = 0.35, and HR acceleration = 0.33. As noted in the preceding subsection, participants comprising twin group A of the MRI test sample were pre-selected from this larger lab-test sample (N = 454) based on biobehavioral THT scores, to ensure effective representation of participants at varying levels on this trait dimension; their cotwins (i.e., twin group B) were tested without constraints. The THT score could not be calculated for one co-twin due to missing physiological data for this case. The two twin groups (A, B) did not differ in average scores on the biobehavioral THT index (t[41] = 1.01, p = .32). Further details regarding pre-selection of participants for MRI testing are provided in the part 1 of the online Supplement, titled “Participant Recruitment and Pre-Selection.”
2.2.2. Diagnostic assessment
Participants were assessed for lifetime occurrence of anxious-depressive (internalizing) disorders and affiliated symptoms using the Structured Clinical Interview for DSM-IV-TR Axis I disorders (SCID-I; First et al., 2002). Each participant was interviewed by a PhD-level clinical psychologist or advanced clinical psychology graduate student trained in administration and scoring of the SCID-I. Symptom ratings (i.e., maximum number met at any time in the participant’s life) and diagnostic classifications (i.e., full diagnostic criteria ever met versus never met) were determined through a consensus process involving meetings of the study interviewers (cf. Iacono et al., 1999), attended by the project PI (Christopher Patrick) and a licensed clinical psychologist who provided expert consultation on ratings and diagnostic decisions.
The internalizing disorders with the highest prevalence rates in the current study sample (N = 44) were major depression, diagnosed in 13 cases (5 in twin group A and 8 in twin group B), and social phobia, diagnosed in 6 cases (3 in group A, 3 in group B). A considerable portion of participants in the sample reported experiencing one or more symptoms of these diagnostic conditions: 43% for major depression, and 45% for social phobia. For these two disorders, we also had questionnaire-based symptom scores from the Inventory of Depression and Anxiety Symptoms (IDAS; Watson et al., 2007), a self-report measure that assesses for symptoms of major depression and anxiety-related conditions that often co-occur with it. Scores for the General Depression and Social Phobia scales of this inventory were combined with corresponding SCID-I symptom counts to enhance the range of score variation in analyses focusing on these two diagnostic conditions. Specifically, an omnibus Depression score was computed as the average of standardized (z- transformed) SCID-I major depression and IDAS General Depression scores, and an omnibus Social Phobia score was computed as the average of standardized (z-transformed) SCID-I social phobia and IDAS Social Phobia scores.
Of note, social phobia and major depression represent disorders from distinct fearful and dysphoric classes of internalizing psychopathology, respectively (Krueger, 1999; Vaidyanathan, Patrick, & Iacono, 2011). The availability of broadly omnibus symptom scores for Social Phobia and Depression with appreciable variability thus allowed us to effectively test for associations of constituent disorders from these two classes with amygdala volume. In addition, to evaluate associations with amygdala volume for disorders of these two types more generally in the current study sample, we created symptom composites for internalizing conditions considered fear disorders and those considered dysphoric disorders (Krueger, 1999). The fear disorder composite was computed by averaging the omnibus symptom score for social phobia with standardized (z- transformed) symptom scores for specific phobia (for which 16/44 = 36% of participants reported one or more symptoms) and panic disorder (for which 6/44 = 14% reported one or more symptoms).1 The dysphoric disorder composite was computed by averaging the above-noted omnibus symptom score for depression with standardized (z-transformed) symptom scores for dysthymic disorder (for which 5/44 = 11% of participants reported one or more symptoms) and generalized anxiety disorder (for which 9/44 = 20% reported one or more symptoms).
2.3. MRI Scanning and Brain Volume Quantification:
The MRI testing session was conducted at the University of Minnesota’s Center for Magnetic Resonance Research, using a 3.0 Tesla Siemens Trio scanner. Structural brain data in in the form of standard Tl-weighted whole-head brain images (176 slices, 1 mm slice thickness, 256 mm field of view, 1 mm3 voxel size) were acquired using a 3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence. Voxel-based morphometry (VBM) analysis, performed using the SPM8 software package (Wellcome Institute of Imaging Neuroscience, London, UK) as implemented in Matlab (MathWorks Inc., Natick, MA, USA), was used to compute brain volume scores. The software package’s segmentation algorithm (‘New Segment’) was used to partition images into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). After this, a sample specific template was generated out of 44 individual images using an iteratively nonlinear registration algorithm (DARTEL; Ashburner, 2007). The generated template was registered to the MNI template to allow for the definition of regions of interest in MNI space. Individual gray and white matter images were normalized to the MNI normalized sample specific template and were smoothed using a Gaussian kernel of 8mm3 (full width at half maximum).
To evaluate volume differences, normalized images were modulated by multiplication with the Jacobian determinant of the spatial normalization function (Good et al., 2001) to correct for volume changes by normalization. In order to rule out artifacts caused by voxels with insufficient GM, we only included voxels with a GM value of 0.1 or higher. This is the threshold recommended in the software manuals for VBM to protect against edge effects (i.e., incorrect classification of voxels around the border between gray and white matter). Threshold masking also protects against the possibility of implausible false positives occurring outside the brain due to the very low variance in voxels with consistently low smoothed tissue density — the extreme limit of the phenomenon described by Reimold et al. (2006; see also Ridgway, et al., 2009). Total intracranial volume (TIV) was assessed by combining the totality of GM, WM, and CSF volumes.
As our study focused on specific hypotheses regarding the relationship between anxiety scores and amygdala volume, scores for this region were quantified - along with scores for the hippocampus, as a control region - by extracting mean voxel values in a region of interest (ROI) as defined by the AAL brain atlas (Tzourio-Mazoyer et al., 2002), implemented in the WFU pickatlas tool (Maldjian et al., 2003). The mean voxel values for the amygdala were the main focus of the analyses reported below, with results reported for the hippocampus as a comparison. To allow for correlational analyses including twin concordance evaluation and regression modeling, we used extracted voxel values instead of a whole brain VBM analysis. Gender, age, and TIV were controlled for in all analyses by partialing these variables out and using standardized residual scores for all further correlations. These scores were calculated separately for left and right amygdala. All procedures described here were also applied to a hippocampus ROI that was derived from the same atlas as the described amygdala ROI. Hippocampal volume was used as a control region for amygdala, as the volumes of these two regions are expected to be highly correlated, but related to different specific functions (cf. Bickart et al., 2011).
As an additional approach to quantifying gray matter volumes for the main structure of interest (the amygdala), we also processed the MRI scan data using the FreeSurfer 5.3 image analysis suite (Fischl, 2012). A description of the procedures for this analysis, along with presentation and interpretation of findings for left and right amygdala GMVs, is provided in part 2 of the Supplement to this article.
2.4. Statistical Analysis:
All statistical analyses were performed using the IBM SPSS Statistics 19 software package, controlling for TIV, age, and sex. Pearson correlations were used to test for bivariate associations of biobehavioral THT scores and diagnostic symptom scores (Social Phobia, Depression) with left and right amygdala (and hippocampal) GMV, and regression analysis was used to evaluate overlap versus uniqueness of observed relations for these predictors. Twin concordances for variables of interest were quantified using intraclass correlations. These analyses were evaluated using a two-tailed significance threshold of .05. Cohen’s (1988) f2 was calculated as a measure of effect size for regression analyses.
In addition, two-way random intraclass correlations (ICC) were used to evaluate whether biobehavioral THT scores for twin-pair members predicted left/right amygdala GMV scores for their co-twins (Hu et al., 1998). Given our a priori hypothesis of a negative directional association, and the reduction in effective sample size (i.e., by 50%) for these cross-twin correlations, we used a one-tailed significance threshold of .05 for these analyses. As noted above, all other analyses utilized a two-tailed .05 threshold.
3. Results
3.1. Overall sample associations
As hypothesized, within the sample as a whole, biobehavioral THT scores showed significant negative correlations with extracted gray matter volume (GMV) scores for both left and right amygdala ROIs (left: r = −.40, p = .01; right: r = −.32, p = .03; see Figures 1 and 2) when controlling for TIV, age, and sex.2 Social Phobia symptoms, which correlated quite strongly with THT scores (r = .65, p < .001), also showed negative associations with GMV for both left and right amygdala (left: r = −.35, p = .02; right: r = −.37, p = .01). Corresponding correlations for the fear disorder composite (i.e., aggregate of symptoms for social phobia, specific phobia, and panic disorder) were also negative, but somewhat lower in magnitude (rs = −.28 and −.29. respectively, ps = .066 and .056) - though not significantly so (Steiger’s [1980] Z values = .55 and .60, respectively, ps = .58 and .56). By contrast, Depression symptoms, which correlated only moderately with THT scores (r = .32, p = .04), did not evidence significant associations with either left or right amygdala GMV (left: r = −.15, p = .33; right: r = −.17, p = .27).3 Scores for the dysphoric disorder composite (i.e., aggregate of symptoms for depression, dysthymic disorder, and generalized anxiety disorder) also showed weak, nonsignificant associations with left and right amygdala volume (rs = −.07 and − .14, respectively, ps = .68 and .36).
Figure 1 :
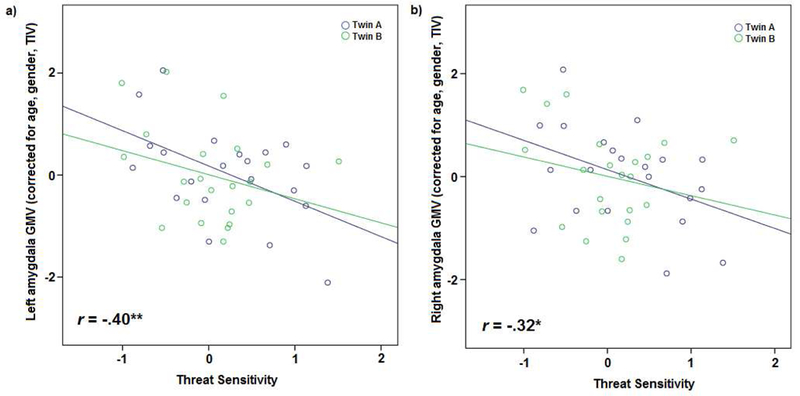
Scatterplots depicting correlations between Biobehavioral Threat Sensitivity score and gray matter volume (GMV) in left (a) and right (b) amygdala for all twin participants (blue: twin A, green: twin B). * = p < .05; ** = p < .01
Figure 2:
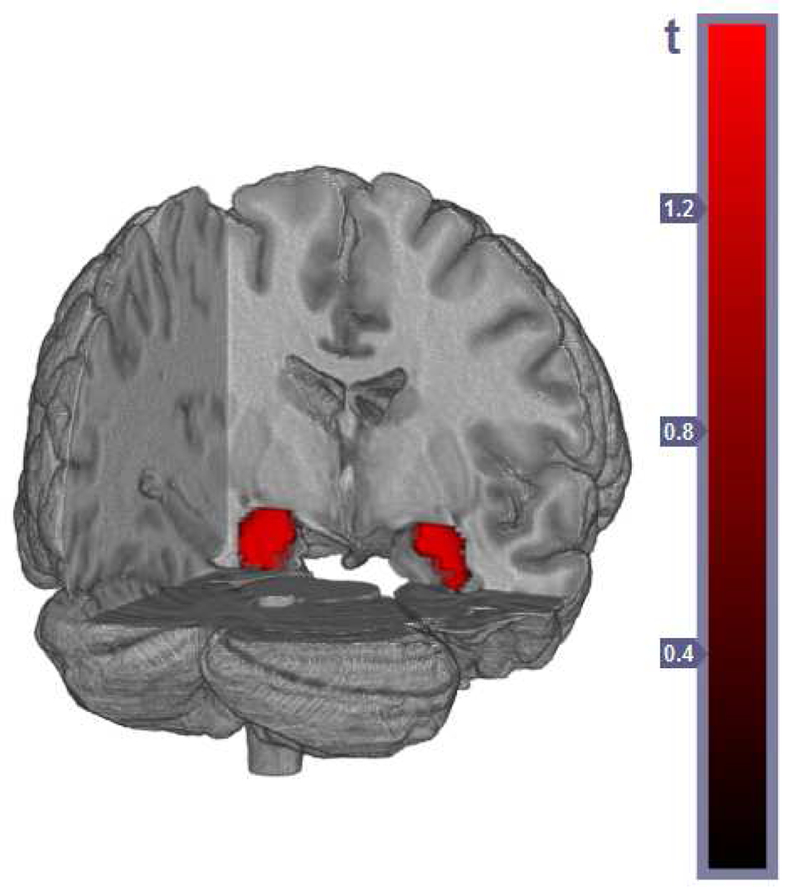
Location of inverse correlation between gray matter concentration in bilateral amygdala and Biobehavioral Threat Sensitivity score for all participants, using total intracranial volume as a covariate. View from posterior.
When Social Phobia and THT scores were included as concurrent predictors of GMV in regression models for left and right amygdala, the overall model emerged as significant in each case (left: F(2, 40) = 4.31, p = .02; right: F(2, 40) = 3.54, p = .04), but with neither variable showing a significant unique predictive association (left amygdala: Bs = −.18 and −.28, respectively, ps = .36 and .14, Cohen’s f2 = .03 and .09; right amygdala: Bs = −.28 and −.14, respectively, ps = .15 and .46, f2 = .09 and .02). The implication is that it was the variance in common between Social Phobia and THT that accounted for the association of each with amygdala volume. Results for regression models utilizing fear disorder composite scores and THT scores as concurrent predictors were similar (i.e., overall models were significant, with overlap evident in the associations for the two predictors), though in the regression model for left amygdala GMV some unique prediction was evident for THT scores (B = −.33, p = .045, f2 = .12) but not fear disorder composite scores (B = - .14, p = .38, f2 = .02).
3.2. Twin concordances
Twin concordances for biobehavioral THT scores and amygdala GMV scores (i.e., extent of similarity between twin-pair members and their co-twins for these measures) were evaluated using intraclass correlations (ICC). Twin concordance was moderate and significant for THT scores (ICC = .43; F(20, 20) = 2.53, p = .02), and strong and significant for amygdala GMV scores (left: ICC = .78; F(21, 21) = 8.00, p < .001; right: ICC = .66; F(21, 21) = 4.84, p < .001).
Given significant observed concordances for both THT and amygdala GMV, we also evaluated whether THT scores would predict GMV scores across twins. The intraclass correlation for THT scores of twin-pair members with amygdala GMV scores for their co-twins was significant for the left amygdala (ICC = .38; F(20, 20) = 2.21, p = .02; see Figure 3), and approached significance for the right amygdala (ICC = .27; F(20, 20) = 1.72, p = .058).
Figure 3:
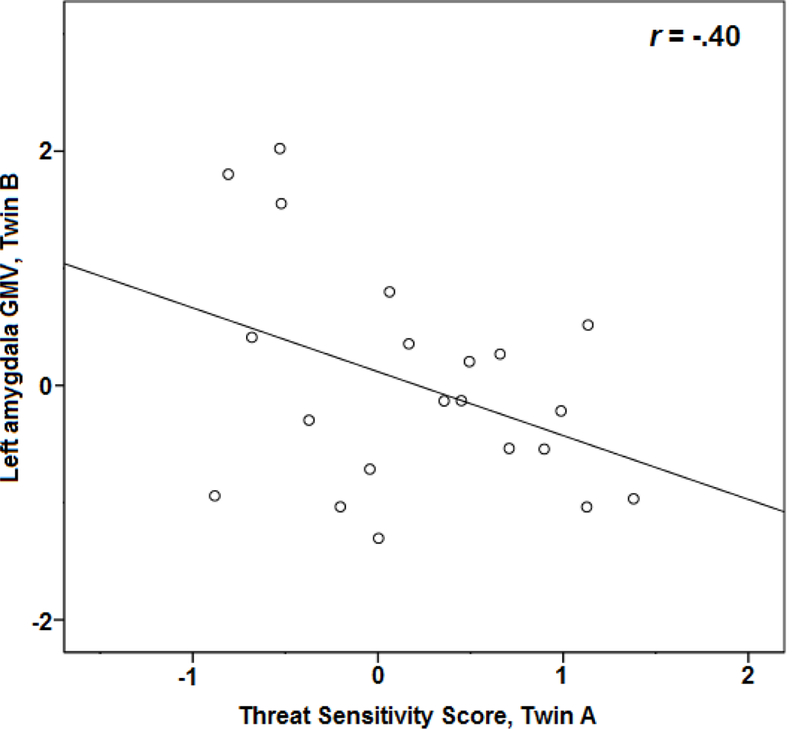
Scatterplot depicting cross-twin correlation between Biobehavioral Threat Sensitivity score and gray matter volume (GMV) in left amygdala.
3.3. Associations for control region (hippocampus)
GMV scores for the left and right hippocampus were correlated appreciably with corresponding amygdala GMV scores (rs = .63 and .55, respectively, ps < .001), but showed small, nonsignificant correlations with biobehavioral THT scores, Social Phobia and Depression symptom scores, and fear and dysphoric disorder composite scores. Regression analyses incorporating both hippocampus and amygdala GMV scores as predictors of either THT, Social Phobia, or fear disorder composite scores revealed further attenuation of associations for hippocampus GMV with these scores, indicating that modest observed relations for this region were attributable to covariation with amygdala GMVs. (For details of these analyses, see part 3 of the online Supplement.)
4. Discussion
The work reported here was undertaken to test for a relationship between dispositional fear (threat sensitivity) and amygdala volume in human subjects. As described at the outset, some studies have reported a positive relationship between these two variables whereas others have reported negative or null associations. Based on relatively more consistent evidence for a negative directional association in animal studies (and a smaller number of human studies) that have quantified threat sensitivity (THT) in terms of fearful behavior or stress reactivity (e.g., Fisler et al., 2013; Hayano et al., 2009; Irle et al., 2010; Park et al., 2015; Rogers et al., 2009), we hypothesized that a biobehavioral index of THT (i.e., composed of aversive physiological-response measures together with a psychological scale measure; Yancey et al., 2016) would show a negative relationship with amygdala volume, consistent with animal study findings.
Consistent with our hypothesis, we found a negative correlation between scores on this biobehavioral index of THT and amygdala GMV scores; correlations were of similar magnitude for the right and left amygdalae, and significant in each case, indicating bilaterality of the association. Also of note, we found negative directional associations for symptoms of social phobia, and fear disorders more broadly (i.e., symptoms of social phobia, specific phobia, and panic disorder combined), with right and left amygdala GMV.4 Importantly, regression-model analyses revealed that these relationships for social phobia and fear disorder symptomatology were attributable to variance in common with biobehavioral THT (r between the two = .65, indicating ~42% shared variance). By contrast, symptoms of major depression - a prototypic dysphoria/distress condition - correlated to a lesser degree with biobehavioral threat sensitivity (r = .21, indicating ~4% shared variance) and evidenced only a weak, nonsignificant negative association with amygdala GMV. Null associations with amygdala volume were also found for symptoms of dysphoric disorders more broadly (i.e., depression, dysthymic disorder, and generalized anxiety disorder combined).
Our use of a participant sample consisting of MZ twins enabled us to evaluate the possibility of a heritable basis to the observed negative association between neurobehavioral THT and amygdala volume. Prior twin research has demonstrated high heritability for amygdala volume. A study by Munn et al. (2007) investigated the relationship between amygdala volume and major depression in a similar-sized sample (N = 47) consisting of twin pairs and unrelated paired control participants. While no relationship between amygdala GMV and depression was found in the sample as a whole (consistent with the results reported here), MZ twin participants demonstrated substantial concordance for amygdala volume. A subsequent molecular genetic study by Montag et al. (2009) found that carriers of a specific gene polymorphism, the Val66Met variant of the BDNF gene, evidenced significantly lower amygdala gray matter volume scores in comparison with individuals lacking this variant. Consistent with this prior evidence for genetic influences, amygdala GMV in the current study showed strong concordance between MZ twins and their co-twins (ICCs > .65 for both right and left amygdala). Twin concordance for biobehavioral THT scores was somewhat lower (ICC = .43), consistent with prior work demonstrating moderate-level heritability for this dispositional variable (Venables et al., 2017). Critically, biobehavioral THT scores covaried significantly with amygdala volume across twin-pair members (i.e., MZ twins’ THT scores were predictive of their co-twins’ amygdala GMV scores). Because our study did not include a comparison sample of dizygotic twins, we are not able to rule out a possible role for shared family environment in this observed cross-twin concordance. However, prior twin work reporting negligible contributions of shared environment either to amygdala volume (van der Schot et al., 2009) or biobehavioral THT (Venables et al., 2017) argues against this as an alternative explanation for the observed concordance.
4.1. Implications for Affective Dispositions and Internalizing Psychopathology
A key innovative feature of this study was its use of a biobehavioral approach to quantify individual differences in threat sensitivity, wherein physiological indices of defensive reactivity to aversive cues were combined with a psychological (i.e., self-report scale) measure of general fearfulness. This approach to quantification is based on the idea of dispositional THT as a brain- based attribute of people that affects their general perceptions of and behavioral stance toward threatening situations and stimuli, and their level of physiological reactivity to such situations/stimuli (Yancey et al., 2016). A distinct advantage of this approach in research examining biological correlates of threat sensitivity is that it connects measurement of this dispositional construct directly to the biological-systems domain. We used this approach in order to parallel prior animal studies (and some human studies) that have quantified THT in biobehavioral terms, and we believe this was important to demonstrating a clear association with brain morphology, mirroring that reported in the animal literature. As such, our findings suggest a potential reason for the heterogeneity of findings from prior human neuroimaging studies: Methods for indexing fearfulness or anxiety-proneness that do not connect clearly to the neurobiological domain may lack the capacity to relate clearly or consistently to brain-structural indicators. Prior published work has in fact shown that biobehavioral assessments of trait constructs like THT and inhibitory control predict criterion measures of physiological reactivity more effectively than standard report-based assessments (Yancey et al., 2016; Patrick et al., 2013; Venables et al., 2018). The current work extends this by demonstrating effective prediction of brain-structural difference from a biobehavioral index of THT.
A key question raised by the current findings is why the direction of association between dispositional threat and amygdala volume appears negative, when (for example) fearfulness or anxiety-proneness tends to be associated with increased amygdala reactivity, and damage to or destruction of the amygdala leads to reduced rather than enhanced fearfulness (cf., Adolphs et al., 2005; Hitchcock & Davis, 1986). In considering this question, it is important to recognize that the relationship between amygdala volume and quantity of neurons (as well as number of non-neuronal cells) is not always clear (cf. Yang et al., 2008; Mozhui et al., 2007), and that changes in amygdala tissue due to lesion or damage have different functional consequences than naturally occurring variations in amygdala volume. Thus, it is conceivable that smaller constitutional volume of the amygdala results, on average, in reduced efficiency of threat-stimulus processing and lesser ability to regulate fear reactivity.
In this context, the current work is able to shed some light on the understanding of how amygdala volume relates to internalizing psychopathology: notably, while amygdala GMV was correlated with trait fear as well as fear disorder symptomatology, the same brain measure showed no relationship with dysphoric disorder symptomatology. This suggests that relationships between amygdala volume and anxious-depressive conditions, which have been reported in prior literature, may depend on the extent to which biobehavioral threat sensitivity is represented in those conditions generally, and within particular participant samples (such as psychiatric inpatients as opposed to clinic outpatients or community participants). This should be clarified in future studies, along with the role that biobehavioral threat sensitivity plays in the relationship between psychological disorders and brain anatomy and physiology. Its influence should be also examined in conjunction with other biobehavioral traits, such as reward sensitivity and inhibitory control, which could also be expected to show associations with brain measures.
4.2. Limitations and Future Directions
The current study has some notable limitations. The size of the participant sample limited statistical power for analyses, so that smaller-sized effects may have gone undetected. Additionally, our use of MZ twins alone precluded direct evaluation of the role of environment in observed concordances. Follow-up research with larger samples that include dizygotic (DZ) as well as MZ twins is needed to corroborate our reported findings. A further limitation is that internalizing disorders other than social phobia and major depression were not well represented due to the size of the sample and pre-selection only for THT scores and not for different forms of psychopathology. To the extent this pre-selection caused the sample to over-represent certain forms of psychopathology, this would limit the study’s generalizability. It will be important in future research to further examine how amygdala volume relates to fear and dysphoric disorder symptomatology in samples containing greater representation of conditions including specific phobia, panic disorder, agoraphobia, dysthymic disorder, generalized anxiety disorder, and posttraumatic stress disorder. To establish the generalizability of the findings, it will also be important to test for associations with amygdala volume in samples that exhibit varying levels of biobehavioral threat but are lacking in diagnosable psychiatric conditions. Apart from issues pertaining to the participant sample, the analysis performed here was unable to distinguish subdivisions of the amygdala (e.g., basolateral-input subdivision versus central-output subdivision; Davis, 1992). It should be noted, however, that this is true of all studies of this type to date that have used this method, and may in fact be a source of inconsistencies in reported findings.
Notwithstanding these limitations, the findings presented here support the view of amygdala gray matter volume as a specific marker for trait fear and threat sensitivity, and the use of biobehavioral or psychoneurometric scores as indicators of intermediate phenotypes. The current work provides evidence for a relationship between threat sensitivity defined in biobehavioral terms and amygdala volume. Our findings also provide some insight into inconsistent prior results for internalizing disorders, suggesting that the degree of involvement of dispositional THT in such conditions may affect the nature of their association with amygdala volume. It bears noting that the approach we used to quantify THT is consistent with initiatives calling for greater use of biological and behavioral variables in quantifying characteristics of relevance to mental health problems (e.g., RDoC; Kozak & Cuthbert, 2016).
Supplementary Material
Highlights.
Higher threat sensitivity correlates with lower amygdala gray matter volume
Both threat sensitivity and amygdala volume correlate between twins
Threat sensitivity of one twin predicts amygdala volume of the other
Amygdala volume also relates to fear disorder symptomatology
This latter relationship is due to variance in common with threat sensitivity
5. Acknowledgments
Data collection for this work was supported by NIH Center grant P50 MH072850, NIH BTRC grant P41 RR008079, and funds from the Hathaway Endowment at the University of Minnesota. Preparation of this article was supported in part by US Army grant W911NF-14–1-0027 and NIMH grant MH089727. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Government, Department of Defense, Department of the Army, Department of Veterans Affairs, or U.S. Recruiting Command. We wish to thank Siri Scott and Megan Lucy for their assistance with MRI data collection, and Max Owens from the University of Georgia for his help with analyses that were performed during the revision process for this manuscript.
Footnotes
Agoraphobia was not included in the fear disorder composite because only two participants in the current study sample endorsed symptoms of this disorder, and in both cases these symptoms were reported in the context of panic disorder.
In response to queries from anonymous reviewers of this report, we also examined correlations separately for self-report and physiological indicators of biobehavioral THT within the current test sample. The TF-55 self-report indicator showed significant negative correlation coefficients with left and right amygdala volume (r = –.38 & –.36, respectively, ps = .01 & .02), comparable to those for biobehavioral THT scores; by contrast, a composite of the three physiological indicators showed weaker, nonsignificant rs with left and right amygdala volume (rs = –.15 & –.06, respectively). Our interpretation is that levels of biobehavioral THT in participants tested in the current MRI study, relative to participants as a whole within the larger (N = 454) project sample from which they were drawn, were reflected more in TF-55 scores than in physiological indicator scores. A detailed account of this interpretive perspective, along with supportiveevidence, is provided in part 4 of the online Supplement.
In a whole-brain analysis, there were no clusters in which the association between GMV and THT exceeded Family- Wise Error (FWE) correction for multiple comparisons at an alpha level of .05. This highlights the importance of a priori hypothesis testing in MRI studies with modest sample sizes (Poldrack, 2007).
Analyses of subsets of items from the TF-55 scale used in the current study provided further evidence for associations of both non-social and social fear with reduced amygdala volume. The TF-55 includes 13 items from an inventory of situation-related fears, the Fear Survey Schedule (FSS; Arrindell et al., 1984); 6 of these 13 items pertain to social situations or stimuli (crowds, strangers, being teased, being watched working, being criticized, rejection by others), the other 7 to non-social situations or stimuli (doctors, riding in elevators, journeys by car, entering strange places, bats, cemeteries, enclosed spaces). Aggregates of these two item sets showed very similar correlations with right and left amygdala volume (rs = –.31 and –.34, respectively, for social fear set, and –.35 and –.37 for non-social fear set, all ps ≤ .04), indicating that fearfulness in general, rather than social fear specifically, relates to reduced amygdala volume.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, & Damasio AR (2005). A mechanism for impaired fear recognition after amygdala damage. Nature, 433(7021), 68–72. [DOI] [PubMed] [Google Scholar]
- Arrindell WA, Emmelkamp PMG, & van der Ende J (1984). Phobic dimensions: I. Reliability and generalizability across samples, gender, and nations. Advances in Behavior Research and Therapy, 6(4), 207–254. [Google Scholar]
- Ashburner J (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, … & Ernst M (2009). Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry, 66(3), 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, & Barrett LF (2011). Amygdala volume and social network size in humans. Nature Neuroscience, 14(2), 163–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, & Yücel M (2012). Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. Journal of Affective Disorders, 138(1), 9–18. [DOI] [PubMed] [Google Scholar]
- Cohen JE (1988). Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Cools R, Calder AJ, Lawrence AD, Clark L, Bullmore E, & Robbins TW (2005). Individual differences in threat sensitivity predict serotonergic modulation of amygdala response to fearful faces. Psychopharmacology, 180(4), 670–679. [DOI] [PubMed] [Google Scholar]
- Davis M (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15(1), 353–375. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, … & Ryan ND (2000). A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry, 48(1), 51–57. [DOI] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, & Liberzon I (2015). Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and Clinical Risk Management, 11, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2002). Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fischl B (2012). FreeSurfer. Neuroimage, 62(2), 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisler MS, Federspiel A, Horn H, Dierks T, Schmitt W, Wiest R, … & Soravia LM (2013). Spider phobia is associated with decreased left amygdala volume: a cross-sectional study. BMC Psychiatry, 13(1), 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, & Hariri AR (2008) Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience, 28(4), 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, & Frackowiak RS (2001). Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage, 14(3), 685–700. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, & Gotlib IH (2008). Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Molecular Psychiatry, 13(11), 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, & Weinberger DR (2002). The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage, 17(1), 317–323. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, & Phelps EA (2011). Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cerebral Cortex, 21(9), 1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, … & Hirayasu Y (2009) .Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry and Clinical Neurosciences, 63(3), 266–276. [DOI] [PubMed] [Google Scholar]
- Hitchcock J, & Davis M (1986). Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience, 100(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Hu FB, Goldberg J, Hedeker D, & Henderson WG (1998). Modelling ordinal responses from co□twin control studies. Statistics in Medicine, 17(9), 957–970. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, & McGue M (1999). Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology, 11(4), 869–900. [DOI] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Nunez-Elizalde AO, Dunn BD, & Bishop SJ (2011). Fear- conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron, 69(3), 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irle E, Ruhleder M, Lange C, Seidler-Brandler U, Salzer S, Dechent P, … & Leichsenring F(2010). Reduced amygdalar and hippocampal size in adults with generalized social phobia. Journal of Psychiatry & Neuroscience, 35(2), 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Filipek PA, Berenji GR, Modahl C, Osann K, & Spence MA (2006). Association between amygdala volume and anxiety level: magnetic resonance imaging (MRI) study in autistic children. Journal of Child Neurology, 21(12), 1051–1058. [DOI] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, … & Blumberg HP (2009). Relation between amygdala structure and function in adolescents with bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 48(6), 636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, & Whalen PJ (2011). The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research, 223(2), 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology, 53(3), 286–297. [DOI] [PubMed] [Google Scholar]
- Kramer MD, Patrick CJ, Krueger RF, & Gasperi M (2012). Delineating physiologic defensive reactivity in the domain of self-report: phenotypic and etiologic structure of dispositional fear. Psychological Medicine, 42(06), 1305–1320. [DOI] [PubMed] [Google Scholar]
- Krueger RF (1999). The structure of common mental disorders. Archives of General Psychiatry, 56(10), 921–926. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Bouchard TJ, McGue M, & Tellegen A (1990). The Minnesota Twin Family Registry: Some Initial Findings. Acta Geneticae Medicae et Gemellologiae: Twin Research, 39(1), 35–70. [DOI] [PubMed] [Google Scholar]
- Machado-de-Sousa JP, Osorio Fde L, Jackowski AP, Bressan RA, Chagas MH, Torro- Alves N, … & Hallak JE (2014). Increased amygdalar and hippocampal volumes in young adults with social anxiety. PloS One, 9(2), e88523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–1239. [DOI] [PubMed] [Google Scholar]
- Merz EC, Tottenham N, & Noble KG (2017). Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. Journal of Clinical Child & Adolescent Psychology, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, Dickstein DS, Leibenluft E, Ernst M, … & Pine DS (2005). Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biological Psychiatry, 57(9), 961–966. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, & Clark LA (1998). Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology, 49(1), 377–412. [DOI] [PubMed] [Google Scholar]
- Montag C, Weber B, Fliessbach K, Elger C, & Reuter M (2009). The BDNF Val66Met polymorphism impacts parahippocampal and amygdala volume in healthy humans: incremental support for a genetic risk factor for depression. Psychological Medicine, 39(11), 1831–1839. [DOI] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Hooper SR, & De Bellis MD (2016). Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology, 41(3), 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, & Rowland D (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383(6603), 812–815. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Hamre KM, Holmes A, Lu L, & Williams RW (2007). Genetic and structural analysis of the basolateral amygdala complex in BXD recombinant inbred mice. Behavior Genetics, 37(1), 223–243. [DOI] [PubMed] [Google Scholar]
- Munn MA, Alexopoulos J, Nishino T, Babb CM, Flake LA, Singer T, … & Botteron KN (2007). Amygdala volume analysis in female twins with major depression. Biological Psychiatry, 62(5), 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LD, Strickland C, Krueger RF, Arbisi PA, & Patrick CJ (2016). Neurobehavioral traits as transdiagnostic predictors of clinical problems. Assessment, 23(1), 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrmann P, Rauch AV, Bauer J, Kugel H, Arolt V, Heindel W, & Suslow T (2007). Threat sensitivity as assessed by automatic amygdala response to fearful faces predicts speed of visual search for facial expression. Experimental Brain Research, 183(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Park MH, Garrett A, Boucher S, Howe M, Sanders E, Kim E, … & Chang K (2015). Amygdalar volumetric correlates of social anxiety in offspring of parents with bipolar disorder. Psychiatry Research: Neuroimaging. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Durbin CE, & Moser JS (2012). Reconceptualizing antisocial deviance in neurobehavioral terms. Development and Psychopathology, 24(3), 1047–1071. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, & Kramer MD (2013). A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology, 122(3), 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza C, Sánchez-López J, Castilla-Ortega E, Rosell-Valle C, Zambrana-Infantes E, García-Fernández M, … & Estivill-Torrús G (2014). Fear extinction and acute stress reactivity reveal a role of LPA1 receptor in regulating emotional-like behaviors. Brain Structure and Function, 219(5), 1659–1672. [DOI] [PubMed] [Google Scholar]
- Phelps EA (2006). Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology, 57, 27–53. [DOI] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106(2), 274–285. [DOI] [PubMed] [Google Scholar]
- Poldrack RA (2007). Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience, 2(1), 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, & Menon V (2014). Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biological Psychiatry, 75(11), 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, & Wright CI (2003). Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences, 985(1), 389–410. [DOI] [PubMed] [Google Scholar]
- Reimold M, Slifstein M, Heinz A, Mueller-Schauenburg W, & Bares R (2006). Effect of spatial smoothing on t-maps: arguments for going back from t-maps to masked contrast images. Journal of Cerebral Blood Flow and Metabolism, 26(6), 751–759. [DOI] [PubMed] [Google Scholar]
- Ridgway GR, Omar R, Ourselin S, Hill DL, Warren JD, & Fox NC (2009). Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage, 44(1), 99–111. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, … & Kasai K (2009). Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Research: Neuroimaging, 174(3), 210–216. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, … & Gorka SM (2013). A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology, 122(2), 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger J (1980). Tests for comparing elements of a correlation matrix. Psychological Bulletin, 87, 245–251. [Google Scholar]
- Syal S, Hattingh CJ, Fouché JP, Spottiswoode B, Carey PD, Lochner C, & Stein DJ (2012). Grey matter abnormalities in social anxiety disorder: a pilot study. Metabolic Brain Disease, 27(3), 299–309. [DOI] [PubMed] [Google Scholar]
- Tian X, Hou X, Wang K, Wei D, & Qiu J (2016). Neuroanatomical correlates of individual differences in social anxiety in a non-clinical population. Social Neuroscience, 11(4), 424–437. [DOI] [PubMed] [Google Scholar]
- Tottenham N, & Sheridan MA (2009). A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience, 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … & Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, & Iacono WG (2011). Patterns of comordibity among common mental disorders: A person-centered approach. Comprehensive Psychiatry, 52, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Plas EA, Boes AD, Wemmie JA, Tranel D, & Nopoulos P (2010). Amygdala volume correlates positively with fearfulness in normal healthy girls. Social Cognitive and Affective Neuroscience, 5(4), 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Schot AC, Vonk R, Brans RG, van Haren NE, Koolschijn PCM, Nuboer V,… & Pol HEH (2009). Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Archives of General Psychiatry, 66(2), 142–151. [DOI] [PubMed] [Google Scholar]
- Venables NC, Hicks BM, Yancey JR, Kramer MD, Nelson LD, Strickland CM, … & Patrick CJ (2017). Evidence of a prominent genetic basis for associations between psychoneurometric traits and common mental disorders. International Journal of Psychophysiology, 115, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Foell J, Yancey JR, Kane MJ, Engle RW, & Patrick CJ (2018). Quantifying inhibitory control as externalizing proneness: A cross-domain model. Clinical Psychological Science, 6, 561–580. [Google Scholar]
- Watson D, O’Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez E, Gamez W, & Stuart S (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological Assessment, 19, 253–268. [DOI] [PubMed] [Google Scholar]
- Yancey JR, Vaidyanathan U, & Patrick CJ (2015). Aversive startle potentiation and fear pathology: Mediating role of threat sensitivity and moderating impact of depression. International Journal of Psychophysiology, 98(2), 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey JR, Venables NC, & Patrick CJ (2016). Psychoneurometric operationalization of threat sensitivity: Relations with clinical symptom and physiological response criteria. Psychophysiology, 53(3), 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, & Holmes A (2008). Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology, 33(11), 2595–2604. [DOI] [PubMed] [Google Scholar]
- Zhao K, Yan WJ, Chen YH, Zuo XN, & Fu X (2013). Amygdala volume predicts interindividual differences in fearful face recognition. PLoS One, 8(8), e74096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


