Abstract
Aging is typically associated with declines in sensorimotor performance. Previous studies have linked some age-related behavioral declines to reductions in network segregation. For example, compared to young adults, older adults typically exhibit weaker functional connectivity within the same functional network but stronger functional connectivity between different networks. Based on previous animal studies, we hypothesized that such reductions of network segregation are linked to age-related reductions in the brain's major inhibitory transmitter, gamma aminobutyric acid (GABA). To investigate this hypothesis, we conducted graph theoretical analyses of resting state functional MRI data to measure sensorimotor network segregation in both young and old adults. We also used magnetic resonance spectroscopy to measure GABA levels in the sensorimotor cortex and collected a battery of sensorimotor behavioral measures. We report four main findings. First, relative to young adults, old adults exhibit both less segregated sensorimotor brain networks and reduced sensorimotor GABA levels. Second, less segregated networks are associated with lower GABA levels. Third, less segregated networks and lower GABA levels are associated with worse sensorimotor performance. Fourth, network segregation mediates the relationship between GABA and performance. These findings link age-related differences in network segregation to age-related differences in GABA levels and sensorimotor performance. More broadly, they suggest a neurochemical substrate of age-related dedifferentiation at the level of large-scale brain networks.
Keywords: Aging, Sensorimotor, Network segregation, GABA, Dedifferentiation
1. Introduction
Advanced age is typically associated with declines in sensorimotor functioning. Such declines affect the ability of older adults to perform activities of daily living and maintain their functional independence (Seidler et al., 2010). Part of this decline is associated with impairments in the peripheral sensorimotor system, including motor unit reorganization (Galganski et al., 1993) or reduced functioning of cutaneous afferents. But evidence also suggests that some of these age-related declines in behavior are related to changes in the brain, including alterations in neurochemistry, gray matter atrophy, and changes in the functional organization of large-scale brain networks (Seidler et al, 2010, 2015; Langan et al., 2010; Raz and Rodrigue, 2006; Goh, 2011; Ferreira and Busatto, 2013). Understanding these brain-behavior relationships is important for our efforts to prolong the functional independence of older adults as our society continues to age.
Studies in young adults have demonstrated the existence of multiple segregated functional brain networks. Regions within these networks exhibit spontaneous yet correlated activity, and thus are thought to be functionally connected (Biswal et al., 1995; Buckner et al., 2013). Studies have found that connections within these functional networks are quite dense, whereas connections between different networks are more sparse. This organization is considered to benefit specialized or segregated information processing in different brain systems (Bullmore and Sporns, 2012). Several studies have investigated the effect of age on functional connectivity by measuring differences in correlated brain activity within and between brain networks at rest. Many of these studies have found that older adults exhibit weaker functional connectivity between brain regions within the same functional network but stronger functional connectivity between regions belonging to different networks. In other words, their functional networks are less segregated (i.e., a type of age-related dedifferentiation) (Chan et al., 2014; Geerligs et al., 2015; Damoiseaux, 2017).
Many studies have also found that less segregated brain networks are associated with worse cognitive performance, independent of age (Chan et al., 2014; Geerligs et al., 2015; Wang et al., 2010; Damoiseaux et al., 2008). Only a few studies have investigated the relationship between network segregation and sensorimotor behavior, but one such study found that reduced segregation of several large-scale resting state brain networks was associated with poorer bimanual motor performance (King et al., 2018). In sum, the evidence to date suggests that age-related changes in functional connectivity may contribute to age-related declines in cognitive and sensorimotor performance.
An important open question is what causes age-related reductions in network segregation, or neural dedifferentiation. Previous animal studies have linked neural dedifferentiation to changes at the neurotransmitter level. In particular, studies by Leventhal and colleagues suggested that age differences in the brain's major inhibitory neurotransmitter, gamma-aminobutyric acid (GABA), may play an important and potentially causal role in age-related dedifferentiation (i.e., reductions in the specificity of neural activity). More specifically, they demonstrated that manipulations of GABA levels led to changes in the orientation-selectivity of neurons in the visual cortex (Leventhal et al., 2003). The application of GABA or a GABA agonist made visual cortex neurons in old monkeys more orientation-selective, thereby making them similar to neurons in young monkeys. In contrast, the application of a GABA antagonist reduced the orientation-selectivity of visual cortex neurons in young monkeys, thereby making them similar to neurons in old monkeys. These results demonstrate that manipulations of GABA cause changes in neural selectivity in animals, raising the possibility that age declines in GABA might contribute to age-related neural dedifferentiation and associated behavioral declines in humans.
Consistent with this hypothesis, more recent studies in humans have linked individual differences in GABA levels to performance variations across healthy young adults (Edden et al., 2009; Boy et al., 2010; Puts et al., 2011). For example, Edden et al. found that orientation discrimination performance is predicted by GABA levels in primary visual cortex (Edden et al., 2009). In addition, Puts et al. demonstrated that GABA levels in sensorimotor cortex correlate with tactile discrimination thresholds (Puts et al., 2011). Previous work has also found that reactivity in the GABA system plays an important role in motor learning and learning-related brain activity. For instance, Stagg et al. (2011) demonstrated that participants in whom motor cortex (M1) GABA levels decreased the most following transcranial direct current stimulation (i.e., greatest GABA reactivity), also exhibited the most learning in a motor task (Stagg et al., 2011).
Studies have also observed a relationship between functional connectivity within specific resting state networks and GABA levels in young adults. For example, Stagg and colleagues demonstrated that functional connectivity strength within the motor resting state network is related to GABA levels in primary motor cortex (Stagg et al., 2014). A similar relationship was also reported between GABA levels in the posteromedial cortex and the strength of default mode network connectivity (Kapogiannis et al., 2013). While these studies linked individual differences in functional connectivity to differences in GABA levels, no studies have investigated whether this relationship varies with age. Moreover, no studies have examined GABA levels, network segregation, and sensorimotor behavior within the same participants in order to explore the potential links between all three levels.
In the present study, we specifically addressed these gaps. We performed graph theoretical analysis of resting state functional MRI data to measure sensorimotor network segregation and used magnetic resonance spectroscopy (MRS) to measure GABA levels in the sensorimotor cortex. We also collected a battery of sensorimotor behavioral measures to determine whether network segregation and/or GABA levels are associated with individual differences in performance. All three datasets were collected within the same participants, making it possible to examine associations between all the measures.
We tested three hypotheses: 1) The sensorimotor resting state brain network would be less segregated and sensorimotor cortex GABA levels would be reduced in older compared to young adults; 2) lower levels of GABA would be associated with less segregated networks, independent of age; and 3) lower levels of GABA and less segregated networks would be associated with worse sensorimotor performance, independent of age.
2. Materials and methods
2.1. Participants
Twenty-two young adults (age range 19–29 years; 13 females) and 23 older adults (age range 65–81; 12 females) were recruited for this study. All participants were right-handed, native English speakers. We screened participants to ensure they were not taking any medications with psychotropic effects, and were free from any other MRI safety contraindications. Participants were also assessed for cognitive impairment using the Montreal Cognitive Assessment (MoCA) and only those with scores ≥23 were included in the study (Brenkel et al., 2017). Data from one younger and one older adult were excluded due to voluntary withdrawal from the study before completion of the protocol. Behavioral data from one additional older adult participant was excluded due to technical issues related to behavioral task assessments. All study procedures were reviewed and approved by the University of Michigan Institutional Review Board. All participants provided detailed written consent for their involvement in this study.
2.2. Study design
Participants completed three separate testing sessions: one resting state fMRI session, one MRS session, and one behavioral session. The order of the fMRI and behavioral sessions was counterbalanced across participants, but the fMRI session always occurred prior to the MRS session so that we could use the fMRI activation to guide the placement of the MRS voxels. All measurements were acquired from participants within an average period of 25 days.
2.3. Sensorimotor assessments
We used a sensorimotor test battery that included both motor and somatosensory components. The motor measures included a 9-hole pegboard dexterity test, grip strength, and a 2-min walk endurance test. The somatosensory (tactile) measures included vibrotactile simple and choice reaction time (RT), static and dynamic vibrotactile detection thresholds, and a functional tactile object recognition test (fTORT). Detailed descriptions of each task are provided in the supplemental materials, as are the age-group means (and standard errors) for each behavioral measure (Table S1). In addition, covariance matrices across all behavioral measures are included in the supplemental materials for all participants (Table S2), for the older adult group alone (Table S3), and for the younger adult group alone (Table S4). Given the wide range of sensorimotor measurements, participant scores for all tests were submitted to a factor analysis to identify behavioral factors that reflect general sensorimotor functioning. Please refer to supplemental material for factor analysis model coefficients across all participants (Table S5), within the older adult group (Table S6), and within the younger adult group (Table S7).
2.4. MRI data acquisition
Anatomical and functional brain images were acquired using a GE Discovery MR750 3-T MRI scanner located at the University of Michigan Functional Magnetic Resonance Imaging Laboratory. A GE 8-channel head coil was used, and participant movement was minimized by stabilizing the head with cushions and Velcro straps. Imaging sessions included the acquisition of T1-weighted anatomical images, high-resolution anatomical images using spoiled 3D gradient-echo acquisition (SPGR), and T2*-weighted functional images. Functional images were acquired using a single-shot gradient-echo (GRE) reverse spiral pulse sequence. The field of view was 220 × 220 mm, with a voxel size of 3×3×4 mm (40 axial slices), a TR (repetition time) of 2 s, and a TE (echo time) of 30 ms. The duration of the resting state scan was 8 min.
2.5. MRS data acquisition
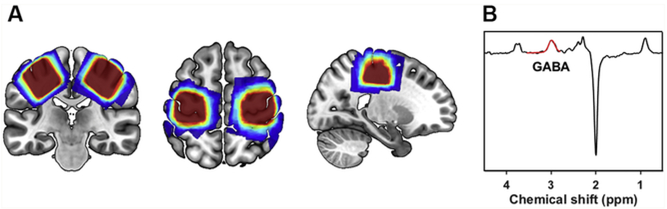
MRS data were acquired on the same scanner on a different day. Data were collected from 30 mm × 30 mm x 30 mm voxels placed in the left and right sensorimotor cortex (See Fig. 1A). The placement of voxels in each participant corresponded to the region of maximal sensorimotor activity in that same individual from their previous task-based fMRI session (not included in the present study). Briefly, participants performed motor (finger tapping on right vs. left hands) and somatosensory (vi-brotactile stimulation to right vs. left hands) tasks in the MRI scanner. The MRS voxel was subsequently placed in each participant to maximize overlap with fMRI activation from both of these tasks. Participants were not instructed to do any task during MRS data acquisition.
Fig. 1.
A) Sensorimotor cortex MRS voxel overlap across all participants, with brighter (red) colors representing more participant overlap and darker (blue) colors representing less overlap. B) Edited MR spectra from a representative younger adult participant (black) demonstrating a clearly resolved peak for GABA+ at 3 ppm, with the fitted GABA+ model in red. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
GABA-edited MR spectra were acquired using a MEGA-PRESS sequence (Mescher et al., 1998; Edden and Barker, 2007) with the following acquisition parameters: TE = 68 ms (TE1 = 15 ms, TE2 = 53 ms); TR = 1.8s; 256 transients (128 ON interleaved with 128 OFF) of 4096 data points; spectral width = 5 kHz; frequency selective editing pulses (14ms) applied at 1.9ppm (ON) and 7.46ppm (OFF); total scan time, approximately 8.5 min per voxel. The MRS voxels were acquired serially.
2.6. Resting state fMRI data preprocessing
Resting state data preprocessing was performed with the Statistical Parametric Mapping 8 software (SPM8; www.fil.ion.ucl.ac.uk/spm). Preprocessing steps included slice-time correction, realignment, segmentation of structural images, normalization into standard Montreal Neurological Institute (MNI) space and spatial smoothing using a Gaussian kernel of 8 mm full width at half-maximum (FWHM). Because functional connectivity measurements are influenced by head motion in the scanner (Van Dijk et al., 2012), we detected and rejected motion artifacts using the artifact detection toolbox (ART; http://www.nitrc.org/projects/artifact_detect). Specifically, an image was defined as an outlier if the head displacement in the x, y, or z direction was greater than 0.5mm from the previous frame, if the rotational displacement was greater than 0.02 radians from the previous frame, or if the global mean intensity of an image was greater than 3 standard deviations from the mean image intensity for the entire resting scan. Outliers in the global mean signal intensity and motion were subsequently included as nuisance covariates in the first level general linear model (GLM). A total of 36vol were removed from four participants during preprocessing (8vol in one older adult, 2vol in another older adult, 20vol in one young adult, and 6vol in a second young adult). The difference between the age groups was not statistically significant, t(41) = 0.76, p = .45).
Additional denoising on the resting state data was performed with the CONN toolbox (Whitfield-Gabrieli et al., 2012). The resting state data were filtered using a temporal band-pass filter of 0.008–0.09 Hz in order to examine the frequency band of interest and to exclude higher frequency sources of noise such as heart rate and respiration. For noise reduction, we used the anatomical component-based noise correction method aCompCor, which models the influence of noise as a voxel-specific linear combination of multiple empirically estimated noise sources by deriving principal components from noise regions of interest (ROIs) and including them as nuisance parameters in the first level GLM (Behzadi et al., 2007). Specifically, the anatomical image for each participant was segmented into white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) masks. To minimize partial voluming effects with GM, the WM and CSF masks were eroded by one voxel. The eroded WM and CSF masks were then used as noise ROIs. Residual head movement parameters (three rotations, three translations, and six parameters that represent their first-order temporal derivatives) and signals from WM and CSF were regressed out during the computation of functional connectivity maps.
2.7. First-level functional connectivity analysis
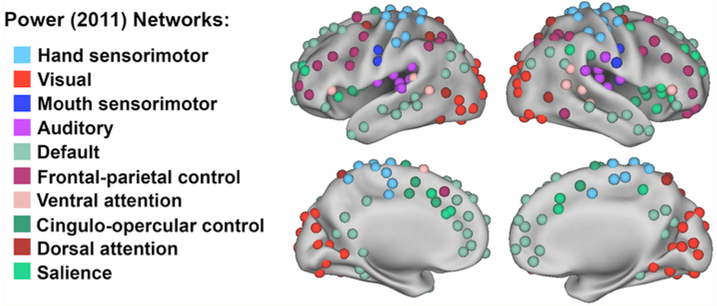
First-level ROI-to-ROI functional connectivity MRI (fcMRI) analysis was performed with the CONN toolbox (Whitfield-Gabrieli et al., 2012). For this analysis, we created ROIs (using 5mm diameter spheres) using coordinates published in Power et al. (See MNI coordinates in Table S8) (Power et al., 2011). We used all coordinates from this previous study except for those belonging to “subcortical” and “undefined” networks, which left us with 214 ROIs. Each ROI was labeled according to this published functional network map, which was defined by consensus of network assignments using community detection of resting state functional connectivity across multiple thresholds. The final network labels of each ROI are depicted in Fig. 2. For each participant, the resting state fMRI time series within each of the 214 ROIs was extracted from the unsmoothed functional images (to avoid potential “spillage” of the BOLD signal from nearby regions/ROIs), and the mean time course was computed. The cross-correlation of each ROI's time course with every other ROI's time course was computed, creating a 214 × 214 ROI-to-ROI correlation matrix. Correlation coefficients (i.e., graph edges) were converted into z-values using Fisher's r-to-z transformation (Zar, 1996). The resulting Fisher z-transformed correlation matrix is a fully connected, relatedness graph. Negatively weighted edges were set to zero in each correlation matrix to avoid potential misinterpretation of negative edge weights (Chan et al., 2014, 2017) (we also performed the same analyses with inclusion of both positively and negatively weighted edges, and observed similar findings (See Table S9)). Thus, the final connectivity matrix for each participant was a 214 × 214 z-matrix with the diagonal removed and the negative values set to zero.
Fig. 2.
214 regions of interest were created using coordinates defined by Power et al (2011) to produce ten resting state networks of interest.
2.8. Graph theoretical analysis
Following Chan et al. (2014), network segregation was defined as the difference of mean within-network connectivity and mean between-network connectivity divided by the mean within-network connectivity:
where Z̄w is the mean Fisher z-transformed correlation between ROIs within each network and Z̄b is the mean Fisher z-transformed correlation between ROIs of one network to all ROIs in other networks (Chan et al., 2014).
2.9. MRS analysis
GABA levels from both right and left sensorimotor cortices were measured using the GABA analysis toolbox, Gannet (Edden et al., 2014). All time domain data were frequency- and phase-corrected using spectral registration and filtered with a 3-Hz exponential line broadening and zero-filled by a factor of 16 (Near et al., 2015). The 3-ppm GABA peak in the difference spectrum was fit using a Gaussian model and quantified relative to water (fit with a Gaussian-Lorentzian model) in institutional units (See Fig. 1B). This editing scheme results in significant excitation of coedited macromolecule (MM) signal, which has been reported to contribute approximately 45% to the edited signal at 3-ppm (Mullins et al., 2014). Thus, all GABA values are reported as GABA+ (i.e., GABA+ MM) in the present study. To control for the differential relaxation constants and water visibility between WM, GM and CSF, a binary mask of the MRS voxel was created using Gannet's integrated voxel-to-image coregistration. Next, segmentation of the anatomical image was performed using the Segment function in SPM and the voxel fractions containing CSF, GM and WM were computed (Harris et al., 2015). From this procedure, a tissue-corrected GABA+ value was calculated for each participant. GABA+ levels from right and left sensorimotor cortices were averaged together before any statistical analyses. Thus, all reported GABA+ measures are reported as the mean value across both right and left sensorimotor cortices. We also collected measures of signal-to-noise (SNR) and fit error values for both voxels and for all participants to examine any age differences in these measures.
To control for age differences in brain structure, we measured the GM volume of bilateral precentral, postcentral, and supramarginal gyri in each participant. FreeSurfer was used to acquire these GM measurements, with each gyrus defined by an automated cortical parcellation technique (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004).
2.10. Statistical analysis
To test for group differences in network segregation, GABA+ levels, and behavior between young and older adults, we performed independent sample t-tests on these data. To explore the relationship between network segregation, GABA+ levels and behavior, we performed partial correlation analyses with bootstrapping (using 1000 samples, 95% confidence intervals) across all participants (controlling for age and GM volume differences). We also investigated this relationship specifically within each age group using bivariate correlation analyses. For all analyses, outliers greater than three standard deviations above or below the mean were excluded. SPSS software was used for all statistical analyses (SPSS Inc., Chicago IL).
3. Results
3.1. Age differences in sensorimotor performance
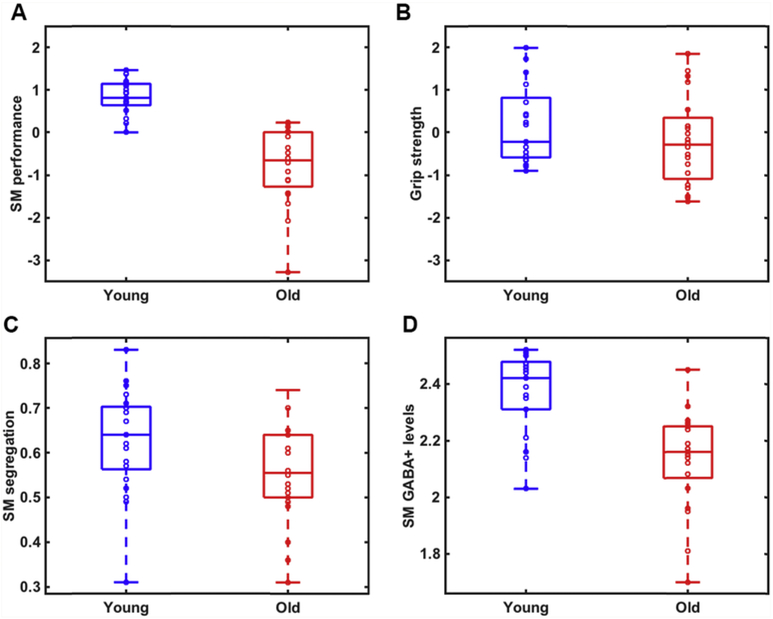
An exploratory factor analysis of the sensorimotor behavioral measures identified two factors, one corresponding to grip strength and one corresponding to all of the other sensorimotor measures (Table S5). These two sensorimotor factors were used in all further statistical analyses. Significant age differences were observed in the general sensorimotor factor (t(39) = 7.24, p < .001; Fig. 3A), whereas there was no significant effect of age on the grip strength factor (t(39) = 1.24, p = .22; Fig. 3B). To test whether gender influenced these results, we performed follow-up ANCOVAs using age group as the independent variable, behavior as the dependent variable, and gender as a covariate. We observed a significant effect of age on both the sensorimotor performance factor (F(1, 37) = 49.89, p < .001) and the grip strength factor F(1, 37) = 4.44, p = .042 after controlling for gender. Because we observed significant age differences in years of education (t(40) = 2.51, p = .017) between the two groups, we tested whether education influenced the observed age effects on behavior. Controlling for education in follow-up ANCOVAs, we still observed a significant effect of age on the sensorimotor performance factor (F(1, 38) = 41.97, p < .001, but not on the grip strength factor, (F(1, 38) = 1.66, p = .21).
Fig. 3.
Age differences in A) a summary measure of general sensorimotor performance (t = 7.24, p < .001); B) grip strength (t = 1.24, p = .22); C) sensorimotor network segregation (t = 2.23, p = .031); and D) sensorimotor GABA+ levels (t = 4.97, p < .001) between young (blue) and older (red) adults. On each box, the central line indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We also performed a factor analysis on each age group separately (see Supplementary Tables S6 and S7). Although the factor structures were somewhat different between the two age groups, the sample sizes in each group were relatively small and we therefore decided to use the factors from the entire sample for most of the subsequent analyses. We also examined the relationship between our neural measures with each individual behavioral measure to ensure that the results were not an artifact of differences in factor structure (see Tables S10 and S11).
3.2. Age differences in network segregation
We found that sensorimotor network segregation was significantly reduced in older compared to younger adults, t(41) = 2.23, p = .031 (See Fig. 3C). As a follow-up analysis, we investigated age differences in mean network segregation (averaged across all 10 networks) and found that this was also significantly reduced in older relative to younger adults, t(40) = 4.84, p < .001. We also examined the effect of age in each network individually and found significant age differences in five out of the ten networks examined. These included the Sensorimotor hand (t(41) = 2.23, p = .031), Auditory (t(41) = 2.23, p = .031), Sensorimotor mouth (t(39) = 4.35, p < .001), Cingulo-opercular control (t(41) = 3.7, p = .001) and Dorsal attention (t(41) = 2.28, p = .028) networks. Furthermore, the p-value for three additional networks was between 0.05 and 0.10 (Visual: p = .099, Ventral attention: p = .059, Salience: p = .057). It is important to note that these post-hoc analyses were not corrected for multiple comparisons, and therefore these findings should be interpreted with caution.
To test whether gender influenced these results, we performed a follow-up ANCOVA using gender as a covariate in the model. We still observed significant age differences in sensorimotor network segregation, even after controlling for gender, F(2, 39) = 4.55, p = .039. We also tested whether level of education influenced the observed age effect on network segregation. Controlling for education in a follow-up ANCOVA, the effect of age on sensorimotor network segregation was not quite significant, F(1, 38) = 3.99, p = .053.
3.3. Age differences in GABA levels
Tissue-corrected GABA+ levels (controlling for the differential relaxation constants and water visibility between WM, GM and CSF in the MRS voxels) in sensorimotor cortex were significantly reduced in older compared to younger adults, t(40) = 4.97, p < .001 (See Fig. 3D). Sensorimotor GABA+ levels were still significantly reduced in older relative to younger adults after controlling for gender (F(1, 38) = 24.80, p < .001) and education (F(1, 39) = 18.64, p < .001). We did not observe any significant age differences in SNR or fit error, either when examining left (SNR: t(40) = 1.43, p = .16; fit error: t(40) = 1.11, p = .28) or right (SNR: t(39) = 1.74, p = .09; fit error: t(39) = −0.21, p = .84) voxels separately, or averaged together (SNR: t(41) = 1.72, p = .093; fit error: t(41) = 0.83, p = .41).
3.4. Relationship between network segregation and GABA levels
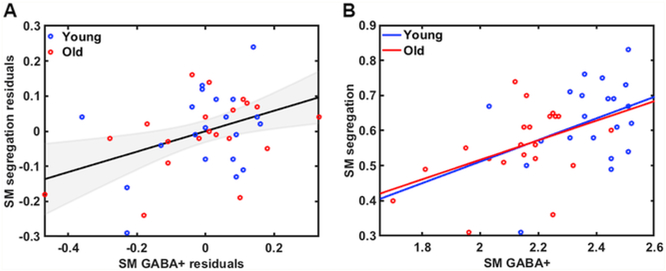
Controlling for age and gray matter (GM) volume differences, we observed a positive relationship between sensorimotor network segregation and GABA+ levels across all subjects, r(38) = 0.41, p = .008, bootstrap confidence interval [0.078, 0.65] (See Fig. 4A). This finding was also observed when examining the older adult group alone (r(21) = 0.45, p = .042; Fig. 4B), but did not reach significance in the younger adult group (r(21) = 0.37, p = .10). To explore whether these correlations were significantly different between the two age groups, we transformed the correlations to z-scores to compare between groups. Results revealed no significant differences between the two groups in the relationship between sensorimotor network segregation and GABA+ levels, p = .77.
Fig. 4.
A) Relationship between sensorimotor GABA+ levels and sensorimotor network segregation across all participants (accounting for age and GM volume differences; r = .41, p = .008) and B) within each age group separately (Old: r = 0.45, p = .042; Young: r = 0.37, p = .10).
To examine whether education level influenced these results, we performed a follow-up partial correlation analysis that included age (years), GM volume, and education level as covariates. Controlling for these variables, we still observed a significant positive relationship between sensorimotor network segregation and GABA+ levels, r(37) = 0.42, p = .008, confidence interval [0.095, 0.65]. We also tested whether the association between sensorimotor network segregation and GABA+ levels was significant when analyzed using a non-parametric permutation test that makes no assumptions about the statistical distribution. To do this, we randomly shuffled the within- and between-network connection labels 10,000 times. We then computed the association between GABA and sensorimotor network segregation based on that random shuffling. We found that the association with the true segregation value (r = 0.496) was within the top 1% (actually in the top 0.15%) of all segregation values.
In order to assess the specificity of the relationship between segregation and GABA+ levels in the sensorimotor system across the whole group, we examined the relationship between mean network segregation (averaged across all 10 networks) and GABA+ levels. Controlling for age and GM volume differences, we observed a significant positive relationship between these measures, r(37) = 0.43, p = .007, confidence interval [0.059, 0.69]. This finding was also observed when examining the older adult group alone (r(22) = 0.54, p = .011), but did not reach significance in the younger adult group (r(21) = 0.15, p = .53) (but the strength of this relationship was not significantly different in the two groups, p = .17). We also examined the relationship between sensorimotor segregation and GABA+ levels measured in ventral visual cortex (VVC) and auditory cortex (controlling for age and GM volume differences). We observed no significant relationships, either between sensorimotor segregation and GABA+ levels in VVC (r(38) = 0.03, p = .86, confidence interval [−0.36, 0.38]), or sensorimotor segregation and GABA+ levels in auditory cortex (r(38) = 0.12, p = .46, confidence interval [−0.30, 0.47]).
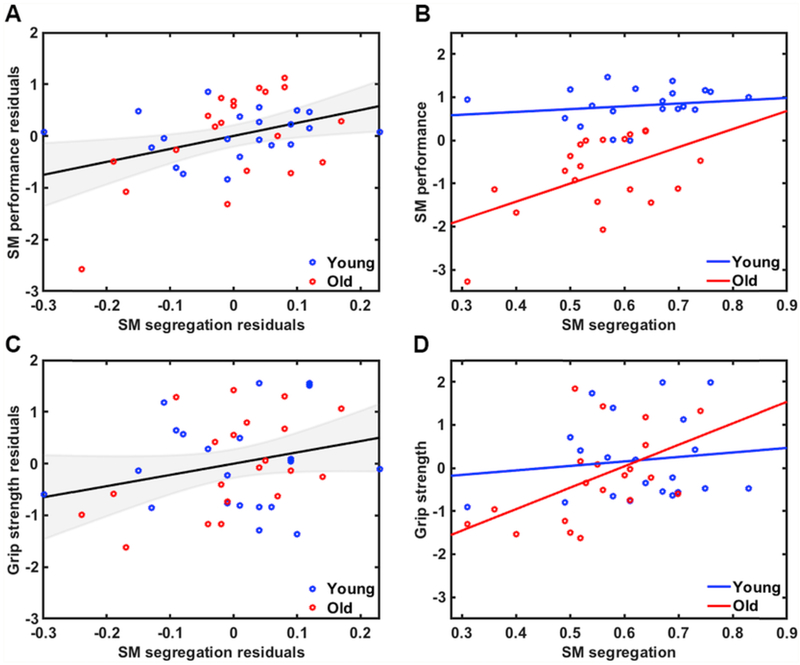
3.5. Relationship between network segregation and behavior
Controlling for age and GM volume differences, we observed a positive relationship between sensorimotor network segregation and sensorimotor performance across all subjects, r(37) = 0.38, p = .016, confidence interval [0.011, 0.67] (See Fig. 5A). These findings were also observed when examining the older adult group alone (r(20) = 0.50, p = .026; See Fig. 5B), but did not reach significance in the younger adult group (r(21) = 0.18, p = .44). There was also a trend toward a positive relationship between sensorimotor network segregation and grip strength across all participants (r(37) = 0.27, p= .093, confidence interval [−0.028, 0.51]; See Fig. 5C). Examining this grip strength relationship within the two age groups separately, we observed a significant relationship within the older adult group (r(20) = 0.51, p = .021; See Fig. 5D), but not within the younger adult group (r(21) = 0.13, p = .59). To explore whether there were significant age differences in these relationships, we again transformed the correlations to z-scores to compare between groups. We found no significant group differences, either on the sensorimotor performance measure (p = .28) or on the grip strength measure (p = .20). Please refer to supplemental materials for (uncorrected) associations between sensorimotor network segregation and individual behavioral measures (Table S10).
Fig. 5.
A) Relationship between sensorimotor network segregation and sensorimotor performance across all participants (accounting for age and GM volume differences; r = .38, p = .016) and B) within each age group separately (Old: r = 0.50, p = .026; Young: r = 0.18, p = .44). C) Relationship between sensorimotor network segregation and grip strength across all participants (accounting for age and GM volume differences; r = .27, p = .093) and D) within each age group separately (Old0: r = 0.51, p = .021; Young: r = 0.13, p = .59).
To examine whether education level influenced these results, we performed follow-up partial correlation analyses that included age (years), GM volume, and education level as covariates. Controlling for these variables, we still observed a significant positive relationship between sensorimotor network segregation and the sensorimotor performance factor (r(36) = 0.39, p = .017, confidence interval [−0.012, 0.67]) but only a trend toward a positive relationship between sensorimotor network segregation and the grip strength factor (r(36) = 0.27, p = .097, confidence interval [−0.028, 0.55])
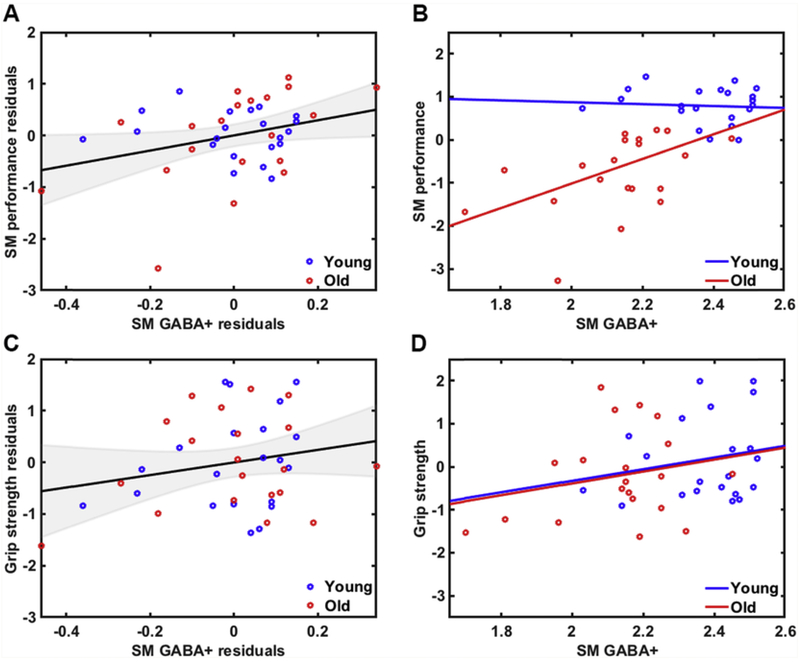
3.6. Relationship between GABA levels and behavior
Controlling for age and GM volume differences, we observed a positive relationship between GABA+ levels and sensorimotor performance across all participants (r(37) = 0.32, p = .046, confidence interval [0.013, 0.56]; Fig. 6A). This finding was also observed when examining the older adult group alone (r(20) = 0.48, p = .031; Fig. 6B), but was not significant in the younger adult group (r(21) = −0.14, p = .55). Follow-up analyses revealed a trend toward an age group difference in this relationship, p = .05. We did not observe an association between GABA+ levels and grip strength within the whole group (r(37) = 0.21, p = .19, confidence interval [−0.12, 0.49]; Fig. 6C) or within either age group separately (Old: r(20) = 0.23, p = .33; Young: r(21) = 0.20, p = .38; Fig. 6D). Not surprisingly, follow-up analyses revealed no significant group differences in this relationship, p = .93. Please refer to supplemental materials for (uncorrected) associations between sensorimotor GABA+ levels and individual behavioral measures (Table S11).
Fig. 6.
A) Relationship between sensorimotor GABA+ levels and sensorimotor performance across all participants (accounting for age and GM volume differences; r = .32, p = .046) and B) within each age group separately (Old: r = 0.48, p = .03; Young: r = −0.14, p = .55). C) Relationship between sensorimotor GABA+ levels and grip strength across all participants (accounting for age and GM volume differences; r = .21, p = .19) and D) within each age group separately (Old: r = 0.23, p = .33; Young: r = 0.20, p = .38).
To test whether education level influenced these results, we performed follow-up partial correlation analyses that included age (years), GM volume, and education level as covariates. Controlling for these variables, we still observed a trend toward a positive relationship between sensorimotor GABA+ levels and the sensorimotor performance factor (r(36) = 0.32, p = .05, confidence interval [−0.022, 0.55]) but no significant relationship between sensorimotor GABA+ levels and the grip strength factor (r(36) = 0.21, p = .20, confidence interval [−0.11, 0.46]).
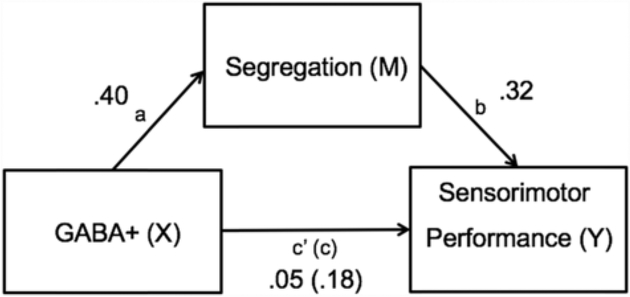
3.7. Mediation analysis
In order to further explore the potential role of network segregation as a mechanistic link between GABA levels and the sensorimotor performance factor, we performed a mediation analysis on the MR and behavioral data (we did not perform this analysis for the grip strength factor because GABA+ was not significantly associated with grip strength). In this analysis, GABA+ levels were the independent variable (X), with sensorimotor network segregation as the mediator (M) and sensorimotor performance as the dependent variable (Y). Mediation was performed using regression with bootstrapping (Hesterberg et al., 2005; Hayes and Scharkow, 2013) to determine whether network segregation accounted for significant variance in the GABA-performance association.
Controlling for age and GM volume differences, we found that network segregation mediates the link between GABA+ and sensorimotor performance, with a percentage mediation (PM) of 70%. This resulted from a significant indirect effect (c) of greater magnitude than the direct effect (c’), which itself was not significant (ab = 0.13, confidence interval [0.0037, 0.41]; See Fig. 7). Performing this analysis within each age group separately, we found no evidence for a mediation effect within either group alone. We also performed another mediation analysis using mean network segregation as the mediator variable, rather than sensorimotor network segregation. Controlling for age and GM volume differences, we found no significant mediation effect (ab = .07, confidence interval [−0.026, 0.29]).
Fig. 7.
Sensorimotor network segregation mediates the link between sensorimotor GABA+ levels and sensorimotor performance across all participants (accounting for age and GM volume differences). The mediator accounted for 70% of the total effect (PM = .70).
4. Discussion
Previous studies have investigated the relationship between network segregation and behavior (Chan et al., 2014; King et al., 2018), GABA+ levels and behavior (Edden et al., 2009; Puts et al., 2011; Stagg et al., 2011), and within-network functional connectivity and GABA+ levels (Stagg et al., 2014; Kapogiannis et al., 2013). In the present study, we investigated whether older and younger adults differ with regard to sensorimotor network segregation, GABA+ levels, and sensorimotor performance, and for the first time we examined the association between all of these variables within the same participants. There were four main findings. First, relative to young adults, old adults exhibited less segregated sensorimotor brain networks and reduced sensorimotor GABA+ levels. Second, less segregated networks were associated with lower GABA+ levels. Third, less segregated networks and lower GABA levels were associated with worse sensorimotor performance. Fourth, network segregation mediated the relationship between GABA+ and performance. These findings suggest that age-related reductions of GABA+ are a neurochemical substrate of age-related dedifferentiation at the level of large-scale brain networks. We now discuss each of these findings in turn.
4.1. Resting state networks are less segregated in older adults
Our results revealed significant age differences in the organization of large-scale resting state brain networks, such that the networks of older adults were less segregated than those of their younger counterparts. This finding was observed both in the sensorimotor network specifically, when segregation was averaged across all ten networks, and in five ot the ten networks individually (uncorrected). Overall, these findings are consistent with several previous studies that also observed less segregated functional networks in older adults (Chan et al., 2014; Geerligs et al., 2015; Damoiseaux, 2017; Song et al., 2014; Cao et al., 2014; Betzel et al., 2014).
The age differences in network segregation observed in this study are consistent with the neural dedifferentiation hypothesis of aging, which posits that aging results in reduced functional specificity of localized brain regions during task performance (Li and Lindenberger, 1999; Park et al, 2004, 2010; Carp et al., 2010, 2011a, 2011b). The present results are consistent with the hypothesis that neural dedifferentiation also occurs on the level of large-scale resting state functional networks. Such age-related dedifferentiation of resting state functional networks could potentially be linked to dedifferentiation of task-based activation patterns; future studies could address this question.
4.2. Sensorimotor GABA levels are reduced in older adults
We also found that GABA+ levels in sensorimotor cortex were reduced in older relative to younger adults. These age differences in GABA+ levels were evident even after accounting for tissue differences in gray matter, white matter, and cerebrospinal fluid, suggesting that the effects were not due simply to cortical atrophy (Hermans et al., 2018; Maes et al). This finding is consistent with previous work that has reported age-related reductions in GABA+ levels in frontal and parietal cortices (Gao et al., 2013; Porges et al., 2017). However, this finding is inconsistent with recent studies that have found no significant age differences in sensorimotor GABA levels (Hermans et al., 2018; Maes et al). For instance, Hermans and colleagues found that M1 GABA levels did not significantly differ between young and older adults. In addition, Maes et al. reported that age-related differences in sensorimotor and occipital GABA levels are driven by bulk tissue changes. However, after correcting for voxel composition, they observed a trend toward an age-related difference in sensorimotor but not occipital GABA levels, suggesting some degree of disproportionality in GABA levels within tissue fractions as a function of age in sensorimotor cortex only (Maes et al). The discrepancy between these findings may be due to differences in the specific cortical region studied, MRS voxel placement, or preprocessing procedures. Future studies should aim to disentangle these inconsistent findings by examining a variety of cortical regions and MRS placement and preprocessing methods in a larger sample size.
The reported decline in GABA levels in older adults may reflect the loss of GABAergic interneurons during normal aging. This is consistent with several animal studies that have shown a reduction in GABAergic neurons with old age (Hua et al., 2008; Stanley et al., 2012). Previous work has also provided evidence for a relationship between GABA levels and genes encoding for glutamic acid decarboxylase (GAD), a transaminase involved in the production of GABA (Marenco et al., 2010). Thus, the current findings may also be indicative of reduced GABA production with aging.
4.3. Network segregation is related to GABA
Our findings revealed a positive relationship between GABA+ levels and sensorimotor network segregation, such that participants with lower GABA+ levels showed less segregated networks. This observation is contrary to some previous reports that have found a negative relationship between GABA+ levels and functional connectivity (Stagg et al., 2014; Kapogiannis et al., 2013; Bachtiar et al., 2015). However, these previous studies only examined within-network functional connectivity in young adult participants. In contrast, our findings demonstrated a positive relationship between GABA+ levels and network segregation that was stronger in the older adult group.
Consistent with our finding, Antonenko et al. found a negative relationship between motor network connectivity and sensorimotor GABA+ levels, but only in young-older adults (i.e., 50–63 years old). In fact, when looking solely within an old-older adult group (i.e., >63 years old), there was a trend toward a positive relationship (Antonenko et al., 2017). Such an age-related dissociation may reflect disrupted neuronal functioning in older adults.
In addition to the relationship between sensorimotor GABA+ and sensorimotor segregation, there was also a significant relationship between sensorimotor GABA+ and mean network segregation (averaged across all 10 networks). It is therefore natural to ask whether the connection between GABA+ and network segregation is regionally specific or not. We believe that the data suggest that it is. For example, sensorimotor segregation was not correlated with GABA+ levels outside of sensorimotor cortex (i.e., VVC and auditory cortex). Furthermore, although sensorimotor segregation significantly mediated the association between sensorimotor GABA and sensorimotor behavior, mean network segregation did not (and sensorimotor GABA+ levels were related to sensorimotor performance whereas GABA+ levels outside of sensorimotor cortex were not (ps > .25)).
But if the GABA-segregation relationship is regionally specific, then why would sensorimotor GABA+ levels be associated with mean network segregation? We believe the answer is simple and relatively uninteresting: It turns out that sensorimotor network segregation is highly correlated with mean network segregation in both age groups (Old: r = .63, p = .002; Young: r = 0.67, p = .001). It is therefore not surprising that a variable like sensorimotor GABA+ that is associated with sensorimotor segregation would also be associated with mean segregation. Nevertheless, future studies should aim to test the regional specificity of the relationship between segregation and GABA in larger sample sizes.
Taken together, our results link, for the first time, network segregation to GABA+ levels, and thereby suggest a neurochemical substrate for age-related dedifferentiation at the level of large-scale resting state brain networks.
4.4. Age differences in segregation & GABA are associated with sensorimotor performance
We also found that less segregated sensorimotor networks were associated with worse sensorimotor performance. This finding is in line with previous investigations that have observed a relationship between segregation and cognitive (Chan et al., 2014) and motor (King et al., 2018) performance. Our study extends these findings by revealing a relationship between sensorimotor performance and segregation within the sensorimotor network. Specifically, sensorimotor network segregation was correlated with a summary measure of somatosensory and motor abilities in addition to grip strength.
Our data also revealed a positive correlation between sensorimotor GABA+ levels and sensorimotor performance. This finding is consistent with previous studies linking local GABA+ levels in motor cortex to motor performance (Boy et al., 2010), GABA+ levels in visual cortex to orientation discrimination performance (Edden et al., 2009), and GABA+ levels in sensorimotor cortex to tactile discrimination thresholds (Puts et al., 2011). Overall, these findings confirm a principal role of sensorimotor GABAergic inhibition in sensorimotor function.
It is also worth noting that nearly all the neural and neural-behavioral associations observed in this study appeared to be stronger within the older compared to the younger adult group, although these effects were not statistically significant. Future research with larger sample sizes will be necessary to determine whether these relationships are indeed driven by the older adult population. Nonetheless, these findings provide preliminary evidence for age-related dissociations in the link between GABA+, network segregation and sensorimotor performance. They also point to the GABA-segregation relationship as a potential factor in explaining why some older adults age gracefully while others do not.
4.5. Segregation mediates the link between GABA and sensorimotor performance
Our findings also demonstrate for the first time that sensorimotor network segregation mediates the relationship between GABA+ levels and sensorimotor performance. One possible explanation for this finding is that age-related reductions in sensorimotor GABA levels lead to less segregated neural networks, which in turn lead to declines in sensorimotor behavior. Such a causal relationship between age declines in GABA and age-related reductions in neural specificity in visual cortex has been reported in previous animal studies (Leventhal et al., 2003). Furthermore, one recent human study reported that GABA+ levels in somatosensory cortex correlate with perceptual acuity, and that this relationship is mediated by the tuning of activity in somatosensory cortex (Kolasinski et al., 2017). Although we cannot make causal inferences from the data in the present study, our findings from the mediation analysis do provide preliminary evidence for directionality in the relationship between GABA, network segregation and behavior.
Given the relationship that we found between GABA+, network segregation and behavior, it is plausible that neuronal inhibition (mediated by GABAergic interneurons) may influence sensorimotor behavior at the network level. For instance, studies have proposed a role for beta and gamma oscillations in facilitating the synchrony of neural firing for somatosensory (Bessaih et al., 2018) and motor (van Wijk et al., 2012) behavior. Moreover, functional connectivity of the motor resting state network is linked to fluctuations in the power of beta oscillations (Brookes et al., 2011), which in turn have been linked to GABA activity (Hall et al., 2011). Thus, GABA may influence sensorimotor performance via modulation in the power of sensorimotor oscillations.
4.6. Limitations
A central challenge in network analyses concerns the different parcellation and/or clustering approaches that are used to obtain different network measures. Although some studies have reported consistent results between parcellation/clustering techniques (Chan et al., 2014; Cao et al., 2014), these comparisons are not comprehensive. Thus, there is currently no consensus regarding what the canonical network organization approach should be. However, using an independent dataset to define ROIs and assign network labels to these ROIs (as performed in the present study) allows an examination of network segregation in a relatively unbiased manner. One problem with this approach is that the Power et al (2011) parcellation scheme used here was based on a sample of young adults, which could potentially influence the results. Chan et al. compared the results across three different parcellation schemes (i.e., using Power et al (2011)-defined, age cohort-defined, and subject-defined communities) and found no significant differences across system label definitions and edge densities (Chan et al., 2014), but this is still an important issue to consider in future studies.
The MRS technique used to measure GABA+ levels also has some limitations. The voxels used for MRS data acquisition, although fairly standard in the MRS literature (Mullins et al., 2014), are quite large (30 × 30 × 30 mm), and were most likely not limited to sensorimotor cortex. In addition, at 3T, the MEGA-PRESS sequence results in significant excitation of coedited macromolecule (MM) signal, which has been shown to contribute ~45% to the edited signal at 3-ppm (Puts et al., 2011; Mullins et al., 2014). Accounting for MM contamination is an area of considerable on-going efforts and therefore will likely be addressed in future work.
Another limitation of the present study is that our findings were derived from cross-sectional comparisons. Therefore, we can only draw inferences about age-related differences, not age-related change (only longitudinal studies can measure change) (Maxwell and Cole, 2007). In addition, cohort and period effects hamper the interpretation of differences in neural and behavioral measures between groups (Damoiseaux, 2017) and the associations between neural and behavioral measures may suffer from the issue of cross-sectional mediation (Shrout, 2011; Cole and Maxwell, 2003; Lindenberger et al., 2011). Longitudinal study designs mitigate these concerns and allow the evaluation of age-related changes in addition to the relationship between neural and behavioral measures, independent of age. Importantly, longitudinal studies also make it possible to identify the order of certain age-related changes, which may help to illuminate causal relationships. Determining directionality is critical for the early detection of age-related neural and behavioral changes and the design of potential targeted interventions.
Finally, we used behavioral factors derived from the entire sample, because the sample size in the two groups was relatively small. Nevertheless, it is worth noting that the covariance matrices were somewhat different in the two groups (See Tables S3 and S4), so it is possible that the factors derived from the whole group were biased toward variance in one group more than the other.
5. Conclusions
Our findings provide evidence that 1) resting state networks are less segregated and sensorimotor GABA+ levels are reduced in older relative to younger adults; 2) less segregated sensorimotor networks are linked to lower GABA+ levels, 3) lower GABA+ levels and less segregated networks are associated with worse sensorimotor performance, and 4) the GABA-performance relationship is mediated by network segregation. Although these relationships were observed across all ages (controlling for age and GM volume difference), these associations appeared to be stronger in the older adult group specifically. These results suggest that resting state network segregation may be an important factor in distinguishing older adults who maintain their sensorimotor abilities from those who do not, and that age-related reductions in GABA may play a critical role.
Supplementary Material
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://doi.org/10.1016/j.neuroimage.2018.11.008.
References
- Antonenko D, et al. , 2017. Tdcs-induced modulation of GABA levels and restlng-state functional connectivity In older adults. J. Neuroscl. 37, 4065–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtiar V, Near J, Johansen-Berg H, Stagg CJ, 2015. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. eLife 4, e08789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Llau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessaih T, Higley MJ, Contreras D, 2018. Millisecond precision temporal encoding of stimulus features during cortically generated gamma oscillations In the rat somatosensory cortex. J. Physiol. 596, 515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, et al. , 2014. Changes In structural and functional connectivity among restlng-state networks across the human lifespan. Neuroimage 102, 345–357. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS, 1995. Functional connectivity In the motor cortex of resting human brain using echo-planar mrl. Magn. Reson. Med. 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Boy F, et al. , 2010. Individual differences In subconscious motor control predicted by GABA concentration In SMA. Curr. Biol. 20, 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenkel M, Shulman K, Hazan E, Herrmann N, Owen AM, 2017. Assessing capacity In the elderly: comparing the MoCA with a novel computerized battery of executive function. Dement. Geriatr. Cogn. Disord. Extra 7, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, et al. , 2011. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc. Natl. Acad. Sci. U. S. A 108, 16783–16788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT, 2013. Opportunities and limitations of Intrinsic functional connectivity MRI Nat. Neurosci. 16, nn.3423. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Spoms O, 2012. The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349. [DOI] [PubMed] [Google Scholar]
- Cao M, et al. , 2014. Topological organization of the human brain functional connectome across the lifespan. Dev. Cogn. Neurosci. 7, 76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Gmeindl L, Reuter-Lorenz PA, 2010. Age differences in the neural representation of working memory revealed by multi-voxel pattern analysis. Front. Hum. Neurosci. 4, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Park J, Polk TA, Park DC, 2011. Age differences in neural distinctiveness revealed by multi-voxel pattern analysis. Neuroimage 56, 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Park J, Hebrank A, Park DC, Polk TA, 2011. Age-related neural dedifferentiation in the motor system. PloS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS, 2014. Decreased segregation of brain systems across the healthy adult lifespan. Proc. Natl. Acad. Sci. U. S. A 111, E4997–E5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Alhazmi FH, Park DC, Savalia NK, Wig GS, 2017. Resting-state network topology differentiates task signals across the adult life span. J. Neurosci. 37, 2734–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DA, Maxwell SE, 2003. Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J. Abnorm. Psychol. 112, 558–577. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, 2017. Effects of aging on functional and structural brain connectivity. Neuroimage https://doi.Org/10.1016/j.neuroimage.2017.01.077. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, et al. , 2008. Reduced resting-state brain activity in the ‘default network’ in normal aging. Cerebr. Cortex 18, 1856–1864. [DOI] [PubMed] [Google Scholar]
- Edden RAE, Barker PB, 2007. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn. Reson. Med. 58, 1276–1282. [DOI] [PubMed] [Google Scholar]
- Edden RAE, Muthukumaraswamy SD, Freeman TCA, Singh KD, 2009. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci. 29, 15721–15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ Gannet, 2014. A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imag 40, 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF, 2013. Resting-state functional connectivity in normal brain aging. Neurosci. Biobehav. Rev. 37, 384–400. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM, 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. Unit. States Am 97, 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, et al. , 2004. Automatically parcellating the human cerebral cortex. Cerebr. Cortex 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Galganski ME, Fuglevand AJ, Enoka RM, 1993. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J. Neurophysiol. 69, 2108–2115. [DOI] [PubMed] [Google Scholar]
- Gao F, et al. , 2013. Magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM, 2015. A brain-wide study of age-related changes in functional connectivity. Cerebr. Cortex 25, 1987–1999. [DOI] [PubMed] [Google Scholar]
- Goh JOS, 2011. Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis. 2, 30–48. [PMC free article] [PubMed] [Google Scholar]
- Hall SD, et al. , 2011. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage 56, 1506–1510. [DOI] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, Barker PB, Edden RAE, 2015. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn. Reson. Med. 74, 1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Scharkow M, 2013. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter?. Psychol. Sci. 24, 1918–1927. [DOI] [PubMed] [Google Scholar]
- Hermans L, et al. , 2018. GABA levels and measures of intracortical and interhemispheric excitability in healthy young and older adults: an MRS-TMS study. Neurobiol. Aging 65, 168–177. [DOI] [PubMed] [Google Scholar]
- Hesterberg T, et al. , 2005. Bootstrap Methods and Permutation Tests, vol. 14. [Google Scholar]
- Hua T, Kao C, Sun Q, Li X, Zhou Y, 2008. Decreased proportion of GABA neurons accompanies age-related degradation of neuronal function in cat striate cortex. Brain Res. Bull. 75, 119–125. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Reiter DA, Willette AA, Mattson MP, 2013. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage 64, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR et al. Age-related declines in motor performance are associated with decreased segregation of large-scale resting state brain networks. Cerebral Cortex, Volume 28, Issue 12, 1 December 2018, Pages 4390–4402, 10.1093/cercor/bhx297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinski J, et al. , 2017. A mechanistic link from GABA to cortical architecture and perception. Curr. Biol 27, 1685–1691.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan J, et al. , 2010. Functional implications of age differences in motor system connectivity. Front. Syst. Neurosci. 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AG, Wang YC, Pu ML, Zhou YF, Ma YY, 2003. GABA and its agonists improved visual cortical function in senescent monkeys. Science 300, 812–815. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, 1999. Cross-level unification: a computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. Cogn. Neurosci. Mem. 103–146. [Google Scholar]
- Lindenberger U, von Oertzen T, Ghisletta P, Hertzog C, 2011. Cross-sectional age variance extraction: what's change got to do with it?. Psychol. Aging 26, 34–47. [DOI] [PubMed] [Google Scholar]
- Maes C et al. Age-related differences in GABA levels are driven by bulk tissue changes. Hum. Brain Mapp. 0,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, et al. , 2010. Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharma col. 35, 1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA, 2007. Bias in cross-sectional analyses of longitudinal mediation. Psychol. Methods 12, 23–44. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R, 1998. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11, 266–272. [DOI] [PubMed] [Google Scholar]
- Mullins PG, et al. , 2014. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 86, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near J, et al. , 2015. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn. Reson. Med. 73, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, et al. , 2004. Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci. U. S. A 101, 13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Hebrank A, Park DC, Polk TA, 2010. Neural specificity predicts fluid processing ability in older adults. J. Neurosci. 30, 9253–9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges EC, et al. , 2017. Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol. Psychiatry Cogn. Neurosci. Neu- roimag. 2, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, et al. , 2011. Functional network organization of the human brain. Neuron 72, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE, Evans CJ, McGlone F, McGonigle DJ, 2011. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J. Neurosci. 31, 16556–16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, 2006. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci. Biobehav. Rev 30, 730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, et al. , 2010. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev 34, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R, et al. , 2015. Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. Neuroimage 108, 47–59. [DOI] [PubMed] [Google Scholar]
- Shrout PE, 2011. Commentary: mediation analysis, causal process, and cross-sectional data. Multivariate Behav. Res. 46, 852–860. [DOI] [PubMed] [Google Scholar]
- Song J, et al. , 2014. Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect. 4, 662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H, 2011. The role of GABA in human motor learning. Curr. Biol. 21, 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, et al. , 2014. Local GABA concentration is related to network-level resting funtional connectivity, elife 3, e01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EM, Fadel JR, Mott DD, 2012. Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol. Aging 33, 431.el–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL, 2012. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BCM, Beek PJ, Daffertshofer A, 2012. Neural synchrony within the motor system: what have we learned so far?. Front. Hum. Neurosci 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. , 2010. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage 51, 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon, Conn A, 2012. A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Zar JH, 1996. Biostatistical Analysis. Prentice Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.