Abstract
Transporter-mediated uptake determines the peak concentration, duration, and physical spread of released monoamines. Most studies of monoamine clearance focus on the presynaptic uptake1 transporters SERT, NET and DAT. However, recent studies have demonstrated the expression of the uptake2 transporter OCT3 (organic cation transporter 3), throughout the rodent brain. In contrast to NET, DAT and SERT, OCT3 has higher capacity and lower affinity for substrates, is sodium-independent, and is multi-specific, with the capacity to transport norepinephrine, dopamine, serotonin and histamine. OCT3 is insensitive to inhibition by cocaine and antidepressant drugs but is inhibited directly by the glucocorticoid hormone corticosterone. Thus, OCT3 represents a novel, stress hormone-sensitive, monoamine transport mechanism. Incorporating this transporter into current models of monoaminergic neurotransmission requires information on: A) the cellular and subcellular localization of the transporter; B) the effects of OCT3 inhibitors on monoamine clearance; and C) the consequences of decreased OCT3-mediated transport on physiology and/or behavior. This review summarizes studies describing the anatomical distribution of OCT3, its cellular and subcellular localization, its contribution to the regulation of dopaminergic signaling, and its roles in the regulation of behavior. Together, these and other studies suggest that both Uptake1 and Uptake2 transporters play key roles in regulating monoaminergic neurotransmission and the effects of monoamines on behavior.
Monoamines, including dopamine, serotonin, epinephrine and norepinephrine, exert powerful modulatory influences on brain function and behavior via actions mediated by receptors on neuronal and glial cells. The magnitude of released monoamine signals is determined not only by diffusion, but also by transporter-mediated clearance. Current models of dopamine signaling have suggested that dopamine concentration is primarily regulated by diffusion immediately surrounding release sites, but that transporter-mediated uptake determines the duration and physical spread of released monoamines (Rice and Cragg, 2008). Thus, uptake processes dictate the extent to which high- and low-affinity monoamine receptors are activated and, thus, the extent to which thousands of surrounding synapses are influenced. For these reasons, understanding the diversity and complexity of uptake processes is critical for a complete understanding of monoaminergic neurotransmission.
Most studies of monoamine uptake have focused on the high-affinity sodium-dependent transporters which constitute the “Uptake1” family of monoamine transporters (DAT – dopamine transporter; NET – norepinephrine transporter; and SERT – serotonin reuptake transporter) and are expressed almost exclusively on the axon terminals of the monoamine releasing neurons. However, it has long been known that an “Uptake2” family of low-affinity, high-capacity transporters also contributes to monoamine clearance (Iversen, 1965). This review focuses on studies examining the localization of one uptake2 transporter, organic cation transporter 3 (OCT3), and its roles in the regulation of dopaminergic neurotransmission and behavior.
Catecholamine Uptake1 and Uptake2
Early studies of catecholamine transport in heart tissue revealed the presence of two distinct uptake processes, termed uptake1 and uptake2, which differed in kinetic properties and sensitivity to inhibitors. Uptake1, a high-affinity (Kd = 0.27 μM), low-capacity (Vmax = 1.22 nmol/min/g tissue) transport process, was inhibited by cocaine and desipramine. Uptake2, a low-affinity (Kd =252 μM ), high-capacity (Vmax = 100 nmol/min/g) process, while insensitive to cocaine and desipramine, was inhibited by normetanephrine and corticosterone (Iversen, 1965) (Iversen and Salt, 1970). Based on its low affinity, uptake2 was originally believed to contribute to catecholamine clearance only when substrates were present at very high concentrations. However, subsequent studies demonstrated that uptake2 contributed to catecholamine clearance at all substrate concentrations (Lightman and Iversen, 1969).
Uptake2-like activity has been attributed to at least four distinct transporters. These transporters, including the organic cation transporters (OCT1, OCT2, and OCT3) and the plasma membrane monoamine transporter (PMAT), all display high-capacity, bidirectional, sodium-independent transport of monoamines, with each transporter displaying a distinct substrate specificity profile for monoamines (Duan and Wang, 2010;Schomig et al., 2006). Although they were all (except for PMAT) originally described in peripheral tissues, all uptake2 transporters have been detected in brain tissue (Amphoux et al., 2006;Engel et al., 2004). All are inhibited by corticosterone, although the sensitivity to corticosterone varies depending on the species examined and the tissue preparation used (reviewed in (Koepsell et al., 2007)). Importantly, corticosterone-induced inhibition of OCT-mediated transport is rapid and involves direct interaction of the steroid with the transporter at specific sites (Volk et al., 2009). OCT3 is the most corticosteroid-sensitive, with IC50s in the physiological range for corticosterone (Gasser et al., 2006;Grundemann et al., 1998;Hill et al., 2011). This property positions OCT3 as a critical mediator of stress and corticosteroid effects on neuronal and glial physiology and behavior independent of the glucocorticoid receptor (see (Gasser and Lowry, 2018), for a review of OCT3 as a mediator of nongenomic corticosteroid actions).
Cellular and subcellular localization of OCT3
Understanding the roles of OCT3 in regulating monoaminergic neurotransmission and in mediating the effects of corticosterone on monoamine signaling and behavior requires knowledge of the regional, cellular and subcellular distribution of the transporter. Immunohistochemical studies with an OCT3-specific antibody have revealed that the transporter is expressed throughout the rat brain, with at least low levels of expression detected in nearly all brain regions (Gasser et al., 2009). The transporter is expressed in neurons (Cui et al., 2009;Graf et al., 2013;Hill and Gasser, 2013;Shang et al., 2003;Vialou et al., 2004), including dopamine neurons (Mayer et al., 2018), and glial cells, including astrocytes (Cui et al., 2009;Gasser et al., 2017;Takeda et al., 2002) and microglia (He et al., 2017). It is also expressed in oligodendrocytes (Gasser et al., 2009), ependymal cells (Gasser et al., 2006;Gasser et al., 2009), and vascular endothelial cells in the brain(Li et al., 2013). The broad expression of this high-capacity monoamine transporter suggests that it plays an essential role in the regulation of extracellular monoamine concentrations in a variety of microenvironments.
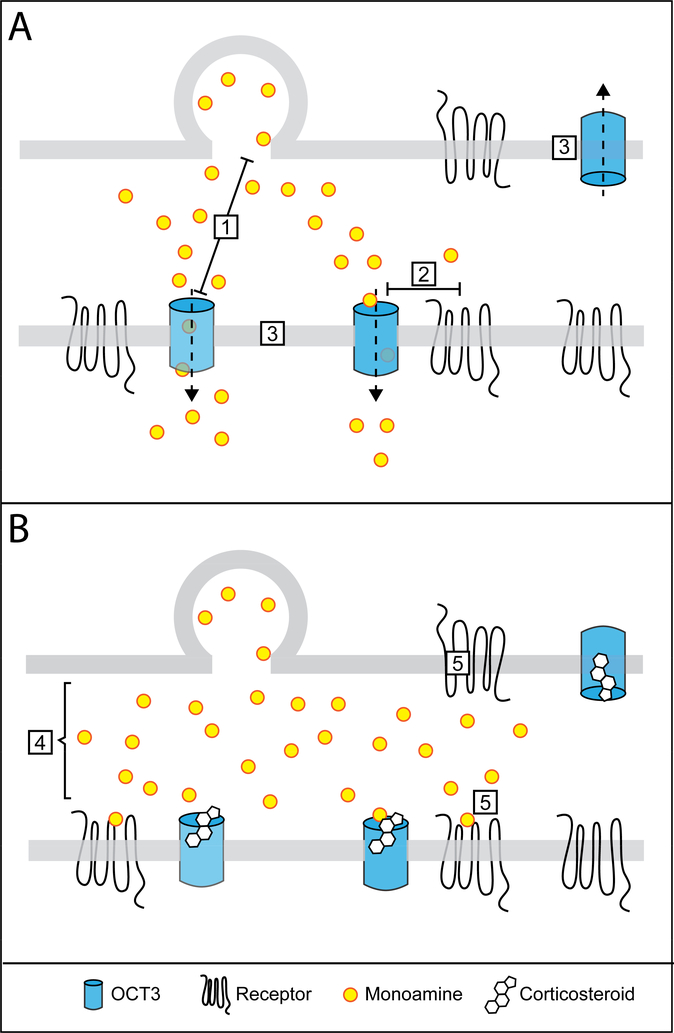
The degree to which OCT3-mediated transport activity determines the activation of monoamine receptors critically depends on the subcellular localization of the transporter, especially its proximity to monoamine release sites and receptors (Figure 1). While there have been no studies directly examining the co-localization of OCT3 and monoamine release sites or receptors, a recent report described the subcellular localization of the transporter in rat brain using immuno-electron microscopy (Gasser et al., 2017). This study demonstrated OCT3 localization to plasma membranes of neuronal somata, as well as to dendritic and axonal profiles. OCT3 was also densely expressed in plasma membranes of astrocyte processes surrounding axodendritic and axospinous profiles. Positioned at these sites, OCT3-mediated transport may exert considerable influence over the duration and spread of released monoamines, and thus over the activation of monoamine receptors on both pre- and post-synaptic cells (Figure 1).
Figure 1.
Schematic model of the potential roles of OCT3-mediated transport, and its inhibition by corticosterone, in regulating monoamine signaling, and highlighting key outstanding questions regarding the localization and actions of OCT3. Monoaminergic terminals are depicted adjacent to a cell expressing plasma membrane receptors for monoamines under conditions of low (A) and high (B) concentrations of corticosterone. OCT3-mediated, high capacity monoamine transport is hypothesized to play important roles in regulating the duration and physical spread of released monoamines. A) The specific roles played by OCT3 in regulating monoaminergic transmission depend on the answers to key questions regarding 1) the localization of OCT3 with respect to monoamine release sites; 2) the proximity of OCT3 to monoamine receptors; and 3) the identity of the OCT3-expressing cells. B) While several studies have demonstrated OCT3-mediated, corticosterone-sensitive monoamine clearance, key unanswered questions remain, including 4) the effects of corticosterone-induced inhibition on the physical spread and duration of released monoamines; and 5) the activation of low- and high-affinity monoamine receptors in target brain regions.
Effects of Corticosterone on dopamine clearance: in vivo studies
The anatomical studies indicating that OCT3 is positioned to make important contributions to the clearance of released monoamines are consistent with ex vivo studies which have demonstrated OCT3-mediated, corticosterone-sensitive uptake of monoamines (Amphoux et al., 2006;Duan and Wang, 2010). This section summarizes studies in which we examined the effects of DAT inhibition (by cocaine or GRB12909) and OCT3 inhibition (by corticosterone) on extracellular dopamine concentrations (by microdialysis) and clearance (using fast-scan cyclic voltammetry). These studies were focused on understanding the mechanisms by which stress, through elevation of glucocorticoid hormones, increases the reinstatement of cocaine seeking in rats with a history of cocaine self-administration. All of these studies examined the regulation of dopamine signaling in the nucleus accumbens, a region in which both DAT and OCT3 are expressed (Gasser et al., 2009), and an important site of dopamine action in the regulation of motivated behavior. Previous studies by Cui and colleagues provided convincing evidence that OCT3 plays a role in striatal dopamine clearance. These studies examined the effects of methamphetamine and the neurotoxin 1-methyl-4-phenylpyridinium (MPP+), two dopamine-releasing agents, on extracellular dopamine levels in the dorsal striatum of wild-type and OCT3-deficient mice. Increases in striatal extracellular dopamine induced by both methamphetamine and MPP+ were significantly larger, and lasted significantly longer, in OCT3-deficient mice than in wild-type mice (Cui et al., 2009).
Studies aimed at examining potential joint roles of OCT3 and DAT in regulating dopamine signaling tested the effects corticosterone and cocaine, alone and in combination, on extracellular dopamine concentrations in the rat nucleus accumbens (Graf et al., 2013). Rats were injected with vehicle or corticosterone (2 mg/kg, ip, a dose that approximates plasma concentrations reached in response to stress), followed by a low dose of cocaine (2.5 mg/kg), during the collection of dialysate samples from the nucleus accumbens. Corticosterone alone had no effect on basal extracellular dopamine concentrations. Surprisingly, low-dose cocaine alone had no effect on extracellular dopamine concentrations when preceded by vehicle injection. However, that same dose of cocaine caused significant increases in extracellular dopamine when it was preceded by an injection of corticosterone (Graf et al., 2013). These results are consistent with the hypothesis that corticosterone-induced potentiation of low-dose cocaine effects are due to inhibition of OCT3-mediated DA clearance, but because microdialysis does not allow direct quantification of dopamine clearance, the results may also be due to corticosterone-induced changes in the release or metabolism of dopamine.
Direct assessment of the effects of corticosterone on DA clearance, required the use of in vivo fast-scan cyclic voltammetry (FSCV), an electrochemical technique that measures dopamine concentrations on a sub-second time-scale, allowing detection of changes in dopamine release and clearance. In studies on anesthetized rats, we examined the effects of corticosterone, in the presence or absence of the DAT inhibitor GBR12909, on the clearance of dopamine released in the nucleus accumbens in response to electrical stimulation of the ventral tegmental area projection (Graf et al, 2013). After basal release and uptake parameters were collected, animals received an injection of the DAT inhibitor GBR12909, which significantly decreased the clearance (increased full width at half height (FWHH), apparent Km, and tau). After the effects of DAT blockade had stabilized, animals received an injection of vehicle or corticosterone (2 mg/kg, ip), and release and clearance parameters were again measured. Injection of corticosterone, but not vehicle, resulted in further decreases in dopamine clearance (further increases in FWHH, apparent Km, and tau), revealing for the first time in an in vivo study, the presence of corticosterone-sensitive, DAT-independent clearance of dopamine in vivo (Graf et al., 2013).
Subsequent studies used FSCV to examine the effects of corticosterone on dopamine clearance in awake and behaving animals. In these studies, animals were treated with corticosterone (2 mg/kg, ip) or low-dose cocaine (2.5 mg/kg, ip), alone or in combination, and the amplitude and duration of naturally-occurring dopamine transients in the nucleus accumbens were measured. Surprisingly, administration of corticosterone alone, but not low-dose cocaine alone, significantly increased the duration and magnitude of spontaneous dopamine transients. Low-dose cocaine, while it had no effect on transient amplitude or duration alone, did induce robust increases in these measures when it was administered after corticosterone (Wheeler et al., 2017). These results indicate that, even in a DAT-rich region like the nucleus accumbens, corticosterone-induced decreases in DA clearance can be observed in the absence of DAT blockade, and that uptake1 (DAT-mediated) and uptake2 (likely OCT3-mediated) transport work in concert to shape dopamine signals in vivo. The relative contributions of uptake1 and uptake2 processes to regulating specific monoamine signaling will likely vary regionally, depending on both the distributions of the transporters and the patterns of monoamine release.
Corticosterone and the regulation of cocaine-seeking behavior: a role for OCT3
The anatomical and neurochemical studies described above suggest that OCT3-mediated clearance may contribute significantly to the regulation of dopamine-dependent physiological processes, such as spike timing-dependent plasticity (in cortical regions) and dopamine-dependent behavioral processes, including reward- and motivation-related behaviors. As it is inhibited by physiological concentrations of corticosterone, it represents a mechanism by which stress, via elevating glucocorticoids, may regulate brain function and behavior. Much work remains to clarify the contribution of OCT3 and other uptake2 transporters in physiological and behavioral processes. The remainder of this review focuses on work we have done to examine a potential role of OCT3 in mediating glucocorticoid-induced alterations in cocaine-seeking behavior in rats.
A series of behavioral studies examined the interaction of stress-levels of corticosterone with subthreshold doses of cocaine in rodent models of addiction (Graf et al., 2013;McReynolds et al., 2017). All studies involved training of rats or mice to associate lever pressing (rat self-administration) or a specific chamber (mouse conditioned place-preference (CPP)) with the administration of cocaine. Acquisition of the task is followed by a maintenance phase, in which drugself-administration is stably expressed, and then by extinction training, in which lever pressing or entry into the cocaine-paired chamber results in the administration of saline instead of cocaine. This leads to the extinction of the cocaine-conditioned behavior. Reinstatement of cocaine-seeking behavior (pressing the lever in the absence of a cocaine reward (rat self-administration studies), or entry into the previously cocaine-paired chamber in the absence of drug reinforcement (mouse CPP studies)) in response to stress, low-dose cocaine, or corticosterone treatment is interpreted as drug-seeking behavior.
The ability of corticosterone to influence reinstatement of drug-seeking behavior was tested in rats which had been trained to self-administer cocaine under short-access conditions (2 hours daily access to the drug)(Graf et al., 2013;McReynolds et al., 2017). Rats trained under short-access conditions, compared to those trained under long-access conditions, are resistant to stress- or cocaineinduced reinstatement of drug seeking behavior. Specifically, these animals do not resume lever pressing during reinstatement tests after either an injection of a low dose of cocaine (2.5 mg/kg), or exposure to 15 minutes of intermittent electric footshock stress. While neither of these two stimuli alone induces significant reinstatement of drug-seeking behavior, combination of the two (low dose cocaine preceded, 40 minutes earlier, by electric footshock) does (Graf et al., 2013;McReynolds et al., 2017). The effects of stress could be mimicked either by intraperitoneal injection of corticosterone at a dose that reproduces stress levels of the hormone (a treatment that did not by itself lead to reinstatement), or by bilateral injection of either corticosterone or the OCT3 inhibitor normetanephrine into the nucleus accumbens. The ability of corticosterone to potentiate low-dose cocaine-induced reinstatement was unaffected by pretreatment with RU-38486, an inhibitor of the glucocorticoid receptor, indicating that the corticosteroid effect was not mediated by the intracellular glucocorticoid receptor. The potentiating effect was blocked by intra-nucleus accumbens injection of fluphenazine, a DA receptor antagonist (Graf et al., 2013;McReynolds et al., 2017). Together, these behavioral studies indicate that corticosterone-induced potentiation of drug seeking is mediated by a GR-independent mechanism involving local striatal dopamine signaling. In combination with the anatomical and neurochemical studies described above, these data are consistent with the hypothesis that corticosterone-induced potentiation of reinstatement involves inhibition of OCT3-mediated clearance. However, due to the lack of mono-specific inhibitors of OCT3, these findings did not rule out the involvement of other cellular mechanisms.
To more definitively demonstrate a role for OCT3 inhibition in corticosterone-induced potentiation of cocaine-primed reinstatement, we examined the reinstatement of cocaine conditioned place preference in wild type mice, and in transgenic OCT3-deficient mice that express a truncated form of OCT3, and lack OCT3-mediated transport activity (Zwart et al., 2001). Both wild-type and OCT3 deficient mice acquired CPP to a high dose of cocaine (15 mg/kg) and extinguished drug-seeking behavior in a similar time course. Reinstatement of CPP was measured in response to a subthreshold dose of cocaine (0.93 mg/kg), which did not lead to significant reinstatement in wild-type mice. In wildtype mice, injection of either corticosterone or normetanephrine potentiated low-dose cocaine-induced reinstatement of CPP, but neither OCT3 inhibitor potentiated reinstatement in OCT3-deficient mice (McReynolds et al., 2017). Together, the rodent reinstatement studies coupled with the neurochemical measures indicate that the ability of stress and corticosterone to influence cocaine-seeking behavior is mediated, at least in part, by corticosterone-induced inhibition of dopamine clearance in the nucleus accumbens.
The anatomical, neurochemical and behavioral studies described here suggest that uptake1 and uptake2 processes work together in a region- and context-specific manner to regulate the amplitude, duration and physical spread of dopamine signals and, thus, to determine the neuromodulatory influence of released dopamine. Although the studies described in this review focused on the role of OCT3 in regulating dopaminergic signaling, similar roles for OCT3 (and other uptake2 transporters) in the regulation of noradrenergic, serotonergic and histaminergic neurotransmission likely exist. Indeed, a significant body of research has demonstrated roles for OCT3 and PMAT in serotonergic transmission (Baganz et al., 2010;Baganz et al., 2008;Dahlin et al., 2007). Similarly, this review focused on our studies examining OCT3-mediated dopamine transport as it relates to the neurochemical and behavioral effects of cocaine. There is a growing body of work examining roles for OCT3-mediated dopamine transport in the actions of other psychostimulant drugs, including amphetamine (Mayer et al., 2018;Vialou et al., 2008) and methamphetamine (Cui et al., 2009;Kitaichi et al., 2003;Nakayama et al., 2007). Clearly, many questions remain to be answered about the roles played by OCT3 and the other uptake2 transporters in regulating monoamine signaling throughout the brain (Gasser and Daws, 2017).
Acknowledgements:
Supported by grant National Institute on Drug Abuse (DA032895) to PJG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Amphoux A, Vialou V, Drescher E, Bruss M, La Cour CM, Rochat C, Millan MJ, Giros B, Bonisch H, Gautron S (2006) Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology 50:941–952. [DOI] [PubMed] [Google Scholar]
- Baganz N, Horton R, Martin K, Holmes A, Daws LC (2010) Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci 30:15185–15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC (2008) Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A 105:18976–18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV, Rappold PM, Hatwar M, Panza J, Jackson-Lewis V, Javitch JA, Ballatori N, Przedborski S, Tieu K (2009) The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc Natl Acad Sci U S A 106:8043–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, Wang J (2007) Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience 146:1193–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J (2010) Selective Transport of Monoamine Neurotransmitters by Human Plasma Membrane Monoamine Transporter and Organic Cation Transporter 3. J Pharmacol Exp Ther 335:743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J (2004) Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem 279:50042–50049. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Daws LC (2017) Extending the family: Roles for uptake2 transporters in regulation of monoaminergic signaling. J Chem Neuroanat 83-84:107–108. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Hurley MM, Chan J, Pickel VM (2017) Organic cation transporter 3 (OCT3) is localized to intracellular and surface membranes in select glial and neuronal cells within the basolateral amygdaloid complex of both rats and mice. Brain Struct Funct 222:1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA (2018) Organic cation transporter 3: A cellular mechanism underlying rapid, nongenomic glucocorticoid regulation of monoaminergic neurotransmission, physiology, and behavior. Horm Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M (2006) Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci 26:8758–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA (2009) Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol 512:529–555. [DOI] [PubMed] [Google Scholar]
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Robble MA, Vranjkovic O, Wheeler DS, Mantsch JR, Gasser PJ (2013) Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci 33:11800–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundemann D, Schechinger B, Rappold GA, Schomig E (1998) Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci 1:349–351. [DOI] [PubMed] [Google Scholar]
- He Q, Wang Q, Yuan C, Wang Y (2017) Downregulation of miR-7116–5p in microglia by MPP+ sensitizes TNF-alpha production to induce dopaminergic neuron damage. Glia 65:1251–1263. [DOI] [PubMed] [Google Scholar]
- Hill JE, Gasser PJ (2013) Organic cation transporter 3 is densely expressed in the intercalated cell groups of the amygdala: anatomical evidence for a stress hormone-sensitive dopamine clearance system. J Chem Neuroanat 52:36–43. [DOI] [PubMed] [Google Scholar]
- Hill JE, Makky K, Shrestha L, Hillard CJ, Gasser PJ (2011) Natural and synthetic corticosteroids inhibit uptake 2-mediated transport in CNS neurons. Physiol Behav 104:306–311. [DOI] [PubMed] [Google Scholar]
- Iversen LL (1965) The uptake of adrenaline by the rat isolated heart. British Journal of Pharmacology 24:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen LL, Salt PJ (1970) Inhibition of catecholamine Uptake-2 by steroids in the isolated rat heart. Br J Pharmacol 40:528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaichi K, Morishita Y, Doi Y, Ueyama J, Matsushima M, Zhao YL, Takagi K, Hasegawa T (2003) Increased plasma concentration and brain penetration of methamphetamine in behaviorally sensitized rats. Eur J Pharmacol 464:39–48. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C (2007) Polyspecific Organic Cation Transporters: Structure, Function, Physiological Roles, and Biopharmaceutical Implications. Pharm Res. [DOI] [PubMed] [Google Scholar]
- Li RW, Yang C, Kwan YW, Chan SW, Lee SM, Leung GP (2013) Involvement of organic cation transporter-3 and plasma membrane monoamine transporter in serotonin uptake in human brain vascular smooth muscle cells. Front Pharmacol 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL, Iversen LL (1969) The role of uptake2 in the extraneuronal metabolism of catecholamines in the isolated rat heart. British Journal of Pharmacology 37:638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer FP, Schmid D, Owens WA, Gould GG, Apuschkin M, Kudlacek O, Salzer I, Boehm S, Chiba P, Williams PH, Wu HH, Gether U, Koek W, Daws LC, Sitte HH (2018) An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Taylor A, Vranjkovic O, Ambrosius T, Derricks O, Nino B, Kurtoglu B, Wheeler RA, Baker DA, Gasser PJ, Mantsch JR (2017) Corticosterone Potentiation of Cocaine-Induced Reinstatement of Conditioned Place Preference in Mice is Mediated by Blockade of the Organic Cation Transporter 3. Neuropsychopharmacology 42:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Kitaichi K, Ito Y, Hashimoto K, Takagi K, Yokoi T, Takagi K, Ozaki N, Yamamoto T, Hasegawa T (2007) The role of organic cation transporter-3 in methamphetamine disposition and its behavioral response in rats. Brain Res. [DOI] [PubMed] [Google Scholar]
- Schomig E, Lazar A, Grundemann D (2006) Extraneuronal monoamine transporter and organic cation transporters 1 and 2: A review of transport efficiency. Handb Exp Pharmacol 175:151–180. [DOI] [PubMed] [Google Scholar]
- Takeda H, Inazu M, Matsumiya T (2002) Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn Schmiedebergs Arch Pharmacol 366:620–623. [DOI] [PubMed] [Google Scholar]
- Vialou V, Amphoux A, Zwart R, Giros B, Gautron S (2004) Organic cation transporter 3 (Slc22a3) is implicated in salt-intake regulation. J Neurosci 24:2846–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Balasse L, Callebert J, Launay JM, Giros B, Gautron S (2008) Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J Neurochem 106:1471–1482. [DOI] [PubMed] [Google Scholar]
- Volk C, Gorboulev V, Kotzsch A, Mueller TD, Koepsell H (2009) Five Amino Acids in the Innermost Cavity of the Substrate Binding Cleft of Organic Cation Transporter 1 Interact with Extracellular and Intracellular Corticosterone. Mol Pharmacol. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Ebben AL, Kurtoglu B, Lovell ME, Bohn AT, Jasek IA, Baker DA, Mantsch JR, Gasser PJ, Wheeler RA (2017) Corticosterone regulates both naturally occurring and cocaine-induced dopamine signaling by selectively decreasing dopamine uptake. Eur J Neurosci 46:2638–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP (2001) Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol Cell Biol 21:4188–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]