INTRODUCTION
In 2017, there is estimated to be almost 13,000 cases of cervical cancer diagnosed, making it the third most common gynecologic malignancy1. Though cervical cancer screening has helped dramatically in identifying disease at an earlier stage and reducing overall mortality, there is still estimated to be over 4,000 women who will die from this disease in the United States this year 1; 2. As a result, it is imperative that we continue to advance our understanding of both the disease process and of the most effective therapeutic practices.
There are a number of risk factors known to be associated with cervical cancer prognosis,including stage at diagnosis, histology, smoking and race1; 3; 4; 5. Age as an independent prognostic factor for cervical cancer is a question that has been debated and evaluated in the literature without a clear, definitive answer. There is some evidence arguing that older age may serve as an independent factor for poor prognosis6; 7; 8, while other studies have failed to find this association9; 10. We aim to evaluate this question using the Surveillance, Epidemiology, and End Results Program (SEER) database and determine the prognostic implication of age at diagnosis in women with cervical cancer. Additionally, we aim to determine the impact of age on treatments received, clinical outcomes and to determine implications for future treatment paradigms.
METHODS AND MATERIALS
Patient population
SEER is an online database11; 12 that aggregates cancer statistic data in the United States based on information from regional cancer registries. Using this resource, we obtained information on the diagnosis, treatment, and outcomes of women diagnosed with cervical cancer between the years of 1973 to 2015 in the United States. This study qualified for institutional review board approval exemption as the SEER data are already de-identified. A SEER program Data-Use agreement was submitted prior to the acquisition of the data set.
Specific data collected included demographics, tumor histology and stage, treatment details and survival outcomes. Age was grouped into 20–49, 50–69, ≥70 years. Stage was classified as localized (FIGO IAIB1), regional (IB2-IVA) and distant (IVB). Histology was grouped as squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma or other (clear cell, small cell). Race was categorized as White, Black, or other. Treatments were classified as “aggressive” (surgery, external beam (X) RT + brachytherapy (BT), surgery + BT, surgery + XRT, or surgery + XRT + BT), “less aggressive” (XRT alone, BT alone) or no treatment.
Exclusion criteria included women with in situ disease, unknown stage, age less than 20 years old, unknown race, and women with a sarcoma or lymphoma-derived tumor histology. After exclusion, a total of 46,350 patients remained.
Statistical Analysis
The Log-Rank test was used to determine whether the distributions of the survival time (months) differ between age groups, stages of disease, histology groups, treatment types and treatment categories. The analyses were performed using the LEFETEST procedure in the SAS (Statistical Analysis System) software (v.9.3).
The Cox Proportional Hazards model was used to test the effect of a set of covariates (age group, race, stage, histology, treatment) on survival time. Since these covariates are all categorical variables, a specific value was chosen as the reference in each variable (race: white; stage: localized; histology: squamous cell carcinoma; treatments: no treatment). It was performed using the PHREG procedure in the SAS (Statistical Analysis System) software (v.9.3). The Chi-square test was used to determine associations between different variables (age, stage, etc). The significance level was set to 5% for all comparisons.
RESULTS
Demographics
46,350 women with cervical cancer were identified. Of these, 54% were ages <50 yrs, 33% 5069, and 13% ≥70 yrs. The majority of patients were white (76%), had a localized stage (50%), and squamous cell histology (70%). Most women received aggressive therapy (75%) (Table 1).
Table 1.
| Frequency | Percent | |
|---|---|---|
| Age | ||
| 20–49 | 25062 | 54.07 |
| 50–69 | 15417 | 33.26 |
| 70+ | 5871 | 12.67 |
| Stage | ||
| Localized | 23053 | 49.74 |
| Regional | 17229 | 37.17 |
| Distant | 6068 | 13.09 |
| Histology | ||
| Squamous | 32373 | 69.84 |
| Adenocarcinoma | 9301 | 20.07 |
| Adenosquamous | 1938 | 4.18 |
| Other | 2738 | 5.91 |
| Race | ||
| White | 35169 | 78.88 |
| Black | 6589 | 14.22 |
| Other | 4592 | 9.91 |
| Treatment | ||
| Aggressive | 34989 | 75.49 |
| Less aggressive | 8100 | 17.48 |
| None | 3261 | 7.04 |
Older women show poorer survival rates, regardless of disease stage
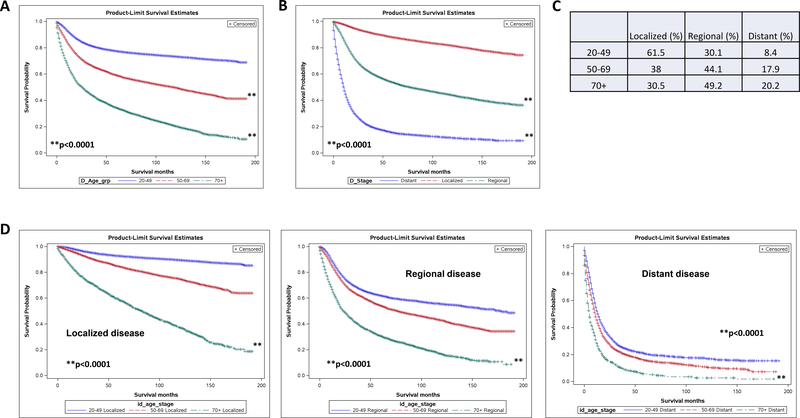
When looking at the total population, overall survival curves for women with cervical cancer tend to separate based on age, with increasingly poorer survival seen as women age (Fig 1A). There are a variety of factors that could theoretically contribute to this finding, including the stage of disease at diagnosis. It is well established that increasing stage (defined here as localized, regional, or distant) correlates with poor survival. This trend is replicated within our study population as well (Fig 1B). To evaluate the question of whether older women simply present with more advanced disease, we looked at the frequency of stages per age group. Indeed, most women over age 70 present with regional or distant disease (49% with regional, 20% with distant) (Fig 1C). Despite this, when survival is evaluated among age groups specifically for individual stages, older women show poorer survival tendencies, regardless of how advanced their disease is at presentation (Fig 1D). This tells us that even though older women may present with more advanced disease, this factor does not account for the survival discrepancy seen between older and younger women.
Fig 1.
Older women with cervical cancer show poorer overall survival regardless of stage of disease at presentation. (A) Kaplan-Meier survival curve stratified by age (A) and stage (B). (C) Frequency of disease stage by age group. (D) Kaplan-Meier survival curves for localized, regional and distant disease stratified by age group (20–40, 50–69, 70+) p<0.0001
Older women show poorer survival for all histologic subtypes
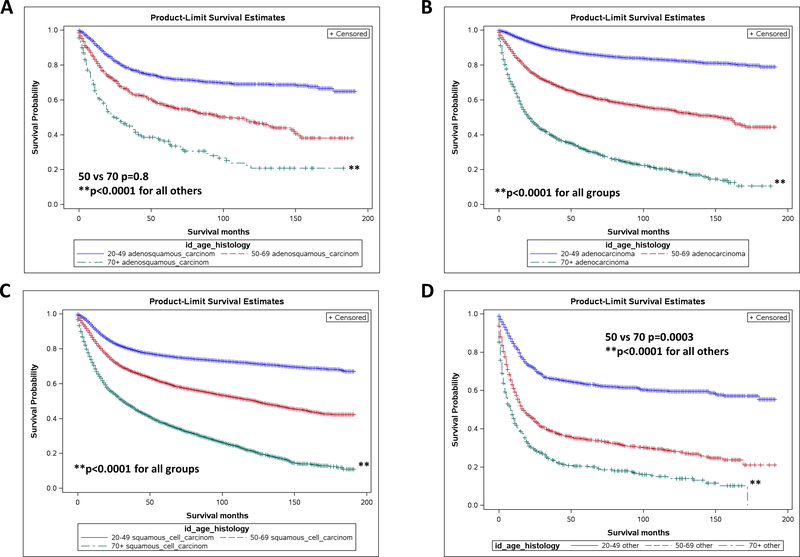
Histology is also a variable known to have prognostic significance in cervical cancer. We found this to be true in our study population as well, with women with clear cell and small cell disease showing the poorest overall survival. When survival was analyzed by age group with respect to histology, older women continued to show a poorer overall survival as compared to younger patients (p<0.0004). For adenosquamous disease, women 50–69 showed no significant survival advantage over women greater than 70 (Fig 2A). Both groups, though, showed decreased survival as compared to women 20–49 (p<0.0001). For all other subtypes, older age was a significantly negative prognostic factor, with women showing decreased survival tendencies regardless of histology (Fig 2B-D).
Fig 2.
Older women with cervical cancer show poorer overall survival regardless of histology of disease. Kaplan-Meier survival curves for adenosquamous (A), adenocarcinoma (B), squamous cell carcinoma (C), and other (clear cell, small cell) (D) histologies, stratified by age. Adenosquamous: 50 vs 70 p=0.99, Other: 50 vs 70 p=0.0008, all others p<0.0001
Increasing age indicative of negative prognostic outcome
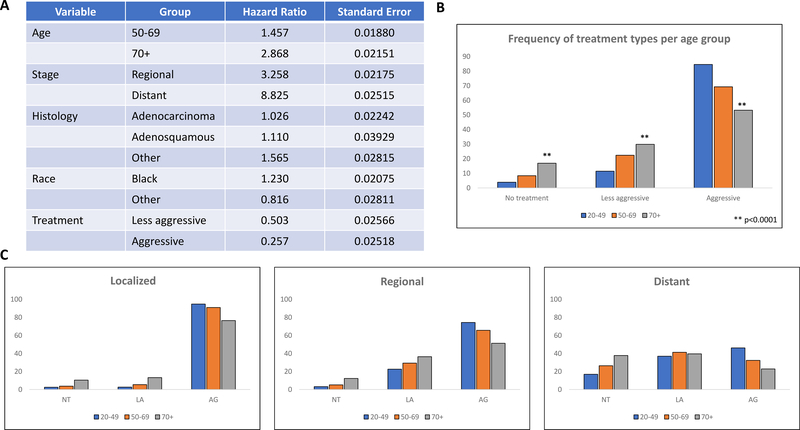
To evaluate these findings further, Cox Proportional Hazard Modeling was used to establish hazard ratios (HR) for various age groups. When accounting for disease stage, histology, race, and treatment, increasing age demonstrates negative prognostic significance with a HR of 1.46 for women ages 50–69 and 2.87 for women over age 70 as compared to women aged 20–49 (Fig 3A). Consistent with the literature, our study population also showed a higher risk association with distant disease (HR 8.83), clear cell and small cell histologies (HR 1.57), and black race (HR 1.23) (Fig 3A). As compared to no treatment, less aggressive treatment regimens showed a HR of 0.50 and aggressive regimens a HR of 0.26 (Fig 3A).
Fig 3.
Age is an independent negative prognostic factor for cervical cancer with regression modeling (A) showing increased risk with increasing age. Reference for Age Group: 20–49; Race: White; Stage: Localized; Histology: Squamous Cell Carcinoma; Treatment: No Treatment. Older women are also less likely to receive treatment. Frequency of treatment by age group (B) and subsequently stratified by stage (C). p<0.0001 vs all groups
Older women receive less aggressive treatment
To evaluate the possible implications of age as a negative prognostic factor, we examined treatment frequency trends by age group and stage. Overall, older women, particularly those over 70, were significantly less likely to receive aggressive treatment regimens (53.2% over 70, 69.3% 50–69, 84.6% 20–49; p<0.0001). They were also significantly more likely to receive less aggressive treatment regimens (p<0.0001 vs 50–69 and 20–49), as well as no treatment at all. While only 3.9% of women 2049 received no treatment for their disease, 8.4% of women aged 50–69 did not receive treatment (p<0.0001) and 16.9% of women over 70 (p<0.0001) (Fig 3B). These trends held true when evaluated by stage as well. The majority of patients with localized disease, regardless of age were treated with an aggressive regimen. Despite this, there were still a significantly lower number of women over 70 in this category as compared to younger patients. In fact, 76.5% of women 70+ received aggressive treatment for localized disease as compared to 90.9% of women 50–69 (p<0.0001) and 94.8% of women aged 2049 (p<0.0001). This was similarly seen in regional disease and distant disease as well with older women, both aged 50–69 and 70+, having significantly higher rates of no treatment, less aggressive treatment, and lower rates of aggressive treatment regimens (p<0.0001 vs women 20–49) (Fig 3C).
Less aggressive treatment associated with improved survival over no treatment, particularly among older women
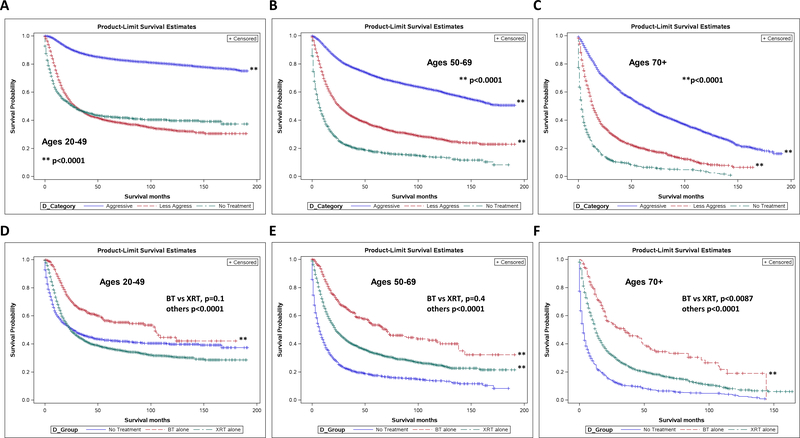
Overall survival was analyzed according to treatment regimen received and stratified by age. For women aged 20–49, aggressive treatment regimens showed a significant survival advantage over less aggressive regimens or no treatment (p<0.0001). Survival differences between the less aggressive and no treatment groups, while statistically significant, did not show clinical significance that is to say that the survival benefit seen was very small and not clinically relevant (Fig 4A). For women aged 50–69, aggressive therapy again showed a significant survival advantage (p<0.0001). Less aggressive treatment also showed a significant survival benefit over no treatment as well (p<0.0001) (Fig 4B). This trend held true for women over 70 as well, with significant survival advantages seen with both less aggressive (p<0.0001) and aggressive treatment strategies (p<0.0001) (Fig 4C).
Fig 4.
Less aggressive treatment associated with improved survival over no treatment, particularly among older women. Kaplan-Meier survival curves for all stage disease stratified by type of treatment for ages 20–49 (A), 50–69 (B), and 70+ (C). p<0.0001 for all groups. Brachytherapy alone showed greatest benefit out of less aggressive therapies, with survival seen versus external beam in women 70+. KaplanMeier survival curves for all stage disease stratified by type of less aggressive treatment for ages 20–49 (D), 50–69 (E), and 70+ (F). Ages 20–49: BT vs XRT p=0.3, others p<0.0001. 50–69: BT vs XRT p=0.8, others p<0.0001. 70+: BT vs XRT p=0.0087, others p<0.0001.
Brachytherapy alone provides survival benefit in women over 70 as compared to external beam alone
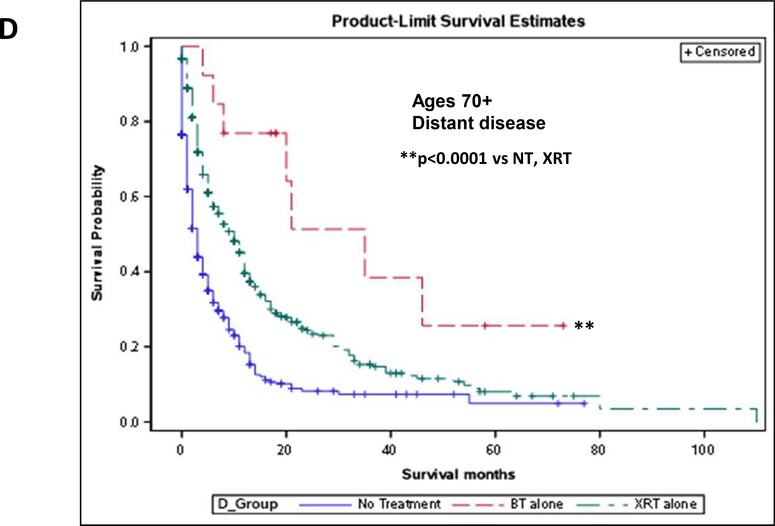
Due to the survival benefit seen with less aggressive therapies and the fact that older women receive less aggressive therapy much more often than their younger counterparts for similar stage disease, we analyzed survival for individual therapies in this group. In women aged 20–49, there was a significant benefit to brachytherapy alone (BT) and external beam alone (XRT) as compared to no treatment (p<0.0001), but no statistical difference between the two therapies (p=0.3) (Fig 4D). For women aged 50–69, treatment of any type again gave a survival advantage over no treatment (p<0.0001), but there was no advantage to either individual treatment (p=0.83) (Fig 4E). For women over 70, however, not only was BT or XRT significantly advantageous for survival (p<0.0001), BT showed a significant survival advantage over XRT in this population (p=0.0087) (Fig 4F).
Brachytherapy alone provides survival benefit in women over 70 with distant disease
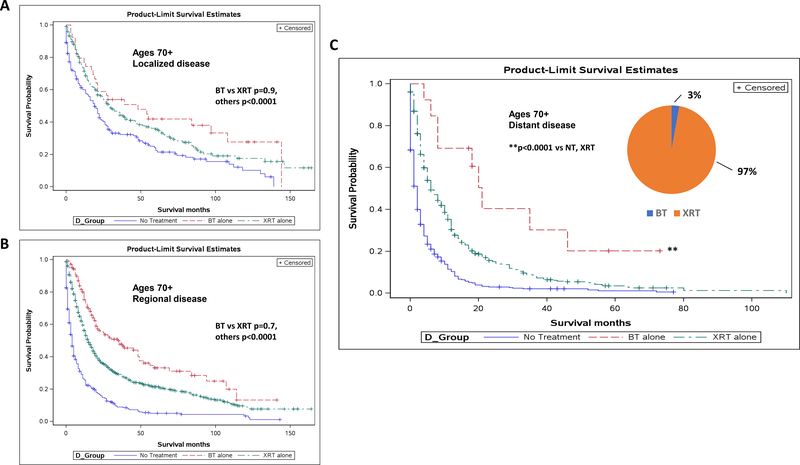
To determine whether stage was playing a role in the survival advantages seen in women over 70, we examined survival curves for each disease stage. For localized (p<0.01) and regional disease (p<0.0001), treatment of either kind showed a survival advantage as compared to no treatment, but there was no difference between BT and XRT (p=0.9 and 0.7, respectively) (Fig 5A-B). However, for women over 70 with distant disease, BT alone showed a significant survival benefit not only as compared to untreated women (p<0.0001), but to women treated with XRT alone as well (p<0.0001), with average survival increasing from 13 months with XRT to 25 months with BT alone (Fig 5C).
Fig 5.
Brachytherapy alone provides survival benefit in women over 70 with distant disease. Kaplan-Meier survival curves for women over 70 with localized (A), regional (B), and distant (C) disease, stratified by type of less aggressive treatment. Localized disease: BT vs XRT p=0.9, others p<0.0001. Regional disease: BT vs XRT p=0.7, others p<0.001. Distant disease: p<0.0001 for BT vs NT, XRT. Causespecific survival in women >70 with distant disease (D). p<0.0001 for BT vs NT, XRT.
Finally, despite the significant survival advantage seen with brachytherapy alone among less aggressive regimens for women over 70 with distant disease, only 2.8% of our population received this therapy, as compared to the 97.3% who received XRT (Fig 5C).
Brachytherapy alone provides cause-specific survival benefit in women over 70 with distant disease
To take this one step further, we wanted to evaluate this same group of women with respect to cause-specific survival. It would easy to argue that older women who received brachytherapy perhaps were just healthier in general and that is why they showed an overall survival benefit. However, similar to overall survival, we found that in women over 70 with distant disease, BT showed a cause-specific survival benefit as compared to both untreated women (p<0.0001) and XRT (p<0.0001)(Fig 5D).
Discussion
While it is well established that the average age of cervical cancer diagnosis ranges from 35–44, with a median age of diagnosis of 49, there is still a significant number of older women who not only are diagnosed with cervical cancer, but who die from it each year1. From 2009–2013, 38% of cervical cancer cases occurred in women 55 and older, with almost 20% of cases found in women greater than age 651;2. This information is particularly relevant given the increasing elderly population in the coming years. The effect of age on a woman’s overall prognosis, however, is something that has been debated in the literature6; 7; 8; 9; 10. To our knowledge, this is the largest study to date looking at age as a prognostic factor in cervical cancer and our results provide new insight into the factors that may mitigate death from this disease specifically in the elderly population.
Regression modeling supported that in our population of over 46,000 women, older women showed a higher HR and ultimately had a poorer overall survival, even when accounting for race, stage at diagnosis, tumor histology, and treatment. This data highlights older women with cervical cancer as a particularly high-risk group. While this information is useful to know, we wanted to use the data we had generated to find a way to make this discovery clinically applicable and be able to affect treatment in a beneficial way for these patients. When looking at the frequency of treatment in our study population, we found that women over 70 were significantly less likely to receive standard of care treatment and much more likely to receive less aggressive (palliative) treatment, or no treatment at all.
It is a well-established occurrence that the elderly population, in general, often receives incomplete or substandard medical treatment. This is especially true in the field of oncology, where it has been shown in multiple studies that older age is a significant risk factor for receiving less treatment or no treatment at all as compared to younger patients with a similar diagnosis6; 13; 14; 15; 16; 17. Often times, this may be due to the presence of comorbidities, which also tend to increase in proportion with age. However, many times, there may be the assumption that the elderly patient is unable to tolerate standard treatment or may not benefit as much from treatment as they have less life to live as compared to younger patients. Unfortunately, many cancers tend to have a worse prognosis in patients of advanced age, independent of other factors such as comorbidities, histology, and stage of disease18; 19; 20; 21; 22; 23. Based on our data, cervical cancer is among these diseases.
The novelty of this study lies in the fact that it goes beyond simply identifying undertreatment of elderly women and evaluates the clinical consequence of this. Not surprisingly, older women, both those in the 50–69 group as well as those 70+, showed a significant survival benefit with aggressive treatment as compared to those treated less aggressively or not at all. However, even women treated with less aggressive therapies, which were defined as single modality radiotherapy, either XRT or BT, still showed a significant survival advantage as compared to women who received no treatment. When compared directly, XRT and BT yielded similar survival outcomes for women under 70. Incredibly, though, brachytherapy showed a significant survival advantage over XRT in women 70 and older. When this subgroup of women was evaluated according to stage, this survival benefit with brachytherapy was seen only in women with distant disease.
This finding may be the consequence of several brachytherapy effects, most likely those affecting quality of life effects24 or those related to potential immune stimulation and indirect effects on more distant sites of disease. Women with distant disease also often have bulky, symptomatic regional disease that can be reduced greatly by brachytherapy. Brachytherapy is well tolerated, often having much less toxicity than XRT, and, therefore, may be able to greatly improve the quality of life of this group. The significant palliative effect may be contributing to the survival benefit seen. The additional survival seen with brachytherapy, though, is significant, about a year, and likely is the result of a more complex biological process. We believe that our data may be evidence of an abscopal effect, wherein palliative brachytherapy is activating a secondary systemic anti-cancer response25; 26; 27. Women with distant disease are the ones most likely to die of their disease and, therefore, would also be the ones most likely to benefit from a systemic response. Additionally, literature supports that abscopal effects are much more pronounced and evident with higher dose per fraction radiotherapy25, such as that typically given with stereotactic body radiotherapy (SBRT), though brachytherapy is the quintessential high dose per fraction, focal, fractionated therapy. This may explain why XRT showed inferior survival benefits as compared to BT. This hypothesis is one that, though very interesting, still requires significant further investigation to evaluate more thoroughly.
The inclusion of brachytherapy in the curative treatment of cervical cancer is essential in improving overall survival8; 15; 28; 29; 30. It is a technique that requires skill, trained personnel, special facilities and the need for volume to maintain quality standards31. As a result, many non-academic medical facilities are not able to offer brachytherapy. It has been reported that many women, especially those not in proximity to a high-volume academic center, are often not offered brachytherapy as a part of their cervical cancer treatment regimen or not offered the resources required to find appropriate brachytherapy elsewhere, despite the evidence supporting its survival benefit.8; 14; 28; 30; 31 Brachytherapy inclusion has been mostly studied in the context of larger treatment regimens, which include surgery and external beam radiation with or without the addition of chemotherapy. We, however, for the first time, demonstrate here the ability of brachytherapy as a single modality treatment to provide a survival advantage for older women with disseminated disease when being used as a palliative treatment. For this population of patients, though, XRT is the most commonly used palliative treatment given.
We acknowledge that due to the nature of the SEER database, our study has limitations. Most notably is the lack of information on patient comorbidities as well as that pertaining to the receipt of chemotherapy. Additionally, information on a patient’s performance status, such as KPS scores, would be useful information to have that is also not available through the SEER database. Patient comorbidities can contribute substantially to a patient’s overall prognosis, affecting their ability to both receive and tolerate treatment. Because we cannot account for this factor, it is possible women who received brachytherapy may have been in better overall health and that why they were offered brachytherapy. The ability to perform propensity-matched analyses would help to control for bias, but unfortunately, the information required to carry out these studies is not available in the SEER database. Despite this, we are still able to show that brachytherapy offered not only an overall survival benefit, but a cause-specific one as well. So even if the women who were offered brachytherapy were healthier than those not offered it, they still had a survival advantage specifically related to their cervical cancer. That is to say, the women who died in our study, those who did not get brachytherapy, even if they were unhealthier, still were dying from their cervical cancer rather than from sequelae of other comorbidities. If anything, this pushes us to further encourage the use of brachytherapy for advanced cervical cancer in older women even more, as it truly appears to offer a survival benefit.
Unfortunately, , we believe that there is a subset of older women with metastatic cervical cancer who could receive brachytherapy, but that are not receiving it and who are represented in our study by those women who did receive it. We show that only 3% of women in this group actually received brachytherapy, but believe that the percentage that could have received it is much higher. Venkatesulu et. al. discussed the decline of brachytherapy use in cervical cancer, particularly in elderly populations, and included an evaluation of reasons why patients did not receive brachytherapy15. They noted that patient refusal was cited in almost 40% of women. This may be due to social or logistical issues, such as transportation, or simply fear of what may sound like a frightening and foreign procedure. Either way, it supports our belief that palliative brachytherapy for metastatic cervical cancer is an option for a significant group of women and that even within our study population, there were most likely many more than 3% of women who could have received and benefitted from brachytherapy. Furthermore, the literature has demonstrated repeatedly that brachytherapy is a well-tolerated procedure, even in elderly patients, and that they have no increased rates of toxicity as compared to their younger counterparts9; 13; 32; 33; 34. Given a survival advantage for those who did actually receive it, we would strongly encourage providers to include brachytherapy in their palliative regimens, even if this means helping the patient seek care outside of their respective facilities at a high-volume brachytherapy center.
Conclusion
As we and others have now shown, advancing age is an independent negative prognostic factor for mortality for women with cervical cancer. It is imperative that we reevaluate our clinical decision making and not compromise standard of care in the setting of an elderly patient. We acknowledge that each patient is unique and presents with their own set of circumstances that may alter their ability to receive standard of care treatment, but we urge clinicians to strive to achieve this standard in older women whenever possible. When this is not an option, we highly encourage palliative treatment to include brachytherapy, especially in those with distant disease.
Acknowledgments
Services and products in support of this research project were generated by the VCU Massey Cancer Center Biostatistics Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.INSTITUTE, N. C. Cervical Cancer Treatment (PDQ®) Bethesda, MD: National Cancer Institute., 2017. [Google Scholar]
- 2.BULLETINS—GYNECOLOGY COP ACOG Practice Bulletin Number 131: Screening for cervical cancer. Obstet Gynecol, v. 120, n. 5, p. 1222–38, November 2012. ISSN 1873–233X. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/23090560 >. [DOI] [PubMed] [Google Scholar]
- 3.BEAVIS AL; GRAVITT PE; ROSITCH AF Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer, v. 123, n. 6, p. 1044–1050, May 2017. ISSN 1097–0142. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/28112816 >. [DOI] [PubMed] [Google Scholar]
- 4.ADAMS SA et al. Racial disparities in cervical cancer mortality in an African American and European American cohort in South Carolina. J S C Med Assoc, v. 105, n. 7, p. 237–44, December 2009. ISSN 0038–3139. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/20108710 >. [PMC free article] [PubMed] [Google Scholar]
- 5.VINH-HUNG V et al. Prognostic value of histopathology and trends in cervical cancer: a SEER population study. BMC Cancer, v. 7, p. 164, August 2007. ISSN 1471–2407. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/17718897 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SHARMA C et al. Patterns of care and treatment outcomes for elderly women with cervical cancer. Cancer, v. 118, n. 14, p. 3618–26, July 2012. ISSN 1097–0142. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/22038773 >. [DOI] [PubMed] [Google Scholar]
- 7.BRUN JL et al. Survival and prognosis of women with invasive cervical cancer according to age. Gynecol Oncol, v. 91, n. 2, p. 395–401, November 2003. ISSN 0090–8258. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/14599872 >. [DOI] [PubMed] [Google Scholar]
- 8.HAN K et al. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys, v. 87, n. 1, p. 111–9, September 2013. ISSN 1879–355X. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/23849695 >. [DOI] [PubMed] [Google Scholar]
- 9.IKUSHIMA H et al. Radiation therapy for cervical cancer in the elderly. Gynecol Oncol, v. 107, n. 2, p. 339–43, November 2007. ISSN 1095–6859. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/17707075 >. [DOI] [PubMed] [Google Scholar]
- 10.MINAGAWA Y et al. The outcome of radiation therapy in elderly patients with advanced cervical cancer. Int J Gynaecol Obstet, v. 58, n. 3, p. 305–9, September 1997. ISSN 0020–7292. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/9286865 >. [DOI] [PubMed] [Google Scholar]
- 11.INSTITUTE, N. C. Surveillance Research Program, SEER*Stat software. [Google Scholar]
- 12.SURVEILLANCE E, AND END RESULTS (SEER) PROGRAM. SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (19732013 varying) - Linked To County Attributes - Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission 2016. [Google Scholar]
- 13.MOORE KN et al. Is age a prognostic biomarker for survival among women with locally advanced cervical cancer treated with chemoradiation? An NRG Oncology/Gynecologic Oncology Group ancillary data analysis. Gynecol Oncol, v. 143, n. 2, p. 294–301, November 2016. ISSN 1095–6859. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/27542967 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SHOWALTER TN et al. Determinants of Quality Care and Mortality for Patients With Locally Advanced Cervical Cancer in Virginia. Medicine (Baltimore), v. 95, n. 8, p. e2913, February 2016. ISSN 1536–5964. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/26937934 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VENKATESULU BP; MALLICK S; RATH GK Patterns of care of cervical cancer in the elderly: A qualitative literature review. J Geriatr Oncol, v. 8, n. 2, p. 108–116, March 2017. ISSN 1879–4076. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/28169196 >. [DOI] [PubMed] [Google Scholar]
- 16.WRIGHT JD et al. Cervical carcinoma in the elderly: an analysis of patterns of care and outcome. Cancer, v. 103, n. 1, p. 85–91, January 2005. ISSN 0008–543X. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/15540239 >. [DOI] [PubMed] [Google Scholar]
- 17.DALE DC Poor prognosis in elderly patients with cancer: the role of bias and undertreatment. J Support Oncol, v. 1, n. 4 Suppl 2, p. 11–7, 2003. Nov-Dec 2003. ISSN 1544–6794. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/15346995 >. [PubMed] [Google Scholar]
- 18.TAS F et al. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett, v. 6, n. 5, p. 1507–1513, November 2013. ISSN 1792–1074. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/24179550 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.URBAN RR et al. Ovarian cancer outcomes: Predictors of early death. Gynecol Oncol, v. 140, n. 3, p. 474–80, March 2016. ISSN 1095–6859. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/26743531 >. [DOI] [PubMed] [Google Scholar]
- 20.OKUMA K et al. Advanced age is a significant determinant of poor prognosis in patients treated with surgery plus postoperative radiotherapy for endometrial cancer. J Obstet Gynaecol Res, v. 36, n. 4, p. 757–63, August 2010. ISSN 1447–0756. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/20666941 >. [DOI] [PubMed] [Google Scholar]
- 21.SYRIGOS KN et al. Skin cancer in the elderly. In Vivo, v. 19, n. 3, p. 643–52, May-Jun 2005. ISSN 0258–851X. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/15875788 >. [PubMed] [Google Scholar]
- 22.LEU Y-S et al. Prognosis of Nasopharyngeal Carcinoma in the Elderly is Worse than in Younger Individuals&-Experience of a Medical Institute. International Journal of Gerontology, v. 8, n. 2, p. 81–84,ISSN 1873–9598. Disponível em: < . [DOI] [Google Scholar]
- 23.PATEL SS et al. Elderly patients with colon cancer have unique tumor characteristics and poor survival. Cancer, v. 119, n. 4, p. 739–47, February 2013. ISSN 1097–0142. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/23011893 >. [DOI] [PubMed] [Google Scholar]
- 24.TEMEL JS et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med, v. 363, n. 8, p. 733–42, August 2010. ISSN 1533–4406. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/20818875 >. [DOI] [PubMed] [Google Scholar]
- 25.CAMPHAUSEN K et al. Radiation abscopal antitumor effect is mediated through p53. Cancer Res, v. 63, n. 8, p. 1990–3, April 2003. ISSN 0008–5472. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/12702593 >. [PubMed] [Google Scholar]
- 26.DEMARIA S; GOLDEN EB; FORMENTI SC Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol, v. 1, n. 9, p. 1325–32, December 2015. ISSN 2374–2445. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/26270858. [DOI] [PubMed] [Google Scholar]
- 27.FORMENTI SC; DEMARIA S Systemic effects of local radiotherapy. Lancet Oncol, v. 10, n. 7, p. 718–26, July 2009. ISSN 1474–5488. Disponível em:https://www.ncbi.nlm.nih.gov/pubmed/19573801 >. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BAGSHAW HP et al. Patterns of care with brachytherapy for cervical cancer. Int J Gynecol Cancer, v. 24, n. 9, p. 1659–64, November 2014. ISSN 1525–1438. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/25251463 >. [DOI] [PubMed] [Google Scholar]
- 29.KARLSSON J et al. Differences in outcome for cervical cancer patients treated with or without brachytherapy. Brachytherapy, v. 16, n. 1, p. 133–140, 2017 Jan-Feb 2017. ISSN 1873–1449. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/27836496 >. [DOI] [PubMed] [Google Scholar]
- 30.TANDERUP K et al. Curative radiation therapy for locally advanced cervical cancer: brachytherapy is NOT optional. Int J Radiat Oncol Biol Phys, v. 88, n. 3, p. 537–9, March 2014. ISSN 1879–355X. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/24411631 >. [DOI] [PubMed] [Google Scholar]
- 31.EIFEL PJ et al. Patterns of radiation therapy practice for patients treated for intact cervical cancer in 2005 to 2007: a quality research in radiation oncology study. Int J Radiat Oncol Biol Phys, v. 89, n. 2, p. 249–56, June 2014. ISSN 1879–355X. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/24411621 >. [DOI] [PubMed] [Google Scholar]
- 32.MAGNÉ N et al. Patterns of care and outcome in elderly cervical cancer patients: a special focus on brachytherapy. Radiother Oncol, v. 91, n. 2, p. 197–201, May 2009. ISSN 1879–0887. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/18954913 >. [DOI] [PubMed] [Google Scholar]
- 33.PIGNON T et al. Age is not a limiting factor for radical radiotherapy in pelvic malignancies. Radiother Oncol, v. 42, n. 2, p. 107–20, February 1997. ISSN 0167–8140. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/9106920 >. [DOI] [PubMed] [Google Scholar]
- 34.ZACHARIAH B et al. Radiotherapy for cancer patients aged 80 and older: a study of effectiveness and side effects. Int J Radiat Oncol Biol Phys, v. 39, n. 5, p. 1125–9, December 1997. ISSN 0360–3016. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/9392554 >. [DOI] [PubMed] [Google Scholar]