Abstract
The dopamine transporter (DAT) plays a critical role in dopamine (DA) homeostasis by clearing transmitter from the extraneuronal space after vesicular release. DAT serves as a site of action for a variety of addictive and therapeutic reuptake inhibitors, and transport dysfunction is associated with transmitter imbalances in disorders such as schizophrenia, attention deficit hyperactive disorder, bipolar disorder, and Parkinson disease. In this review, we describe some of the model systems that have been used for in vitro analyses of DAT structure, function and regulation, and discuss a potential relationship between transporter kinetic values and membrane cholesterol.
Keywords: kinetic parameters, neuronal cultures, CFT, cholesterol, striatum, nucleus accumbens
1. Introduction
In the central nervous system the neurotransmitter dopamine (DA) controls many physiological and psychological processes including reward, motivation, attention, and motor activity (Iversen and Iversen, 2007). Dopaminergic neurons located in the substantia nigra and ventral tegmental area regions of the basal gangia project to motor, reward, and cognitive areas such as striatum (Str), nucleus accumbens (NAc), and frontal cortex, where they release DA into synaptic and perisynaptic spaces to mediate these functions. Reuptake of transmitter back into presynaptic neurons serves as the major regulator of DA signaling (Giros et al., 1996) and is mediated by the dopamine transporter (DAT), a plasma membrane protein found exclusively on DA neurons (Nirenberg et al., 1996).
Defects in DA signaling and homeostasis are associated with several neurological disorders including Parkinson disease (PD), schizophrenia, bipolar disorder, depression, and attention-deficit hyperactive disorder (Kristensen et al., 2011; Pramod etal., 2013), and dysregulated DAT function resulting in imbalanced DA levels has been invoked as a potential factor in their etiologies (Hahn and Blakely, 2002; Kristensen et al., 2011; Pramod et al., 2013). DAT is a target for many psychostimulant drugs that suppress reuptake and elevate synaptic DA levels, including addictive compounds such as cocaine and amphetamine (AMPH), and therapeutic drugs prescribed for DA disorders such as Ritalin® (methylphenidate) and Wellbutrin® (bupropion) (Blakely and Bauman, 2000; German et al., 2012; Iversen and Iversen, 2007). DAT can also transport neurotoxic compounds including 6-hydroxydopamine (Miller et al., 1999; Nass et al., 2002), 1-methyl-4-phenylpyridinium (Gainetdinov et al., 1997; Javitch and Snyder, 1984), and environmental chemicals such as paraquat, serving as a gateway for their entry into neurons, a postulated mechanism for dopaminergic neurodegeneration in PD (Miller et al., 1999).
The central role of DAT in signaling, addiction, and disease has made the transporter a focus of intense efforts to elucidate its transport and regulatory mechanisms (Foster and Vaughan, 2017; Kristensen et al., 2011; Rastedt et al., 2017). Such studies are largely undertaken in vitro using model systems such as synaptosomes and brain slices, primary neuronal cultures, and cell expression systems (Kristensen et al., 2011; Pramod et al., 2013). In this minireview, we briefly describe the characteristics and scientific and technical advantages and disadvantages of many of these systems, including photomicrographs of several of the described cell lines (Fig. 1), a tabulation of transport and cocaine analog kinetic parameters (Tables 1-4), and discussion of a potential mechanistic link between transporter kinetics and membrane cholesterol (Figs. 2-4).
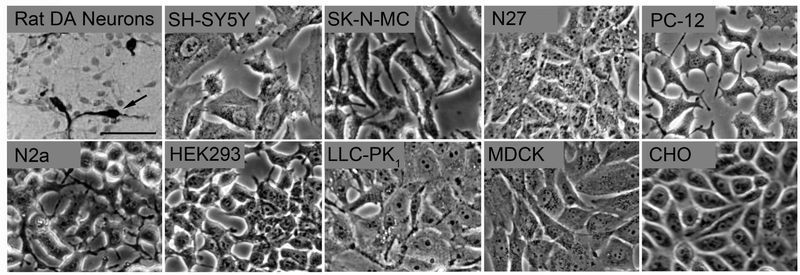
Fig. 1. Morphology of cell systems used to study DAT function and regulation.
Panels show phase contrast microscopic images of several cell systems studied in our labs. Images were acquired using a 20X objective on an inverted Olympus microscope equipped with a digital camera. The scale bar indicates 50 μm and is the same for all panels. The dopaminergic neuron image showing tyrosine hydroxylase positive midbrain neurons (arrow) is modified from (Branton and Clarke, 1999).
Table 1:
Vmax and Km values determined for DA transport in various cell lines.
pmol/min/105 cells
fmol/sec
Mouse multipotent neural progenitor cells
Some FLAG tagged
Table 4:
Bmax and Kd values for β-CFT binding determined for DAT in animal tissues.
| Tissue Type/Region | CFT Bmax (fmol/mg) |
CFT Kd (nM) |
Reference |
|---|---|---|---|
| Rat | |||
| Striatal Synaptosomes (including NAc and CPu) | 61-1950 | 7 - 63 | (Foster et al., 2008; Hong and Amara, 2010; Jones et al., 2017; Kimmel et al., 2000; Moritz et al., 2013; Woolverton et al., 2000; Zhen et al., 2005) |
| Mouse | |||
| Striatal Synaptosomes | 3700 ± 800 | 5 ± 1 | (Rao et al., 2013) |
| Rhesus Monkeys (Macaca mulatta) | |||
| Striatal Synaptosomes (including NAc and CPu) | 753 -1850 | 1 – 27 | (Beveridge et al., 2009; Kaufman and Madras, 1993; Letchworth et al., 2001; Madras et al., 1989; Woolverton et al., 2000) |
| Human | |||
| Frozen CPu Synaptosomes | 10,000 ± 400 | 4 ± 1 | (Staley et al., 1994) |
| Frozen CPu Synaptosomes | 6 ± 4a | 12 ± 2a | (Little et al., 1993) |
| Frozen NAc Synaptosomes | 6 ± 3a | 8 ± 1a | (Little et al., 1993) |
nCi/mg
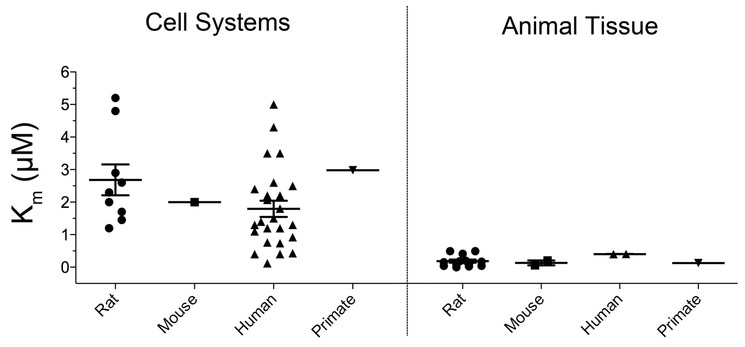
Fig. 2. Distribution of Km(DA) values across model systems.
Scatterplot representation of reported Km(DA) values for rat, mouse, human, and nonhuman primate DATs expressed in cell or brain tissue systems.
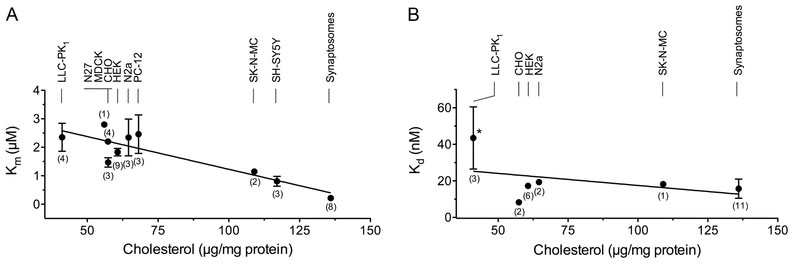
Fig. 4. Inverse correlation between Km and membrane cholesterol content.
(A) Plot of pooled Km(DA) values (means ± SEM) vs. membrane cholesterol for the indicated cell lines and tissues. The line indicates the linear regression analysis (r2=0.78; Pearson correlation coefficient of r = −0.88 ;*** p= 0.0008).(B) Plot of Kd(CFT) values (means ± SEM) vs. membrane cholesterol for the indicated cell lines and tissues. The line indicates linear regression analysis (r2=0.16, Pearson correlation coefficient of r = ‒0.4; p = 0.43). Asterisk indicates significant difference (*, p<0.05) between Kd(CFT) LLC-PK1 vs synaptosomes. Numbers in parentheses indicate the number of reports for each system and where absent error bars lie within the symbol.
2. Model systems for studying DAT function and regulation
2.1. Striatal slices
This ex vivo system provides the advantage of replicating many aspects of the in vivo environment such as tissue architecture, synaptic circuitry, and regulatory machinery. Brains of freshly dispatched animals (commonly rodents) are typically harvested at postnatal days (PND) 3-15, and desired regions of the brain are sliced into 350-500 μm sections that may include cell bodies, projection axons, and terminal regions (Cho et al., 2007; Kearns etal., 2006). However, thicker slices (up to 1 mm) and those from adult rodents have successfully been used in several studies (Block et al., 2015; Inyushin et al., 2013; Wheeler et al., 2015). The slices are bathed in artificial cerebrospinal fluid and remain viable for many hours, allowing for examination of long-term treatments or outcomes. Sections can be mounted to permeable tissue culture membrane inserts that provide fine access and control, allowing uptake and neuronal functions to be analyzed by high-resolution procedures such fast scan cyclic voltammetry (FSCV) and electrophysiology. Tissue from slices can also be minced or processed into synaptosomes for biochemical analyses.
2.2. Striatal synaptosomes
In this model system desired DA projection areas (usually striatum or NAc) are dissected from brains of freshly dispatched animals and homogenized using a procedure that generates pinched-off, resealed nerve terminals. Circuit connections are severed, but the structures retain capacity for transmitter uptake and release, and contain many nerve terminal components including cytoplasm, synaptic vesicles (SVs), mitochondria, receptors, and signaling molecules (Evans, 2018; Whittaker et al., 1964). In terms of monitoring DAT function, synaptosomes have a limited viability (30-60 min), especially after suspension in isotonic medium at physiologic temperature. Transport activity decays considerably across this time frame, possibly due to loss of electrochemical gradient, making synaptosomes suitable primarily for shortterm experiments.
2.3. Primary dopaminergic neurons in culture
Primary dopaminergic neurons (typically obtained from mouse or rat embryo midbrain), provide a cell culture system that presumably most closely resembles neurons in vivo. The cells express DA markers including tyrosine hydroxylase (TH), DA receptors, and DAT, and contain synaptic vesicles and exocytotic release machinery (Chinta and Andersen, 2005; Choi et al., 2013; Greene, 2006). However, although the harvested neurons are cultured in vitro several days prior to use, this may not recapitulate the maturation that occurs in vivo. This is an important point for DAT, which undergoes significant developmental regulation. In rats, DAT expression at birth is only ~20-30% of that at PNDs 14-60, and N-linked glycosylation, which regulates DAT processing and targeting, is also negligible at birth (Patel et al., 1994). These properties have not been examined at embryonic stages of development, including those commonly used to obtain primary neurons, and additional DAT regulatory properties including other post-translational modifications (PTMs) and interactome profile have not been characterized at early life stages. In addition, midbrain DA neurons are low in number, constitute only a small fraction of the total neurons present in these regions (see Fig. 1) (Hegarty et al., 2013), and do not undergo cell division, factors which reduce their utility for analyses that require large amounts of uniform source material. However, primary cultures of dopaminergic neurons isolated from C. elegans, which can be grown in abundance, have been successfully employed in radiolabeled DA uptake/efflux experiments (Carvelli et al., 2004; Safratowich et al., 2014).
2.4. Human Neuroblastoma (SH-SY5Y) cells
The human SH-SY5Y line was subcloned (SK-N-SH → SH-SY → SH-SY5 → SH-SY5Y) from the original line, SK-N-SH, which was isolated in 1970 from a bone marrow biopsy of a 4-year-old girl with neuroblastoma (Biedler et al., 1973). The neuroblast-like cells are slow-growing and only loosely adherent to standard tissue culture plates, making protocols with repeated manipulations such as washing steps problematic due to cell loss. The cells are positive for TH, and produce DA and norepinephrine (NE), but only appear to store DA (Biedler et al., 1978). They also express DAT, the vesicular monoamine transporter 2 (VMAT2), and D2 and D3 dopamine receptors, making them a good model for studying the neurotoxic and neuroprotective effects of DA-related compounds (Xicoy et al., 2017). These cells express the norepinephrine transporter (NET), which can also transport DA, necessitating its selective blockade when investigating DAT-mediated DA uptake. The cells also possess cholinergic and GABAergic properties in that they produce acetylcholine and GABA and express muscarinic and nicotinic acetylcholine receptors (Biedler et al., 1978; Kovalevich and Langford, 2013). SH-SY5Y cells express immature neuronal markers, and can be differentiated using a number of methods including retinoic acid, phorbol esters, and dibutyryl cyclic adenosine monophosphate (dbcAMP), with retinoic acid induction being the most commonly used and best-characterized method (Kovalevich and Langford, 2013). After differentiation, SH-SY5Y cells show reduced proliferation, express neuronal markers and become morphologically more similar to primary neurons, with development of neurite processes reminiscent of axons and dendrites (Kovalevich and Langford, 2013).
2.5. Human Neuroepithelioma (SK-N-MC) cells
This human neural epithelial line was derived in 1971 from the neuroepithelioma tumor of a 14-year-old girl (Biedler et al., 1973). These neuroblast-like cells are TH-positive and display both catecholaminergic and cholinergic properties (e.g. production of DA, NE, and acetylcholine) (Biedler et al., 1973). They express D1 but not D5 DA receptors (Sidhu, 1997), and do not express DAT or NET (Pifl et al., 1996, 1993). The cells grow at a moderate rate and are adherent to standard tissue culture plates. They have a good transfection efficiency and stable expressing clones can be isolated.
2.6. Immortalized Rat Dopaminergic Neurons (N27) cells
The N27 cell line was derived from dopaminergic neurons isolated from embryonic day 14 rat mesencephalon and immortalized with SV40 large T antigen (Prasad et al., 1994). The original lines have been recloned to isolate line 1RB3AN27, with cells that are >95% TH-positive (Fig. 1) (Prasad et al., 1994). These cells are reported to express TH and DAT mRNA and protein via RT-PCR and immunocytochemistry (Clarkson et al., 1998; Gao et al., 2016). However, they do not display [3H]DA uptake or DAT protein by immunoblotting (Foster and Vaughan, unpublished results), indicating that any endogenous transporter expression is very low and insufficient to support function detectable by typical methodology. They also synthesize and store DA, but lack dopamine-beta-hydroxylase activity and do not synthesize NE (Gao et al., 2016). N27 cells can be differentiated using dbcAMP plus dehydroepiandrosterone (Clarkson et al., 1998). After differentiation, the cells become morphologically more similar to primary neurons, with production of neurite processes and increased TH and DAT mRNA levels. It is reported that after many passages TH expression becomes highly variable (Gao et al., 2016), and these cells require further analysis to assess their dopaminergic character. The N27 line has recently been recloned, producing line N27-A, in which nearly 100% of the cells express TH, and DAT levels increase 3-4 fold (Gao et al., 2016). N27-A cells also express VMAT2, dopaminergic transcription factors Nurr1, En1, FoxA2 and Pitx3 (Gao et al., 2016), and show DA release under basal and depolarizing conditions (Gao et al., 2016). The cells grow at a moderate rate and are strongly adherent to standard tissue culture plates. They have a good transfection efficiency and stable expressing clones can be isolated.
2.7. Rat Adrenal Gland Pheochromocytoma (PC-12) cells.
This cell line was derived from a rat pheochromocytoma adrenal gland transplantable tumor (Greene and Tischler, 1976). They are small, irregularly shaped cells (see Fig. 1) that are slow-growing and weakly adherent to standard tissue culture plates, making protocols with repeated manipulations problematic. The cells are catecholaminergic, producing and storing DA and NE but not epinephrine (Greene and Rein, 1977; Greene and Tischler, 1976).PC-12 cells respond reversibly to nerve growth factor (NGF), which induces neurite outgrowth when plated on collagencoated culture flasks (Greene and Tischler, 1976). The cells express little to no endogenous DAT, but transporter expression increases upon differentiation (Greene and Tischler, 1976). The cells also express NET, which must be selectively blocked for analysis of DAT-dependent DA uptake. They have a moderate transfection efficiency and stable expressing clones can be isolated.
2.8. Mouse Neuroblastoma Neuro-2a (N2a) cells
This neuronal line was derived from a mouse neuroblastoma tumor and has been widely used for studies of neurite outgrowth and neurotoxicity (Salto et al., 2015). They do not endogenously express DAT or other monoamine transporters and are not known to synthesize or store monoamine neurotransmitters, but can be differentiated using dbcAMP into cells possessing neuronal properties such as neurite extensions, increased TH expression, and ability to synthesize DA (Tremblay et al., 2010). The cells are fast-growing and adherent to standard tissue culture plates, have a good transfection efficiency, and stable expressing clones can be isolated.
2.9. Human Embryonic Kidney 293 (HEK293) cells
This line was derived from adenovirus transformation of human embryonic kidney cells. They have an endothelial morphology (Fig. 1), although the cells possess some properties of immature neurons such as expression of neurofilament proteins (Louis et al., 1997; Shaw et al., 2002). They are a widely used, fast-growing line with very high transfection efficiency, but are weakly adherent to standard tissue culture plates and easily dislodged, making protocols with repeated manipulations problematic. To overcome this issue the more tightly adherent GripTite™ 293 MSR (Invitrogen) cell line was developed in which the HEK293 line was engineered to express the human macrophage scavenger receptor to increase cell adherence (Robbins and Horlick, 1998).
2.10. Lilly Laboratories Cell Porcine Kidney (LLC-PK1) cells
In recent years, this LLC cell line has been commonly misidentified as a Lewis Lung Carcinoma, but was established in 1958 at Lilly Research Laboratories from the kidneys of a domestic male Hampshire pig (Hull et al., 1976). They are a spontaneously-immortalized epithelial line that is fast-growing and strongly adherent to standard tissue culture plates, making them advantageous for use in protocols with repeated manipulations. They are also very stable, undergoing little to no transformation or neoplastic change after numerous passages. In fact, the original line was passaged 88 times before first being frozen and stored in liquid nitrogen (Hull et al., 1976). The cells have a moderate transfection efficiency and stable expressing clones can be isolated.
2.11. Madin Darby Canine Kidney (MDCK) cells
This cell line was established in 1958 from the kidney of a normal adult female cocker spaniel by S.H. Madin and N.B. Darby (Leighton et al., 1970). They are a spontaneously-immortalized epithelial cell line that is fast-growing and strongly adherent to standard tissue culture plates making them advantageous for protocols with repeated manipulations. These cells have a moderate transfection efficiency and are a widely used heterologous expression model (Leighton et al., 1970).
2.12. CV-1 in Origin carrying SV40 (COS-7) cells
This fibroblast-like cell line was derived in the 1980s from transformation of normal CV-1 cells obtained from the kidney of an African green monkey. CV-1 cells were transformed using a mutant strain of the SV40 virus which produces large wild-type T-antigen resulting in the COS-7 line (Gluzman, 1981). They are fast-growing, adherent cells that have good transfection efficiency and are commonly used for recombinant mammalian protein expression (Aruffo, 2002).
2.13. Chinese Hamster Ovary (CHO) cells
These epithelial cells were derived from ovaries of Chinese hamsters. They are a commonly used model with multiple distinct lines (CHO-K1, CHO-DXB11, CHO-pro3) possessing different attributes derived from the original (Lai et al., 2013; Wurm, 2004). They are fast-growing adherent cells having high transfection efficiency and the ability to produce significant levels of recombinant proteins (Lai et al., 2013; Wurm, 2004).
3. Transport and binding kinetic parameters in various model systems
In Tables 1-4 we have collated kinetic parameters for DAT transport (Km and Vmax) and cocaine analog binding (Kd and Bmax) reported from many of these commonly used model systems. Most of the reported findings were obtained from studies using mouse, rat, monkey, and human brain tissues, or cell systems heterologously expressing cloned transporters from these species. The tables include only results obtained with WT or epitope-tagged transporters under control conditions, and do not include outcomes related to experimental manipulations or mutations. We acknowledge that this tabulation may not be fully comprehensive but hope that the information will serve as a useful resource for DAT investigators.
3.1. Km and Vmax for DA transport
DAT transport parameters are typically determined in vitro by [3H]-DA uptake saturation analysis, although kinetic constants can also be obtained non-isotopically by rotating disc electrode voltammetry (Jones et al., 2012; Volz et al., 2009). Uptake activity in live animals or brain slices can be measured with FSCV (Cremona et al., 2011; Ferris et al., 2014; Garcia-Olivares et al., 2013; Speed et al., 2011; Wheeler et al., 2017), microdialysis (Howell and Wilcox, 2002), or assessed indirectly by detection of substrate-driven ion currents (Sulzer et al., 2005; Tang et al., 2015), but these methods are less amenable for saturation analyses needed to derive kinetic constants.
A compilation of Km and Vmax values for DA transport in various cell lines and tissue preparations is shown in Tables 1 and 2, with Km values displayed as scatterplots in Figure 2. It can be seen that even for studies performed with the same species or in the same system there is a certain amount of variability. However, a major pattern that emerges is that Km for DATs expressed in the brain is up to 10-fold lower (30-500 nM) than for transporters expressed heterologously (0.1-5 μM) (Fig. 2).
Table 2:
Vmax and Km values determined for DA transport in animal tissues and brain regions.
| Tissue Type/Region | Vmax (pmol/min/mg) |
Km (μM) |
Reference |
|---|---|---|---|
| Rat | |||
| Striatal Synaptosomes (including NAc and CPu) | 6 – 159 | 0.03 – 0.50 | (Afonso-Oramas et al., 2009; Carvelli et al., 2002; Foster and Vaughan, 2011; Hong and Amara, 2010; Jones et al., 2017; Moritz et al., 2013; Morón et al., 2003; Richards and Zahniser, 2009; Vaughan et al., 1997; Zahniser et al., 1999) |
| VTA Slices | 0.33 ± 0.03a | (Mebel et al., 2012) | |
| Striatum (in vivo) | 0.7a | 10 – 12 (KT) | (Sabeti et al., 2002; Zahniser et al., 1999) |
| Mouse | |||
| Striatal Synaptosomes | 38 ± 7 | 0.06 ± 0.01 | (Rao et al., 2013) |
| NAc Slices | 1.0a | 0.2 | (Lu et al., 2015) |
| Human | |||
| Striatal Synaptosomes | 0.41 | (Mash et al., 2002) | |
| Drosophila | |||
| Channelrhodospin2 (ChR2) DA neurons | 0.11 ± 0.02a | 1.3 ± 0.6 | (Vickrey et al., 2013) |
μM/s
For studies performed with brain tissue there were no significant Km differences between species, brain regions, or tissue preparation method. The few values available for human DAT were similar to those obtained from other species, although these studies may include caveats such as differential post-mortem intervals and tissue freezing that could impact uptake parameters. In cell culture systems there were no clear differences in Km values between cell types, although we cannot exclude that minor differences might be obscured by variability.
Maximal uptake velocities, Vmax, also show considerable variability. In this case it is virtually impossible to mechanistically interpret the differences, as velocity depends on surface expression of the transporter, which can vary greatly between systems and studies. Interpretation of Vmax values can be performed within studies if velocities are normalized for DAT surface expression, and in controlled characterizations Vmax has been shown to be responsive to regulatory manipulations (Bermingham et al., 2017; Foster and Vaughan, 2011; Kivell et al., 2014; Moritz et al., 2015).
3.2. Kd and Bmax forβ-CFT binding
Several radioligands based on transporter blockers have been used to assess DAT binding functionality, including [3H]GBR 12909, [3H]mazindol, and [125I]RTI 55, but arguably the most commonly used ligand for these types of analysis is the cocaine analog [3H] β-CFT (2-beta-carbomethoxy-3-beta-(4-fluorophenyl) tropane). A compilation of Kd and Bmax values for [3H]β-CFT binding is shown in Tables 3 and 4. Kd(β-CFT) ranged from 4-77 nM in cell lines and from 1-63 nM in brain tissue. Comparison of mean values obtained from synaptosomes (16 ± 5 nM) to those of cell systems showed statistical difference only for LLC-PK1 cells (44 ±17 nM, p<0.05).
Table 3:
Bmax and Kd values for CFT binding determined for DAT in various cell lines.
| Cell Lines | CFT Bmax (fmol/mg) |
CFT Kd (nM) |
Reference |
|---|---|---|---|
| rDAT | |||
| LLC-PK1 | 681 - 804a | 32 - 77 | (Moritz et al., 2013) |
| COS-7 | 7000 | 4 - 22 | (Eshleman et al., 1995; Kitayama et al., 1992) |
| hDAT | |||
| LLC-PK1 | 2000 ± 200 | 22 ± 3 | (Zhen and Reith, 2016) |
| CHO hDAT | 5 – 10b | 8 - 9 | (Midde et al., 2015; Slusher et al., 1997) |
| HEK | 1000 – 13,000 | 16 - 22 | (Berfield et al., 1999; Chen et al., 2000; Wang et al., 2003; Zhen et al., 2015,2005) |
| N2a | 2500 ± 300 | 19 - 22 | (Little et al., 2002; Zhang et al., 1998) |
| SK-N-MC | - | 18 ± 4 | (Sweeney et al., 2017) |
fmol/well
pmol/105 cells
The maximal number of β-CFT binding sites, Bmax, vary widely between studies, and as for Vmax, are virtually impossible to compare unless the experiments are conducted under identical conditions and values normalized for DAT expression.
4. Potential mechanisms for kinetic differences between systems
The general similarities of DAT kinetic and biochemical properties in these model systems supports their utility for many types of analyses. However, the finding that kinetic parameters differ for native and heterologously expressed DATs suggests subtle but detectable impacts of system properties on the conformational equilibrium of the protein. During the transport process DAT cycles through multiple conformational states, including outwardly-facing forms that bind extracellular DA, occluded forms generated by extracellular and intracellular gates that control the directionality of substrate movement, inward forms that release substrate to the cell interior, and reorientation of the empty protein back to the outward form for another round of transport (Forrest and Rudnick, 2010; Krishnamurthy et al., 2009; Shimamura et al., 2010). The overall rate of these events defines Vmax, and Km represents the summation of the kinetic rates between these and other likely intermediate forms. These events also pertain to actions of uptake inhibitors, as the outwardly-facing transporter is the preferred conformation for cocaine analog binding (Beuming et al., 2006; Dahal et al., 2014; Krout et al., 2017), while atypical inhibitors may prefer inwardly facing forms (Loland et al., 2008; Reith et al., 2012; Schmitt and Reith, 2011)
Multiple processes control these events, including interactions with regulatory binding partners, post-translational modifications, and cholesterol and membrane raft partitioning (Kristensen et al., 2011; Vaughan and Foster, 2013), and it is likely that differences between brain and cell systems in one or more of these parameters could underlie differential transport kinetics.
For example, several proteins including syntaxin 1A, flotillin 1, DA receptors, and G protein subunits bind to cytoplasmic domains of DAT mechanistically linked to the permeation pathway to regulate forward and reverse transport, and cell models, especially those with few neuronal characteristics, may lack appropriate expression of these partners (Kristensen et al., 2011; Vaughan and Foster, 2013). It has also been postulated that in neurons, translocation of DA across the plasma membrane is directly coupled to transmitter loading into SVs by VMAT2 (Cartier et al., 2010; Egana et al., 2009). This could enhance transport and/or suppress constitutive efflux by reducing levels of free intracellular DA that could re-bind to inwardly-facing transporter forms, with differential impacts on these properties in cells that lack VMAT2 or SVs.
In addition, DAT is tightly regulated by multiple kinase pathways, with N- and C-terminal domains containing sites of phosphorylation and palmitoylation that mediate some of these events (Foster and Vaughan, 2017; Kristensen et al., 2011; Ramamoorthy et al., 2011; Rastedt et al., 2017). Signaling pathways are known to be alterated in immortalized and cancer cell lines (Hanahan and Weinberg, 2011), and model systems may lack the repertoire of enzymes and signals that perform these functions in neurons. We have previously noted differences in DAT glycosylation, phosphorylation, and palmitoylation characteristics between different brain regions and between brain and cell systems (Foster et al., 2003; Foster and Vaughan, 2011; Lew et al., 1992), supporting this idea.
Membrane cholesterol is also key to DAT function, with transport activity being reduced or enhanced, respectively, by depletion or supplementation of cholesterol (Adkins et al., 2007; Foster et al., 2008; Hong and Amara, 2010; Jones et al., 2012). These effects may be exerted by direct sterol interaction with DAT (Penmatsa et al., 2015; Wang et al., 2015; Zeppelin et al., 2018) or by impacts on DAT partitioning to cholesterol-rich membrane rafts that control DAT interactome, phosphorylation, and conformational equilibrium (Adkins et al., 2007; Cremona et al., 2011; Foster et al., 2008; Hong and Amara, 2010; Jones et al., 2012).
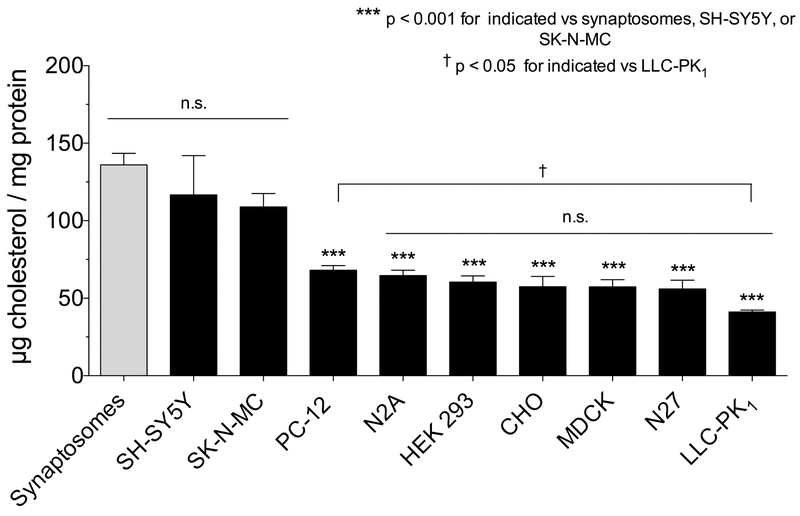
To examine the possibility that the kinetic differences reported between brain and cell systems relate to cholesterol/DAT relationships, we measured membrane cholesterol from several of the model systems available in our labs (Fig. 3). The results show about a 3-fold range in cholesterol content, with highest levels in rat striatal synaptosomes and the neuronally-derived cell lines SH-SY5Y and SK-N-MC (109-136 μg/mg protein; p<0.001 vs LLC-PK1 cells), an intermediate level in PC-12 cells (68 μg/mg protein p<0.05 vs LLC-PK1 cells, p<0.001 vs striatal synaptosomes), and lowest levels in the remaining cells (41-57 μg/mg protein, p<0.001 vs striatal synaptosomes) (Fig. 3). Correlation analysis (Fig. 4A) revealed a statistically significant inverse relationship between membrane cholesterol and Km(DA) (r2=0.78, **p=0.0008), consistent with enhancement of transport. We found no significant correlation across all cell lines between cholesterol and Kd(β-CFT) (r2=0.16, p=0.43) (Fig. 4B), although binding affinities did differ between LLC-PK1 cells and synaptosomes, the systems with the greatest difference in cholesterol content. The apparent ability of cholesterol to affect Km(DA) but not Kd(β-CFT) could indicate that the impact follows from alteration of conformational movements during the transport cycle rather than stabilization of a particular transporter form. These findings thus identify an endogenous biochemical difference between model cells and native tissues that may relate to transport mechanisms.
Fig 3. Membrane cholesterol content from DAT model systems.
Membranes were prepared as previously described from cells (Moritz et al., 2013) or rat striatal synaptosomes (Foster and Vaughan, 2011) and assayed for total cholesterol using a colorimetric total cholesterol kit from Fujifilm Wako Diagnostics (Richmond, VA) and total protein using the BCA method as previously described (Foster et al., 2008). Values shown are means ± SEM (n=3-6). Statistical analysis was performed by ANOVA with a Dunnett’s multiple comparison post-hoc test. ***, p<0.001 indicated cells vs. synaptosomes; †, p<0.05 PC-12 cells vs. LLC-PK1 cells; n.s. no statistical difference for indicated groupings.
5.0. Conclusions
The relative similarities of transport and binding values obtained in these model systems indicates their general usefulness for many types of studies on DAT structure and function. Primary neurons and cells that endogenously express DAT and other DA machinery may be more suited for regulatory studies and analyses that use approaches such as imaging and electrophysiology that do not require large numbers of cells. Heterologous expression systems that can generate higher levels of the transporter may be more suited for biochemical or protein isolation analyses but may not faithfully recapitulate neuronal regulatory events that can impact functional outcomes. Such issues are key for interpretation of transporter alterations in disease and drug abuse, and will be aided by continuing elucidation of DAT characteristics.
Highlights:
In the CNS DAT performs crucial functions in DA neurotransmission where it mediates functions such as movement, reward, and cognition.
Dysregulation of DAT function is implicated in mood disorders and psychostimulant drug addiction.
Here we discuss advantages, disadvantages, and potential differential regulatory processes in several commonly-used model systems for in vitro analysis of DAT structure, function, and regulation.
Acknowledgements
Work from the authors’ laboratories was supported by grants from the National Institutes of Health DA 031991 (JDF), DA13147 and 5P20-104360 (RAV), P30-GM103329 (UND), P20-GM12345 (UND).
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, Ramamoorthy S, Gether U, 2007. Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry 46, 10484–10497. [DOI] [PubMed] [Google Scholar]
- Afonso-Oramas D, Cruz-Muros I, de la Rosa DÁ, Abreu P, Giráldez T, Castro-Hernández J, Salas-Hernández J, Lanciego JL, RodrÍguez M, González-Hernández T, 2009. Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson’s disease. Neurobiol. Dis 36, 494–508. [DOI] [PubMed] [Google Scholar]
- Aruffo A, 2002. Expression of Proteins in Mammalian Cells: Transient Expression of Proteins. Basic Protoc. Protein Expr 1–7. [Google Scholar]
- Berfield JL, Wang LC, Reith MEA, 1999. Which Form of Dopamine Is the Substrate for the Human Dopamine Transporter: the Cationic or the Uncharged Species. J Biol Chem 274, 4876–4882. [DOI] [PubMed] [Google Scholar]
- Bermingham DP, Hardaway JA, Refai O, Marks CR, Snider SL, Sturgeon SM, Spencer WC, Colbran RJ, Miller DM, Blakely RD, 2017. The Atypical MAP Kinase SWIP-13/ERK8 Regulates Dopamine Transporters through a Rho-Dependent Mechanism. J. Neurosci 37, 9288–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Shi L, Javitch J, Weinstein H, 2006. A Comprehensive Structure-Based Alignment of Prokaryotic and Eukaryotic Neurotransmitter/Na+ Symporters (NSS) Aids in teh Use of the LeuT Structure to Probe NSS Structure Function. Mol. Pharmacol 70, 1630–1642. [DOI] [PubMed] [Google Scholar]
- Beveridge TJR, Smith HR, Nader MA, Porrino LJ, 2009. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology 34, 1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J, Helson L, Spengler B, 1973. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 33, 2643–2652. [PubMed] [Google Scholar]
- Biedler J, Roffler-Tarlov S, Schachner M, Freedman L, 1978. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 38, 3751–3757. [PubMed] [Google Scholar]
- Blakely RD, Bauman AL, 2000. Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol 10, 328–36. [DOI] [PubMed] [Google Scholar]
- Block ER, Nuttle J, Balcita-Pedicino JJ, Caltagarone J, Watkins SC, Sesack SR, Sorkin A, 2015. Brain Region-Specific Trafficking of the Dopamine Transporter. J. Neurosci 35, 12845–12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton RL, Clarke DJ, 1999. Apoptosis in Primary Cultures of E14 Rat Ventral Mesencephala: Time Course of Dopaminergic Cell Death and Implications for Neural Transplantation. Exp. Neurol 98, 88–98. [DOI] [PubMed] [Google Scholar]
- Cartier E, Hamilton PJ, Belovich AN, Shekar A, Campbell NG, Saunders C, Andreassen TF, Gether U, Veenstra-Vanderweele J, Sutcliffe JS, Ulery-Reynolds PG, Erreger K, Matthies HJG, Galli A, 2015. Rare Autism-Associated Variants Implicate Syntaxin 1 (STX1 R26Q) Phosphorylation and the Dopamine Transporter (hDAT R51W) in Dopamine Neurotransmission and Behaviors. EBioMedicine 2, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier EA, Parra LA, Baust TB, Quiroz M, Salazar G, Faundez V, Egaña L, Torres GE, 2010. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J. Biol. Chem 285, 1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, McDonald PW, Blakely RD, DeFelice LJ, 2004. Dopamine Transporters Depolarize Neurons by a Channel Mechanism. Proc. Natl. Acad. Sci 101, 16046–16051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeblundberg FLM, Merrill G.a, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A, 2002. PI 3-kinase regulation of dopamine uptake. J Neurochem 81, 859–869. [DOI] [PubMed] [Google Scholar]
- Chen N, Appell M, Berfield JL, Reith MEA, 2003. Inhibition by arachidonic acid and other fatty acids of dopamine uptake at the human dopamine transporter. Eur. J. Pharmacol 478, 89–95. [DOI] [PubMed] [Google Scholar]
- Chen N, Ferrer JV, Javitch JA, Justice JB, 2000. Transport-dependent accessibility of a cytoplasmic loop cysteine in the human dopamine transporter. J. Biol. Chem 275, 1608–1614. [DOI] [PubMed] [Google Scholar]
- Chen N, Trowbridge CG, Justice JB, 1999. Cationic modulation of human dopamine transporter: dopamine uptake and inhibition of uptake. J. Pharmacol. Exp. Ther 290, 940–949. [PubMed] [Google Scholar]
- Chinta SJ, Andersen JK, 2005. Dopaminergic neurons. Int. J. Biochem. Cell Biol 37, 942–946. [DOI] [PubMed] [Google Scholar]
- Cho S, Wood A, Bowlby M, 2007. Brain Slices as Models for Neurodegenerative Disease and Screening Platforms to Identify Novel Therapeutics. Curr. Neuropharmacol 5, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W-S, Kim H-W, Xia Z, 2013. Preparation of Primary Cultured Dopaminergic Neurons from Mouse Brain. Neural Dev. 61–69. [DOI] [PubMed] [Google Scholar]
- Clarkson ED, Rosa FG, Edwards-Prasad J, Weiland DA, Witta SE, Freed CR, Prasad KN, 1998. Improvement of neurological deficits in 6-hydroxydopamine-lesioned rats after transplantation with allogeneic simian virus 40 large tumor antigen gene-induced immortalized dopamine cells. Proc Natl Acad Sci 95, 1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona LM, Matthies HJG, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, Rothman JE, Galli A, Javitch JA, Yamamoto A, 2011. Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat. Neurosci 14, 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal RA, Pramod AB, Sharma B, Krout D, Foster JD, Cha JH, Cao J, Newman AH, Lever JR, Vaughan RA, Henry LK, 2014. Computational and biochemical docking of the irreversible cocaine analog RTI 82 directly demonstrates ligand positioning in the dopamine transporter central substrate-binding site. J. Biol. Chem 289, 29712–29727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar DE, Mayo C, Uhl GR, 2005. The interaction of methylphenidate and benztropine with the dopamine transporter is different than other substrates and ligands. Biochem. Pharmacol 70, 461–469. [DOI] [PubMed] [Google Scholar]
- Dar DE, Metzger TG, Vandenbergh DJ, Uhl GR, 2006. Dopamine uptake and cocaine binding mechanisms: The involvement of charged amino acids from the transmembrane domains of the human dopamine transporter. Eur. J. Pharmacol 538, 43–47. [DOI] [PubMed] [Google Scholar]
- Doolen S, Zahniser NR, 2001. Protein tyrosine kinase inhibitors alter human dopamine transporter activity in Xenopus oocytes. J Pharmacol Exp Ther 296, 931–938. [PubMed] [Google Scholar]
- Egana LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S, Pena K, Quiroz M, Hong WC, Dorostkar MM, Janz R, Sitte HH, Torres GE, 2009. Physical and Functional Interaction between the Dopamine Transporter and the Synaptic Vesicle Protein Synaptogyrin-3. J. Neurosci. 29, 4592–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Neve RL, Janowsky A, 1995. Characteritzation of a recombinant human dopamine transporter in multiple cell lines. J. Pharmacol. Exp. Ther 274, 276–283. [PubMed] [Google Scholar]
- Evans GJO, 2018. Subcellular Fractionation of the Brain: Preparation of Synaptosomes and Synaptic Vesicles. CSH Protoc. 6. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, España RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, Jones SR, 2014. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc. Natl. Acad. Sci 111, E2751–E2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LR, Rudnick G, 2010. The Rocking Bundle: A Mechanism for Ion-Coupled Solute Flux by Symmetrical Transporters. Physiol. 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Adkins S, Lever J, Vaughan R, 2008. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J. Neurochem. 105, 1683–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Pananusorn B, Cervinski MA, Holden HE, Vaughan RA, 2003. Dopamine transporters are dephosphorylated in striatal homogenates and in vitro by protein phosphatase 1. Mol. Brain Res 110, 100–108. [DOI] [PubMed] [Google Scholar]
- Foster JD, Vaughan RA, 2017. Phosphorylation mechanisms in dopamine transporter regulation. J Chem Neuroanat 83–84, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Vaughan RA, 2011. Palmitoylation controls dopamine transporter kinetics, degradation, and protein kinase C-dependent regulation. J. Biol. Chem 286, 5175–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Yang JW, Moritz AE, ChallaSivaKanaka S, Smith MA, Holy M, Wilebski K, Sitte HH, Vaughan RA, 2012. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J. Biol. Chem 287, 29702–29712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG, 1997. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J. Neurochem 69, 1322–1325. [DOI] [PubMed] [Google Scholar]
- Gao L, Zhou W, Symmes B, Freed CR, 2016. Re-Cloning the N27 Dopamine Cell Line to Improve a Cell Culture Model of Parkinson’s Disease. PLoS One 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Olivares J, Torres-Salazar D, Owens WA, Baust T, Siderovski DP, Amara SG, Zhu J, Daws LC, Torres GE, 2013. Inhibition of Dopamine Transporter Activity by G Protein βγ Subunits. PLoS One 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German CL, Hanson GR, Fleckenstein AE, 2012. Amphetamine and methamphetamine reduce striatal dopamine transporter function without concurrent dopamine transporter relocalization. J. Neurochem 123, 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jarber M, Jones SR, Wightman M, Caron MG, 1996. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612. [DOI] [PubMed] [Google Scholar]
- Gluzman Y, 1981. SV-40 Transformed Simian Cells Support the Replication of SV40 Mutants. Cell 23, 175–182. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos TN, Jones SM, Yoshimura M, Hoover BR, Das M, Snyder EY, Larson G. a, Zahniser NR, Tabakoff B, Zawada WM, 2010. Neurotransplantation of stem cells genetically modified to express human dopamine transporter reduces alcohol consumption. Stem Cell Res. Ther 1, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JG, 2006. Gene expression profiles of brain dopamine neurons and relevance to neuropsychiatric disease. J. Physiol 575, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Rein G, 1977. Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheo-chromocytoma cells. Brain Res 129, 247–263. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS, 1976. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73, 2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Wall SC, Rudnick G, 1994. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J. Biol. Chem 269, 7124–7130. [PubMed] [Google Scholar]
- Guptaroy B, Fraser R, Desai A, Zhang M, Gnegy ME, 2011. Site-directed mutations near transmembrane domain 1 alter conformation and function of norepinephrine and dopamine transporters. Mol. Pharmacol. 79, 520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MK, Blakely RD, 2002. Monoamine transporter gene structure and polymorphisms in relation to psychiatric and other complex disorders. Pharmacogenomics J. 2, 217–235. [DOI] [PubMed] [Google Scholar]
- Hamilton PJ, Belovich AN, Khelashvili G, Saunders C, Erreger K, Javitch JA, Sitte HH, Weinstein H, Matthies HJG, Galli A, 2015. PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat. Chem. Bio 10, 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2011. Hallmarks of Cancer: The Next Generation. Cell 144, 646–647. [DOI] [PubMed] [Google Scholar]
- Hara S, Arawaka S, Sato H, Machiya Y, Cui C, Sasaki A, Koyama S, Kato T, 2013. Serine 129 phosphorylation of membrane-associated -synuclein modulates dopamine transporter function in a G protein-coupled receptor kinase-dependent manner. Mol. Biol. Cell 24, 1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty SV, Sullivan AM, O’Keeffe GW, Keeffe GWO, 2013. Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev. Biol 379, 123–138. [DOI] [PubMed] [Google Scholar]
- Hong WC, Amara SG, 2010. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J. Biol. Chem 285, 32616–32626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM, 2002. Functional imaging and neurochemical correlates of stimulant self-administration in primates. Psychopharmacology (Berl). 163, 352–361. [DOI] [PubMed] [Google Scholar]
- Huang CL, Chen HC, Huang NK, Yang DM, Kao LS, Chen JC, Lai HL, Chern Y, 1999. Modulation of dopamine transporter activity by nicotinic acetylcholine receptors and membrane depolarization in rat pheochromocytoma PC12 cells. J Neurochem 72, 2437–2444. [DOI] [PubMed] [Google Scholar]
- Hull RN, Cherry WR, Weaver GW, 1976. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro 12, 670–677. [DOI] [PubMed] [Google Scholar]
- Inyushin MU, Arencibia-Albite F, Cruz A.de la, Vazquez-Torres R, Colon K, Sanabria P, Jimenez-Rivera CA, 2013. New method to visualize neurons with DAT in slcies of rat VTA using fluorescent substrate for DAT, ASP+. J Neurosci Neuroeng. 6, 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL, 2007. Dopamine: 50 years in perspective. Trends Neurosci 30, 188–193. [DOI] [PubMed] [Google Scholar]
- Javitch JA, Snyder SH, 1984. Uptake of MPP(+) by dopamine neurons explains selectivity of parkinsonism-inducing neurotoxin, MPTP. Eur J Pharmacol 106, 455–456. [DOI] [PubMed] [Google Scholar]
- Jiang H, Jiang Q, Feng J, 2004. Parkin Increases Dopamine Uptake by Enhancing the Cell Surface Expression of Dopamine Transporter. J. Biol. Chem 279, 54380–54386. [DOI] [PubMed] [Google Scholar]
- Jones KT, Woods C, Zhen J, Antonio T, Carr KD, Reith MEA, 2017. Effects of diet and insulin on dopamine transporter activity and expression in rat caudateputamen, nucleus accumbens, and midbrain. J. Neurochem 140, 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Zhen J, Reith MEA, 2012. Importance of cholesterol in dopamine transporter function. J. Neurochem 123, 700–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MC, Madras BK, 1993. [3H]CFT ([3H]win 35,428) accumulation in dopamine regions of monkey brain: comparison of a mature and an aged monkey. Brain Res. 611, 322–325. [DOI] [PubMed] [Google Scholar]
- Kearns SM, Scheffler B, Goetz AK, Lin DD, Baker HD, Roper SN, Mandel RJ, Steindler DA, 2006. A method for a more complete in vitro Parkinson’s model: Slice culture bioassay for modeling maintenance and repair of the nigrostriatal circuit. J. Neurosci. Methods 157, 1–9. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Carroll FI, Kuhar MJ, 2000. Dopamine transporter synthesis and degradation rate in rat striatum and nucleus accumbens using RTI-76. Neuropharmacology 39, 578–85. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Shimada S, Xu H, Markham L, Donovan DM, Uhl GR, 1992. Dopamine transporter site-directed mutations differentially alter substrate transport and cocaine binding. Proc. Natl. Acad. Sci. USA 89, 7782–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivell B, Uzelac Z, Sundaramurthy S, Rajamanickam J, Ewald A, Chefer V, Jaligam V, Bolan E, Simonson B, Annamalai B, Mannangatti P, Prisinzano TE, Gomes I, Devi LA, Jayanthi LD, Sitte HH, Ramamoorthy S, Shippenberg TS, 2014. Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1 / 2-dependent mechanism. Neuropharmacology 86, 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Langford D, 2013. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Piscitelli CL, Gouaux E, 2009. Unlocking the molecular secrets of sodium-coupled transporters. Nature 459, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jørgensen TN, Sørensen L, Eriksen J, Loland CJ, Strømgaard K, 2011. SLC6 Neurotransmitter Transporters: Structure, Function, and Regulation. Pharmacol. Rev 63, 585–640. [DOI] [PubMed] [Google Scholar]
- Krout D, Rodriquez M, Brose SA, Golovko MY, Henry LK, Thompson BJ, 2017. Inhibition of the Serotonin Transporter Is Altered by Metabolites of Selective Serotonin and Norepinephrine Reuptake Inhibitors and Represents a Caution to Acute or Chronic Treatment Paradigms. ACS Chem. Neurosci 8, 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T, Yang Y, Ng SK, 2013. Advances in Mammalian cell line development technologies for recombinant protein production. Pharm. 6, 579–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJS, Pristupa ZB, Ciliax BJ, Levey AI, Niznik HB, 1996. The dopamine transporter Carboxyl-terminal Tail truncation/substitution mutants selectively confer high affinity dopamine uptake while attenuating recognition of the ligand binding domain 20885–20894. [DOI] [PubMed] [Google Scholar]
- Leighton J, Estes LW, Mansukhani S, Brada Z, 1970. A cell line derived from normal dog kidney (MDCK) exhibiting qualities of papillary adenocarcinoma and of renal tubular epithelium. Cancer 26, 1022–1028. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino L, 2001. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci 21, 2799–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew R, Patel A, Vaughan RA, Wilson A, Kuhar MJ, 1992. Microheterogeneity of dopamine transporters in rat striatum and nucleus accumbens. Brain Res 584, 266–71. [DOI] [PubMed] [Google Scholar]
- Li Y, Mayer FP, Hasenhuetl PS, Burtscher V, Schicker K, Sitte HH, Freissmuth M, Sandtner W, 2017. Occupancy of the zinc-binding site by transition metals decreases the substrate affinity of the human dopamine transporter by an allosteric mechanism. J. Biol. Chem 292, 4235–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Itokawa M, Uhl GR, 2000. Dopamine transporter proline mutations influence dopamine uptake, cocaine analog recognition, and expression. FASEB J. 14, 715–28. [DOI] [PubMed] [Google Scholar]
- Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L, 2002. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol. Pharmacol 61, 436–445. [DOI] [PubMed] [Google Scholar]
- Little KY, Kirkman JA, Carroll FI, Clark B, Duncan GE, 1993. Cocaine use increases [3H]WIN 35428 binding sites in human striatum. Brain Res. 628, 17–25. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou M, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U, 2008. Relationship between Conformational Changes in the Dopamine Transporter and Cocaine-Like Subjective Effects of Uptake Inhibitors. Mol. Pharmacol 73, 813–823. [DOI] [PubMed] [Google Scholar]
- Louis N, Evelegh C, Graham FL, 1997. Cloning and sequencing of the cellular-viral junctions from the human adenovirus type 5 transformed 293 cell line. Virology 233, 423–429. [DOI] [PubMed] [Google Scholar]
- Lu Y, Driscoll N, Ozden I, Yu Z, Nurmikko AV, 2015. Modulating dopamine release by optogenetics in transgenic mice reveals terminal dopaminergic dynamics. Neurophotonics 2, 031207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Spealman D, Fahey MA, Neumeyer JL, Saha JK, Milius RA, 1989. Cocaine Receptors Labeled by [3H]2B-carbomethoxy-3B-(1-fluorophenyl)tropane. Mol. Pharmacol 518–524. [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasse S, 2002. Dopamine transport function is elevated in cocaine users. J. Neurochem 81, 292–300. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Maiya R, Keller D, Zahniser NR, 2001. Ethanol potentiates the function of the human dopamine transporter expressed in Xenopus oocytes. J Neurochem 79, 1070–1079. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Blakely RD, 2005. Expression studies of naturally occurring human dopamine transporter variants identifies a novel state of transporter inactivation associated with Val382Ala. Neuropharmacology 49, 737–749. [DOI] [PubMed] [Google Scholar]
- Mebel DM, Wong JCY, Dong YJ, Borgland SL, 2012. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur. J. Neurosci 36, 2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikian HE, Buckley KM, 1999. Membrane trafficking regulates the activity of the human dopamine transporter. J. Neurosci 19, 7699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Yuan Y, Quizon PM, Sun WL, Huang X, Zhan CG, Zhu J, 2015. Mutations at Tyrosine 88, Lysine 92 and Tyrosine 470 of Human Dopamine Transporter Result in an Attenuation of HIV-1 Tat-Induced Inhibition of Dopamine Transport. J. Neuroimmune Pharmacol. 10, 122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikelman SR, Guptaroy B, Gnegy ME, 2017. Tamoxifen and its active metabolites inhibit dopamine transporter function independently of the estrogen receptors. J. Neurochem 141, 31–36. [DOI] [PubMed] [Google Scholar]
- Miller GW, Gainetdinov RR, Levey AI, Caron MG, 1999. Dopamine transporters and neuronal injury. Trends Pharmacol Sci 20, 424–429. [DOI] [PubMed] [Google Scholar]
- Moritz AE, Foster JD, Gorentla BK, Mazei-Robison MS, Yang JW, Sitte HH, Blakely RD, Vaughan RA, 2013. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. J. Biol. Chem 288, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz AE, Rastedt DE, Stanislowski DJ, Shetty M, Smith MA, Vaughan RA, Foster JD, 2015. Reciprocal phosphorylation and palmitoylation control dopamine transporter kinetics. J. Biol. Chem 290, 29095–29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morón J. a, Zakharova I, Ferrer JV, Merrill G. a, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch J. a, Galli A, Shippenberg TS, 2003. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J. Neurosci 23, 8480–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM 3rd, Blakely RD, 2002. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci USA 99, 3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickeli VM, 1996. The Dopamine Transporter Is Localized to Dendritic and Axonal Plasma Membranes of Nigrostriatal Dopaminergic Neurons. J. Neurosci 76, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AP, Cerruti C, Vaughan RA, Kuhar MJ, 1994. Developmentally regulated glycosylation of dopamine transporter. Brain Res Dev 83, 53–8. [DOI] [PubMed] [Google Scholar]
- Penmatsa A, Wang KH, Gouaux E, 2015. X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat Struct Mol Biol 22, 506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifl C, Giros B, Caron MG, 1993. Dopamine transporter expression confers cytotoxicity to low doses of the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium. J. Neurosci 13, 4246–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifl C, Hornykiewicz O, Giros B, Caron MG, 1996. Catecholamine transporters and 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine neurotoxicity: Studies comparing the cloned human noradrenaline and human dopamine transporter. J. Pharmacol. Exp. Ther 277. [PubMed] [Google Scholar]
- Pramod AB, Foster J, Carvelli L, Henry LK, 2013. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Asp. Med 34, 197–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KN, Carvalho E, Kentroti S, Edwards-Prasad J, Freed C, Vernadakis A, 1994. Establishment and characterization of immortalized clonal cell lines from fetal rat mesencephalic tissue. Vitr. Cell Dev Biol Anim 30A, 596–603. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD, 2011. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol. Ther 129, 220–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Sorkin A, Zahniser NR, 2013. Mice expressing markedly reduced striatal dopamine transporters exhibit increased locomotor activity, dopamine uptake turnover rate and cocaine responsiveness. Synapse 67, 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastedt DE, Vaughan RA, Foster JD, 2017. Palmitoylation mechanisms in dopamine transporter regulation. J Chem Neuroanat 83–84, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith MEA, Ali S, Hashim A, Sheikh IS, Theddu N, Gaddiraju NV, Mehrotra S, Schmitt KC, Murray TF, Sershen H, Unterwald EM, Davis FA, 2012. Novel C-1 Substituted Cocaine Analogs Unlike Cocaine or Benztropine. J. Pharmacol. Exp. Ther 343, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Zahniser NR, 2009. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J. Neurochem 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A, Horlick R, 1998. Macrophage scavenger receptor confers an adherent phenotype to cells in culture. Biotechniques 2, 240–244. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Adams CE, Burmeister J, Gerhardt GA, Zahniser NR, 2002. Kinetic analysis of striatal clearance of exogenous dopamine recorded by chronoamperometry in freely-moving rats. J. Neurosci. Methods 121, 41–52. [DOI] [PubMed] [Google Scholar]
- Safratowich BD, Hossain M, Bianchi L, Carvelli L, 2014. Amphetamine Potentiates the Effects of B-Phenylethylamine through Activation of an Amine-Gated Chloride Channel. J. Neurosci 34, 4686–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salto R, Vilchez JD, Giron MD, Cabrera E, Campos N, Manzano M, Rueda R, Lopez-Pedrosa JM, 2015. beta-Hydroxy-beta-Methylbutyrate (HMB) Promotes Neurite Outgrowth in Neuro2a Cells. PLoS One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith MEA, 2011. The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Morse S, Ararat M, Graham FL, 2002. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB 16, 869–871. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MSP, Iwata S, Henderson PJF, Cameron AD, 2010. Molecular Basis of Alternating Access Membrane Transport by the Sodium-Hydantoin Transporter Mhp1. Science (80-.). 328, 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu A, 1997. Regulation and expression of D-1 but not D-5, dopamine receptors in human SK-N-MC neuroblastoma cells. J. Recept Signal Transduct Res 5, 777–784. [DOI] [PubMed] [Google Scholar]
- Slusher BS, Tiffany CW, Olkowski JL, Jackson PF, 1997. Use of identical assay conditions for cocaine analog binding and dopamine uptake to identify potential cocaine antagonists. Drug Alcohol Depend. 48, 43–50. [DOI] [PubMed] [Google Scholar]
- Speed N, Saunders C, Davis AR, Owens WA, Matthies HJG, Saadat S, Kennedy JP, Vaughan RA, Neve RL, Lindsley CW, Russo SJ, Daws LC, Niswender KD, Galli A, 2011. Impaired striatal akt signaling disrupts dopamine homeostasis and increases feeding. PLoS One 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC, 1994. High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. J. Pharmacol. Exp. Ther. 271, 1678–1685. [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A, 2005. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 75, 406–433. [DOI] [PubMed] [Google Scholar]
- Sweeney CG, Tremblay BP, Stockner T, Sitte HH, Melikian HE, 2017. Dopamine transporter amino and carboxyl termini synergistically contribute to substrate and inhibitor affinities. J. Biol. Chem. 292, 1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QY, Kolanos R, De Felice LJ, Glennon RA, 2015. Structural Analysis of Dopamine- and Amphetamine-Induced Depolarization Currents in the Human Dopamine Transporter. ACS Chem. Neurosci 6, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay RG, Sikorska M, Sandhu JK, Lanthier P, Ribecco-Lutkiewicz M, Bani-Yaghoub M, 2010. Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci. Methods 186, 60–67. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Foster JD, 2013. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci 34, 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan RA, Huff RA, Uhl GR, Kuhar MJ, 1997. Protein kinase C-mediated phosphorylation and functional regulation of dopamine transporters in striatal synaptosomes. J. Biol. Chem 272, 15541–6. [DOI] [PubMed] [Google Scholar]
- Vickrey TL, Xiao N, Venton BJ, 2013. Kinetics of the dopamine transporter in Drosophila larva. ACS Chem. Neurosci 4, 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, Hanson GR, Fleckenstein AE, 2009. Measurement of plasmalemmal dopamine transport, vesicular dopamine transport, and K+- stimulated dopamine release in frozen rat brain tissue. J. Neurosci. Methods 180, 317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Cui XN, Chen N, Reith MEA, 2003. Binding of cocaine-like radioligands to the dopamine transporter at 37°C: Effect of Na+and substrates. J. Neurosci. Methods 131, 27–33. [DOI] [PubMed] [Google Scholar]
- Wang W, Runkle KB, Terkowski SM, Ekaireb RI, Witze ES, 2015. Protein depalmitoylation is induced by Wnt5a and promotes polarized cell behavior. J. Biol. Chem 290, 15707–15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DS, Ebben AL, Kurtoglu B, Lovell ME, Bohn AT, Jasek IA, Baker DA, Mantsch JR, Gasser PJ, Wheeler RA, 2017. Corticosterone regulates both naturally occurring and cocaine-induced dopamine signaling by selectively decreasing dopamine uptake. Eur. J. Neurosci 46, 2638–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DS, Underhill SM, Stolz DB, Murdoch GH, Thiels E, Romero G, Amara SG, 2015. Amphetamine activates Rho GTPase signaling to mediate dopamine transporter internalization and acute behavioral effects of amphetamine. PNAS 112, E7138–E7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V, Michaelson I, Kirkland R, 1964. The separation of synaptic vesicles from nerve-ending particles (‘synaptosomes’). Biochem. J 90, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Rowlett JK, Wilcox KM, Paul IA, Kline RH, Newman AH, Katz JL, 2000. 3’- and 4’-Chloro-Substituted Analogs of Benztropine: Intravenous Self-Administration and in Vitro Radioligand Binding Studies in Rhesus Monkeys. Psychopharmacology (Berl). 147, 426–35. [DOI] [PubMed] [Google Scholar]
- Wu S, Fagan RR, Uttamapinant C, Lifshitz LM, Fogarty KE, Ting AY, Melikian HE, 2017. The Dopamine Transporter Recycles Via A Retromer-Dependent Post-Endocytic Mechanism: Tracking Studies Using A Novel Fluorophore-Coupling Approach. J. Neurosci 37, 3885–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gu HH, 1999. Molecular cloning of the mouse dopamine transporter and pharmacological comparison with the human homologue. Gene 233, 163–170. [DOI] [PubMed] [Google Scholar]
- Wurm FM, 2004. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 22, 1393–1398. [DOI] [PubMed] [Google Scholar]
- Xicoy H, Wieringa B, Martens GJ, 2017. The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener 12, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Quizon PM, Sun WL, Yao J, Zhu J, Zhan CG, 2016. Role of Histidine 547 of Human Dopamine Transporter in Molecular Interaction with HIV-1 Tat and Dopamine Uptake. Sci. Rep 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA, 1999. In Vivo Dopamine Clearance Rate in Rat Striatum: Regulation by Extracellular Dopamine Concentration and Dopamine Transporter Inhibitors. J. Pharmacol. Exp. Ther 289, 266–277. [PubMed] [Google Scholar]
- Zeppelin T, Ladefoged LK, Sinning S, Periole X, Schiøtt B, 2018. A direct interaction of cholesterol with the dopamine transporter prevents its out-to-inward transition. PLoS Comput. Biol 14, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Elmer L, Little KY, 1998. Expression and regulation of the human dopamine transporter in a neuronal cell line. Brain Res Mol Brain Res 59, 66–73. [DOI] [PubMed] [Google Scholar]
- Zhen J, Antonio T, Cheng SY, Ali S, Jones KT, Reith MEA, 2015. Dopamine transporter oligomerization: Impact of combining protomers with differential cocaine analog binding affinities. J. Neurochem 133, 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Chen N, Reith MEA, 2005. Differences in interactions with the dopamine transporter as revealed by diminishment of Na+ gradient and membrane potential: Dopamine versus other substrates. Neuropharmacology 49, 769–779. [DOI] [PubMed] [Google Scholar]
- Zhen J, Reith MEA, 2016. Impact of disruption of secondary binding site S2 on dopamine transporter function. J. Neurochem 138, 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SJ, Kavanaugh MP, Sonders MS, Amara SG, Zahniser NR, 1997. Activation of protein kinase C inhibits uptake, currents and binding associated with the human dopamine transporter expressed in Xenopus oocytes. J Pharmacol Exp Ther 282, 1358–1365. [PubMed] [Google Scholar]