Abstract
Objective
To investigate factors associated with delay in initiation of initial disease-modifying antirheumatic drug (DMARD) in patients newly diagnosed with rheumatoid arthritis (RA).
Methods
We performed a retrospective cohort descriptive study using administrative data from the US military’s TRICARE program (2007–2012). We identified incident RA cases using billing codes and initial DMARD receipt using prescription fill date. We quantified the time between RA presentation and initial DMARD receipt, evaluated temporal changes in delay over the study period, and investigated predictors of treatment delay (>90 days) using logistic regression.
Results
We identified 16,680 patients with incident RA that were prescribed DMARDs and mean age was 47.2 (SD 13.5) years. The mean time from initial RA presentation to first DMARD prescription receipt was 125.3 days (SD 175.4). Over one-third (35.6%) of incident RA patients initiated DMARD >90 days after presentation. There was less treatment delay in later years of the study (mean days to DMARD of 144.7 days in 2007; 109.7 days in 2012). Patients prescribed opioids had mean time to DMARD of 212.8 days (SD 207.4) compared to mean of 77.3 days (SD 132.3) for those who did not use opioids (p<0.0001). Patients prescribed opioids between RA presentation and initial DMARD receipt were more likely to have delay in initial DMARD (OR 4.07, 95% CI: 3.78–4.37).
Conclusion
In this large US nationwide study, delays in initial DMARD receipt for incident RA were common but time to treatment improved in recent years. While further analysis using clinical data is warranted, these findings suggest that limiting opioid use in patients newly presenting with RA may decrease delay in initiating DMARDs.
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by polyarthritis, typically of the small joints of the hands and feet. Long-term detrimental outcomes of RA include bone erosions, joint deformities, disability, multimorbidity, and early mortality (1–3). Overall prevalence of RA in the US has been estimated to be as high as 1%, (4) affecting up to 1.5 million individuals in the United States (2,3,5).
Guidelines from both the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) recommend that DMARD treatment should commence as soon as possible once RA is diagnosed (6, 7). Early DMARD treatment is associated with significant reduction of long-term joint damage (8–13). Conversely, treatment delay has been associated with greater risk of chronic joint destruction, disability, lower rates of DMARD-free remission, and greater likelihood of chronic pain (13). However even given these findings, delays in commencement of DMARD therapy persist (14–17). Concurrent and/or intervening prescriptions for other drugs might play a role (18, 19) in lengthening delays.
Given this abundance of evidence supporting early DMARD initiation upon diagnosis of RA, this study sought to assess delays in treatment, i.e., time from initial presentation to commencement of DMARD therapy. We analyzed beneficiaries with RA who are insured by the US military’s TRICARE program, a large, previously unexamined, universally insured population. We also sought to analyze the factors associated with delay in DMARD. We hypothesized that delay in DMARD receipt would improve over time and that use of analgesics, such as opioids and NSAIDs, after initial presentation for RA would be associated with delay in DMARD receipt. Additionally, because timeliness of care is a continual focus of the Military Health System (20), information regarding the extent of and predictors for treatment delays is important for its decision makers.
1. METHODS
1.1 Sample population and data source
We performed a retrospective cohort descriptive study to quantify delays and evaluate temporal trends in prescribing DMARDS for newly-diagnosed cases of RA. We used administrative claims from the US military’s TRICARE program for fiscal years 2007–2012. TRICARE is the health care program for approximately 9.5 million military active duty members, retirees, and their dependents: it is separate from the Veterans Health Administration healthcare system. The sample for this study was drawn from the approximately 5 million beneficiaries enrolled in either TRICARE Prime or TRICARE Plus. Both of these programs are similar to health maintenance organizations, where beneficiaries are assigned a primary care manager. TRICARE Plus allows Medicare-eligible beneficiaries to continue using TRICARE Prime for primary care. These enrollees receive care in both military-managed facilities (direct care) and civilian sector facilities (purchased care), so administrative data were available for both settings. Additionally, care is well documented since these enrollees are assigned a primary care manager, and thus are likely to receive most of their care within TRICARE. Finally, low or absent patient copayments/deductibles suggest that cost of care is less likely to be a factor in seeking care for this population. The study was performed under the purview of the Comparative Effectiveness and Provider Induced Demand Collaboration, which partners the Uniformed Services University of Health Sciences and Brigham and Women’s Hospital. Data for this study were obtained under a data sharing agreement with the Defense Health Agency along with Institutional Review Board approval to use TRICARE program data that is maintained in the Military Health System Data Repository, including the Prescription Transaction Data System.
The primary inclusion criteria for the study population were: 1) age 18 years or older; 2) initial RA billing code (ICD-9CM) of 714.XX documented in any care setting (inpatient/outpatient; purchased/direct care); and 3) prescription for first DMARD within 24 months after appearance of an initial RA billing code. We defined the date of the initial RA billing code as the index date. We only analyzed those who were prescribed a DMARD since individuals who received RA billing codes but no RA-related medications may not have been diagnosed with RA. Use of RA billing codes and DMARD receipt has been validated as accurate for identifying RA in administrative databases (21).
To ensure we captured only initial RA presentations, we excluded RA presentations when either a previous DMARD prescription or a prior RA code was found in the preceding 180 days. Our definition of DMARD was broadened slightly to include key anti-inflammatory drugs often used in RA. We identified the following DMARDs by American Hospital Formulary Service (AHFS) therapeutic class 082000, 923600, 924400, and 081824: abatacept, adalimumab, anakinra, auranofin, azathioprine, cyclosporine, etanercept, gold sodium thiomalate, golimumab, hydroxychloroquine, infliximab, leflunomide, methotrexate, mycophenolate mofetil, mycophenolic acid, penicillamine, rituximab, sulfasalazine, tocilizumab, and tofacitinib. Hereafter in the paper, the term DMARD will refer to these drugs.
1.2 Primary outcome: days to initial DMARD receipt
Our outcome of interest was time between initial presentation for RA (index date) and first DMARD receipt. We quantified this as time in days between initial RA billing code and the first filled prescription of DMARD.
1.3 Secondary outcome: delay in initial DMARD receipt (>90 days)
We defined delay in treatment as receipt of DMARD >90 days after initial RA billing code to investigate variables associated with longer delay using logistic regression. We chose this dichotomous cutoff based on established guidelines recommending early treatment within 3 months of presentation (22, 23).
1.4 Covariates
Demographic and clinical covariates including and up to 12 months prior to the index date were: age [investigated in categories (18–24, 25–34, 35–44, 45–64, and 65+)], sex, sponsor military rank (junior/senior officer, junior/senior enlisted), dependent status, patient relationship to sponsor (self, spouse, dependent), care setting (purchased, direct), US geographic region (north, south, west), fiscal year, Charlson Comorbidity Index (CCI) (24) (used as a categorical variable: 0, 1, >1), and number of medical visits (0, 1, 2–5, 6+). The rank covariate represents rank of the sponsor, meaning that a dependent of a senior enlisted would be categorized as such: this can be considered a proxy for SES. We considered the 180 days prior to the index date to determine the CCI and number of prior medical visits. A missing indicator variable was used as necessary to maintain sample size of the analysis. We also considered presence of a prescription for 1) NSAIDs (AHFS 280804) or 2) opioids (AHFS 280808 and 280812) prescribed after initial RA presentation but before commencement of DMARD therapy. Glucocorticoid use was not precisely captured in AHFS categories in this data, and thus their use was not focus of this analysis.
1.5 Statistical Analysis
We reported descriptive statistics for both the primary outcome variable (time from index RA visit to DMARD commencement) and baseline covariates using frequencies for categorical variables and mean and standard deviation (SD) for continuous variables. Kaplan-Meier curves for DMARD-free survival (time until DMARD commencement) were created for key categorical variables, and we tested whether curves were statistically different using the log-rank test. Bivariate analyses were performed to examine both length of time to DMARD and presence of delay >90 days in time to initiation of DMARD therapy by 1) care setting and 2) the presence (or absence) of an intervening NSAID or opioid prescription. Logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for having a delay in treatment >90 days between index date and initial DMARD receipt. The multivariable logistic regression model assessed all examined variables: care setting (purchased/direct), age, sex, rank [as a proxy for socioeconomic status]), CCI, health care utilization, year, TRICARE US region, and intervening prescriptions of NSAIDs and opioids. All analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, North Carolina, USA). We considered a two-sided p value of <0.05 as statistically significant in all analyses.
2. RESULTS
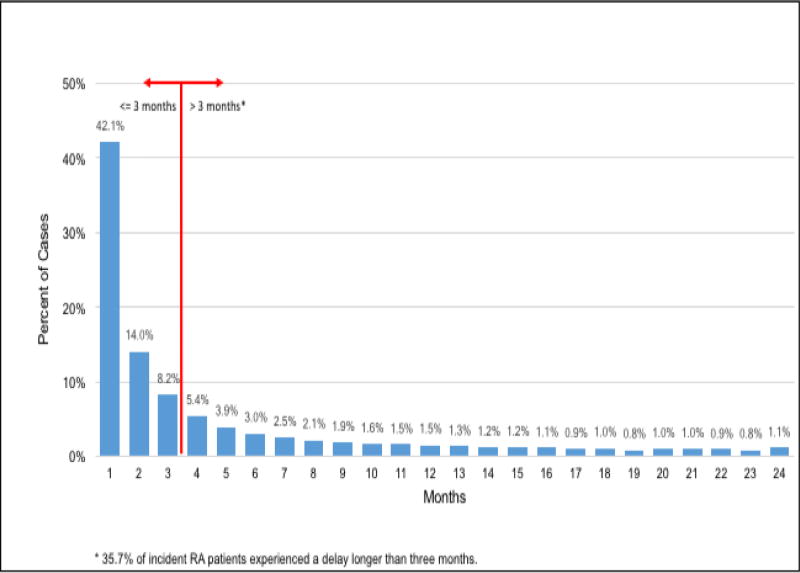
Figure 1 displays the findings for length of time between initial RA presentation until DMARD therapy initiation. The mean time from initial RA presentation to commencement of DMARD therapy was 125.3 days (SD 175.4): the median was 44 days (IQR:10 to 163 days), and approximately 35.7% commenced DMARD therapy three months or more after therapy initiation.
Figure 1.
Times from Initial RA Presentation to DMARD Therapy Commencement
Baseline covariate descriptive statistics are shown in Table 1. There were 16,680 patients with incident RA identified. Approximately 79.4% of incident RA patients were seen in the purchased care setting. The majority of patients in this sample were in the 45- to 64-year age range, while less than five percent were over 65 (since many beneficiaries switch to Medicare past this age). Approximately 77.6% were female, as expected for a population with RA. Spouses of active duty members (not serving in the military) represented much (44.4%) of the sample. Senior enlisted members and their dependents comprised 71.3% of the sample. Opioid prescriptions between RA presentation and initial DMARD receipt occurred in 35.5% of patients; intervening NSAID prescriptions occurred in 42.2% of patients.
Table 1.
Baseline characteristics of TRICARE Prime patients with incident rheumatoid arthritis receiving DMARDs (n = 16,680)
| N (%) | ||
|---|---|---|
| Age (Mean 47.16 (s.d. 13.5)) | ||
| 18 – 24 | 1,184 (7.1) | |
| 25 – 34 | 2,049 (12.3) | |
| 35 – 44 | 3,268 (19.6) | |
| 45 – 64 | 9,428 (56.5) | |
| 65+ | 751 (4.5) | |
| Sex | ||
| Female | 12,949 (77.6) | |
| Sponsor/Dependent Status | ||
| Sponsor | 3,928 (23.5) | |
| Spouse | 7,402 (44.4) | |
| Other family members | 432 (2.6) | |
| Missing | 4,918 (29.5) | |
| Rank (of patient or patient’s sponsor) | ||
| Enlisted, Senior | 11,893 (71.3) | |
| Enlisted, Junior | 1,334 (8.0) | |
| Officer, Senior | 1,194 (7.2) | |
| Officer, Junior | 1,936 (11.6) | |
| Missing | 323 (1.9) | |
| Care Setting | ||
| Direct (military) | 3,437 (20.6) | |
| Purchased (civilian) | 13,243 (79.4) | |
| Tricare US Region | ||
| North | 3,224 (19.3) | |
| South | 5,022 (30.1) | |
| West | 3,483 (20.9) | |
| Missing | 4,951 (29.7) | |
| Fiscal Year of Index Date | ||
| 2007 | 2,807 (16.8) | |
| 2008 | 2,784 (16.7) | |
| 2009 | 2,809 (16.8) | |
| 2010 | 2,738 (16.4) | |
| 2011 | 2,798 (16.8) | |
| 2012 | 2,744 (16.5) | |
| Charlson Comorbidity Index | ||
| 0 | 14,484 (86.8) | |
| 1 | 1,636 (9.8) | |
| >2 | 560 (3.4) | |
| Clinic Visits | ||
| 0 | 7,447 (44.7) | |
| 1 | 1,244 (7.5) | |
| 2–5 | 2,371 (14.2) | |
| 6+ | 5,618 (33.7) | |
| Opioid Use After Index Date | ||
| Yes | 5,916 (35.5) | |
| NSAID Use After Index Date | ||
| Yes | 7,031(42.2) | |
2.1 Days to initial DMARD receipt
Bivariate analyses were performed to examine time until DMARD initiation by 1) care setting, 2) intervening opioid use, and 3) intervening NSAID use as displayed in Table 2. We found no significant difference between care settings for DMARD (p=0.976). The mean duration between presentation and first DMARD for direct care was 125.2 days ((SD 173.0) (median: 48) (IQR: 11 to 157 days)): the mean for purchased care was 125.3 days ((SD 176.0) (median: 43) (IQR: 10 to 165 days)). Statistically significant differences (p<0.0001) were found for intervening opioid (mean: 212.8 days (SD 207.4) vs 77.3 days (SD 132.3) without opioid use) or NSAIDs (mean: 189.4 days (SD 199.3) vs 78.6 days (SD 138.0) without NSAID use).
Table 2.
Time until DMARD and delays in DMARD receipt by care setting and intervening opioids/NSAIDs.
| Category | Count | Mean days until DMARD (SD) |
p-value | % with Delay >90 days |
|---|---|---|---|---|
| Care Setting | ||||
| Direct | 3,437 | 125.2 (173.0) | 36.0 | |
| Purchased | 13,243 | 125.3 (176.0) | .98 | 35.3 |
| Intervening Opioids | ||||
| Yes | 5916 | 212.8 (207.4) | 49.1 | |
| No | 10,764 | 77.3 (132.3) | <.01 | 25.5 |
| Intervening NSAIDs | ||||
| Yes | 7031 | 189.4 (199.3) | 63.6 | |
| No | 9,649 | 78.6 (138.0) | <.01 | 19.9 |
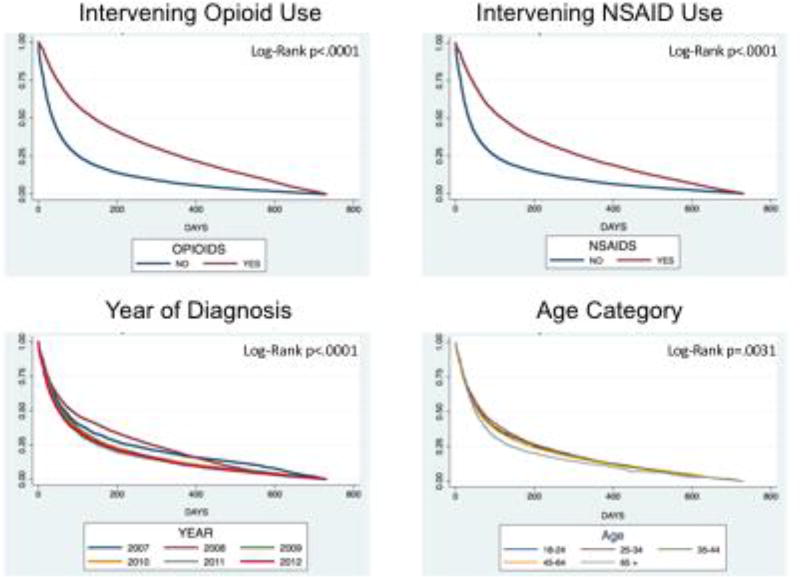
Figure 2 shows Kaplan-Meier curves for opioid use, NSAID use, fiscal year, and age categories. A steeper curve represents quicker time to commencement of DMARD therapy. When compared with patients not receiving NSAIDs or opioids, the curves for DMARD-free survival times of patients with either intervening NSAID or opioid prescriptions were statistically significant different (log-rank p<0.0001). The curves for categories of age showed that the older category of patients tended to receive a DMARD prescription quicker than other age categories (log-rank p=0.0031). The curves for year of initial presentation showed a quicker time to DMARD prescription receipt for later years in the study compared to earlier years (log-rank p<0.0001).
Figure 2.
Kaplan-Meier Survival Curves for DMARD Initiation
2.2 Delays in DMARD receipt
Table 2 shows the percentage of patients that experienced delays greater than 90 days by care setting, presence of intervening opioid prescription and presence of intervening NSAID prescription. Treatment delays were experienced similarly in the direct (35.3%) and purchased care settings (36.0%). Treatment delays occurred more frequently for patients who had intervening prescriptions of opioids (49.1%) than those who did not (25.5%). Delays also occurred more frequently for patients who had intervening prescriptions of NSAIDs (63.5%) than those who did not (19.9%).
2.3 Predictors of delay in initial DMARD receipt
Results of modeling for the binary outcome of delay in DMARD receipt (>90 days) are reported in Table 3. Odds ratios <1 were interpreted as being less likely to have a delay in initial DMARD receipt. Unadjusted logistic regression models for each covariate are displayed on the left side of the table. Statistically significant increased odds of delayed DMARD prescription were found for Junior Enlisted rank of patient or sponsor (compared to Senior Enlisted), south region (compared to north), intervening NSAID use (compared to non-use), and intervening opioid use (compared to non-use). Junior Enlisted rank was no longer statistically significant in the multivariable model but the other factors remained statistically associated with increased odds of treatment delay. Characteristics with statistically significant decreased odds of delayed (>90 days) receipt of a DMARD prescription included: male sex (compared to female), age 65+ (compared to 18–24), 2010, 2011, or 2012 year of index date (compared to 2007), and both 2–5 and 6+ visits prior to first RA billing code (compared to 0 visits). Statistical significance for age 65+ and for 2–5 prior visits were no longer detectable in the multivariable model but all other factors remained independently associated with decrease in treatment delay.
Table 3.
Odds ratio for delay in DMARD receipt >90 days after initial RA presentation.
| Care setting | Unadjusted Odds Ratio 95% Confidence Interval |
Multivariable Odds Ratio 95% Confidence Interval |
|
|---|---|---|---|
| Direct (military) | 1.0 Ref | 1.0 Ref | |
| Purchased (civilian) | 0.97 (0.897–1.049) | 0.92 (0.836–1.018) | |
| Sex | |||
| Female | 1.0 Ref | 1.0 Ref | |
| Male | 0.81* (0.748–0.874) | 0.89* (0.819–0.975) | |
| Age | |||
| 18 – 24 | 1.0 Ref | 1.0 Ref | |
| 25 – 34 | 1.11 (0.960–1.291) | 1.03 (0.869–1.220) | |
| 35 – 44 | 0.97 (0.843–1.112) | 0.94 (0.799–1.114) | |
| 45 – 64 | 0.93 (0.820–1.054) | 0.95 (0.811–1.108) | |
| 65+ | 0.73* (0.595–0.882) | 0.80 (0.632–1.022) | |
| Rank (of patient or sponsor) | |||
| Enlisted, Senior | 1.0 Ref | 1.0 Ref | |
| Enlisted, Junior | 1.26* (1.122–1.414) | 1.06 (0.915–1.230) | |
| Officer, Senior | 0.88 (0.777–1.001) | 1.13 (0.983–1.307) | |
| Officer, Junior | 0.96 (0.867–1.061) | 1.07 (0.953–1.195) | |
| Missing | 1.38 (1.104–1.736) | 1.56* (1.197–2.047) | |
| Tricare US Region | |||
| North | 1.0 Ref | 1.0 Ref | |
| South | 1.23* (1.210–1.350) | 1.12* (1.004–1.240) | |
| West | 1.11 (1.003–1.231) | 1.11 (0.990–1.244) | |
| Missing | 1.36* (1.240–1.495) | 1.31* (1.171–1.463) | |
| Fiscal Year of index date | |||
| 2007 | 1.0 Ref | 1.0 Ref | |
| 2008 | 1.29* (1.161–1.438) | 1.28* (1.132–1.438) | |
| 2009 | 0.91 (0.813–1.011) | 0.90 (0.799–1.019) | |
| 2010 | 0.81* (0.728–0.908) | 0.84* (0.739–0.947) | |
| 2011 | 0.77* (0.692–0.863) | 0.78* (0.689–0.883) | |
| 2012 | 0.79* (0.705–0.880) | 0.78* (0.691–0.890) | |
| Charlson Comorbidity Index | |||
| 0 | 1.0 Ref | 1.0 Ref | |
| 1 | 1.01 (0.908–1.124) | 1.00 (0.880–1.127) | |
| >2 | 0.91 (0.763–1.091) | 1.10 (0.886–1.338) | |
| Visits prior to 1st RA billing code | |||
| 0 | 1.0 Ref | 1.0 Ref | |
| 1 | 0.97 (0.856–1.098) | 0.88 (0.760–1.008) | |
| 2–5 | 0.89* (0.810–0.984) | 0.90 (0.800–1.005) | |
| 6+ | 0.85* (0.793–0.917) | 0.77* (0.700–0.841) | |
| NSAID use after 1st RA billing code | |||
| No | 1.0 Ref | 1.0 Ref | |
| Yes | 4.02* (3.760–4.300) | 3.32* (3.092–3.570) | |
| Opioid use after 1st RA billing code | |||
| No | 1.0 Ref | 1.0 Ref | |
| Yes | 4.74* (4.422–5.072) | 4.07* (3.781–4.374) | |
p<0.05
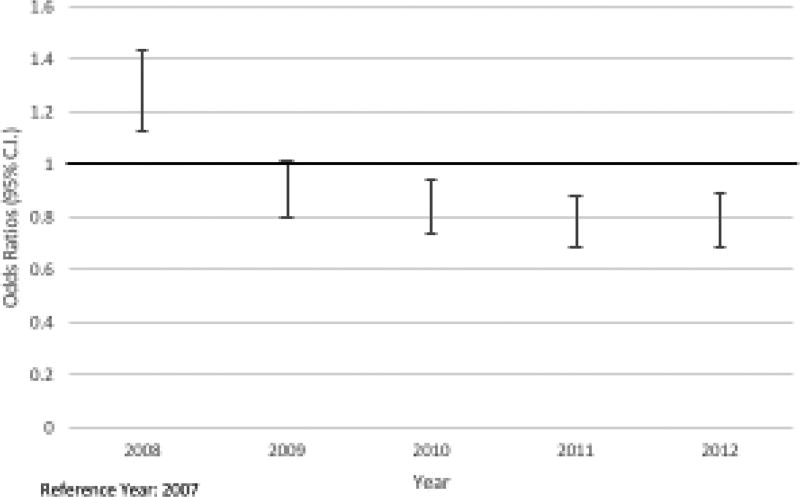
The multivariable logistic regression model assessed the effects of care setting (purchased/direct setting), patient characteristics (age, gender, sponsor rank (as a proxy for income level), CCI and health care utilization), year, geographic region, and intervening prescriptions of NSAIDs and opioids. The following characteristics had statistically significant increased odds of delayed (>90 days) receipt of a DMARD prescription: receiving care in the south region (compared to north) (OR 1.12; CI: 1.00–1.24), an index RA visit in 2008 (compared to 2007) (OR 1.28; CI: 1.13–1.44) and having received either an opioid (OR 4.07; CI: 3.78–4.37) or NSAID (OR 3.32; CI 3.09–3.57) prescription after initial presentation. Intervening opioid and NSAID prescriptions after initial presentation for RA had the strongest association with delay in DMARD use. The following characteristics had statistically significantly lower odds of delayed (>90 days) receipt of a DMARD prescription: being male (OR 0.89; CI: 0.82–0.98), having presented with incident RA in years 2010 through 2012 (compared to 2007) ([2010 OR 0.84; CI: 0.74–0.95], [2011 OR 0.78; CI: 0.69–0.88], [2012 OR 0.78; CI: 0.69–0.89]), and health care utilization in the 180 days prior to initial RA presentation (6 or more previous visits, as compared to no previous visits (OR 0.77; CI: 0.70–0.84). Figure 3 displays the decrease in odds ratios from the multivariable model for delay by year over the study period (FY 2007–2012).
Figure 3.
Odds Ratios for DMARD Delay >90 Days by Year of Incident RA
3. DISCUSSION
In this nationwide study of TRICARE beneficiaries, we found persistent delays in receipt of initial DMARD for newly diagnosed RA patients. The characteristics most significantly associated with delay were use of opioids after the first RA billing code, and use of NSAIDs after the first RA billing code. Age, sex, TRICARE US region, year of index date, number of visits prior to the first RA billing code were also significantly associated with greater delays. Variables pertaining to prescribing behaviors may be modifiable, indicating that DMARD initiation and perhaps subsequent RA disease outcomes may be improved through increased awareness of and attention to evidence-based treatment recommendations among providers. Favorable trends over time of less delay in initial DMARD suggest this may already be occurring but there is still significant room for improvement in decreasing time to initial DMARD prescription.
The mean time from initial RA presentation to DMARD prescription was approximately four months (125.3 days), which is comparable to delays documented elsewhere in the literature. Edwards et al. (14) found a median time from diagnosis to methotrexate initiation of 119 days and a median time to any DMARD initiation of 59 days among incident patients with RA in the United Kingdom. At least two international studies found that only approximately 20% of patients began treatment for RA within three months (16, 17). Additionally, Pappas et al. (15) found an average time from registry enrollment to initial drug therapy of any kind of 12.1 months for RA patients in the US Corrona Registry with no prior therapy at time of enrollment.
Reduced odds for delay during the later years in the study are suggestive of overall improvements in adherence to guidelines regarding prompt commencement of DMARD therapy. Over the six-year period of the study, mean time from initial RA presentation to start of DMARD therapy decreased by about 35 days. However, even in the later years, delay in treatment remained quite common, indicating the need for continued improvement in promptly prescribing DMARDs to patients newly diagnosed with RA.
Delays in DMARD prescription were especially common in patients receiving intervening prescriptions for analgesics, either opioids or NSAIDs. The mean time to DMARD treatment for patients receiving an intervening opioid was 212.8 days (median 131 days) while the mean time for those without opioid prescription was 77.3 days (median 24 days). Since opioids are unlikely to improve the underlying disease process of RA, this finding highlights another potential detrimental effect of opioids during the current “opioid epidemic”. It is possible that a masking analgesic effect of opioids may result in delayed DMARD commencement. Additionally, this window of exposure to opioids in the very early RA period when pain and disease activity are often most pronounced may be a time when opioid dependence may commence. Recent research has concluded that up to one-third of patients with RA are chronic opioid users (19). Chronic pain opioid prescribing guidelines (25) recommend that opioid prescription for analgesia in RA patients should be a course of last resort, not initial treatment. These findings therefore highlight the importance of this very early RA period for prescribers and patients to consider carefully the risks and benefits of opioid use at this vulnerable period.
We observed similar results for patients with intervening prescription NSAID use (median time to DMARD of 189.4 days vs. median of 78.6 days without NSAID use). Treatment for analgesia may also be delaying appropriate care, because of either difficulty detecting active synovitis or reluctance of patients to escalate therapy after pain is successfully treated. NSAIDs and corticosteroids typically have a more immediate impact on reducing pain and stiffness than DMARDs, and thus DMARD initiation might not be deemed essential by a patient whose symptoms are relatively controlled. This finding may also reflect the continued use of a pyramid approach to treating pain by some prescribers, where NSAIDs or other analgesics are first prescribed and DMARD therapy is started after NSAIDs alone are not clinically effective and inflammatory arthritis persists (26). Subsequent studies have provided evidence that more aggressive DMARD treatment improved outcomes (8, 18), and thus clinical practice and guidelines changed. Notably, we found that intervening prescriptions decreased by 6.9% for opioids and 16.2% for NSAIDs from the beginning to the end of the study period, an encouraging finding suggestive of a decreased focus on analgesia alone and increased focus on therapeutic benefit after the initial presentation for RA in the more recent years.
Time from initial RA presentation to treatment did not vary significantly between direct (military) and purchased (civilian) care settings. This suggests that adherence to evidence-based standards for RA is comparable for all types of providers in this analysis and that results may be generalizable to other populations. Ensuring adequacy of care in military facilities is important, as is ensuring that military beneficiaries are receiving appropriate care when seen in civilian facilities. The Defense Health Agency is required to report annually to Congress on its performance in providing care to TRICARE beneficiaries: access and satisfaction are key areas of focus (27). Additionally, this was a major objective of our study in part because other recent studies of TRICARE beneficiaries in different diseases and therapies have found differences by care setting (28, 29).
Additional factors investigated in this study were also found to be associated with delays in treatment. A small but significant lower odds ratio was found for the age category of 65+ years; however, this association did not remain significant in the multivariate model. Increased health care utilization prior to incident RA was associated with less delay. Patients who visit the health care system regularly may see more providers and be assessed more frequently, leading to prompter diagnosis and treatment as well as being more likely to take prescribed medications. Women were more likely to experience delays than men, which is an important finding, since most patients with RA are women. It may be that RA diagnosis is relatively more complicated by the presence or possibility of other conditions, such as fibromyalgia, in women, which may make inflammatory arthritis harder to discern. Finally, significant differences based on rank were noted. These differences are likely related to socioeconomic status and further studies in diverse populations are needed to understand the effect of psychosocial factors on DMARD delay.
Strengths of this study include its design, which was based on nationwide administrative data reflective of typical clinical care, and its focus on a less frequently studied but still representative population of TRICARE enrollees. The dataset included detailed records of care (both visits and prescriptions) received both before and after incident RA visits, thereby improving accuracy of diagnosis (21). Because of the use of PCMs for TRICARE Prime and Plus patients, the records of care contained in the data used for this study can be assumed to be reasonably complete compared to private insurance claims databases, and the data enabled incorporation of a long study period to examine temporal trends.
Limitations of this study are typical of other observational studies of administrative datasets. Neither assessment of disease activity nor reasons why medications might have been prescribed were available for review. First appearance of a billing code for RA does not necessarily correspond to actual clinical diagnosis and may vary based on patient and prescriber characteristics. Prescriptions in this dataset represent those that were actually filled: they are not a precise representation of physician prescribing patterns and is not a guarantee that the medication was actually taken. The time between the clinician writing a prescription and the actual fill date by the patient may have extended the overall delay measured in this study. The majority of subjects in the study were dependents, spouses, and retirees – as opposed to service members - and much of the care was in the civilian setting. Even so, it is possible that our findings may not be generalizable to other populations, particularly patients older than 65 since many older patients do not remain on TRICARE past this age. Finally, residual confounding from unmeasured variables is always possible, but rich sociodemographic, temporal, and clinical covariates were available. We used AHFS classification to capture intervening prescriptions. In this dataset, this strategy did not allow for accurate capturing of glucocorticoid use. Future analysis focused on use of glucocorticoids will be important to produce a more complete picture of pharmacological factors contributing to delays. Further study using clinical records would address some of these weaknesses.
4. CONCLUSION
In conclusion, this study of a universally insured population of TRICARE beneficiaries found that delays in commencement of treatment for RA persist, and that use of opioids and NSAIDs was associated with significantly greater delays in receiving DMARD therapy. These results provide rationale for further research on factors affecting prescribing patterns in early RA. While alleviating pain is important for RA patients, doing so may result in delayed commencement of more definitive DMARD therapy. These findings should be considered as the MHS develops/updates clinical practice guidelines to standardize and promote appropriate and timely treatment for RA patients.
Acknowledgments
Supported by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants K23 AR069688 and L30 AR066953 - Dr. Sparks) and a grant by the Henry M. Jackson Foundation for the Advancement of Military Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mayo Clinic. Rheumatoid arthritis. 2017 URL: http://www.mayoclinic.org/diseases-conditions/rheumatoid-arthritis/home/ovc-20197388.
- 2.National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Handout on health: Rheumatoid arthritis. 2016 URL: https://www.niams.nih.gov/health-topics/rheumatoid-arthritis.
- 3.Murphy J. American College of Rheumatology: Rheumatoid arthritis: Fast facts. 2017 URL: http://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Rheumatoid-Arthritis.
- 4.Gabriel SE, Crowson CS, O'Fallon M. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999;42:415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwoh CK, Simms RW, Anderson LG, Erlandson DM, Greene JM, Moncur C, et al. Guidelines for the management of rheumatoid arthritis: American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996;39:713–22. [PubMed] [Google Scholar]
- 8.Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta-analysis. Arthritis Rheum. 2006;55:864–72. doi: 10.1002/art.22353. [DOI] [PubMed] [Google Scholar]
- 9.Kim G, Barner JC, Rascati K, Richards K. Examining time to initiation of biologic disease-modifying antirheumatic drugs and medication adherence and persistence among Texas Medicaid recipients with rheumatoid arthritis. Clin Ther. 2016;38:646–54. doi: 10.1016/j.clinthera.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Kyburz D, Gabay C, Michel BA, Finckh A. The long-term impact of early treatment of rheumatoid arthritis on radiographic progression: A population-based cohort study. Rheumatology. 2011;50:1106–10. doi: 10.1093/rheumatology/keq424. [DOI] [PubMed] [Google Scholar]
- 11.Nell VPK, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology. 2004;43:906–14. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- 12.Raza K. The Michael Mason prize: Early rheumatoid arthritis - the window narrows. Rheumatology. 2010;49:406–10. doi: 10.1093/rheumatology/kep392. [DOI] [PubMed] [Google Scholar]
- 13.van der Linden MPM, le Cessi S, Raza K, van der Woude D, Knevel R, Huizinga TWJ, et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum. 2010;62:3537–46. doi: 10.1002/art.27692. [DOI] [PubMed] [Google Scholar]
- 14.Edwards CJ, Campbell J, van Staa T, Arden NK. Regional and temporal variation in the treatment of rheumatoid arthritis across the UK: A descriptive register-based cohort study. BMJ Open. 2012;2:1–7. doi: 10.1136/bmjopen-2012-001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappas DA, Kent JD, Greenberg JD, Mason MA, Kremer JM, Holt RJ. Delays in initiation of disease-modifying therapy in rheumatoid arthritis patients: Data from a US-based registry. Rheumatol Ther. 2015;2:153–64. doi: 10.1007/s40744-015-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Thurah A, Norgaard M, Johansen M, Stengaard-Pedersen K. Time to methotrexate treatment in patients with rheumatoid arthritis referred to hospital. Scand J Rheumatol. 2010;39:19–25. doi: 10.3109/03009740903185987. [DOI] [PubMed] [Google Scholar]
- 17.De Cock D, Meyfroidt S, Joly J, Van der Elst K, Westhovens R, Verschueren P. A detailed analysis of treatment delay from the onset of symptoms in early rheumatoid arthritis patients. Scand J Rheumatol. 2014;43:1–8. doi: 10.3109/03009742.2013.805242. [DOI] [PubMed] [Google Scholar]
- 18.Verstappen SM, Jacobs JW, Bijlsma JW, Heurkens AH, van Booma-Frankfort C, Borg EJT, et al. Five-year follow-up of rheumatoid arthritis patients after early treatment with disease-modifying antirheumatic drugs versus treatment according to the pyramid approach in the first year. Arthritis Rheumatol. 2003;48:1797–1807. doi: 10.1002/art.11170. [DOI] [PubMed] [Google Scholar]
- 19.Zamora-Legoff JA, Achenbach SJ, Crowson CS, Krause ML, Davis JM, Matteson EL. Opioid use in patients with rheumatoid arthritis 2005–2014: A population-based comparative study. Clin Rheumatol. 2016;35:1137–44. doi: 10.1007/s10067-016-3239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department of Defense: Final Report to the Secretary of Defense Military Health System Review. Washington DC: 2014. [Google Scholar]
- 21.Kim S, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, Solomon DH. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13.1:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Combe B, Landewe R, Daien CI, Hua C, Aletaha D, Alvaro-Gracia JM, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis. 2017;76:948–59. doi: 10.1136/annrheumdis-2016-210602. [DOI] [PubMed] [Google Scholar]
- 23.Deighton C, O’Mahony R, Tosh J, Turner C, Rudolf M. Management of rheumatoid arthritis: Summary of NICE guidance. BMJ. 2009;338:b702. doi: 10.1136/bmj.b702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J chronic dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilske KR, Healey LA. Remodeling the pyramid: A concept whose time has come. J Rheumatol. 1989;16:565–7. [PubMed] [Google Scholar]
- 27.Defense Health Agency. Evaluation of the TRICARE program: Fiscal year 2017 report to Congress. 2017 URL: http://www.health.mil/Military-Health-Topics/Access-Cost-Quality-and-Safety/Health-Care-Program-Evaluation/Annual-Evaluation-of-the-TRICARE-Program.
- 28.Nguyen LL, Smith AD, Scully RE, Jiang W, Learn PA, Lipsitz SR, et al. Provider induced demand in the treatment of carotid artery stenosis. JAMA Surg. 2017;152:565–72. doi: 10.1001/jamasurg.2017.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranjit A, Jiang W, Haider AH, Witkop CT, Little SE, Robinson JN. Obstetric care in the US military: Comparison of direct and purchased care system within TRICARE [11R] Obstet Gynecol. 2016;127:150S. [Google Scholar]