Abstract
The field of spinal cord stimulation is expanding rapidly, with new waveform paradigms asserting supraspinal sites of action. The scope of treatment applications is also broadening from chronic pain to include cerebral ischemia, dystonia, tremor, multiple sclerosis, Parkinson’s disease, neuropsychiatric disorders, memory, addiction, cognitive function, and other neurologic diseases. The role of neurostimulation as an alternative strategy to opioids for chronic pain treatment is under robust discussion in both scientific and public forums. An understanding of the supraspinal mechanisms underlying the beneficial effects of spinal cord stimulation will aid in the appropriate application and development of optimal stimulation strategies for modulating pain signaling pathways. In this review, we focus on clinical and preclinical studies that indicate the role of supraspinal mechanisms in spinal cord stimulation-induced pain inhibition, and explore directions for future investigations.
Introduction
According to the American Association of Neurological Surgeons, approximately 50,000 spinal cord stimulators are implanted per year, worldwide.1 Growth of this field is moving at a rapid pace with an estimate of the worldwide neuromodulation systems market reaching over 7 billion United States dollars by 2020.2 As we pass the 50th anniversary since Norman Shealy implanted the first spinal cord stimulation system in 1967, the clinical effectiveness of spinal cord stimulation has made steady progress, with more significant advancements in the last decade.3 In 1993, a 7 year follow-up of 320 consecutive patients who had spinal cord stimulation placement for chronic intractable pain and found that 52% still reported at least 50% relief of pain.4 More than a decade later, it was reported that 48% of patients with failed back surgery syndrome who received conventional, paresthesia-guided spinal cord stimulation treatment had more than 50% pain relief at 6 months.5 Considerable progress has been made since that time with reports of clinical effectiveness ranging from 60 to 85%.6–10 Technological advancements in lead design, refinements of anatomical targeting (including structures outside the cord itself), and novel waveforms such as burst spinal cord stimulation and high frequency paresthesia-free spinal cord stimulation are likely to have contributed to this continuous improvement.10–12 Additionally, improved patient selection criteria likely amplified these results. Nevertheless, much room remains to enhance the success rate and expand the clinical application of spinal cord stimulation.
An important step in optimizing stimulation paradigms is to enhance our understanding of spinal cord stimulation mechanisms. Preclinical studies of electrical spinal stimulation can be broadly grouped based on their anatomical focus into three groups: (1) peripheral, distal to the dorsal root ganglion, (2) spinal/segmental, spinal cord and dorsal root ganglion; and (3) involvement of supraspinal structures. Although early studies tended to focus on the peripheral and spinal/segmental mechanisms of spinal cord stimulation, the study of supraspinal pathways will aid in the development of optimal stimulation paradigms for modulating neural activity in the pain signaling pathways and may help to characterize the links between pain, emotions, reward, and other higher functions in the brain. Additionally, the lacking clinical effectiveness of conventional spinal cord stimulation in acute nociceptive pain, pain inhibition extending beyond the stimulation period, the cumulative duration-dependent treatment effect size, and alleviation of pain from non-noxious stimuli (i.e. allodynia) cannot be readily explained by the spinal/segmental mechanism as proposed by Gate Control Theory alone.13
Methods
This review was conducted using a search of MEDLINE/PubMed, Medical Subject Headings, Cochrane Review, and Google Scholar. No date limits were applied and the search was limited to the English language. Both preclinical and clinical sources were included if they were related to supraspinal mechanisms of spinal cord stimulation. The reference lists of the sources selected were also examined to identify the additional studies not found from the original search. We used discretion in this process with preference towards clinical and preclinical peer-reviewed articles in indexed medical journals in settings. This search did not identify any review that specifically concentrated on supraspinal pathways that may be involved in mechanisms of action of spinal cord stimulation for pain treatment. Therefore, in this review, we examine the historical trend of clinical and preclinical studies that indicate a role for supraspinal mechanisms in spinal cord stimulation-induced pain inhibition, first in conventional spinal cord stimulation, then in newer spinal cord stimulation waveforms, and explore directions for future investigations.
1960–80s: Conventional Spinal Cord Stimulation
We use the inclusive term spinal cord stimulation throughout this review rather than the historical term of dorsal column stimulation, which excluded involvement of neighboring neuroanatomical structures (Table 1). Dorsal column stimulation restricted the stimulatory mechanism to those evoked by activation of the dorsal columns reaching the dorsal horns. “Central control” was briefly mentioned as an expression for supraspinal influences, but the earlier focus was primarily on the spinal/segmental mechanisms.14 The Gate Control Theory originally hypothesized that a combination of presynaptic inhibition and the actions of inhibitory interneurons within the spinal cord is activated by large diameter afferent fibers.15 Thus, electrical activation of large diameter afferents, A-beta fibers, produces an inhibitory effect on the processing of signals from small diameter, A-delta & C fibers, afferents.16 Although this theory was the foundation for the development of conventional spinal cord stimulation (paresthesia-inducing tonic waveforms in which stimuli are delivered at a continuous frequency, pulse width, and amplitude), many gaps in our understanding could not be explained by this mechanism.14,17
Table 1.
Glossary of neuroanatomical structures studied in relation to supraspinal mechanisms of spinal cord stimulation.
| Anterior Pretectal Nucleus | Located in the pretectal midbrain near the thalamus, it is considered part of the reticular formation and is thought to exert descending mechanisms of pain control. |
| Cerebellar Fastigial Nucleus | A deep cerebellar nuclei involved in motor coordination. |
| Cingulate Cortex | Involved with memory, learning, and emotion. |
| Diencephalon | An embryonic structure that develops into multiple forebrain structures including the thalamus, hypothalamus, epithalamus (includes pineal gland), and the pituitary gland. |
| Dorsal Column | Ascending pathways relaying sensations of touch, vibration, and proprioception from the periphery |
| Dorsolateral Column | Also known as Lissauer’s tract, a narrow axon tract located at the tip of the dorsal horn close to the entering posterior nerve roots. |
| Dorsolateral Striatum | Involved in habitual behavior. |
| Gracile Nucleus | A dorsal column nucleus located in the medulla that receives input from touch and proprioceptive neurons from the lower body. |
| Locus Coeruleus | Located in the pons, it is main site for norepinephrine production in the brain. |
| Mediodorsal Nucleus of the Thalamus | Associated with memory and cognitive processes. |
| Nucleus of the Solitary Tract | Sensory nuclei located in the medulla that receives input from viscera such as the respiratory, cardiovascular, and gastrointestinal systems. |
| Parafascicular Nucleus of the Thalamus | Involved in goal directed behavior. |
| Parietal Association Area | Integrates information mostly involved with somatosensory and visual association sensory modalities. |
| Periaqueductal Grey | Located in the midbrain surrounding the cerebral aqueduct, it serves multiple functions including descending pain inhibition and enkephalin production. |
| Prefrontal Cortex | Involved in personality and higher cognitive functions. |
| Raphe Nuclei | Midline brainstem nuclei that function to release serotonin and include the raphe obscurus, raphe magnus, median and paramedian raphe, raphe pontis, and dorsal raphe nuclei. |
| Rostral Ventromedial Medulla | Involved in the incorporation of descending signals to the spinal cord, it includes the nucleus raphe magnus, nucleus reticularis gigantocellularis, nucleus reticularis paragigantocellularis lateralis, and nucleus gigangtocellularis pars alpha. |
| Spinothalamic Column | An anterolateral or ventrolateral ascending tract that convey sensations of touch, pressure, pain, and temperature to the thalamus from the periphery. |
| Ventral Posterolateral Nucleus of the Thalamus | The caudal nucleus processes visceral and nociceptive input, rostral nucleus processes proprioception, and the intermediate nucleus processes cutaneous input. |
| Ventromedial Nucleus of the Thalamus | Involved in motor control. |
Clinical
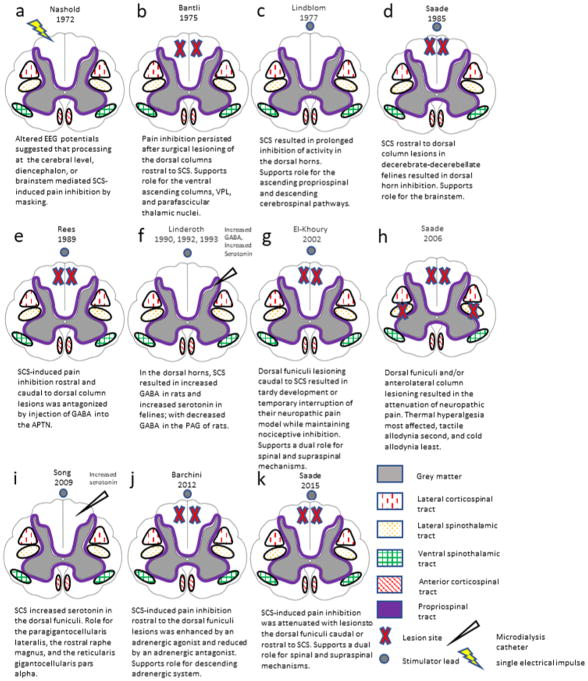
Supraspinal involvement was suggested by Nashold et al.18 in their early work measuring electroencephalogram potentials evoked by stimulation of dorsal column in humans (Figure 1a). This was performed with subdural electrodes delivering stimulation with a pulse duration from 0.1–0.3 milliseconds, intensity from 0.1–30 volts, and frequencies below 200 hertz. They suggested that spinal cord stimulation selectively “masks” neuropathic but not nociceptive pain as a result of processing at the cerebral level, diencephalon, or brainstem, rather than the spinal cord.18,19 They also noted that when compared to direct stimulation of the ventral posterolateral thalamic nucleus or sensory cortex, stimulation with single pulses at the dorsal column was consciously perceived at much lower intensities.18 Prior to this study, researchers assumed that direct brain stimulation, as compared to peripheral stimulation, required lower amplitude intensities for patients to consciously perceive stimulation. These findings suggested the stimulation differs in mechanisms at the two sites examined. Significant reductions in somatosensory evoked potentials that correlate with pain inhibition were reported by Larson et al.20 in humans receiving spinal cord stimulation, and many of these patients also developed hyperactive reflexes, which could indicate a weakening of tonic descending sensorimotor inhibition. This was also performed with subdural electrodes providing stimulation at frequencies of 70 to 100 hertz, pulse width of 0.25 milliseconds, and an estimated pulse current of 0.5 to 1.0 milliamps.
Figure 1.
Cross sectional representations of experiments that have examined supraspinal mechanisms of spinal cord stimulator therapy in chronological order. EEG: electroencephalogram, SCS: spinal cord stimulation, VPL: ventral posterolateral nucleus of the thalamus, GABA: γ-amino-butyric acid, APTN: anterior pretectal nucleus, PAG: periaqueductal grey.
Preclinical
Although Larson et al. were not were not able to replicate their human somatosensory evoked potential findings when studying primates,20 the authors postulated that the extended duration of both pain inhibition and reduced evoked potentials after stimulation involved supraspinal mechanisms. The ventral posterolateral and parafascicular nucleus of the thalamus were implicated in another primate study.21 Investigators in this study measured evoked potentials within these nuclei after removing the dorsal cord rostral to the stimulation site so that only the ventral pathways ascended. The results implied that the dorsal columns may not mediate pain inhibition alone since evoked potentials in these nuclei remained consistent despite dorsal column absence (Figures 1b, 2, and 3).21 While primarily studying the spinal mechanisms of spinal cord stimulation in the inhibition of spinothalamic neurons in primates, Foreman et al.22 secondarily speculated that an ascending dorsal column signal may trigger inhibitory interactions at higher levels of the central nervous system. The involvement of supraspinal (e.g., ascending propriospinal and descending cerebrospinal) systems was suggested based on observations in cats that spinal cord stimulation resulted in prolonged inhibition of a subpopulation of dorsal horn neurons,23 which could not be explained by a spinal mechanism (Figures 1c, 3, and 4).24,25
Figure 2.
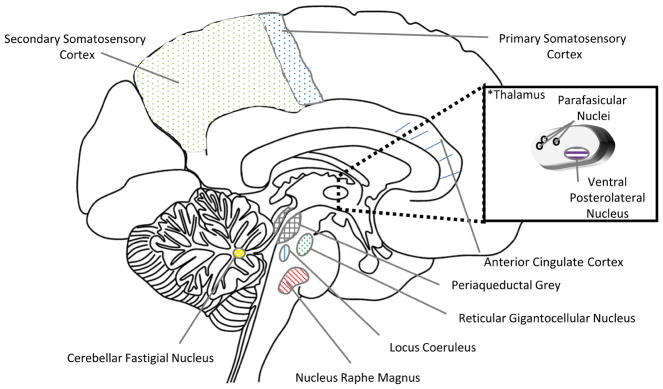
Sagittal brain anatomy of regions involved in supraspinal mechanisms of spinal cord stimulation. *The thalamus insert depicts the thalamus corpus which is situated laterally to the midsagittal section as illustrated.
Figure 3.
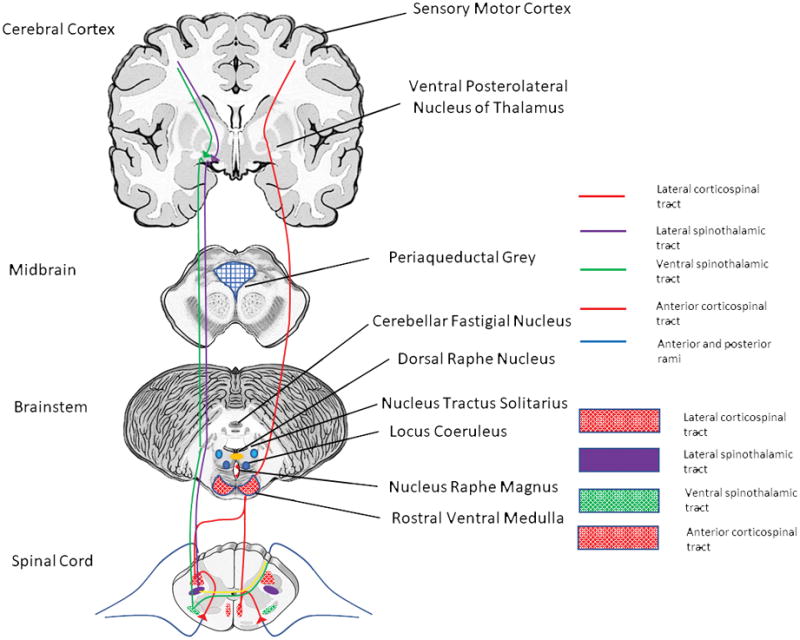
Cross sectional neuroanatomy of potential supraspinal pathways mediating spinal cord stimulation induced pain inhibition.
Figure 4.
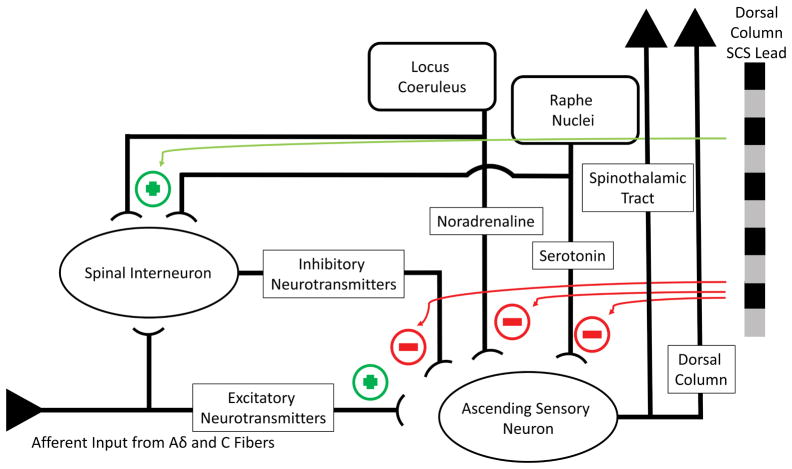
Schematic of historical perspective on pain modulation by spinal cord stimulation. Dorsal column stimulation results in direct presynaptic inhibition of small diameter sensory neurons and the activation of inhibitory interneurons producing an inhibitory effect on these neurons. Dorsal column stimulation also activates descending pain inhibitory pathways originating from the locus coeruleus and raphe nuclei.
Further characterization of the supraspinal pathways by Saade et al.26 occurred with decerebrate-decerebellate cats through spinal cord stimulation rostral to surgical lesions of the dorsal columns. Despite the dorsal column interruption, dorsal horn neuronal inhibition occurred below the lesioned level with multiple modalities of nociceptive stimuli. The brainstem was implicated as the supraspinal source of these effects because the specific preparation used excluded participation of the diencephalon, cerebral cortex, or cerebellum (Figures 1d, 2, and 3).26 In a similar study, the same investigators observed inhibition of pain with dorsal column stimulation rostral to dorsal column lesions in an awake rat model.27 The ascending pathway was attributed only to the dorsal columns because the low intensity stimulation used did not spread beyond this region.27 In their previous studies, the same group of investigators demonstrated the links between the dorsal column and the periaqueductal grey, nucleus raphe magnus, and reticular gigantocellular nucleus (Figures 2 and 3).28–30 They also showed that unilateral spinal cord stimulation could modulate the activity of cochlear neurons bilaterally through direct projections, implying the potential for supraspinal mechanisms with widespread effects.31
The supraspinal effects of stimulation both rostral and caudal to dorsal column lesions in rats were further examined by Rees and Roberts32, particularly the possible involvement of the anterior pretectal nucleus (Figure 1e). They postulated that the short- and long-term inhibition of pain may result from two separate mechanisms. Long-term inhibition was antagonized by injection of γ-aminobutyric acid into the anterior pretectal nucleus, and was attenuated with stimulation caudal to the dorsal column lesion or lesioning of the ipsilateral dorsal column.32 Thus, long-term inhibition was thought to be mediated by an ascending dorsal column pathway to the anterior pretectal nucleus which then spurred descending inhibition, whereas short-term inhibition was thought to be mediated by antidromic spinal segmental mechanisms.32–37
There are perceivable limitations to the use of small animal models for spinal cord stimulation study, which may reduce translatability to the clinical setting. It is also difficult to determine which areas of the spinal cord are stimulated as the electrode to spinal cord size ratio is typically larger than that used in humans. Some models utilized subdural stimulation as opposed to the epidural location of human electrodes. Nevertheless, these models often serve as an important starting point for developing hypotheses to be further examined in large animals (e.g., sheep) and in clinical trials. Although rat models of spinal cord stimulation tend to be most utilized, studies using larger animals may more closely resemble spinal cord stimulation in humans.
1990s: Broadening Applications of Conventional Spinal Cord Stimulation
Clinical
The 1990s brought much discussion regarding the effects of spinal cord stimulation on blood flow, including the mechanisms by which it altered cerebral blood flow.38–42 Clinical applications included not only peripheral vascular disease and angina, but also the prevention of cerebral ischemia, for which high cervical stimulation (C3 and rostrally) had the most profound effects.38,39 Coupling of cerebral blood flow to the sensorimotor regions activated by spinal cord stimulation was proposed as one possible mechanism contributing to these effects.43 The use of neuroimaging approaches, such as positron emission tomography, allowed identification of specific regional cerebral blood flow changes with spinal cord stimulation in clinical studies.40 In patients with chronic angina pectoris, spinal cord stimulation altered regional cerebral blood flow in multiple areas associated with cardiovascular control and nociception.40 Regional cerebral blood flow was shown to differ across patient populations, anatomical locations of stimulation, and mode of stimulation.40,43
Preclinical
Rodent models of spinal cord stimulation-induced increase in cerebral blood flow were attributed to rostral activation of the medullary vasomotor centers or the cerebellar fastigial nucleus, which is known to influence cerebral blood flow (Figures 2 and 3).44,45 During this same period, preclinical studies began revealing supraspinal neurochemical mechanisms of conventional spinal cord stimulation. Supraspinal mechanisms of spinal cord stimulation were examined by Linderoth and colleagues, using microdialysis catheter techniques (Figure 1f).46–49 Catheters placed stereotactically in the periaqueductal grey revealed significantly decreased levels of gamma-aminobutyric acid (GABA) in freely moving rats receiving spinal cord stimulation (Figure 2 and 3).47 An spinal cord stimulation-associated increase in GABA levels in the dorsal horn coupled with decreased levels in the periaqueductal grey may involve enhanced descending inhibition.47,50 In an earlier study, it was found that increased levels of serotonin but unaltered levels of substance P with spinal cord stimulation were present in the dorsal horns of decerebrate cats.46 In intact cats, however, the substance P levels were instead increased both during spinal cord stimulation with “clinical parameters” and after pinch or noxious electrical nerve stimulation of a hindpaw.51 This finding suggested that both orthodromic dorsal column activation and activation of the spinothalamic tract could result in substance P release in the dorsal horn, probably after activation of quite different neuronal circuitry (Figures 1f and 3).51
Surrogate markers of neural activity with spinal cord stimulation were investigated by DeJongste et al.52 through measurement of the rapidly transcribed oncoprotein c-Fos and the stress-induced heat shock protein 72. Though heat shock protein 72 levels were not detectable in neurons with or without spinal cord stimulation, limiting stress as a potential mechanism, c-Fos expression was increased in the spinal cord stimulation group within regions of the limbic system known to modulate emotions and pain.52 Despite these findings, the authors were not in favor of a supraspinal mechanism because c-Fos expression was not increased in the ventrolateral medulla, the nucleus of the solitary tract, or the nucleus raphe magnus (Figures 2 and 3). However, their conclusion may not be entirely valid because many other supraspinal structures are likely involved and sites of central nervous system activation may differ based on the parameters of spinal cord stimulation application.52 c-Fos expression is a nonspecific measure of neuronal activation with numerous limitations. For example, 1) A wide variety of stimuli cause nonspecific c-Fos expression; 2) Expression is transient and lacks differentiating strength of activation; 3) Activated neurons may not always express c-Fos; 4) There is no differentiation between activation of excitatory and inhibitory circuitry; and 5) Neuronal inhibition is not measured.53 Other neuronal activation markers such as phosphorylated extracellular signal-regulated kinase, a more dynamic marker and better indicator of central sensitization, may be worth examining in future studies.54–56
Contrary to a supraspinal hypothesis and findings by Saade and colleagues,57 a later study showed that the flexor reflex attenuation by spinal cord stimulation was mediated via spinal mechanisms, as complete cord transections rostral to the spinal cord stimulation application site did not significantly alter this attenuation.57–59 The contradictory results may be the result of varying stimulation intensities used in the different studies.57–59 Similar to studies by Rees and Roberts36, a model that surgically lesioned the dorsolateral column caudal to spinal cord stimulation found that pain inhibition was diminished, but not absent, which suggested a dual and additive role of spinal and supraspinal mechanisms.60 Revisiting their earlier study of flexion reflexes, Saade et al.61 found that nociceptive flexion reflexes are mediated by both spinal and supraspinal mechanisms. However, long-term inhibition may be particularly potentiated by a pons-brainstem-spinal loop. These studies are in contrast to a mechanistic review at the end of this decade, which concluded that the dorsal columns and the paresthesia elicited through them were a requirement for pain relief by conventional spinal cord stimulation in neuropathic pain.62 Since then, some new spinal cord stimulation paradigms have shown that paresthesia may not be critical or indispensable to the success of spinal cord stimulation.63–65
2000s: New Tools for Elucidating Mechanisms
Clinical
The use of somatosensory evoked potential analysis with conventional spinal cord stimulation was revisited in a study of 9 patients with failed back surgery syndrome undergoing concurrent tibial or sural nerve stimulation.66 This analysis revealed that spinal cord stimulation attenuated somatosensory evoked potential signals in both the primary and secondary somatosensory cortices; however, somatosensory evoked potentials from the mid-cingulate cortex could decrease or increase depending on the parameters of the peripheral stimulation (Figures 2 and 3).66 Consequently, spinal cord stimulation-induced pain inhibition may depend on the type of stimulus applied.66 In a more extensive neurophysiologic assessment, plantar sympathetic skin response, F-wave, somatosensory evoked potentials, H-reflex, and nociceptive flexion reflexes were assessed in a series of 20 patients receiving spinal cord stimulation for failed back surgery syndrome.67 Particularly relevant to supraspinal mechanisms, the somatosensory evoked potential signals had reduced amplitudes, independent of nociceptive flexion- and H-reflexes, and increased latency during spinal cord stimulation.67
Using magnetic resonance spectroscopy in a group of 20 failed back surgery syndrome patients, an increase in GABA and a decrease in glucose concentrations in the ipsilateral thalamus was observed during spinal cord stimulation.68 The increase in GABA was postulated to be due to effects on the spino-reticulo-thalamic-cortical pathway, part of the ascending reticular arousal system, that when modulated, can interfere with the affective components of pain.68 Poor responders also exhibited noticeable, yet less robust, changes in the GABA and glucose concentrations of the ipsilateral thalamus, calling into question the utility of this modality for predicting therapeutic response to spinal cord stimulation.68 Although earlier work used positron emission tomography to study spinal cord stimulation for angina pectoris, a recent study appears to be the first to investigate whether positron emission tomography can be used to determine neuronal activity before and after spinal cord stimulation for neuropathic pain.40,69 Increases in regional cerebral blood flow, a marker of neuronal activity, was noted in the contralateral thalamus, bilateral parietal association area, anterior cingulate cortex, and prefrontal regions.69 In contrast to the common methodology, neuronal activity, regional cerebral blood flow, was measured after rather than during spinal cord stimulation. They surmised that activation of thalamic and parietal association areas modulated pain thresholds while anterior cingulate cortex and prefrontal regions modulated the affective component of pain.69 A recent review of the neurophysiologic and functional neuroimaging literature emphasized the need for large-scale controlled studies, but identified the thalamus and anterior cingulate cortex as key structures in the supraspinal mechanisms (Figures 2 and 3).70
Preclinical
Using microdialysis and immunohistochemical techniques, serotonin levels were examined with conventional spinal cord stimulation in nerve-injured rats.71 The investigators observed increased serotonin in the dorsal horns of spinal cord stimulation responders immediately after stimulation, but not in responders prior to stimulation or non-responders at either time point. Furthermore, pain inhibition was enhanced in non-responders with the exogenous administration of a serotonin agonist.71 Yet, this effect was partially attenuated by concurrent administration of a GABA receptor type B antagonist. Increased serotonin levels may be attributable to a descending dorsolateral column pathway originating from the paragigantocellularis lateralis, the rostral raphe magnus, and the reticularis gigantocellularis pars alpha (Figures 1i, 2, and 4).71,72 Focusing on the descending serotonergic inhibitory mechanism, the Karolinska group further sought to characterize which specific serotoninergic receptor subtypes mediate pain inhibition by conventional spinal cord stimulation.73 They found that S-induced serotonin release mediated pain inhibition through multiple spinal serotonin receptors, including serotonin 2A, serotonin HT3, and serotonin HT4.73 Activation of each of these receptors had differing effects on heat, cold, and mechanical hypersensitivity.73
Using immunohistochemical methods, a separate group also examined the role of serotonin in descending inhibition; however, they looked at the dorsal raphe nucleus, another source of serotonin, in the ventral periaqueductal grey matter rather than the nucleus raphe magnus or rostral ventromedial medulla (Figures 2, 3, and 4).74 While additionally examining the role of the noradrenergic system via the locus coeruleus, they determined that spinal cord stimulation-induced antinociception is mediated by both serotonin and norepinephrine, with increased synthesis of these monoamines observed in the dorsal raphe nucleus and locus coeruleus, respectively (Figures 2, 3, and 4).74 In another study, the Saade team concurrently lesioned the dorsal columns and administered antagonists known to inhibit the effects of descending pain pathway activation.75 Pain inhibition by conventional spinal cord stimulation applied rostral to the lesion was partially attenuated by an adrenergic antagonist, and enhanced by an adrenergic agonist, suggesting that the supraspinal neurochemical mechanisms for spinal cord stimulation-induced pain inhibition at least partially involve the adrenergic system (Figure 1j).75 In a rat model, a comparison of 100, 60, and 4 hertz spinal cord stimulation indicated that only the 4 hertz frequency increased expression of neural activity indicator c-Fos in the nucleus raphe magnus.76 Contrary to the earlier c-Fos study, no changes in expression level were noted in the periaqueductal grey (Figure 2 and 3).76 Because the authors did not observe changes with 100 hertz stimulation in the supraspinal structures examined, they inferred that higher frequencies could alternatively mediate pain inhibition through spinal mechanisms.76
Conflicting results were noted by El-Khoury and colleagues in examination of spinal cord stimulation mechanisms with selective bilateral dorsal column lesioning.77 They showed that pain inhibition was maintained after dorsal column lesioning caudal to spinal cord stimulation, which was applied with the electrodes on the dorsal aspect of the medulla at the level of the dorsal column nuclei. As in previous studies, these results demonstrated a role of descending supraspinal inhibitory influences. However, they noted a possible additional role of the dorsal columns in ascending nociceptive signaling as the lesioning itself produced tardy development or temporary interruption of their neuropathic pain model, a phenomenon called “spinal shock,” which is frequently observed after manipulation in animal experiments (Figure 1g).77 Members of the same group followed this study with investigations into selective unilateral and bilateral spinal cord lesioning of the dorsolateral column and/or spinothalamic column (Figure 1h).78 Interruption of any combination of these tracts resulted in the attenuation of neuropathic pain, with thermal hyperalgesia most affected, tactile allodynia secondly, and cold allodynia least.78 The effects of these lesions were normalized within 2–3 weeks, illustrating the plasticity of the nervous system.78 These results oppose the hypothesis of supraspinal inhibitory influence of either tract because lesioning caused nociception attenuation, not facilitation.78
Building on previous lesioning studies, Saade and coworkers, in collaboration with the Karolinska group, applied spinal cord stimulation rostrally over the dorsal column nuclei or at the lumbar level. They similarly found attenuated spinal cord stimulation effects after dorsolateral column lesions, regardless of whether stimulation was applied rostral or caudal to the lesion (Figure 1k).60,78,79 This finding supports the notion of a dual role for supraspinal and spinal mechanisms, as some antinociceptive effect was preserved after quite extensive lesions. The investigators observed that the suppressive effect of spinal cord stimulation on cold hypersensitivity was eliminated with these lesions, suggesting that cold hypersensitivity is alleviated via a supraspinal mechanism. Yet, this observation conflicts with a previous study, which suggested that an antidromic dorsal column mechanism mediates spinal cord stimulation-induced suppression of cold hypersensitivity.75,79
Because the spinal cord has limited numbers of serotonergic cell bodies, a previous study chose to examine the rostral ventromedial medulla,80 which is known to be the main source of descending serotoninergic pathways (Figure 2, 3, and 4). The investigators conducted microelectrode recordings in the rostral ventromedial medulla and quantified the activity of the ON-cells, OFF-cells, “serotonin-like,” and neutral cells with spinal cord stimulation.80 When they compared spinal cord stimulation responders and non-responders, spinal cord stimulation selectively increased activity of the serotonin-like cells and OFF-cells (antinociceptive) in responders.80 Therefore, responsiveness to spinal cord stimulation may be related to variable properties of the rostral ventromedial medulla in each individual patient. Microinjection of a GABA receptor type A agonist, but not an opioid antagonist, into the rostral ventromedial medulla partially inhibited the spinal cord stimulation-induced activation of these cells, indicating possible GABAergic control that may be related to the periaqueductal grey.80 They also examined the role of the locus coeruleus in supraspinal descending inhibition by comparing locus coeruleus activation in spinal cord stimulation responders and non-responders.81 Although they noted a marked increase in activity of locus coeruleus neurons in spinal cord stimulation responders, noradrenergic concentration in the dorsal horn did not differ between groups, and neither adrenergic alpha 1–2 antagonists administered intrathecally, nor “silencing” by microinjection of lidocaine into the locus coeruleus, reversed spinal cord stimulation-induced pain inhibition.81 Therefore, they concluded that although there may be a supraspinal role (thalamus, periaqueductal grey, or rostral ventromedial medulla) for locus coeruleus neurons in spinal cord stimulation antinociception, it is not mediated by a direct descending spinal projection (Figure 2 and 3).81 Another study in rats showed that anodal and cathodal spinal cord stimulation parameters had differing effects on somatosensory evoked potentials, suggesting that supraspinal mechanisms may be differentially engaged, depending on spinal cord stimulation parameters.82,83
Recent Developments: Continued Expansion of Applications and Novel Waveforms
Spinal cord stimulation has been successfully applied for the treatment of neurologic disorders other than pain, when the disease generator has strong indices for a cerebral dysfunction, thereby strengthening the argument for a supraspinal site of action. Spinal cord stimulation in vegetative and minimally conscious states has been studied for many years in Japan without evoking much interest in the Western world.84 A particularly exciting new role for spinal cord stimulation has been in the treatment of movement disorders such as dystonia, tremor, multiple sclerosis, Parkinson’s disease, and painful legs and moving toes syndrome.85–91 Seminal work in 2009 revealed that spinal cord stimulation restored locomotion in both dopamine-depleted and 6-hydroxydopamine lesioned rat models of Parkinson’s disease.86 They hypothesized that spinal cord stimulation disrupts the pathologic, synchronous low-frequency, oscillatory local field potential and neuronal patterns that are characteristic of the dorsolateral striatum and primary motor cortex in Parkinson’s disease.86 This disruption occurs through spinal cord stimulation-induced activation of large cortical areas, which increases cortical and thalamic input to the striatum.86 Spinal cord stimulation improved motor function similarly in a non-human primate model of Parkinson’s disease concurrently with neuronal activity desynchronization in the corticobasal ganglia circuitry.91
A recent study revisited the potential of spinal cord stimulation to treat cerebral ischemia using radiotracer techniques to extrapolate flow.92 Removal of the superior cervical ganglion before stimulation did not attenuate stimulation-induced cerebral blood flow; however, profound attenuation occurred after spinal cord transection.92 Thus, the effects on cerebral blood flow were attributed to a spinal ascending pathway to central vasomotor centers rather than a direct spinal effect via the superior cervical ganglion.92
Burst Spinal Cord Stimulation
Clinical
The burst stimulation application of spinal cord stimulation described by De Ridder et al.64,93–95 has recently emerged as a waveform technology with a potential supraspinal mechanism. This modality employs bursts of five pulses, with an intraburst frequency of 500 hertz and a repetition frequency of 40 hertz.64 Burst delivery was similarly used in transcutaneous electrical nerve stimulation in the 1970s.96,97 The postulated supraspinal mechanism is based on electroencephalographic evidence from patients, which revealed activation of the dorsal anterior cingulate and dorsolateral prefrontal cortex.94 Because the dorsal anterior cingulate cortex was activated, investigators inferred that burst spinal cord stimulation additionally modulates the medial pain pathways ascending to these regions (via the mediodorsal and ventromedial nucleus of the thalamus), which mediate pain-related affect and attention.94,95 They acknowledged that this ascending pathway does not seem to involve the dorsal columns, as a recent study found that gracile nucleus activity is unaltered by burst spinal cord stimulation but markedly enhanced by conventional spinal cord stimulation parameters.95,98 Instead, they proposed that burst spinal cord stimulation modulates the activity of C fibers terminating on lamina I dorsal horn neurons. A recent multicenter, randomized, unblinded, crossover study examining burst stimulation interestingly found an improvement in affect along with pain relief that could also support a mechanism involving medial thalamic activity.99
Preclinical
Much of the preclinical work regarding burst spinal cord stimulation was carried out after the human studies by De Ridder and colleagues.64,93–95 The effects of burst and conventional spinal cord stimulation on neuronal activity in the lumbosacral spinal cord and gracile nucleus and visceromotor reflexes were compared in an animal model of neuropathic pain.98 From these findings, the investigators hypothesized that the absence of paresthesia reported in patients who receive burst spinal cord stimulation corresponded well with the lack of increasing spontaneous activity in neurons of the gracile nucleus found in their animal study.98 Burst spinal cord stimulation attenuated visceral nociception better than conventional spinal cord stimulation when they measured visceromotor reflexes responses to noxious colorectal distention.98 Because a component of visceromotor reflexes involves supraspinal center modulation, they surmised that additional investigation into these supraspinal sites might elucidate the spinal cord stimulation mechanisms.98 The parameters of burst spinal cord stimulation were studied by examining the effect of varying intra-burst frequency (pulse frequency), burst frequency, burst width, burst amplitude, and pulses per burst (pulse number) on neuronal activity in rats.100 The overall charge delivered to the spinal cord per burst, the integral of the current delivered with a single burst, positively correlated with increased efficacy of spinal cord stimulation.100 Efficacy was measured by a reduction of wide-dynamic range neuronal firing in rats, which was influenced by the parameters of pulse number, pulse width, and amplitude.100 They subsequently discovered that, unlike conventional spinal cord stimulation, burst spinal cord stimulation does not increase spinal GABA release, as they observed no GABA elevation in peripheral blood. Furthermore, the effect of burst spinal cord stimulation was not abolished by a GABA receptor type B antagonist that did attenuate the effect of conventional spinal cord stimulation.101
Paresthesia-free High Frequency Spinal Cord Stimulation
Clinical
Since 2010, much attention has been paid to high frequency spinal cord stimulation. The high frequency stimulation paradigm is typically not programmed (e.g., pulse width, amplitude) to produce paresthesia and has a frequency that is far beyond that of the endogenous central nervous system. Clinical trials have shown that this subparesthetic stimulation paradigm may be superior to conventional spinal cord stimulation for treating low-back and leg pain.8,63 Using higher frequencies requires that the amplitudes and pulse widths be low because paresthetic stimulation beyond 800 hertz may be perceived as uncomfortable by patients.102 High frequency spinal cord stimulation encompasses an arbitrary range of frequencies; however, the commonly accepted boundary appears to be any frequency over 1 kilohertz. An exact definition of high-frequency spinal cord stimulation may eventually be refined as the mechanism of this waveform is further elucidated.
Preclinical
Most preclinical work to date has indicated that the primary mechanism of pain inhibition may occur at the spinal/segmental level.103–108 A recent review of spinal cord stimulation summarized the working hypotheses that high frequency stimulation 1) induces depolarization blockade (which occurs if high-frequency stimulation is applied to a single peripheral nerve), 2) causes desynchronization of afferent neural signaling, and 3) causes “membrane integration” whereby each individual stimulus is inadequate to depolarize a neuron but multiple stimuli delivered over a length of time may cause depolarization.96,109–113 To date, these hypotheses have received no support from computer simulation studies or preclinical experiments.104,106 We found no published study that investigated a supraspinal mechanism of paresthesia-free high frequency spinal cord stimulation. However, recent work has suggested that the variable preclinical results, sustained clinical effectiveness, and paresthesia-free stimulation with different combinations of stimulation parameters warrant investigation into whether possible supraspinal mechanisms participate in the creation of a pain-relieving effect.10,96,114
Earlier studies of high frequency spinal cord stimulation in rat models showed that intensities below the paresthesia threshold have an inhibitory effect on stimulus-evoked pain and that this stimulation paradigm does not activate or block transmission in the dorsal columns.103,104 Furthermore, recent work has highlighted that a certain amount of electric charge transmission from the stimulator lead to the nervous tissue is essential for effect and that the waveform itself may not be critical.65,83 Unpublished findings from preclinical research conducted by McMahon et al. at King’s College London have not included participation of supraspinal mechanisms.115,116 [Our knowledge of these unpublished findings is restricted to: 1) low-intensity 10 kilohertz spinal cord stimulation (20% of motor threshold) in rat models of persistent pain does not alter the excitability of normal myelinated primary sensory neurons or dorsal column neurons, 2) this stimulation may inhibit ectopic firing, in that recordings from lamina I neurons in the dorsal horn have shown that an inhibitory effect appears after 90 minutes of continuous stimulation, and 3) 60 minutes of low-intensity 10 kilohertz spinal cord stimulation suppressed response of deep dorsal horn neurons to wind-up stimuli.]
CONCLUSION
Understanding the supraspinal mechanisms of spinal cord stimulation will not only have important implications for improving the clinical effectiveness of spinal cord stimulation for pain treatment, but also for extending the indications beyond pain inhibition. For example, neuropsychiatric disorders, memory, addiction, behavior, cognitive function, other neurologic diseases, and performance enhancement are all being explored as new targets for spinal cord stimulation. Knowledge of spinal cord stimulation mechanisms will also enhance our overall understanding of the multiple unique and specialized areas in the brain.117 The brain presents a challenge to researchers because invasive techniques require a high degree of skill, noninvasive imaging lacks spatial detail and temporal resolution, and in vivo experimentation with brain manipulation is problematic. Emerging technologies in neuroscience, such as single-cell RNA sequencing, optogenetics, dynamic imaging, and brain recording may help overcome these obstacles. Initial preclinical attention may focus on the superficially located structures of the brain, as these are more readily accessible for experimental investigation. In the clinic, transcranial magnetic stimulation may serve as a bridge to knowledge of what deep brain stimulation and spinal cord stimulation could achieve in the future.118 This appears to be the moment for a new discussion of the potential supraspinal influences of spinal cord stimulation.
Summary Statement.
This article reviews clinical and preclinical studies about supraspinal mechanisms of spinal cord stimulation. An understanding of the mechanisms underlying spinal cord stimulation will help clinicians and researchers to optimize stimulation paradigms for pain treatment.
Acknowledgments
Funding Statement
This study was subsidized by grants from the National Institutes of Health (Bethesda, Maryland, USA): NS070814 (Y.G.) and NS099879 (Y.G.), NS026363 (SR), a grant (Y.G) from Neurosurgery Pain Research Institute at Johns Hopkins University, and by a Stimulating and Advancing ACCM Research (StAAR) seed grant (E.S.) from the Department of Anesthesiology and Critical Care Medicine at Johns Hopkins University.
Footnotes
Clinical trial number and registry URL
Not applicable
Prior Presentations
Not applicable
Acknowledgments
Not applicable
Author contributions. E.S. and D.M. performed most of the literature research and were involved in preparing the figures and writing a draft manuscript. S.N.R. and B.L. were involved in the discussion of the outline and content of the review and contributed to manuscript writing. Y.G. oversaw the study and wrote the final manuscript with E.S. All authors were involved in the revision of the manuscript based on reviews of the initial draft.
Conflicts of Interest
Y.G. and S.R. received research grant support from Medtronic, Inc. B.L. is a consultant for Medtronic Inc., St Jude Medical, Boston Scientific, and Elekta AB. All other authors declare no competing interests.
References
- 1.Surgeons AAoN. Spinal cord stimulation. 2017 Sep 24; doi: 10.1038/sc.2016.81. Available from: http://www.aans.org/Patients/Neurosurgical-Conditions-and-Treatments/Spinal-Cord-Stimulation. [DOI] [PubMed]
- 2.Neuromodulation: Comprehensive textbook of principles, technologies, and therapies, Second Edition edition. Academic Press; 2018. [Google Scholar]
- 3.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg. 1967;46:489–491. [PubMed] [Google Scholar]
- 4.North RB, Kidd DH, Zahurak M, James CS, Long DM. Spinal cord stimulation for chronic, intractable pain: Experience over two decades. Neurosurgery. 1993;32:384–395. doi: 10.1227/00006123-199303000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Kumar K, North R, Taylor R, Sculpher M, den Abeele C, Gehring M, Jacques L, Eldabe S, Meglio M, Molet J. Spinal cord stimulation vs. Conventional medical management: A prospective, randomized, controlled, multicenter study of patients with failed back surgery syndrome (process study) Neuromodulation. 2005;8:213–218. doi: 10.1111/j.1525-1403.2005.00027.x. [DOI] [PubMed] [Google Scholar]
- 6.Liem L, Russo M, Huygen FJ, Buyten V, Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. 2015;18:41–49. doi: 10.1111/ner.12228. [DOI] [PubMed] [Google Scholar]
- 7.Russo M, Verrills P, Mitchell B, Salmon J, Barnard A, Santarelli D. High frequency spinal cord stimulation at 10 khz for the treatment of chronic pain: 6-month australian clinical experience. Pain physician. 2016;19:267–280. [PubMed] [Google Scholar]
- 8.Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, Amirdelfan K, Morgan DM, Brown LL, Yearwood TL. Novel 10-khz high-frequency therapy (hf10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg painthe senza-rct randomized controlled trial. Anesthesiology. 2015;123:851–860. doi: 10.1097/ALN.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 9.Buyten V, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: Results of a prospective multicenter european clinical study. Neuromodulation. 2013;16:59–66. doi: 10.1111/ner.12006. [DOI] [PubMed] [Google Scholar]
- 10.Al-Kaisy A, Buyten V, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10 khz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15:347–354. doi: 10.1111/pme.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schu S, Slotty PJ, Bara G, Knop M, Edgar D, Vesper J. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation. 2014;17:443–450. doi: 10.1111/ner.12197. [DOI] [PubMed] [Google Scholar]
- 12.Wille F, Breel JS, Bakker EW, Hollmann MW. Altering conventional to high density spinal cord stimulation: An energy dose-response relationship in neuropathic pain therapy. Neuromodulation. 2017;20:71–80. doi: 10.1111/ner.12529. [DOI] [PubMed] [Google Scholar]
- 13.Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210–216. doi: 10.1016/j.pain.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melzack R, Wall P. Pain mechanisms: A new theory. Science. 1965;150:971. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 15.Melzack R, Wall PD. Pain mechanisms: A new theory. Survey of Anesthesiology. 1967;11:89–90. [Google Scholar]
- 16.Randich ANT. Modulation of spinal nociceptive processing. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 17.Shealy CN, Taslitz N, Mortimer JT, Becker DP. Electrical inhibition of pain: Experimental evaluation. Anesth Analg. 1967;46:299–305. [PubMed] [Google Scholar]
- 18.Nashold B, Somjen G, Friedman H. Paresthesias and eeg potentials evoked by stimulation of the dorsal funiculi in man. Exp Neurol. 1972;36:273–287. doi: 10.1016/0014-4886(72)90023-4. [DOI] [PubMed] [Google Scholar]
- 19.Nashold BS, Jr, Friedman H. Dorsal column stimulation for control of pain: Preliminary report on 30 patients. J Neurosurg. 1972;36:590–597. doi: 10.3171/jns.1972.36.5.0590. [DOI] [PubMed] [Google Scholar]
- 20.Larson SJ, Sances A, Jr, Riegel DH, Meyer GA, Dallmann DE, Swiontek T. Neurophysiological effects of dorsal column stimulation in man and monkey. J Neurosurg. 1974;41:217–223. doi: 10.3171/jns.1974.41.2.0217. [DOI] [PubMed] [Google Scholar]
- 21.Bantli H, Bloedel JR, Thienprasit P. Supraspinal interactions resulting from experimental dorsal column stimulation. J Neurosurg. 1975;42:296–300. doi: 10.3171/jns.1975.42.3.0296. [DOI] [PubMed] [Google Scholar]
- 22.Foreman R, Beall J, Coulter J, Willis W. Effects of dorsal column stimulation on primate spinothalamic tract neurons. J Neurophysiol. 1976;39:534–546. doi: 10.1152/jn.1976.39.3.534. [DOI] [PubMed] [Google Scholar]
- 23.Lindblom U, Tapper DN, Wiesenfeld Z. The effect of dorsal column stimulation on the nociceptive response of dorsal horn cells and its relevance for pain suppression. Pain. 1977;4:133–144. doi: 10.1016/0304-3959(77)90127-0. [DOI] [PubMed] [Google Scholar]
- 24.Denny-Brown D, Kirk E, Yanagisawa N. The tract of lissauer in relation to sensory transmission in the dorsal horn of spinal cord in the macaque monkey. J Comp Neurol. 1973;151:175–199. doi: 10.1002/cne.901510206. [DOI] [PubMed] [Google Scholar]
- 25.Handwerker H, Iggo A, Zimmermann M. Segmental and supraspinal actions on dorsal horn neurons responding to noxious and non-noxious skin stimuli. Pain. 1975;1:147–165. doi: 10.1016/0304-3959(75)90099-8. [DOI] [PubMed] [Google Scholar]
- 26.Saadé NE, Tabet MS, Banna NR, Atweh SF, Jabbur SJ. Inhibition of nociceptive evoked activity in spinal neurons through a dorsal column-brainstem-spinal loop. Brain Res. 1985;339:115–118. doi: 10.1016/0006-8993(85)90627-4. [DOI] [PubMed] [Google Scholar]
- 27.Saade NE, Tabet MS, Soueidan SA, Bitar M, Atweh SF, Jabbur SJ. Supraspinal modulation of nociception in awake rats by stimulation of the dorsal column nuclei. Brain Res. 1986;369:307–310. doi: 10.1016/0006-8993(86)90540-8. [DOI] [PubMed] [Google Scholar]
- 28.Saadé NE, Salibi NA, Banna NR, Towe AL, Jabbur SJ. Spinal input pathways affecting the medullary gigantocellular reticular nucleus. Exp Neurol. 1983;80:582–600. doi: 10.1016/0014-4886(83)90309-6. [DOI] [PubMed] [Google Scholar]
- 29.Saade NE, Jundi AS, Jabbur SJ, Banna NR. Dorsal column input to inferior raphe centralis neurons. Brain Res. 1982;250:345–348. doi: 10.1016/0006-8993(82)90428-0. [DOI] [PubMed] [Google Scholar]
- 30.Jabbur S, Saadé N. Interactions between dorsal column and ventral spinal inputs in somatosensory thalamocortical areas and in various brainstem nuclei in the cat. In: Willis D, Rowe MJ, editors. Development, Organization ond Processing in Somatosensory Pathways. 1985. pp. 223–229. [Google Scholar]
- 31.Saade NE, Frangieh AS, Atweh SF, Jabbur SJ. Dorsal column input to cochlear neurons in decerebrate-decerebellate cats. Brain Res. 1989;486:399–402. doi: 10.1016/0006-8993(89)90532-5. [DOI] [PubMed] [Google Scholar]
- 32.Rees H, Roberts M. Antinociceptive effects of dorsal column stimulation in the rat: Involvement of the anterior pretectal nucleus. J Physiol. 1989;417:375–388. doi: 10.1113/jphysiol.1989.sp017807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rees H, Prado W, Rawlings S, Roberts M. The effects of intraperitoneal administration of antagonists and development of morphine tolerance on the antinociception induced by stimulating the anterior pretectal nucleus of the rat. Br J Pharmacol. 1987;92:769–779. doi: 10.1111/j.1476-5381.1987.tb11380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rees H, Roberts M. Anterior pretectal stimulation alters the responses of spinal dorsal horn neurones to cutaneous stimulation in the rat. J Physiol. 1987;385:415–436. doi: 10.1113/jphysiol.1987.sp016499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rees H, Roberts M, Sherwood C. Antinociceptive effects of micro injection of d,l-homocysteic acid into the anterior pretectal nucleus of the rat. J Physiol. 1987;394:103–103. [Google Scholar]
- 36.Rees H, Roberts M. Dorsal column inhibition of multireceptive dorsal horn neurons in the anesthesized rat. J Physiol. 1989;412:19–19. [Google Scholar]
- 37.Roberts M, Rees H. The antinociceptive effects of stimulating the pretectal nucleus of the rat. Pain. 1986;25:83–93. doi: 10.1016/0304-3959(86)90011-4. [DOI] [PubMed] [Google Scholar]
- 38.Visocchi M, Cioni B, Pentimalli L, Meglio M. Increase of cerebral blood flow and improvement of brain motor control following spinal cord stimulation in ischemic spastic hemiparesis. Stereotact Funct Neurosurg. 1994;62:103–107. doi: 10.1159/000098604. [DOI] [PubMed] [Google Scholar]
- 39.Visocchi M, Cioni B, Vergari S, Marano G, Pentimalli L, Meglio M. Spinal cord stimulation and cerebral blood flow: An experimental study. Stereotact Funct Neurosurg. 1994;62:186–190. doi: 10.1159/000098616. [DOI] [PubMed] [Google Scholar]
- 40.Hautvast RW, Horst GJ, DeJong BM, DeJongste MJ, Blanksma PK, Paans AM, Korf J. Relative changes in regional cerebral blood flow during spinal cord stimulation in patients with refractory angina pectoris. Eur J Neurosci. 1997;9:1178–1183. doi: 10.1111/j.1460-9568.1997.tb01472.x. [DOI] [PubMed] [Google Scholar]
- 41.Underwood MD, Iadecola C, Sved A, Reis D. Stimulation of c1 area neurons globally increases regional cerebral blood flow but not metabolism. J Cereb Blood Flow Metab. 1992;12:844–855. doi: 10.1038/jcbfm.1992.116. [DOI] [PubMed] [Google Scholar]
- 42.Hosobuchi Y. Treatment of cerebral ischemia with electrical stimulation of the cervical spinal cord. Pacing Clin Electrophysiol. 1991;14:122–126. doi: 10.1111/j.1540-8159.1991.tb04056.x. [DOI] [PubMed] [Google Scholar]
- 43.Hautvast RW, Blanksma PK, DeJongste MJ, Pruim J, van der Wall EE, Vaalburg W, Lie K. Effect of spinal cord stimulation on myocardial blood flow assessed by positron emission tomography in patients with refractory angina pectoris. Am J Cardiol. 1996;77:462–467. doi: 10.1016/s0002-9149(97)89338-1. [DOI] [PubMed] [Google Scholar]
- 44.Sagher O, Huang D-L. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg Spine. 2000;93:71–76. doi: 10.3171/spi.2000.93.1.0071. [DOI] [PubMed] [Google Scholar]
- 45.Nakai M, Iadecola C, Reis DJ. Global cerebral vasodilation by stimulation of rat fastigial cerebellar nucleus. Am J Physiol. 1982;243:H226–H235. doi: 10.1152/ajpheart.1982.243.2.H226. [DOI] [PubMed] [Google Scholar]
- 46.Linderoth B, Gazelius B, Franck J, Brodin E. Release of neuromediators in cat dorsal horn by dorsal column stimulation: Studies using microdialysis. Pain. 1990;41:S228. [Google Scholar]
- 47.Stiller C-O, Linderoth B, O’Connor WT, Franck J, Falkenberg T, Ungerstedt U, Brodin E. Repeated spinal cord stimulation decreases the extracellular level of γ-aminobutyric acid in the periaqueductal gray matter of freely moving rats. Brain Res. 1995;699:231–241. doi: 10.1016/0006-8993(95)00911-9. [DOI] [PubMed] [Google Scholar]
- 48.Linderoth B, Stiller C-O, O’Connor W, Hammarström G, Ungerstedt U, Brodin E. An animal model for the study of brain transmittor release in response to spinal cord stimulation in the awake, freely moving rat: Preliminary results from the periaqueductal grey matter. Springer; 1993. [DOI] [PubMed] [Google Scholar]
- 49.Linderoth B, Stiller C, Gunasekera L, O’connor W, Franck J, Gazelius B, Brodin E. Release of neurotransmitters in the cns by spinal cord stimulation: Survey of present state of knowledge and recent experimental studies. Stereotact Funct Neurosurg. 1993;61:157–170. doi: 10.1159/000100634. [DOI] [PubMed] [Google Scholar]
- 50.Cui J-G, O’Connor TW, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a gabaergic mechanism. Pain. 1997;73:87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 51.Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance p in the cat dorsal horn. Neurosurgery. 1992;31:289–297. doi: 10.1227/00006123-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 52.DeJongste MJ, Hautvast RW, Ruiters MH, Ter Horst GJ. Spinal cord stimulation and the induction of c-fos and heat shock protein 72 in the central nervous system of rats. Neuromodulation. 1998;1:73–84. doi: 10.1111/j.1525-1403.1998.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 53.Appleyard SM. Lighting up neuronal pathways: The development of a novel transgenic rat that identifies fos-activated neurons using a red fluorescent protein. Endocrinology. 2009;150:5199–5201. doi: 10.1210/en.2009-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji R-R, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of erk in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999:2. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 55.Gao Y-J, Ji R-R. C-fos and perk, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Extracellular signal-regulated kinase (erk) and protein kinase b (akt) pathways involved in spinal cord stimulation (scs)-induced vasodilation. Brain Res. 2008;1207:73–83. doi: 10.1016/j.brainres.2007.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saadé NE, Atweh SF, Tabet MS, Jabbur SJ. Inhibition of nociceptive withdrawal flexion reflexes through a dorsal column-brainstem-spinal loop. Brain Res. 1985;335:306–308. doi: 10.1016/0006-8993(85)90482-2. [DOI] [PubMed] [Google Scholar]
- 58.Ren B, Linderoth B, Meyerson BA. Effects of spinal cord stimulation on the flexor reflex and involvement of supraspinal mechanisms: An experimental study in mononeuropathic rats. J Neurosurg. 1996;84:244–249. doi: 10.3171/jns.1996.84.2.0244. [DOI] [PubMed] [Google Scholar]
- 59.Meyerson B, Ren B, Herregodts P, Linderoth B. Spinal cord stimulation in animal models of mononeuropathy: Effects on the withdrawal response and the flexor reflex. Pain. 1995;61:229–243. doi: 10.1016/0304-3959(94)00171-A. [DOI] [PubMed] [Google Scholar]
- 60.Yakhnitsa V, Linderoth B, Meyerson B. Modulation of dorsal horn neuronal activity by spinal cord stimulation in a rat model of neuropathy: The role of the dorsal funicles. Neurophysiology. 1998;30:424–427. [Google Scholar]
- 61.Saadé NE, Atweh SF, Privat A, Jabbur SJ. Inhibitory effects from various types of dorsal column and raphe magnus stimulations on nociceptive withdrawal flexion reflexes. Brain Res. 1999;846:72–86. doi: 10.1016/s0006-8993(99)02003-x. [DOI] [PubMed] [Google Scholar]
- 62.Linderoth B, Foreman RD. Physiology of spinal cord stimulation: Review and update. Neuromodulation. 1999;2:150–164. doi: 10.1046/j.1525-1403.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 63.Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, Amirdelfan K, Morgan DM, Yearwood TL, Bundschu R. Comparison of 10-khz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79:667–677. doi: 10.1227/NEU.0000000000001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation: Toward paresthesia-free pain suppression. Neurosurgery. 2010;66:986–990. doi: 10.1227/01.NEU.0000368153.44883.B3. [DOI] [PubMed] [Google Scholar]
- 65.Song Z, Meyerson BA, Linderoth B. High-frequency (1 khz) spinal cord stimulation—is pulse shape crucial for the efficacy? A pilot study. Neuromodulation. 2015;18:714–720. doi: 10.1111/ner.12344. [DOI] [PubMed] [Google Scholar]
- 66.Poláček H, Kozák J, Vrba I, Vrána J, Stančák A. Effects of spinal cord stimulation on the cortical somatosensory evoked potentials in failed back surgery syndrome patients. Clin Neurophysiol. 2007;118:1291–1302. doi: 10.1016/j.clinph.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 67.de Andrade DC, Bendib B, Hattou M, Keravel Y, Nguyen J-P, Lefaucheur J-P. Neurophysiological assessment of spinal cord stimulation in failed back surgery syndrome. Pain. 2010;150:485–491. doi: 10.1016/j.pain.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Moens M, Mariën P, Brouns R, Poelaert J, De Smedt A, Buyl R, Droogmans S, Van Schuerbeek P, Sunaert S, Nuttin B. Spinal cord stimulation modulates cerebral neurobiology: A proton magnetic resonance spectroscopy study. Neuroradiology. 2013;55:1039–1047. doi: 10.1007/s00234-013-1200-7. [DOI] [PubMed] [Google Scholar]
- 69.Kishima H, Saitoh Y, Oshino S, Hosomi K, Ali M, Maruo T, Hirata M, Goto T, Yanagisawa T, Sumitani M. Modulation of neuronal activity after spinal cord stimulation for neuropathic pain; H215O PET study. Neuroimage. 2010:2564–2569. doi: 10.1016/j.neuroimage.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 70.Bentley L, Duarte R, Furlong P, Ashford RL, Raphael JH. Brain activity modifications following spinal cord stimulation for chronic neuropathic pain: A systematic review. Eur J Pain. 2016;20:499–511. doi: 10.1002/ejp.782. [DOI] [PubMed] [Google Scholar]
- 71.Song Z, Ultenius C, Meyerson BA, Linderoth B. Pain relief by spinal cord stimulation involves serotonergic mechanisms: An experimental study in a rat model of mononeuropathy. Pain. 2009;147:241–248. doi: 10.1016/j.pain.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 72.Zhuo M, Gebhart G. Characterization of descending facilitation and inhibition of spinal nociceptive transmission from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. J Neurophysiol. 1992;67:1599–1614. doi: 10.1152/jn.1992.67.6.1599. [DOI] [PubMed] [Google Scholar]
- 73.Song Z, Meyerson BA, Linderoth B. Spinal 5-ht receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain. 2011;152:1666–1673. doi: 10.1016/j.pain.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Tazawa T, Kamiya Y, Kobayashi A, Saeki K, Takiguchi M, Nakahashi Y, Shinbori H, Funakoshi K, Goto T. Spinal cord stimulation modulates supraspinal centers of the descending antinociceptive system in rats with unilateral spinal nerve injury. Mol Pain. 2015;11:36. doi: 10.1186/s12990-015-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barchini J, Tchachaghian S, Shamaa Fa, Jabbur S, Meyerson B, Song Z, Linderoth B, Saade N. Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: An experimental study in a rat model of neuropathy. Neuroscience. 2012;215:196–208. doi: 10.1016/j.neuroscience.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 76.Maeda Y, Ikeuchi M, Wacnik P, Sluka KA. Increased c-fos immunoreactivity in the spinal cord and brain following spinal cord stimulation is frequency-dependent. Brain Res. 2009;1259:40–50. doi: 10.1016/j.brainres.2008.12.060. [DOI] [PubMed] [Google Scholar]
- 77.El-Khoury C, Hawwa N, Baliki M, Atweh S, Jabbur S, Saade N. Attenuation of neuropathic pain by segmental and supraspinal activation of the dorsal column system in awake rats. Neuroscience. 2002;112:541–553. doi: 10.1016/s0306-4522(02)00111-2. [DOI] [PubMed] [Google Scholar]
- 78.Saadé NE, Al Amin H, Chalouhi S, Baki SA, Jabbur SJ, Atweh SF. Spinal pathways involved in supraspinal modulation of neuropathic manifestations in rats. Pain. 2006;126:280–293. doi: 10.1016/j.pain.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 79.Saadé N, Barchini J, Tchachaghian S, Chamaa F, Jabbur S, Song Z, Meyerson B, Linderoth B. The role of the dorsolateral funiculi in the pain relieving effect of spinal cord stimulation: A study in a rat model of neuropathic pain. Exp Brain Res. 2015;233:1041–1052. doi: 10.1007/s00221-014-4180-x. [DOI] [PubMed] [Google Scholar]
- 80.Song Z, Ansah O, Meyerson B, Pertovaara A, Linderoth B. The rostroventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain. Neuroscience. 2013;247:134–144. doi: 10.1016/j.neuroscience.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 81.Song Z, Ansah O, Meyerson B, Pertovaara A, Linderoth B. Exploration of supraspinal mechanisms in effects of spinal cord stimulation: Role of the locus coeruleus. Neuroscience. 2013;253:426–434. doi: 10.1016/j.neuroscience.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 82.Aguilar J, Pulecchi F, Dilena R, Oliviero A, Priori A, Foffani G. Spinal direct current stimulation modulates the activity of gracile nucleus and primary somatosensory cortex in anaesthetized rats. J Physiol. 2011;589:4981–4996. doi: 10.1113/jphysiol.2011.214189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: A review. Neuromodulation. 2016;19:373–384. doi: 10.1111/ner.12438. [DOI] [PubMed] [Google Scholar]
- 84.Yamamoto T, Watanabe M, Obuchi T, Kobayashi K, Oshima H, Fukaya C, Yoshino A. Trends in reconstructive neurosurgery. Springer; 2017. Spinal cord stimulation for vegetative state and minimally conscious state: Changes in consciousness level and motor function; pp. 37–42. [DOI] [PubMed] [Google Scholar]
- 85.Yadav AP, Nicolelis MA. Electrical stimulation of the dorsal columns of the spinal cord for parkinson’s disease. Mov Disord. 2017 doi: 10.1002/mds.27033. [DOI] [PubMed] [Google Scholar]
- 86.Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA. Spinal cord stimulation restores locomotion in animal models of parkinson’s disease. Science. 2009;323:1578–1582. doi: 10.1126/science.1164901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krauss J, Weigel R, Blahak C, Bäzner H, Capelle H, Grips E, Rittmann M, Wöhrle J. Chronic spinal cord stimulation in medically intractable orthostatic tremor. J Neurol Neurosurg Psychiatry. 2006;77:1013–1016. doi: 10.1136/jnnp.2005.086132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abboud H, Hill E, Siddiqui J, Serra A, Walter B. Neuromodulation in multiple sclerosis. Mult Scler. 2017;23:1663–1676. doi: 10.1177/1352458517736150. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi H, Saitoh C, Iwata O, Nanbu T, Takada S, Morita S. Epidural spinal cord stimulation for the treatment of painful legs and moving toes syndrome. Pain. 2002;96:343–345. doi: 10.1016/S0304-3959(01)00479-1. [DOI] [PubMed] [Google Scholar]
- 90.Raina GB, Piedimonte F, Micheli F. Posterior spinal cord stimulation in a case of painful legs and moving toes. Stereotact Funct Neurosurg. 2007;85:307–309. doi: 10.1159/000107366. [DOI] [PubMed] [Google Scholar]
- 91.Santana MB, Halje P, Simplício H, Richter U, Freire MAM, Petersson P, Fuentes R, Nicolelis MA. Spinal cord stimulation alleviates motor deficits in a primate model of parkinson disease. Neuron. 2014;84:716–722. doi: 10.1016/j.neuron.2014.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel S, Huang D-L, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery. 2004;55:201–206. doi: 10.1227/01.neu.0000126949.28912.71. [DOI] [PubMed] [Google Scholar]
- 93.De Ridder D, Lenders MW, De Vos CC, Dijkstra-Scholten C, Wolters R, Vancamp T, Van Looy P, Van Havenbergh T, Vanneste S. A 2-center comparative study on tonic versus burst spinal cord stimulation: Amount of responders and amount of pain suppression. Clin J Pain. 2015;31:433–437. doi: 10.1097/AJP.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 94.De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013;80:642–649.e1. doi: 10.1016/j.wneu.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 95.De Ridder D, Vanneste S. Burst and tonic spinal cord stimulation: Different and common brain mechanisms. Neuromodulation. 2016;19:47–59. doi: 10.1111/ner.12368. [DOI] [PubMed] [Google Scholar]
- 96.Linderoth B, Foreman RD. Conventional and novel spinal stimulation algorithms: Hypothetical mechanisms of action and comments on outcomes. Neuromodulation. 2017 doi: 10.1111/ner.12624. [DOI] [PubMed] [Google Scholar]
- 97.Eriksson MB, Sjölund BH, Nielzén S. Long term results of peripheral conditioning stimulation as an analgesic measure in chronic pain. Pain. 1979;6:335–347. doi: 10.1016/0304-3959(79)90052-6. [DOI] [PubMed] [Google Scholar]
- 98.Tang R, Martinez M, Goodman-Keiser M, Farber JP, Qin C, Foreman RD. Comparison of burst and tonic spinal cord stimulation on spinal neural processing in an animal model. Neuromodulation. 2014;17:143–151. doi: 10.1111/ner.12117. [DOI] [PubMed] [Google Scholar]
- 99.Deer T, Slavin KV, Amirdelfan K, North RB, Burton AW, Yearwood TL, Tavel E, Staats P, Falowski S, Pope J. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018:56–66. doi: 10.1111/ner.12698. [DOI] [PubMed] [Google Scholar]
- 100.Crosby ND, Goodman Keiser MD, Smith JR, Zeeman ME, Winkelstein BA. Stimulation parameters define the effectiveness of burst spinal cord stimulation in a rat model of neuropathic pain. Neuromodulation. 2015;18:1–8. doi: 10.1111/ner.12221. [DOI] [PubMed] [Google Scholar]
- 101.Crosby ND, Weisshaar CL, Smith JR, Zeeman ME, Goodman-Keiser MD, Winkelstein BA. Burst and tonic spinal cord stimulation differentially activate gabaergic mechanisms to attenuate pain in a rat model of cervical radiculopathy. IEEE Trans Biomed Eng. 2015;62:1604–1613. doi: 10.1109/TBME.2015.2399374. [DOI] [PubMed] [Google Scholar]
- 102.Abejón D, Rueda P, Vallejo R. Threshold evolution as an analysis of the different pulse frequencies in rechargeable systems for spinal cord stimulation. Neuromodulation. 2016;19:276–282. doi: 10.1111/ner.12401. [DOI] [PubMed] [Google Scholar]
- 103.Shechter R, Yang F, Xu Q, Cheong Y-K, He S-Q, Sdrulla A, Carteret AF, Wacnik PW, Dong X, Meyer RA. Conventional and kilohertz-frequency spinal cord stimulation produces intensity-and frequency-dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology. 2013;119:422–432. doi: 10.1097/ALN.0b013e31829bd9e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Song Z, Viisanen H, Meyerson BA, Pertovaara A, Linderoth B. Efficacy of kilohertz-frequency and conventional spinal cord stimulation in rat models of different pain conditions. Neuromodulation. 2014;17:226–235. doi: 10.1111/ner.12161. [DOI] [PubMed] [Google Scholar]
- 105.Arle JE, Mei L, Carlson KW, Shils JL. High-frequency stimulation of dorsal column axons: Potential underlying mechanism of paresthesia-free neuropathic pain relief. Neuromodulation. 2016;19:385–397. doi: 10.1111/ner.12436. [DOI] [PubMed] [Google Scholar]
- 106.Lempka SF, McIntyre CC, Kilgore KL, Machado AG. Computational analysis of kilohertz frequency spinal cord stimulation for chronic pain management. Anesthesiology. 2015;122:1362–1376. doi: 10.1097/ALN.0000000000000649. [DOI] [PubMed] [Google Scholar]
- 107.Crosby ND, Janik JJ, Grill WM. Modulation of activity and conduction in single dorsal column axons by kilohertz-frequency spinal cord stimulation. J Neurophysiol. 2017;117:136–147. doi: 10.1152/jn.00701.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cuellar JM, Alataris K, Walker A, Yeomans DC, Antognini JF. Effect of high-frequency alternating current on spinal afferent nociceptive transmission. Neuromodulation. 2013;16:318–327. doi: 10.1111/ner.12015. [DOI] [PubMed] [Google Scholar]
- 109.Kilgore KL, Bhadra N. Nerve conduction block utilising high-frequency alternating current. Med Biol Eng Comput. 2004;42:394–406. doi: 10.1007/BF02344716. [DOI] [PubMed] [Google Scholar]
- 110.Kilgore KL, Bhadra N. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation. 2014;17:242–255. doi: 10.1111/ner.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Litvak LM, Smith ZM, Delgutte B, Eddington DK. Desynchronization of electrically evoked auditory-nerve activity by high-frequency pulse trains of long duration. J Acoust Soc Am. 2003;114:2066–2078. doi: 10.1121/1.1612492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rubinstein J, Wilson B, Finley C, Abbas P. Pseudospontaneous activity: Stochastic independence of auditory nerve fibers with electrical stimulation. Hear Res. 1999;127:108–118. doi: 10.1016/s0378-5955(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 113.Reilly JP, Freeman VT, Larkin WD. Sensory effects of transient electrical stimulation-evaluation with a neuroelectric model. IEEE Trans Biomed Eng. 1985:1001–1011. doi: 10.1109/TBME.1985.325509. [DOI] [PubMed] [Google Scholar]
- 114.Chakravarthy K, Richter H, Christo PJ, Williams K, Guan Y. Spinal cord stimulation for treating chronic pain: Reviewing preclinical and clinical data on paresthesia-free high-frequency therapy. Neuromodulation. 2017 doi: 10.1111/ner.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McMahon S. Effect of different frequencies of spinal cord stimulation on pain-model rodent superficial dorsal horn neuronal excitability. North American Neuromodulation Society’s 21st Annual Meeting; Las Vegas, Nevada, USA. 2018. [Google Scholar]
- 116.McMahon S, Jones M, Lee D, Bradley K. Effects of 10khz spinal cord stimulation on pain-model rodent deep dorsal horn neuronal excitability. North American Neuromodulation Society’s 21st Annual Meeting; Las Vegas, NV, USA. 2018. [Google Scholar]
- 117.Lozano AM, Hutchison WD, Kalia SK. What have we learned about movement disorders from functional neurosurgery? Annu Rev Neurosci. 2017;40:453–477. doi: 10.1146/annurev-neuro-070815-013906. [DOI] [PubMed] [Google Scholar]
- 118.Doñamayor N, Baek K, Voon V. Distal functional connectivity of known and emerging cortical targets for therapeutic noninvasive stimulation. Cereb Cortex. 2017:1–14. doi: 10.1093/cercor/bhx331. [DOI] [PubMed] [Google Scholar]